Abstract
Purpose
The aim of this study was to analyze the effect of azithromycin (AZM) 1% ophthalmic solution in DuraSite® (AzaSite®) on biofilm formation by Staphylococcus aureus and coagulase-negative staphylococci in vitro.
Methods
Susceptible and resistant clinical strains (n = 8) of S. aureus and coagulase-negative staphylococci were challenged with serial dilutions of AzaSite® and its components: AZM, benzalkonium chloride (BAK), and the DuraSite drug delivery vehicle. After 20 h of incubation, bacterial growth was quantified using a spectrophotometer (A = 600 nm). Plates were stained with crystal violet and biofilm formation was quantified spectrophotometrically at A = 590 nm.
Results
AzaSite® and AZM inhibited bacterial growth (P < 0.05) and biofilm formation (P < 0.05) in AZM-susceptible strains at all studied dilutions. AZM-resistant strains treated with AzaSite® exhibited a significant reduction in biofilm formation (P < 0.05) at subinhibitory concentrations (1.25%–5%). AZM had no effect on bacterial growth in resistant strains but conferred a small reduction in biofilm formation at concentrations from 1.25 to 10 mg/mL in most strains. DuraSite® inhibited biofilm formation at concentrations between 10% and 2.5% in all studied strains (P < 0.05), without affecting bacterial growth. BAK inhibited bacterial growth and biofilm formation in all strains between concentrations of 0.042 and 0.375 mg/mL (P < 0.05).
Conclusions
AzaSite®, AZM, or BAK prevented biofilm formation by inhibiting growth of AZM-susceptible strains. AzaSite®, AZM, and DuraSite® also reduced biofilm formation at subinhibitory concentrations for growth. Our data indicate that AZM has a moderate inhibitory effect on biofilm formation, whereas DuraSite® appears to play a greater role in the inhibition of staphylococcal biofilm formation by AzaSite®.
Introduction
Blepharitis is a chronic ocular disease characterized by an inflammatory reaction on the eyelids.1 Symptoms include redness, itching, burning, and foreign body sensation of the eyes resulting in significant discomfort and visual disturbances.1 Infectious blepharitis is commonly associated with ocular pathogens such as Staphylococcus epidermidis and Staphylococcus aureus, which reside on the eyelid margin2; however, the role of bacterial infection in blepharitis has yet to be fully elucidated. Treatment involves topical antibiotics as well as lid hygiene including warm compresses and eyelid scrubs.3,4 However, many individuals with blepharitis require long-term therapy as blepharitis can be a chronic affliction and relief of symptoms may be temporary. Biofilm formation on the eyelids and eyelashes may contribute to the difficulty in treating infectious blepharitis.5–7
Biofilms are organized communities of microorganisms that adhere to both abiotic and biotic surfaces including heart valves, prosthetic joints, catheters, contact lenses, and punctal plugs.8–12 Microorganisms residing in biofilms are often between 20 and 1,000 times more resistant to antibiotics than genetically identical bacteria living as free floating cells; they are more resistant to host defenses, often difficult to culture, and associated with chronic diseases.6,13,14 Both S. aureus and coagulase-negative staphylococci (CNS) have been shown to form biofilms on a variety of biotic and abiotic surfaces and are associated with blepharitis.2,15,16
Azithromycin (AZM) is a broad-spectrum azalide antibiotic effective against Gram-positive, Gram-negative, and atypical bacteria and has anti-inflammatory characteristics.17 Topical AZM ophthalmic solution 1% in DuraSite® (AzaSite®; Inspire Pharmaceuticals, Durham, NC) has been shown to be effective in treating posterior blepharitis and bacterial conjunctivitis.18,19 DuraSite® is a crosslinked polymer of polyacrylic acid, with a molecular weight of >1 million Da.20 AzaSite® also contains benzalkonium chloride (BAK), a cationic surface-acting agent used as a preservative.
Previous studies with Gram-negative organisms have shown that AZM can inhibit biofilm formation at subinhibitory doses, suggesting that AZM actively inhibits the ability of bacteria to attach to surfaces.21–23 This effect has not been reported for Staphylococcus species that frequently cause ocular and prosthetic-associated infections.5,9,15
In this study, we test the effect of a commercially available preparation of AzaSite® and its individual components on biofilm formation by clinical blepharitis isolates of S. aureus and CNS, including AZM-resistant strains.
Methods
Study drugs
AZM ophthalmic solution 1% in DuraSite® (AzaSite®; pH 6.3) and AZM 1% in 15 mM sodium citrate buffer (pH 8.0) were supplied for the study by Inspire Pharmaceuticals. The proprietary ocular drug delivery vehicle DuraSite® was supplied by InSite Vision (Alameda, CA). BAK was obtained from Sigma-Aldrich (St. Louis, MO; product No. B6295).
Bacterial strains and culture methods
Four de-identified clinical ocular strains of S. aureus and 4 de-identified clinical ocular strains of S. epidermidis were obtained from the Charles T. Campbell Ophthalmic Microbiology Laboratory (UPMC Eye Center, Pittsburgh, PA). The bacterial isolates were part of a clinical tissue bank used for the validation of antibiotic susceptibility testing as mandated by laboratory certification. The only criteria for choice of strain from among a collection of conjunctivitis and blepharitis isolates were the bacterial species and AZM resistance status. Strains and their minimum inhibitory concentrations as determined by E-tests (bioMérieux, Durham, NC) for AZM are shown in Table 1.
Table 1.
Minimum Inhibitory Concentrations of Staphylococcus Isolates to Azithromycin
| Strain | Species | MIC (μg/mL) |
|---|---|---|
| B1487 | Staphylococcus aureus | >256 |
| B1493 | S. aureus | >256 |
| B1412 | S. aureus | 1.5 |
| B1391 | S. aureus | 0.75 |
| B1468 | CNS | >256 |
| B1483 | CNS | >256 |
| B1379 | CNS | 1.5 |
| B1472 | CNS | 1.0 |
MIC, minimum inhibitory concentration; CNS, coagulase-negative Staphylococcus.
Bacteria were streaked on trypticase soy agar plates supplemented with 5% sheep blood (BBL; Sparks, MD) and incubated overnight at 37°C. Individual colonies were isolated, inoculated in 5 mL of brain heart infusion (BD, Franklin Lakes, NJ) medium, and grown overnight for 15 h in a 30°C incubator. Bacterial inocula were standardized using a Beckman DU 70 spectrophotometer detecting absorbance at 600 nm, and 0.01 optical density units of bacteria were suspended in brain heart infusion + 0.2% glucose to promote bacterial growth and biofilm formation. Two-fold serial dilutions of the 4 study drugs used in this study were performed in 96-well flat-bottomed cell culture plates (Costar 3595; Corning, Corning, NY). Using a multichannel pipette, 10 μL of each dilution or control was plated in its corresponding well with 90 μL of bacteria standardized to A600nm = 0.01. Plates were incubated at 37°C for 20 h in a moisture chamber to minimize evaporation. The final concentrations of AzaSite® to which the bacteria were exposed were 0.156%–10% of the commercial preparation. Bacteria were exposed to AZM at 0.156–10 mg/mL. The concentration range of the proprietary vehicle for AzaSite®, DuraSite®, to which bacteria were exposed was 0.0156%–1%. The final concentrations of BAK to which the bacteria were exposed were 12–375 μg/mL.
Plates were analyzed using the Synergy 2 Microplate Reader (BioTek Instruments, Winooski, VT) detecting absorbance at 600 nm to determine total cell density in each well, including both biofilm and planktonic bacteria. As staphylococcal biofilms are formed predominantly on the bottom of the wells and not on the sides, we reasoned that this approach is appropriate to measure total culture density.
Biofilm formation experiments were performed using previously published methods.24 After washing the plates in water to remove all planktonic bacteria, 80 μL of 0.1% crystal violet was added to the wells and incubated for 10 min at room temperature to stain biofilms that had formed at the bottom of each well. The plates were washed in water and dried overnight. The wells were then treated with 150 μL of 33% glacial acetic acid for 15 min at room temperature to solubilize the dried crystal violet adherent to any biofilms. Each well was then analyzed using the Synergy 2 Microplate Reader detecting absorbance at 590 nm to determine crystal violet density in each well to measure biofilm formation. Each data point represents the mean of at least 5 separate experiments performed on different days.
Statistical analysis
Data were analyzed using 1-way ANOVA with a Tukey posttest, with the level of statistical significance set at P < 0.05 comparing untreated to treated samples, using Prizm 5 software (GraphPad Software, La Jolla, CA).
Results
AzaSite® inhibits the in vitro formation of staphylococcal biofilms
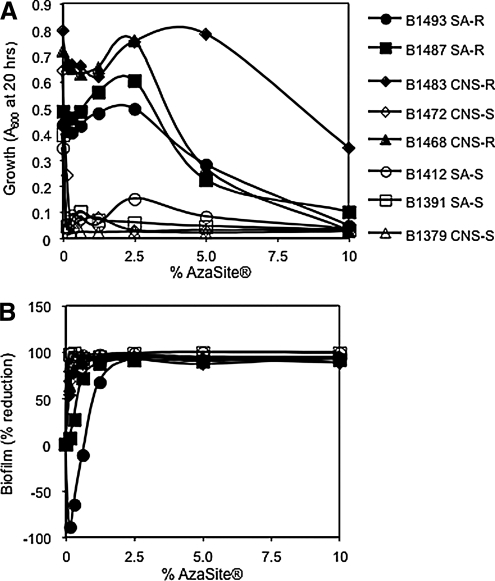
AzaSite® significantly inhibited the growth of 2 AZM-susceptible S. aureus and 2 CNS strains at all studied dilution strengths (P < 0.05; Fig. 1A). A corresponding statistically significant >90% reduction in biofilm formation compared with the no AzaSite® control was observed in the same treatment groups (P < 0.05; Fig. 1B). AZM-resistant strains all exhibited a >90% reduction in biofilm formation at AzaSite® concentrations of ≥0.5% (Fig. 1A, B); at these concentrations, growth was also inhibited. More interestingly, AzaSite® concentrations of 1.25%–2.5% (representing 125–250 μg/mL of AZM plus other ingredients) conferred no significant growth differences from the no AzaSite® control, but a significant reduction (67%–98%, P < 0.05) in biofilm formation (Fig. 1A, B). One strain of AZM-resistant S. aureus, B1493, exhibited significantly (P < 0.05) elevated biofilm formation at further reduced AzaSite® concentrations (0.156%–0.625%—representing 15.6–62.5 μg/mL of AZM plus other ingredients), although its biofilm formation was strongly inhibited at higher concentrations (Fig. 1B). As the active ingredient of AzaSite® is AZM, we tested whether AZM was responsible for the reduction in biofilm formation by AZM-susceptible and -resistant strains.
FIG. 1.
The impact of AzaSite® on end point growth and biofilm formation of ocular staphylococcal isolates. These data are the combination of at least 5 independent experiments performed on different days; the mean is shown. Note: the maximum AZM and BAK concentrations from AzaSite® in this experiment are 100 and 0.3 μg/mL, respectively. (A) Planktonic growth as a function of AzaSite® concentration. (B) Percentage of reduction in biofilm formation as a function of AzaSite® concentration. SA, Staphylococcus aureus; AZM, azithromycin; CNS, coagulase negative staphylococci; R, AZM-resistant; S, AZM-susceptible; BAK, benzalkonium chloride.
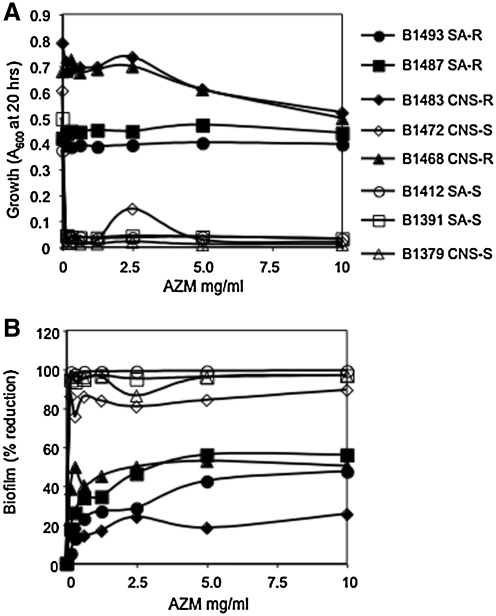
For all susceptible strains, AZM prevented biofilm formation (89%–99% reduction) and growth (Fig. 2A, B). Most AZM-resistant strains did exhibit significant reduction (P < 0.05) in biofilm formation (up to 55%), but it is apparently independent of a change in growth (Fig. 2A, B). This reduction was surprising smaller than the near complete biofilm reduction associated with higher concentrations of AzaSite®. AZM-resistant CNS strains B1483 and B1463 exhibited a significant reduction in growth at the highest concentration of AZM (10,000 μg/mL) (Fig. 2A).
FIG. 2.
The impact of AZM on growth and biofilm formation of ocular staphylococcal isolates. These data are the combination of at least 5 independent experiments performed on different days; the mean is shown. (A) Planktonic growth as a function of AZM concentration. (B) Percentage of reduction in biofilm formation as a function of AZM concentration.
Inhibition of staphylococcal biofilm formation by DuraSite® and BAK
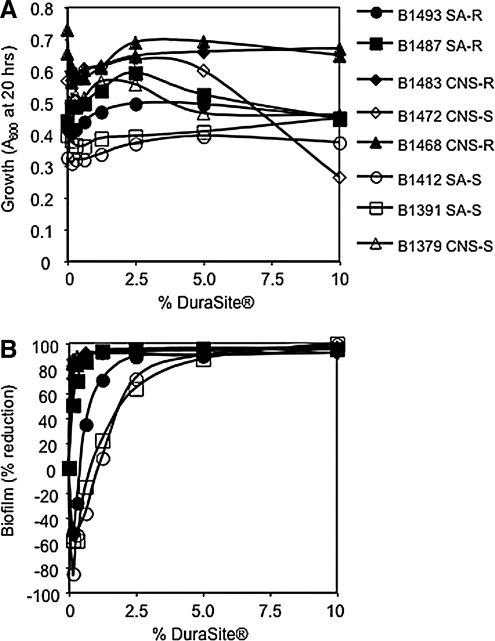
Because the inhibitory effect of AzaSite® was greater than that of AZM, the contribution of additional components of AzaSite was tested for biofilm inhibitory effects. DuraSite® was noted to have caused no significant inhibition of planktonic growth (Fig. 3A); however, biofilm formation was significantly reduced (up to 99%, P < 0.005) when the DuraSite® was included at concentrations of 10%, 5%, and 2.5% (P < 0.05) in all studied strains (Fig. 3B). Notably, 3 strains of S. aureus displayed an increase in biofilm formation in medium supplemented with DuraSite® at very low concentrations (Fig. 3B). Note that DuraSite® does not contain BAK.
FIG. 3.
The impact of DuraSite® on growth and biofilm formation of ocular staphylococcal isolates. These data are the combination of at least 5 independent experiments performed on different days; the mean is shown. (A) Planktonic growth as a function of DuraSite® concentration. (B) Percentage of reduction in biofilm as a function of DuraSite® concentration.
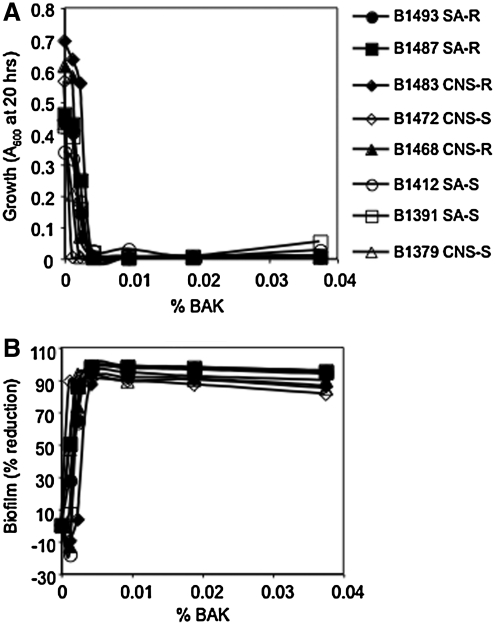
BAK is a preservative in AzaSite® at 30 μg/mL (0.003%). BAK inhibited both planktonic growth (Fig. 4A) and biofilm formation by all strains between concentrations of 42 and 375 μg/mL (P < 0.005). At concentrations of 12 μg/mL, most strains (7 of the 8 strains) exhibited growth that was indistinguishable from the no BAK control, with an 89% reduction in biofilm formation in the 1 strain that did not grow well (B1472). At BAK concentrations of 23 μg/mL, there was a severe growth defect and a 60%–90% reduction in biofilm formation in 7 of the 8 strains, suggesting that BAK inhibits biofilm formation through prevention of bacterial growth (Fig. 4B).
FIG. 4.
The impact of BAK on growth and biofilm formation of ocular staphylococcal isolates. These data are the combination of at least 5 independent experiments performed on different days; the mean is shown. (A) Planktonic growth as a function of BAK concentration. (B) Percentage of reduction in biofilm as a function of BAK concentration.
Discussion
Blepharitis is a chronic multifactorial ocular disease characterized by inflammation of the eyelids. Despite its widespread prevalence and extensive research elucidating its pathophysiology, there is still no optimal treatment strategy to control this disease process. The standard therapy of warm compresses and lid scrubs offer relief from symptoms but often take several weeks before significant improvement is noted. Infectious forms of blepharitis, commonly associated with staphylococcal species,2 complicate the treatment. It is possible that some of this chronic inflammation derives from difficult-to-treat staphylococcal biofilms. In situations such as this, an antibiotic that is efficacious against Gram-positive organisms and has anti-inflammatory properties as well as an ability to inhibit biofilm formation may play a significant role in therapy. Although AZM has been shown to have both antimicrobial effects on S. aureus and CNS as well as anti-inflammatory effects, its effect on biofilm formation is yet to be studied. Here we show that AzaSite® and its components can reduce biofilm formation through a mechanism that acts at concentrations that do not inhibit growth but simply prevent bacterial growth.
The present study was designed to determine whether AZM ophthalmic solution in DuraSite® (AzaSite®) could inhibit biofilm formation. The results show that AzaSite® at 0.25%–1.0% of the concentration in the commercial preparation can inhibit biofilm formation regardless of AZM susceptibility or resistance in all studied strains of S. aureus and CNS. The tested AzaSite® concentrations sufficient to inhibit biofilms were 1:100 to 1:400 of the clinical dose, so the antibiofilm activity may be retained for some time after topical application on the eye. Using the same study protocol for the 3 major components of AzaSite®—AZM, DuraSite®, and BAK—we were able to elucidate which components contributed to this inhibitory effect.
In AZM-susceptible strains of S. aureus and CNS, AZM inhibited both planktonic growth and biofilm formation at all studied concentrations. This inhibition of biofilm formation cannot be separated from the bacteriostatic effect of AZM on the growth of susceptible strains. Therefore, the simplest model to explain these observations is that this inhibition of biofilm formation is a function of reduced growth rather than any active mechanism to prevent biofilm formation.
In AZM-resistant strains of S. aureus and CNS, there was little to no differences in planktonic bacterial growth between groups treated with dilutions of AZM and the control. However, there was a moderate and statistically significant decrease of biofilm formation by both S. aureus strains as well as 1 strain of CNS. Based on these findings in the AZM-resistant strains, there may be an additional specific mechanism of biofilm inhibition (not growth-related). In support of this hypothesis, previous studies have shown that S. aureus virulence factor production and attachment to epithelial cells can be inhibited by AZM.25–27 The inhibition of S. aureus biofilm formation by AZM may have been also present in the AZM-susceptible strains but was masked by the strong bacteriostatic effects of AZM. The mechanism of this biofilm inhibition remains the subject of future studies.
BAK is a commonly used antiseptic with a multitude of applications including in hygienic towelettes and as preservatives in numerous ocular medications. As expected, higher concentrations of BAK effectively inhibited planktonic growth and biofilm formation in all strains. This pattern is similar to that of AZM in susceptible strains. Thus, we cannot conclude that BAK is able to directly inhibit biofilm formation, and the lack of biofilms is likely to be secondary to bacterial growth inhibition.
Interestingly, a pattern of biofilm inhibition matching that of AzaSite® was seen in the same concentrations of DuraSite® alone. DuraSite® is the proprietary ocular drug delivery vehicle developed by InSite Vision. DuraSite® is a synthetic polymer of crosslinked polyacrylic acid that stabilizes small molecules in an aqueous matrix, allowing for increased contact time with the ocular surface resulting in increased absorption. DuraSite® was able to inhibit biofilm formation in all studied strains while having no significant effect on planktonic growth at any studied concentration.
It has been previously noted that antibiotics and other compounds can increase biofilm formation at very low or subinhibitory concentrations.24,28,29 We noted that in 1 strain of S. aureus, AzaSite® at concentrations of <0.625%, a 160-fold dilution of the commercial preparation, significantly increased biofilm formation, as did DuraSite® with all 4 S. aureus strains at very low concentrations.
In summary, our results suggest that the ability of AzaSite® to inhibit staphylococcal biofilm formation stems from 3 factors: (1) inhibition of bacterial growth by AZM and BAK, (2) a moderate, not growth-related, inhibition of biofilm formation by AZM, and (3) a major, not growth-related, inhibition of biofilm formation by DuraSite®. The results presented here suggest that the antibiofilm action of AzaSite® may prove beneficial for inhibition of biofilms on abiotic ocular implants such as intraocular lenses.5,13
Acknowledgments
Special thanks to Nicholas Stella for technical support in this study. This work was supported by an unrestricted grant from Inspire Pharmaceuticals, NEI Core Grant EY08098, NIH AI085570, and a Research to Prevent Blindness Career Development award to R.M.Q.S.
Author Disclosure Statement
This study was funded by Inspire Pharmaceuticals with a research contract through the University of Pittsburgh, Pittsburgh, PA. The authors R.M.Q.S., E.G.R., F.S.M., and R.P.K. have been paid independent consultant fees by Inspire Pharmaceuticals and these fees were deemed to not produce a conflict of interest for the authors by the University of Pittsburgh, Pittsburgh, PA. Inspire Pharmaceuticals did not design, participate, collect data, or analyze the data for this study. Inspire Pharmaceuticals approved the design and was allowed to review the manuscript prior to journal submission. Eric Wu has no competing financial interests. There are no proprietary interests.
References
- 1.Smith R.E. Flowers C.W., Jr. Chronic blepharitis: a review. CLAO J. 1995;21:200–207. [PubMed] [Google Scholar]
- 2.Smolin G. Okumoto M. Staphylococcal blepharitis. Arch. Ophthalmol. 1977;95:812–816. doi: 10.1001/archopht.1977.04450050090009. [DOI] [PubMed] [Google Scholar]
- 3.Carter S.R. Eyelid disorders: diagnosis and management. Am. Fam. Physician. 1998;57:2695–2702. [PubMed] [Google Scholar]
- 4.Jackson W.B. Blepharitis: current strategies for diagnosis and management. Can. J. Ophthalmol. 2008;43:170–179. doi: 10.1139/i08-016. [DOI] [PubMed] [Google Scholar]
- 5.Behlau I. Gilmore M.S. Microbial biofilms in ophthalmology and infectious disease. Arch. Ophthalmol. 2008;126:1572–1581. doi: 10.1001/archopht.126.11.1572. [DOI] [PubMed] [Google Scholar]
- 6.Elder M.J. Stapleton F. Evans E., et al. Biofilm-related infections in ophthalmology. Eye (Lond.) 1995;9(Pt 1):102–109. doi: 10.1038/eye.1995.16. [DOI] [PubMed] [Google Scholar]
- 7.Zegans M.E. Shanks R.M. O'Toole G.A. Bacterial biofilms and ocular infections. Ocul. Surf. 2005;3:73–80. doi: 10.1016/s1542-0124(12)70155-6. [DOI] [PubMed] [Google Scholar]
- 8.Darouiche R.O. Treatment of infections associated with surgical implants. N. Engl. J. Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 9.Trautner B.W. Darouiche R.O. Catheter-associated infections: pathogenesis affects prevention. Arch. Intern. Med. 2004;164:842–850. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donlan R.M. Biofilms and device-associated infections. Emerg. Infect. Dis. 2001;7:277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita J. Yokoi N. Fullwood N.J., et al. The detection of bacteria and bacterial biofilms in punctal plug holes. Cornea. 2001;20:362–365. doi: 10.1097/00003226-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Yokoi N. Okada K. Sugita J., et al. Acute conjunctivitis associated with biofilm formation on a punctal plug. Jpn. J. Ophthalmol. 2000;44:559–560. doi: 10.1016/s0021-5155(00)00214-8. [DOI] [PubMed] [Google Scholar]
- 13.Zegans M.E. Becker H.I. Budzik J., et al. The role of bacterial biofilms in ocular infections. DNA Cell Biol. 2002;21:415–420. doi: 10.1089/10445490260099700. [DOI] [PubMed] [Google Scholar]
- 14.Aslam S. Effect of antibacterials on biofilms. Am. J. Infect Control 36:S175.e. 2008:9–e11. doi: 10.1016/j.ajic.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 15.O'Gara J.P. Humphreys H. Staphylococcus epidermidis biofilms: importance and implications. J. Med. Microbiol. 2001;50:582–587. doi: 10.1099/0022-1317-50-7-582. [DOI] [PubMed] [Google Scholar]
- 16.Otto M. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillis R.J. Iglewski B.H. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 2004;42:5842–5845. doi: 10.1128/JCM.42.12.5842-5845.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abelson M.B. Heller W. Shapiro A.M., et al. Clinical cure of bacterial conjunctivitis with azithromycin 1%: vehicle-controlled, double-masked clinical trial. Am. J. Ophthalmol. 2008;145:959–965. doi: 10.1016/j.ajo.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Luchs J. Efficacy of topical azithromycin ophthalmic solution 1% in the treatment of posterior blepharitis. Adv. Ther. 2008;25:858–870. doi: 10.1007/s12325-008-0096-9. [DOI] [PubMed] [Google Scholar]
- 20.Friedlaender M.H. Protzko E. Clinical development of 1% azithromycin in DuraSite, a topical azalide anti-infective for ocular surface therapy. Clin. Ophthalmol. 2007;1:3–10. [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura A. Ara T. Imamura Y., et al. The effects of antibiotics on in vitro biofilm model of periodontal disease. Eur. J. Med. Res. 2008;13:439–445. [PubMed] [Google Scholar]
- 22.Starner T.D. Shrout J.D. Parsek M.R., et al. Subinhibitory concentrations of azithromycin decrease nontypeable Haemophilus influenzae biofilm formation and diminish established biofilms. Antimicrob. Agents Chemother. 2008;52:137–145. doi: 10.1128/AAC.00607-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichimiya T. Takeoka K. Hiramatsu K., et al. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy. 1996;42:186–191. doi: 10.1159/000239440. [DOI] [PubMed] [Google Scholar]
- 24.Shanks R.M. Sargent J.L. Martinez R.M., et al. Catheter lock solutions influence staphylococcal biofilm formation on abiotic surfaces. Nephrol. Dial. Transplant. 2006;21:2247–2255. doi: 10.1093/ndt/gfl170. [DOI] [PubMed] [Google Scholar]
- 25.Braga P.C. Piatti G. Interference by subinhibitory concentrations of azithromycin with the mechanism of bacterial adhesion to human epithelial cells. Chemotherapy. 1993;39:432–437. doi: 10.1159/000238989. [DOI] [PubMed] [Google Scholar]
- 26.Moneib N.A. Shibl A.M. el-Said M.A., et al. Macrolides induced suppression of virulence factors produced by Staphylococcus aureus. J. Chemother. 1993;5:289–292. doi: 10.1080/1120009x.1993.11739246. [DOI] [PubMed] [Google Scholar]
- 27.Yanagihara K. Morinaga Y. Nakamura S., et al. Subinhibitory concentrations of telithromycin, clarithromycin and azithromycin reduce methicillin-resistant Staphylococcus aureus coagulase in vitro and in vivo. J. Antimicrob. Chemother. 2008;61:647–650. doi: 10.1093/jac/dkm507. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman L.R. D'Argenio D.A. MacCoss M.J., et al. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;436:1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- 29.Nucleo E. Steffanoni L. Fugazza G., et al. Growth in glucose-based medium and exposure to subinhibitory concentrations of imipenem induce biofilm formation in a multidrug-resistant clinical isolate of Acinetobacter baumannii. BMC Microbiol. 2009;9:270. doi: 10.1186/1471-2180-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]