Abstract
Background
A standardized protocol is used to administer recombinant human thyrotropin (rhTSH) in preparation for diagnostic studies and treatment in patients with thyroid cancer. The expectation is that serum TSH concentrations will peak on the day after the second injection and will be sufficiently elevated to stimulate uptake of radioiodine. We wished to test the hypothesis that TSH concentrations achieved after rhTSH injection are influenced by age.
Methods
Patients with thyroid cancer undergoing diagnostic radioiodine scanning were identified by chart review. Serum TSH concentrations were documented 24 and 72 hours after two rhTSH injections (days 3 and 5, respectively). Responses were subdivided into four ascending patient age groups: <35, 35–49, 50–64, and >64 years. TSH concentrations after rhTSH administration were documented according to patient age.
Results
There was a significant correlation between the serum TSH concentrations at both days 3 and 5 and patient age (p < 0.0001). None of the other factors examined (gender, menopausal status, weight, body mass index, baseline TSH, serum creatinine, and estimated glomerular filtration rate) were significant in multivariate analyses. The mean TSH concentration on day 3 increased significantly when patients were divided into the aforementioned groups of ascending age (96, 107, 142, and 196 mIU/L, p < 0.0001). Day 5 concentrations increased in a similar manner.
Conclusions
Both days 3 and 5 TSH concentrations were higher in older individuals after rhTSH administration. This finding did not appear to be related to body weight, body mass index, or glomerular filtration rate in a simple manner. The TSH concentration achieved may be a result of complex interactions between distribution within fat and muscle body compartments, hepatic function, and renal function. Prospective studies could examine whether the magnitude of the TSH elevation after rhTSH administration affects diagnostic or therapeutic efficacy.
Introduction
Use of recombinant human thyrotropin (rhTSH) has become an integral part of the management of differentiated thyroid cancer (1–3). After intramuscular administration of 0.9 mg of rhTSH on 2 successive days (designated as days 1 and 2), serum TSH concentrations generally peak during the next 24 hours on day 3 (4,5). Serum TSH levels usually have fallen considerably by day 5. After rhTSH administration, radioiodine can be administered for either diagnostic or therapeutic purposes. Radioiodine is usually given on day 3 (6–10), although other timing protocols are sometimes employed, particularly if both diagnosis and treatment are planned with a single series of rhTSH injections (11,12). In addition, rhTSH-stimulated serum thyroglobulin levels have diagnostic significance (8,13–15). Serum thyroglobulin is generally measured on day 5 in diagnostic protocols (8,13,14).
Three previous studies, all conducted in Italy, have examined the serum TSH concentrations achieved after rhTSH administration as a function of patient characteristics. A 2003 study of 112 patients showed that day 3 TSH concentrations were affected by body weight, body mass index (BMI), and body surface area (BSA) in univariate analyses (16). Only BSA remained significant in multivariate analyses, with an inverse relationship between BSA and TSH. Patient age did not influence day 3 TSH values in either univariate or multivariate analyses. In another study of 105 patients day 3 TSH concentrations were similarly not impacted by age, but were lower in men than women (17). However, in multivariate analyses only lean body mass was independently associated with peak TSH concentrations, which were reached on day 3 in 94% of patients. These investigators also found that the area under the TSH curve was independently associated with lean body mass. A study performed in a population of 311 patients showed that day 5 TSH levels were affected by age, with the magnitude of the logarithmically transformed TSH value increasing with increasing age (18). No correlations between BMI, BSA, or glomerular filtration rate (GFR) and TSH levels were identified. In the same study day 5 TSH concentrations were higher in women than in men. The age of the two gender groups was not reported, but analyses showed that age and gender were independent predictors of the log-transformed TSH. This study did not report day 3 TSH concentrations. The study methodology did not describe whether day 3 TSH levels were analyzed and not found to be significant, or whether these data were either not collected or not subject to analysis. Consolidating the results of these three studies, it appears that serum TSH concentrations after administration of rhTSH are affected by demographic and anthropometric parameters, but is not clear whether the effect is mediated solely by body composition or whether other parameters are also important. The authors are not aware of any data previously reported by the manufacturers of rhTSH that would serve to clarify theses issues.
Patients with differentiated thyroid cancer undergoing rhTSH-assisted diagnostic studies at Washington Hospital Center typically have full laboratory testing during the week of their evaluation. Available laboratory data generally include serum creatinine, days 3 and 5 TSH concentrations, and day 5 thyroglobulin levels. In addition, complete anthropometric data are available for most patients. We conducted a retrospective analysis of these patients to determine whether TSH concentrations achieved on days 3 and 5 were associated with age, gender, body composition, or renal function.
Methods
Patient data
A group of 112 patients was obtained through chart review after approval of the project by the Institutional Review Board. The review included medical records available from 2003 through 2009 at Washington Hospital Center. A search was conducted for all patients with a diagnosis of thyroid cancer. The paper and electronic records of these patients were reviewed to determine if the patient had undergone a diagnostic whole body scan using rhTSH stimulation. If they had, their records were carefully reviewed to determine if sufficient data had been collected to satisfy the study requirements. In addition to the requirement for documentation of serum TSH levels 24 and 72 hours after rhTSH administration, corresponding to days 3 and 5 of the rhTSH protocol, patients were also required to have a serum creatinine concentration documented within 10 days of the diagnostic protocol. Complete demographic and anthropometric parameters were also required. Any patients without the required data were excluded. Patients receiving rhTSH for remnant ablation were also excluded to ensure that body weight or serum creatinine had not been transiently affected by recent hospitalization for thyroidectomy and recent initiation of levothyroxine therapy.
Patients with all stages of thyroid cancer were included. Although patients with metastases were included, to our knowledge, no patient had functioning metastases that were secreting thyroid hormone. All patients continued to receive their prescribed levothyroxine therapy. The following data were collected from patient records: patient age, gender, height, weight, BMI, menopausal status, mean TSH value during the preceding year, baseline (day 1) TSH value, TSH concentration on days 3 and 5, serum creatinine concentration obtained within 10 days of TSH determination, clinical laboratory utilized, year of protocol, month of protocol, thyroglobulin concentration on day 5, thyroglobulin antibody titer on day 5, presence of distant metastases, presence of residual neck uptake, type of differentiated thyroid cancer, years elapsed since thyroidectomy, and years elapsed since last radioiodine therapy.
Laboratory assessments and GFR estimation
Laboratory testing was performed by the clinical laboratory designated by the patient's insurance company. TSH was analyzed by Quest Diagnostics, LabCorp, or Washington Hospital Center Laboratory. TSH assays used by these clinical laboratories employed a third-generation ultra-sensitive immunochemiluminometric assay with a sensitivity of 0.01 mIU/L (laboratory reference ranges ∼0.4–4.5 mIU/L). GFR was estimated using the abbreviated Modification of Diet in Renal Disease study equation [GFR = 186 × Serum creatinine−1.154 × Age−0.203 × Sex × Race] and reported in mL/min/1.73 m2) (19,20). Sex adjustments were 1 for men and 0.742 for women; adjustments for race were 1.21 for black individuals and 1 for nonblack individuals.
Statistical analysis
For pairs of continuous variables, scatter-plots were constructed to visually display the respective relations. If found to be linear with both variables normally distributed, then the Pearson correlation was computed to statistically describe the significance, strength, and nature of the relation. If the assumption of linearity was violated, then the Spearman rank correlation was used instead.
An independent two samples t-test was used to examine the existence of a statistically significant difference between the means of two groups. The independent two samples t-test assumed that the variable being tested was normally distributed in each of the two independent groups. When the normality assumption was violated in either or both groups, the Wilcoxon-Mann-Whitney test was used.
A one-way analysis of variance was used to examine the difference in means of normally distributed variables between groups of three or more. When the normality assumption was violated, the Kruskal–Wallis test was used. The values of the variables examined were subsequently ranked. A multifactorial analysis of variance was then used to examine statistically significant differences among the ranks of the study groups. The analysis of covariance was used to test for statistically significant differences in the slopes of multiple regression lines. A multiple linear regression was used to model the relation between the dependent variable and a varying combination of predictor variable. SAS 9.1.3 was used for all analyses.
Results
The characteristics of the patient group are shown in Tables 1 and 2. The identified patients had a mean age of 49 years and were 77% women. Their mean BMI was 29. Thirty-nine percent of patients were overweight and 35% were obese. The mean baseline TSH before rhTSH administration was 0.35 mIU/L. The mean TSH concentration on day 3 was 128 mIU/L, and had declined to a mean of 16 mIU/L on day 5. The mean serum creatinine was 0.7 mg/dL; the mean estimated GFR was 111 mL/min/1.73 m2). The median thyroglobulin concentration after rhTSH stimulation was undetectable. Eighteen percent of patients had thyroglobulin antibodies and 17% had distant metastases. Nine percent had residual thyroid bed uptake, with a fractional uptake of <0.1% in most cases. The mean time elapsed since patients had undergone thyroidectomy and received radioiodine therapy were 5 and 3 years, respectively, as some patients had received treatment with radioiodine on more than one occasion.
Table 1.
Demographic and Laboratory Characteristics of Patients Receiving Recombinant Human Thyrotropin for Diagnostic Radioiodine Procedures (Number of Patients = 112)
| Characteristic | Mean (SD) | Percentage |
|---|---|---|
| Age (years) | 49 (13) | |
| Gender | ||
| Female | — | 77 |
| Male | — | 23 |
| Height (m) | 1.67 (0.09) | |
| Weight (kg) | 80 (19) | |
| BMI (kg/m2) | 29 (6) | |
| <25 | — | 26 |
| ≥25 to <30 | — | 39 |
| ≥30 | — | 35 |
| Menopausal status | ||
| Premenopausal | — | 45 |
| Postmenopausal | — | 32 |
| N/a | — | 23 |
| Serum creatinine (mg/dL) | ||
| All ages | 0.7 (0.14) | |
| <35 years | 0.6 (0.09) | |
| 35–49 years | 0.7 (0.12) | |
| 50–64 years | 0.7 (0.15) | |
| >64 years | 0.8 (0.14) | |
| Glomerular filtration rate (mL/min/1.73 m2) | ||
| All ages | 111 (23) | |
| <35 years | 139 (21) | |
| 35–49 years | 115 (21) | |
| 50–64 years | 103 (17) | |
| >64 years | 91 (11) | |
BMI, body mass index; SD, standard deviation; n/a, not applicable.
Table 2.
Thyroid Cancer-Related Characteristics of Patients Receiving Recombinant Human Thyrotropin for Diagnostic Radioiodine Procedures (Number of Patients = 112)
| Characteristic | Mean (SD) | Percentage |
|---|---|---|
| Mean TSH (within last 12 months) | ||
| All ages | 0.34 (0.81) | |
| <35 years | 0.17 (0.53) | |
| 35–49 years | 0.25 (0.64) | |
| 50–64 years | 0.31 (0.57) | |
| >64 years | 0.55 (1.1) | |
| Day 1 TSH (mIU/L) | ||
| All ages | 0.35 (0.45) | |
| <35 years | 0.48 (0.55) | |
| 35–49 years | 0.37 (0.52) | |
| 50–64 years | 0.28 (0.33) | |
| >64 years | 0.34 (0.27) | |
| Day 3 TSH (mIU/L) | 128 (58) | |
| Day 5 TSH (mIU/L) | 16 (11) | |
| Thyroglobulin (ng/mL) | 181.6 (1260) median 0 | |
| Presence of thyroglobulin antibodies | ||
| No | — | 82 |
| Yes | — | 18 |
| Distant metastases | ||
| No | — | 83 |
| Yes | — | 17 |
| <35 years | — | 0.9 |
| 35–49 years | — | 4.5 |
| 50–64 years | — | 7.1 |
| >64 years | — | 4.5 |
| Presence of residual neck uptake | ||
| Yes | — | 9 |
| No | — | 91 |
| <35 years | — | 1.7 |
| 35–49 years | — | 2.7 |
| 50–64 years | — | 2.7 |
| >64 years | — | 1.7 |
| Type of differentiated thyroid cancer | ||
| Papillary | — | 78 |
| Follicular | — | 14 |
| Other variant | — | 8 |
| Years elapsed since thyroidectomy | 5 (6) | |
| Years elapsed since last radioiodine therapy | 3 (3) | |
TSH, thyrotropin.
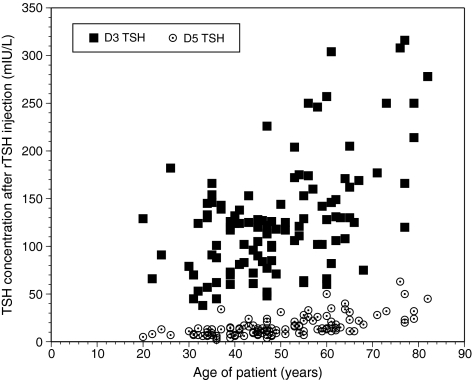
There was a statistically significant positive correlation between TSH values on day 3 and the age of the patient (Spearman correlation: coefficient = 0.433, p-value <0.0001) (see Table 3). The slope of the relationship was 2.2 ± 0.348 (see Fig. 1). There was a 1.95 mIU/L increase in day 3 TSH concentration for every year increase in age. There was also a statistically significant positive correlation between the TSH values on day 5 and patient age (Spearman correlation: coefficient = 0.635, p-value <0.0001) (see Table 3). The slope of the relationship was 0.505 ± 0.348 (see Fig. 1).
Table 3.
Thyrotropin Values, Difference in Thyrotropin Values, Mean Drop in Thyrotropin Per Hour, and Mean Percentage Changes in Thyrotropin Values on Days 3 and 5 After Recombinant Human Thyrotropin Administration, with Patients Divided According to Age (Number of Patients = 112)
|
Patients |
TSH-derived parameters |
|||
|---|---|---|---|---|
| Age groups | Number | Mean | SD | p-Value for correlation between mean TSH parameter and age |
| Day 3 TSH concentration (mIU/L) | ||||
| <35 | 14 | 96 | 44 | p < 0.0001 |
| 35–49 | 49 | 107 | 35 | |
| 50–64 | 34 | 142 | 57 | |
| >64 | 15 | 196 | 72 | |
| Day 5 TSH concentration (mIU/L) | ||||
| <35 | 14 | 9 | 3 | p < 0.0001 |
| 35–49 | 49 | 11 | 6 | |
| 50–64 | 34 | 20 | 10 | |
| >64 | 15 | 28 | 14 | |
| Days 3–5 TSH concentrations (mIU/L) | ||||
| <35 | 14 | 87 | 43 | p < 0.0001 |
| 35–49 | 49 | 96 | 32 | |
| 50–64 | 34 | 122 | 52 | |
| >64 | 15 | 167 | 70 | |
| Mean drop in TSH concentration/hour (days 3–5/48 hours) | ||||
| <35 | 14 | 1.82 | 0.87 | p < 0.0001 |
| 35–49 | 49 | 2.00 | 0.67 | |
| 50–64 | 34 | 2.55 | 1.07 | |
| >64 | 15 | 3.49 | 1.20 | |
| Mean percentage change in TSH concentrations (%) (day 5/day 3 × 100) | ||||
| <35 | 14 | 10 | 4 | p = 0.001 |
| 35–49 | 49 | 11 | 7 | |
| 50–64 | 34 | 15 | 7 | |
| >64 | 15 | 15 | 4 | |
FIG. 1.
Day 3 and day 5 serum thyrotropin (TSH) concentrations displayed according to patient age.
None of the following variables were associated with day 3 TSH values or were significant predictors in the model examining the relationship between TSH values and age: gender, height, weight, menopausal status, baseline TSH (mean during the prior year or day 1), serum creatinine, laboratory utilized, year of rhTSH administration, month of rhTSH administration, thyroglobulin value, thyroglobulin antibody titer, presence of distant metastases, presence of residual neck uptake, histology of differentiated thyroid cancer, years since thyroidectomy, or years since last radioiodine therapy. BMI and GFR were significantly associated with day 3 TSH values (p-values = 0.0033 and 0.0012, respectively), but were no longer significant predictors when age was included in the model. In the case of day 5 TSH values none of the following variables were associated with the day 5 TSH values or were significant predictors in the model examining the relationship between day 5 TSH values and age: gender, height, weight, BMI, menopausal status, baseline TSH (mean during the prior year or day 1), year of rhTSH administration, month of rhTSH administration, thyroglobulin value, thyroglobulin antibody titer, presence of distant metastases, presence of residual neck uptake, histology of differentiated thyroid cancer, years since thyroidectomy, or years since last radioiodine therapy. Serum creatinine and GFR were significantly associated with day 5 TSH values (p-values = 0.027 and 0.001, respectively), but were no longer significant when age was included in the model. The fall in TSH between days 3 and 5 was affected by age (p-value = 0.0001) and GFR (p-value = 0.002), with only age being significant in multivariate analyses. The same was true of the mean drop in TSH concentration per hour. The day 5 TSH as a percentage of day 3 TSH was affected by age only (p-value = 0.0001).
Patients were divided into four age groups as follows: <35 years (n = 14), 35–49 years (n = 49), 50–64 years (n = 34), and >64 years (n = 15). For each of these age groups, the following five parameters were examined: (i) day 3 TSH, (ii) day 5 TSH, (iii) days 3–5 TSH, (iv) days 3–5 TSH/48 hours, and (v) day 5/day 3 TSH (see Table 3). When examining day 3 TSH concentrations, there was a statistically significant difference in mean TSH values among the four age groups (p-value <0.0001). Statistically significant differences in mean TSH levels on day 3 were found between age groups 35–49 and 50–64 (p-value = 0.00660), age groups older than 64 and <35 (p-value <0.0001), age groups 35–49 and older than 64 (p-value <0.0001), and between age group 50–64 and <35 (p-value = 0.0257). There was also a statistically significant difference in mean TSH levels on day 5 among the four age groups (p-value <0.0001). Statistically significant differences in mean TSH levels on day 5 were found between age groups 35–49 and 50–64 (p-value <0.0001), age groups >64 and <35 (p-value <0.0001), age groups 35–49 and <64 (p-value <0.0001), and age groups <35 and 50–64 (p-value <0.0001). Additionally, there were statistically significant differences in the mean of the variables days 3–5 TSH, drop in serum TSH per hour, and day 5/day 3 TSH among the four age groups (p-values <0.0001, <0.0001, and 0.001, respectively). There were significant correlations between both mean days 3–5 TSH values and day 3 TSH values (Spearman correlation coefficient = 0.985, p-value <0.0001) and between mean days 3–5 TSH values and age (Spearman correlation coefficient = 0.381, p-value <0.0001).
When dividing the study patients into the aforementioned age groups, there was no difference between the four age groups with respect to the number of patients with metastases (p-value = 0.09), the number of patients with residual neck uptake (p-value = 0.79), the average TSH concentration during the preceding year (p-value = 0.54), and the mean day 1 TSH concentration (p-value = 0.36). Nevertheless, there was a significantly older age in patients with metastases (mean age 57 years), compared with those without (mean age 45 years) (p-value = 0.005). However, there was no correlation between age and either the presence of residual radioiodine uptake in the neck, or day 1 TSH concentration.
Discussion
We have shown that the serum TSH concentrations achieved in patients between ages 20 and 82 years undergoing diagnostic radioiodine imaging after rhTSH administration were significantly and positively correlated with the patient's age. This phenomenon was such that day 3 TSH concentration ranged from 38 to 316 mIU/L. Day 5 TSH concentrations ranged from 2 to 63 mIU/L. BMI and GFR affected day 3 TSH and serum creatinine and GFR affected day 5 TSH, but only in univariate analyses. Endogenous TSH concentrations did not impact the response to rhTSH, regardless of whether the TSH concentrations immediately before rhTSH administration or the average TSH concentrations for the preceding year were examined. The only factor remaining significant in multivariate analyses was age.
Some of our findings confirm those of previous investigators. However, components of our findings appear to be different from previously reported findings. Montesano et al. showed that day 5 TSH concentrations were affected by both age and gender (18). Our current findings could only confirm the relationship for age, but not gender. Castagna et al. showed that the peak TSH achieved after rhTSH administration was only affected by lean body mass measured by dual-energy X-ray absorptiometry scan in multivariate analyses (17). BMI was only significant in univariate analyses, and age was not mentioned as a factor that was considered in the analysis. Unfortunately lean body mass was not one of the parameters that was recorded in the medical records of our present group of patients. BMI only significantly affected day 3 TSH concentrations in univariate analyses in our study, but was no longer significant in multivariate analyses incorporating age. Vitale et al. reported that while body weight, BMI, and BSA were negatively associated with peak TSH levels, the only factor that remained significant in multivariate analyses was BSA (16). Age was considered in this analysis, but was not found to predict TSH levels. In our particular analysis, neither body weight not height were significant factors in the analysis.
We acknowledge several limitations of the present study. We were unable to measure body composition or examine diagnostic outcomes as these measures were beyond the scope of this unfunded, endocrine fellow-initiated study. Additionally, serum TSH concentrations were not measured in a central laboratory, but were analyzed in the laboratory mandated by the patient's insurance company. However, the laboratory utilized did not significantly affect TSH concentrations in our statistical analysis. All of these shortcomings could be avoided in a future prospective study.
The differences between our findings and those of these other investigators could have several potential explanations. The latter two studies reported correlations between the various factors examined and peak TSH values, rather than day 3 values (16,17). However, this is unlikely to explain any difference in findings, as in the Castagna et al. study 94% of peak values actually fell on day 3 (17). The Vitale et al. study did not give a break-down of whether peak TSH values fell on day 3 versus 4 (16). However, it seems reasonable to assume the distribution between days 3 and 4 would be similar. Other possible differences between our study and the Castagna et al. study (17) could be a different percentage of overweight subjects and/or the fact that the effect of age was not evaluated. In their study 33% of patients were of normal weight, 33% were overweight, and 33% were obese, compared with a breakdown of 26% normal weight subjects, 39% overweight subjects, and 35% obese subjects in our study. These differences, however, do not appear to be of sufficient magnitude to account for the different results. It is possible that lean body mass had an effect on TSH values in their study because age affects lean body mass, and age was not a factor that was included in their analysis. However, this line of reasoning is not supported by the Vitale et al. study (16) in which age was considered, but not found to be a significant factor.
TSH is cleared by both renal and hepatic metabolism (21,22) and endogenous TSH has a half-life of about 1 hour (23). The half-life of rhTSH is reported by the manufacturer as being 25 ± 10 hours, with a median time to peak of 10 hours (range: 3–24 hours). In our study both days 3 and 5 TSH levels were higher in older individuals. A reasonable initial hypothesis would be that this was due to decreased renal clearance. Decreasing GFR values were seen with advancing age. In addition, day 3 TSH and day 5 TSH were both correlated with GFR, increasing as GFR decreased.
However, day 3 TSH values were also associated with BMI, with TSH values decreasing as BMI increased. Perhaps this raises the issue of different metabolism or distribution of TSH within various body compartments within different age groups. It has been shown that rhTSH is a hydrophilic drug (24), perhaps suggesting that it is metabolized in the lean body compartment. Despite the fact that GFR decreased with age, days 3–5 TSH values and the hourly decline in TSH concentrations were of greater magnitude in the older age groups. Days 3–5 TSH values were also positively correlated with age. If the rhTSH half-life were solely determined by renal function opposite observations would be expected. Hepatic metabolism would not be expected to be more rapid in older individuals either. Therefore, the greater fall in TSH levels with time in older patients could either be due to different kinetics of release from the intramuscular injection site (which could have an age-related change in its muscle to fat ratio), or more efficient TSH clearance associated with higher absolute TSH concentrations. The latter hypothesis is supported by the fact that day 3 and days 3–5 TSH values were correlated with each other.
In the final analysis, only age remained a significant predictor of TSH values. We therefore hypothesize that any effects apparently exerted through renal function (reflected in GFR) or metabolism in fat or lean mass compartments (reflected in BMI) had age as the final common denominator. If TSH levels were also being impacted by release from the site of intramuscular injection or by subsequent hepatic metabolism, this could potentially be age related also. These hypotheses would account for only age remaining significant in multivariate analyses.
The different disposal of rhTSH within different age groups is interesting as a stand-alone observation, as it sheds light upon alterations that may help understand the physiology of normal aging. However, if TSH peaks are of different magnitude in different age groups, the additional key clinical question is whether this affects the sensitivity of diagnostic scanning or the efficacy of administered radioiodine therapy. The efficacy of radioiodine therapy is believed to depend on the amount of radiotracer that accumulates in tissue and the length of time that it remains there. The accumulation of tracer would in turn depend on the administered activity, tumor mass, and fractional uptake (25,26). Tracer uptake can, in theory, be improved by stimulating the activity or expression of the sodium iodide symporter (27). Manipulations that are generally believed to achieve this are dietary iodine depletion (28,29) and TSH elevation (30,31). Despite the fact that these two manipulations are commonly employed, not all studies demonstrate the soundness of these approaches. For example, a recent study was unable to show a benefit of a low urinary iodine with respect to the success of ablation (32). The length of time that tracer remains in thyroid tissue, on the other hand, depends on the effective half-time (25,26). The transient nature of the TSH peak seen with rhTSH, in contrast to the sustained TSH elevation seen with withdrawal from thyroid hormone, may be important for retention of radioiodine within thyroid cells also, by virtue of not promoting release of organified radioiodine from remnant tissue (33). The effective half-time can also be affected by agents such as lithium (34).
With respect to TSH concentrations, the impact of the peak TSH concentration achieved after the administration of rhTSH on the sensitivity of diagnostic studies or the efficacy of therapy has rarely been examined. In one study of remnant ablation, the peak TSH value achieved was not different in successfully ablated patients compared with those in whom ablation was deemed unsuccessful (17). However, both groups had mean peak TSH values of >120 mIU/L, which may have reached a plateau above the range at which TSH concentrations proportionally influence iodine uptake. In a 1977 study of a small number of patients undergoing a withdrawal protocol, it was suggested that TSH concentrations above 30 mIU/L were necessary to promote radioiodine uptake (35). However, studies examining a continuum of peak TSH values achieved after rhTSH administration and diagnostic sensitivity or rates of successful ablation would appear to be lacking. Two studies have recently reported similar short-term recurrence rates in patients who underwent remnant ablation with rhTSH rather than thyroid hormone withdrawal as the stimulus (12,36). However, neither of these studies correlated the peak serum TSH concentrations achieved with clinical outcomes. On the basis of our study, we were unable to comment on rates of successful remnant ablation as all patients were undergoing diagnostic studies only.
The effect of age on peak TSH values could be studied more rigorously in a prospective study. It is possible that modified dosing of rhTSH may be indicated to achieve therapeutic TSH concentrations in individuals under age 40–50 years in whom TSH levels are generally lower. Alternatively TSH values may be higher than necessary in older individuals. The TSH level necessary to maximize diagnostic and therapeutic efficacy could also be studied in a prospective study.
Footnotes
Presented in part at the 92nd Annual Meeting of the Endocrine Society.
Acknowledgments
Supported by Grant M01-RR020359 from the General Clinical Research Center Program of the National Center for Research Resources, National Institutes of Health. This analysis constitutes part of the fellowship research project of Dr. Over.
Disclosure Statement
R.O., H.N.M., and J.J. have nothing to disclose. The Washington Hospital Center receives funding to support clinical thyroid cancer protocols from Pfizer and Exelixis. K.D.B. has written articles for Medscape and UpToDate. He is on the Board of the American Thyroid Association, Deputy Editor of the Journal of Clinical Endocrinology and Metabolism, and on the FDA Endocrine Advisory Committee.
References
- 1.Pacini F. Castagna MG. Diagnostic and therapeutic use of recombinant human TSH (rhTSH) in differentiated thyroid cancer. Best Pract Res Clin Endocrinol Metab. 2008;22:1009–1021. doi: 10.1016/j.beem.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Luster M. Lippi F. Jarzab B. Perros P. Lassmann M. Reiners C. Pacini F. rhTSH-aided radioiodine ablation and treatment of differentiated thyroid carcinoma: a comprehensive review. Endocr Relat Cancer. 2005;12:49–64. doi: 10.1677/erc.1.00830. [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferri EL. Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;9:227–247. doi: 10.1677/erc.0.0090227. [DOI] [PubMed] [Google Scholar]
- 4.Meier CA. Braverman LE. Ebner SA. Veronikis I. Daniels GH. Ross DS. Deraska DJ. Davies TF. Valentine M. DeGroot LJ, et al. Diagnostic use of recombinant human thyrotropin in patients with thyroid carcinoma (phase I/II study) J Clin Endocrinol Metab. 1994;78:188–196. doi: 10.1210/jcem.78.1.8288703. [DOI] [PubMed] [Google Scholar]
- 5.Davies TF. Analysis of the results of phase III controlled clinical trials with recombinant human thyrotropin: developing a clinical guide. Endocr Pract. 2000;6:391–395. doi: 10.4158/EP.6.5.391. [DOI] [PubMed] [Google Scholar]
- 6.Robbins RJ. Tuttle RM. Sharaf RN. Larson SM. Robbins HK. Ghossein RA. Smith A. Drucker WD. Preparation by recombinant human thyrotropin or thyroid hormone withdrawal are comparable for the detection of residual differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:619–625. doi: 10.1210/jcem.86.2.7189. [DOI] [PubMed] [Google Scholar]
- 7.Ladenson PW. Braverman LE. Mazzaferri EL. Brucker-Davis F. Cooper DS. Garber JR. Wondisford FE. Davies TF. DeGroot LJ. Daniels GH. Ross DS. Weintraub BD. Comparison of administration of recombinant human thyrotropin with withdrawal of thyroid hormone for radioactive iodine scanning in patients with thyroid carcinoma. N Engl J Med. 1997;337:888–896. doi: 10.1056/NEJM199709253371304. [DOI] [PubMed] [Google Scholar]
- 8.Haugen BR. Pacini F. Reiners C. Schlumberger M. Ladenson PW. Sherman SI. Cooper DS. Graham KE. Braverman LE. Skarulis MC. Davies TF. DeGroot LJ. Mazzaferri EL. Daniels GH. Ross DS. Luster M. Samuels MH. Becker DV. Maxon HR., 3rd Cavalieri RR. Spencer CA. McEllin K. Weintraub BD. Ridgway EC. A comparison of recombinant human thyrotropin and thyroid hormone withdrawal for the detection of thyroid remnant or cancer. J Clin Endocrinol Metab. 1999;84:3877–3885. doi: 10.1210/jcem.84.11.6094. [DOI] [PubMed] [Google Scholar]
- 9.Pacini F. Ladenson PW. Schlumberger M. Driedger A. Luster M. Kloos RT. Sherman S. Haugen B. Corone C. Molinaro E. Elisei R. Ceccarelli C. Pinchera A. Wahl RL. Leboulleux S. Ricard M. Yoo J. Busaidy NL. Delpassand E. Hanscheid H. Felbinger R. Lassmann M. Reiners C. Radioiodine ablation of thyroid remnants after preparation with recombinant human thyrotropin in differentiated thyroid carcinoma: results of an international, randomized, controlled study. J Clin Endocrinol Metab. 2006;91:926–932. doi: 10.1210/jc.2005-1651. [DOI] [PubMed] [Google Scholar]
- 10.Luster M. Lassmann M. Haenscheid H. Michalowski U. Incerti C. Reiners C. Use of recombinant human thyrotropin before radioiodine therapy in patients with advanced differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:3640–3645. doi: 10.1210/jcem.85.10.6903. [DOI] [PubMed] [Google Scholar]
- 11.Pacini F. Molinaro E. Castagna MG. Lippi F. Ceccarelli C. Agate L. Elisei R. Pinchera A. Ablation of thyroid residues with 30 mCi (131)I: a comparison in thyroid cancer patients prepared with recombinant human TSH or thyroid hormone withdrawal. J Clin Endocrinol Metab. 2002;87:4063–4068. doi: 10.1210/jc.2001-011918. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle RM. Brokhin M. Omry G. Martorella AJ. Larson SM. Grewal RK. Fleisher M. Robbins RJ. Recombinant human TSH-assisted radioactive iodine remnant ablation achieves short-term clinical recurrence rates similar to those of traditional thyroid hormone withdrawal. J Nucl Med. 2008;49:764–770. doi: 10.2967/jnumed.107.049072. [DOI] [PubMed] [Google Scholar]
- 13.Pacini F. Molinaro E. Castagna MG. Agate L. Elisei R. Ceccarelli C. Lippi F. Taddei D. Grasso L. Pinchera A. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–3673. doi: 10.1210/jc.2002-021925. [DOI] [PubMed] [Google Scholar]
- 14.Mazzaferri EL. Robbins RJ. Spencer CA. Braverman LE. Pacini F. Wartofsky L. Haugen BR. Sherman SI. Cooper DS. Braunstein GD. Lee S. Davies TF. Arafah BM. Ladenson PW. Pinchera A. A consensus report of the role of serum thyroglobulin as a monitoring method for low-risk patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:1433–1441. doi: 10.1210/jc.2002-021702. [DOI] [PubMed] [Google Scholar]
- 15.Pacini F. Molinaro E. Lippi F. Castagna MG. Agate L. Ceccarelli C. Taddei D. Elisei R. Capezzone M. Pinchera A. Prediction of disease status by recombinant human TSH-stimulated serum Tg in the postsurgical follow-up of differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2001;86:5686–5690. doi: 10.1210/jcem.86.12.8065. [DOI] [PubMed] [Google Scholar]
- 16.Vitale G. Lupoli GA. Ciccarelli A. Lucariello A. Fittipaldi MR. Fonderico F. Panico A. Lupoli G. Influence of body surface area on serum peak thyrotropin (TSH) levels after recombinant human TSH administration. J Clin Endocrinol Metab. 2003;88:1319–1322. doi: 10.1210/jc.2002-020953. [DOI] [PubMed] [Google Scholar]
- 17.Castagna MG. Pinchera A. Marsili A. Giannetti M. Molinaro E. Fierabracci P. Grasso L. Pacini F. Santini F. Elisei R. Influence of human body composition on serum peak thyrotropin (TSH) after recombinant human TSH administration in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2005;90:4047–4050. doi: 10.1210/jc.2005-0534. [DOI] [PubMed] [Google Scholar]
- 18.Montesano T. Durante C. Attard M. Crocetti U. Meringolo D. Bruno R. Tumino S. Rubello D. Al-Nahhas A. Colandrea M. Maranghi M. Travascio L. Ronga G. Torlontano M. Age influences TSH serum levels after withdrawal of l-thyroxine or rhTSH stimulation in patients affected by differentiated thyroid cancer. Biomed Pharmacother. 2007;61:468–471. doi: 10.1016/j.biopha.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS. Coresh J. Greene T. Stevens LA. Zhang YL. Hendriksen S. Kusek JW. Van Lente F. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 20.Levey A. Greene T. Kusek J. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11 A0828 (abstract). [Google Scholar]
- 21.Cuttelod S. Lemarchand-Beraud T. Magnenat P. Perret C. Poli S. Vannotti A. Effect of age and role of kidneys and liver on thyrotropin turnover in man. Metabolism. 1974;23:101–113. doi: 10.1016/0026-0495(74)90107-3. [DOI] [PubMed] [Google Scholar]
- 22.Constant RB. Weintraub BD. Differences in the metabolic clearance of pituitary and serum thyrotropin (TSH) derived from euthyroid and hypothyroid rats: effects of chemical deglycosylation of pituitary TSH. Endocrinology. 1986;119:2720–2727. doi: 10.1210/endo-119-6-2720. [DOI] [PubMed] [Google Scholar]
- 23.Faglia G. Beck-Peccoz P. Piscitelli G. Medri G. Inappropriate secretion of thyrotropin by the pituitary. Horm Res. 1987;26:79–99. doi: 10.1159/000180687. [DOI] [PubMed] [Google Scholar]
- 24.Thotakura NR. Desai RK. Bates LG. Cole ES. Pratt BM. Weintraub BD. Biological activity and metabolic clearance of a recombinant human thyrotropin produced in Chinese hamster ovary cells. Endocrinology. 1991;128:341–348. doi: 10.1210/endo-128-1-341. [DOI] [PubMed] [Google Scholar]
- 25.Maxon HR. Quantitative radioiodine therapy in the treatment of differentiated thyroid cancer. Q J Nucl Med. 1999;43:313–323. [PubMed] [Google Scholar]
- 26.Maxon HR. Thomas SR. Hertzberg VS. Kereiakes JG. Chen IW. Sperling MI. Saenger EL. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983;309:937–941. doi: 10.1056/NEJM198310203091601. [DOI] [PubMed] [Google Scholar]
- 27.Castro MR. Bergert ER. Goellner JR. Hay ID. Morris JC. Immunohistochemical analysis of sodium iodide symporter expression in metastatic differentiated thyroid cancer: correlation with radioiodine uptake. J Clin Endocrinol Metab. 2001;86:5627–5632. doi: 10.1210/jcem.86.11.8048. [DOI] [PubMed] [Google Scholar]
- 28.Maxon HR. Thomas SR. Boehringer A. Drilling J. Sperling MI. Sparks JC. Chen IW. Low iodine diet in I-131 ablation of thyroid remnants. Clin Nucl Med. 1983;8:123–126. doi: 10.1097/00003072-198303000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Pluijmen MJ. Eustatia-Rutten C. Goslings BM. Stokkel MP. Arias AM. Diamant M. Romijn JA. Smit JW. Effects of low-iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2003;58:428–435. doi: 10.1046/j.1365-2265.2003.01735.x. [DOI] [PubMed] [Google Scholar]
- 30.Kogai T. Endo T. Saito T. Miyazaki A. Kawaguchi A. Onaya T. Regulation by thyroid-stimulating hormone of sodium/iodide symporter gene expression and protein levels in FRTL-5 cells. Endocrinology. 1997;138:2227–2232. doi: 10.1210/endo.138.6.5189. [DOI] [PubMed] [Google Scholar]
- 31.Schlumberger M. Tubiana M. De Vathaire F. Hill C. Gardet P. Travagli JP. Fragu P. Lumbroso J. Caillou B. Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 32.Tala Jury HP. Castagna MG. Fioravanti C. Cipri C. Brianzoni E. Pacini F. Lack of association between urinary iodine excretion and successful thyroid ablation in thyroid cancer patients. J Clin Endocrinol Metab. 2010;95:230–237. doi: 10.1210/jc.2009-1624. [DOI] [PubMed] [Google Scholar]
- 33.Hanscheid H. Lassmann M. Luster M. Thomas SR. Pacini F. Ceccarelli C. Ladenson PW. Wahl RL. Schlumberger M. Ricard M. Driedger A. Kloos RT. Sherman SI. Haugen BR. Carriere V. Corone C. Reiners C. Iodine biokinetics and dosimetry in radioiodine therapy of thyroid cancer: procedures and results of a prospective international controlled study of ablation after rhTSH or hormone withdrawal. J Nucl Med. 2006;47:648–654. [PubMed] [Google Scholar]
- 34.Koong SS. Reynolds JC. Movius EG. Keenan AM. Ain KB. Lakshmanan MC. Robbins J. Lithium as a potential adjuvant to 131I therapy of metastatic, well differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1999;84:912–916. doi: 10.1210/jcem.84.3.5527. [DOI] [PubMed] [Google Scholar]
- 35.Edmonds CJ. Hayes S. Kermode JC. Thompson BD. Measurement of serum TSH and thyroid hormones in the management of treatment of thyroid carcinoma with radioiodine. Br J Radiol. 1977;50:799–807. doi: 10.1259/0007-1285-50-599-799. [DOI] [PubMed] [Google Scholar]
- 36.Elisei R. Schlumberger M. Driedger A. Reiners C. Kloos RT. Sherman SI. Haugen B. Corone C. Molinaro E. Grasso L. Leboulleux S. Rachinsky I. Luster M. Lassmann M. Busaidy NL. Wahl RL. Pacini F. Cho SY. Magner J. Pinchera A. Ladenson PW. Follow-up of low-risk differentiated thyroid cancer patients who underwent radioiodine ablation of postsurgical thyroid remnants after either recombinant human thyrotropin or thyroid hormone withdrawal. J Clin Endocrinol Metab. 2009;94:4171–4179. doi: 10.1210/jc.2009-0869. [DOI] [PubMed] [Google Scholar]