Abstract
Purpose
To determine if a peptide, TAT-Cd0, inhibits Herpes simplex virus type 1 infection of human corneal epithelial cells.
Methods
TAT-Cd0 and a control peptide, E50,51TAT-Cd0, were added at various times throughout infection with the lacz-expressing hrR3 virus, and viral replication was measured by β-galactosidase activity. Toxicity was assessed using a dye reduction assay.
Results
The CC50 value for TAT-Cd0 was ∼100 μM. In assays with peptide present at all times, TAT-Cd0 was 150-fold more active than E50,51TAT-Cd0 (EC50 0.2 vs. 30.0 μM). The EC50 values of TAT-Cd0 for entry inhibition, cell protection, virus inactivation, and inhibition of attachment were 0.1, 0.4, 9.5, and 3.0 μM, respectively. TAT-Cd0 was less effective when added 1 h postinfection (EC50 = 30.0 μM).
Conclusions
TAT-Cd0 is an effective inhibitor of Herpes simplex virus type 1 infection in human corneal epithelial cells and affects multiple steps before, or very early, in infection. The peptide has potential as an antiviral and further studies are warranted.
Introduction
Herpes simplex virus type 1 (HSV-1) is a significant human pathogen, infecting 70%–90% of the population.1 An estimated 400,000 people in the United States suffer from HSV ocular infection, with 50,000 new and recurrent cases each year. HSV keratitis is the leading cause of blindness due to infection in developed countries.2,3 On the basis of the Herpetic Eye Disease trial, the current recommended therapy is topical corticosteroid with a prophylactic antiviral.4 In addition, prophylaxis with oral acyclovir reduces the rate of recurrence.5 Although these therapies are effective, they are not always successful and there are problems associated with the use of steroids.4 As a result, there is a need for additional therapeutic approaches.
HSV rapidly establishes a latent infection, and this presents significant problems in dealing with the virus. Reactivation can lead to recrudescence, increasing the damage to the cornea with each event. Currently available antivirals can reduce the establishment of latency but only when added within the first few hours of the primary infection, which is difficult to achieve in practice.6,7 Therapeutic strategies that prevent the primary infection or eliminate latent virus would be useful.
We have previously identified several peptides that are broad-spectrum inhibitors of virus infection. These peptides act to either inhibit attachment of virus to cells or inhibit entry at low micromolar concentrations.8–13 In addition, we have shown that these peptides have little, if any, toxicity for a number of cell types, including a human trabecular meshwork cell line and primary keratocyte cultures at antiviral concentrations.14 Moreover, they were not toxic when applied topically to mouse corneas at concentrations up to 1,000 times the EC50 values.14 These studies raise the possibility that these antiviral peptides may be therapeutically useful for ocular HSV infections. One of our cationic peptides, TAT-Cd0, is particularly attractive as a candidate as it contains only 11 residues and is highly soluble and stable in aqueous solutions.11
In another study testing the in vivo efficacy of a modified cationic θ-defensin, RC-2, we found that the peptide had modest activity.15 One potential explanation for the modest efficacy is that θ-defensin and other cationic antivirals may have had reduced antiviral activity in corneal epithelial cells. Therefore, we believed that it was necessary to show that the TAT-C peptide was an effective antiviral in corneal epithelial cells before conducting studies in animal models of ocular HSV infection. The goals of this study were to determine if the TAT-Cd0 peptide was toxic for a corneal epithelial cell line and to confirm that the peptide acts to inhibit the entry of HSV-1 into these cells.
Methods
Cells and viruses
An immortalized human corneal epithelial (HCE) cell line16 was grown in 50% Dulbecco's modified Eagle's medium: 50% F12 medium supplemented with 5% fetal bovine serum, 5% calf serum, penicillin (100 U/mL), streptomycin (0.1 mg/mL), and amphotericin B (2.5 μg/mL). Assays were done with HEPES buffered medium to which no fungicide or antibiotic was added (S+, medium with serum; S−, medium without serum). S+ and S− Dulbecco's modified Eagle's medium without carbonate buffer was used if the plate was incubated at room temperature, at 4°C, or at 37°C in the absence of CO2 (Attachment, entry blocking and virus inactivation assays). High titer stocks of the HSV-1 KOS mutant hrR3, which expresses Escherichia coli β-galactosidase from the early ICP6 promoter,17 were prepared in Vero cells18 and further purified on sucrose gradients as described previously.19
Peptide synthesis and analysis
The TAT-Cd0 and the E50,51TAT-Cd0 (2 glutamic acid residues substituted for lysine at positions 50 and 51) peptides were synthesized in the Peptide Synthesis Facility at the University of Wisconsin–Madison Biotechnology Center (>95% purity) as described previously.8,9,11 Peptide concentrations were determined by measuring absorbance at 215 and 225 nm and are reported as the monomeric peptide concentration.20
MTS assay
Peptide cytotoxicity was determined using a commercially available assay (Celltiter 96® AQqueous One Solution cell proliferation assay reagent, #3582; Promega) as described previously.14 Briefly, HCE cells (4.0 × 104 cells/well) were seeded in a 96-well plate and incubated for 24 h at 37°C. After incubation, the medium was aspirated from each well and replaced with 40 μL of S+ medium and the plate incubated at 37°C. Fifty microliters of S+ medium with the desired concentration of peptide was added to each well. Control wells received the medium only. The plate was incubated at 37°C for 6 h. After incubation, the cells were examined visually for cytotoxicity and refed with 100 μL S+ medium and 20 μL of the 96 AQqueous One Solution cell proliferation assay reagent per well. The plate was then incubated for 1.5 h at 37°C, and at the end of incubation 25 μL of lysis buffer (2.5% IGEPAL CA-630; Sigma, #I3021) was added to each well. Absorbance at 490 nm was determined using a 96-well plate reader (Biotek Instruments, Inc.).
Antiviral assays
HCE cells (2 × 104 cells/well), seeded in 96-well plates and incubated for 2 days at 37°C, were infected with hrR3 at a multiplicity of infection (MOI) of 0.5. All rinses with S+ medium, 1 × phosphate-buffered saline (PBS), and citrate buffer treatment used 100 μL/well. At 6 h postinfection, β-galactosidase activity was measured by ELISA as we described previously.12 After lysing the cells with 0.5% NP-40 (Sigma Chemical Co.), 2 mM MgCl2 in PBS (50 μL/well) 10 mM Chlorophenol Red-β-D-galactopyranoside, 50 μL/well (Roche Applied Science) was added to each well and the absorbance was measured at 570 nm in a 96-well plate reader (Biotek Instruments). All assays were done in duplicate or triplicate. Note that the peptides tested were oxidized and therefore dimers. We have previously shown that dimerization improves the antiviral activity.11
Comprehensive antiviral assay
Various concentrations of the TAT-Cd0 or the E50,51TAT-Cd0 peptides were mixed with virus (1 × 106 PFU/mL) in 70 μL of S+ medium and incubated for 1 h at 37°C. HCE cells in 96-well plates were treated with various concentrations of peptide (40 μL/well) and incubated for 1 h at 37°C. After incubation, the medium was aspirated from the plate, the virus/peptide mixture was added (40 μL/well), and the plate was incubated at 37°C. At 6 h postinfection, β-galactosidase activity was measured as described above.
Virus inactivation assay
Twenty-five microliters of virus (1 × 106 PFU/mL) was mixed with various concentrations of peptide in 25 μL of S− medium and incubated for 1 h at 37°C. The virus/peptide mixture (40 μL) was then added to 1 mL of ice-cold S+ medium and spun at 13,200 RPM in an Eppendorf 5415 microcentrifuge for 90 min at 4°C. The supernatant was aspirated and the pellet was resuspended in 200 μL of ice-cold S+ medium. Virus was resuspended by sonication using 10 pulses from a microtip at 30% duty cycle (Branson SLPe cell disruptor; Branson Corporation). Fifty microliters of the sonicated solution was diluted in 150 μL of S+ medium and 40 μL of the dilution was applied to a 96-well plate seeded with HCE cells. The plate was incubated at 37°C for 1 h, the cells were washed with 1 × PBS, and extracellular virus was inactivated by treatment with citrate buffer (pH 3.0) for 1 min. The cells were rinsed with 1 × PBS, fed with S+ medium, and incubated at 37°C. The β-galactosidase activity was measured 6 h later.
Cell protection assay
HCE cells were treated with various concentrations of peptide (40 μL/well) and incubated at 37°C for 1 h. The cells were then washed with S+ medium, infected with 40 μL/well of hrR3 (MOI 0.5), and incubated at 37°C. After 1 h, the cells were rinsed with S+ and washed with 1 × PBS, and any remaining extracellular virus was inactivated by treatment with citrate buffer (pH 3.0) for 1 min. The infected cells were rinsed again with 1 × PBS, fed with S+ medium, and placed in a 37°C incubator, and the β-galactosidase activity was measured 6 h later.
Entry inhibition assay
HCE cells were fed with S+ medium and precooled at 4°C for 30 min. The medium was removed, and hrR3 (40 μL/well, MOI 0.5) was allowed to attach to cells at 4°C. After 1 h, cells were rinsed with S+ medium. Various concentrations of peptide were then added (40 μL/well) and the cultures were incubated at 4°C for 15 min before shifting them to 37°C. After 1 h, the cells were washed with 1 × PBS and extracellular virus was inactivated by treatment with citrate buffer (pH 3.0) for 1 min. The infected cells were rinsed with 1 × PBS, fed with S+ medium, incubated at 37°C, and β-galactosidase activity was measured 6 h later.
Attachment assay
HCE cells were fed with S+ medium and precooled at 4°C for 30 min. The hrR3 virus (5 × 107 PFU/mL) was mixed with various concentrations of peptide in S+ and 40 μL of the mixture was added to each well. The plate was then incubated at 4°C for 1 h. After rinsing with 100 μL S−, the cells were fixed with 4% formalin in PBS (100 μL/well) and left at room temperature for 1 h. The cells were washed 3 × with S− medium (100 μL/well) and blocked for 1 h with 3% bovine serum albumin (BSA) in S− medium (100 μL/well). Absorbed virus was detected with a 1:100 dilution of polyclonal rabbit anti-HSV-1 IgG (#B0014; Dako, 90 μL per well) in 0.05% BSA S− medium. After 1 h incubation at room temperature, the cells were rinsed 3 × with 5 min washes of 0.05% BSA in S− medium (100 μL/well). An alkaline phosphatase-conjugated goat-anti rabbit IgG (#A3687; Sigma-Aldrich) was diluted 1:2,000 in 0.05% BSA S− medium and 90 μL was added per well. After 1 h incubation at room temperature, the cells were rinsed 3 × with 5 min washes of 0.05% BSA in S− medium (100 μL/well). The medium was aspirated and an alkaline-phosphatase substrate (BCIP tablet dissolved in 10 mL ddH2O, #B5655; Sigma) was added (50 μL/well). Absorbance (405 nm) was read in a 96-well plate reader (Biotek Instruments, Inc.) for 20 min at 1 min intervals. The amount of binding was determined from the slope of the initial rate, and the signal from mock-infected cultures was subtracted from the data.
Post-entry assay
HCE wells were infected with hrR3 (40 μL/well, MOI 0.5) and incubated at 37°C. After 1 h, cells were rinsed with S+ medium and washed with 1 × PBS, and extracellular virus was inactivated by treatment with citrate buffer (pH 3.0) for 1 min. The infected cells were rinsed again with 1 × PBS, fed with S+ medium, and incubated at 37°C for 1 h. S+ medium was aspirated and various concentrations of peptide in S+ medium were then added (50 μL/well) and the plate was incubated at 37°C. The β-galactosidase activity was measured 6 h later.
Results
Toxicity
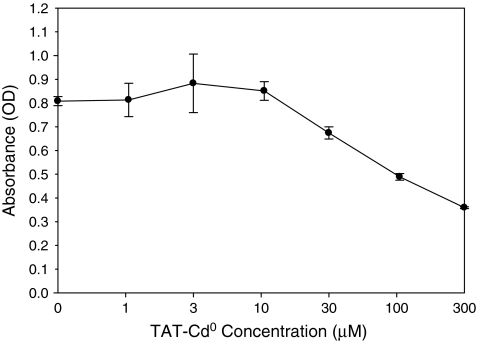
To determine if TAT-Cd0 was toxic, HCE cells were incubated with peptide for 6 h and toxicity was measured using a dye reduction assay. As shown in Fig. 1, the CC50 value was ∼100 μM, indicating little, if any, toxicity. This result was in the same range as other cell lines tested previously with TAT peptides.14
FIG. 1.
MTS assay. The TAT-Cd0 peptide is not cytotoxic to HCE cells. Monolayers of HCE cells in a 96-well plate were treated with various concentrations of the peptides for 6 h at 37°C. After incubation, 20 μL of Celltiter 96 AQqueous One Solution cell proliferation assay reagent (Promega) was added into each well, and 90 min later, the absorbance at 490 nm was read in a 96-well plate reader. Each point and error bar represents the mean ± standard error of triplicate samples. HCE, human corneal epithelial.
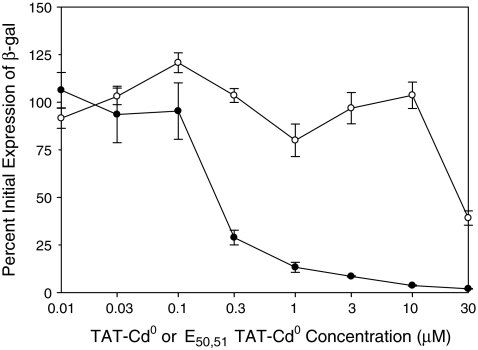
Comprehensive antiviral assay
Antiviral activity was first tested in a comprehensive assay where peptides were incubated with virus before addition of the cells and were present for the entire assay. The sequences and EC50 values of the TAT-Cd0 and E50,51TAT-Cd0 control peptides are shown in Table 1 and the data are shown in Fig. 2. The EC50 value for the control peptide was ∼30 μM compared to 0.20 μM for the TAT-Cd0 peptide. Thus, the antiviral activity was sequence specific and the peptide was effective in preventing the infection of HCE cells by HSV-1. On the basis of these results, the control peptide was not included in subsequent assays.
Table 1.
Peptide Sequences and EC50 Values of the TAT Peptides in the Comprehensive Assay
| Peptide | Sequence | EC50 (μM) |
|---|---|---|
| TAT-Cd0 | NH2-GRKKRRQRRRC-amide | 0.20 |
| E50,51TAT-Cd0 | NH2-GREERRQRRRC-amide | ∼30.0 |
Numbers are means of the EC50 values determined from the comprehensive antiviral assay.
FIG. 2.
Comprehensive antiviral assay. The antiviral activity of TAT-Cd0 (•) and E50,51TAT-Cd0 (○) in the comprehensive assay. Cells and virus were incubated with various concentrations of peptides for 1 h at 37°C. After incubation, the medium was replaced with virus/peptide mixtures and the cultures were incubated further. Six hours later, β-galactosidase activity was determined as a measure of infectivity. Each point and error bar represents the mean ± standard error of triplicate samples.
TAT-Cd has multiple antiviral activities in HCE cells
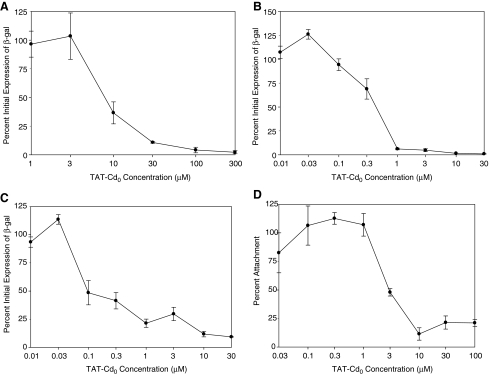
Previous studies with TAT peptides showed that, depending on the peptide, they could be virucidal, inhibit entry, inhibit infection when cells were pretreated with the peptide, and interfere with attachment to cells. To determine if TAT-Cd0 was virucidal, virus was incubated with various concentrations of peptide for 1 h at 37°C and then added to cells. As shown in Fig. 3A and Table 2, TAT-Cd0 was virucidal with an EC50 value of 9.5 μm. Pretreatment of HCE cells with peptide followed by exposure to virus revealed that TAT-Cd0 could induce a state of resistance to infection (Fig. 3B and Table 2) with an EC50 value of 0.4 μM. To determine if TAT-Cd0 was a specific entry inhibitor, virions were attached to cells at 4°C, peptide was added, and the cells were shifted to 37°C. Viral titers were then assayed. As shown in Fig. 3C and Table 2, TAT-Cd0 blocked entry with an EC50 value of 0.1 μM. The TAT-Cd0 peptide also inhibited attachment of virus to cells (Fig. 3D and Table 2). At lower concentrations, there appeared to be a slight enhancement of attachment, but inhibition was evident at concentrations above 1.0 μM. The EC50 value for inhibition of attachment was ∼3.0 μM. When considered together, the results indicate that TAT-Cd0 has multiple activities that inhibit infection of HCE cells by HSV-1.
FIG. 3.
Antiviral assays. Antiviral activities of the TAT-Cd0 peptides. (A) Inactivation of virions in solution. Virus was incubated with various concentrations of peptide at 37°C for 1 h. The mixture was then spun for 90 min; the pellet was resuspended and sonicated and then applied to HCE cells in a 96-well plate. After 1 h of incubation at 37°C, the cells were washed with PBS, treated with citrate buffer (pH 3.0), washed with PBS, and refed with the medium. After 6 h at 37°C, β-galactosidase activity was measured. (B) Cell protection assay. HCE cells were treated with various concentrations of peptide and incubated at 37°C for 1 h. They were then infected with hrR3 (MOI 0.5) and incubated at 37°C for 1 h. The cells were then rinsed with the medium, washed with PBS, treated with citrate buffer (pH 3.0), washed with PBS, and refed with the medium. After 6 h at 37°C, β-galactosidase activity was measured. (C) Entry inhibition assay. HCE cells were precooled at 4°C for 30 min and hrR3 (MOI 0.5) was allowed to attach to cells at 4°C. After 1 h, cells were rinsed with S+ medium and various concentrations of peptide were then added. The cultures were incubated at 4°C for 15 min before shifted to 37°C. After 1 h, the cells were washed with PBS, treated with citrate buffer (pH 3.0), washed with PBS, and refed with the medium. After 6 h at 37°C, β-galactosidase activity was measured. (D) Viral attachment assay. HCE cells were cooled at 4°C for 1 h. The cultures were fixed with 4% formalin and blocked with 3% bovine serum albumin in the medium for 1 h at room temperature. Primary and secondary antibodies were polyclonal rabbit anti-Herpes simplex virus type 1 antibody (Dako) and goat anti-rabbit IgG conjugated with alkaline phosphatase (Sigma), respectively. Absorbance at 405 nm was read and recorded as percent of viral attachment. Each data point and error bar represents the mean ± standard error of duplicate or triplicate samples for all panels. MOI, multiplicity of infection; PBS, phosphate-buffered saline.
Table 2.
EC50 Values (μM) of TAT-Cd0 Peptides in Various Antiviral Assays
| Peptide | Entry block | Postentry | Cell protection | Virus inactivation | Attachment |
|---|---|---|---|---|---|
| TAT-Cd0 | 0.1 | ∼30.0 | 0.4 | 9.5 | 3.0 |
Numbers are means of the EC50 values determined for each of the assays.
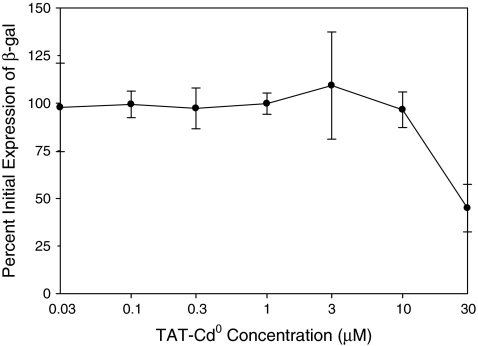
TAT-Cd does not act at a post-entry step
To confirm that the peptide acted at an early step in infection, cells were infected with virus and peptide was added at the end of the adsorption period (1 h after addition of virus). Figure 4 shows that the peptide was not effective when added after virus had entered cells. The EC50 value in the post-entry assay was ∼30 μM, which was over 150-fold higher than the EC50 for the comprehensive assay (Table 1), suggesting that TAT-Cd0 was predominantly acting before entry of virus into the cells.
FIG. 4.
TAT-Cd0 is not active post-infection. HCE wells were infected with hrR3 (MOI 0.5) and incubated at 37°C. After 1 h, cells were rinsed with the medium, washed with PBS, treated with citrate buffer (pH 3.0), washed with PBS, and refed with the medium for 1 h. Various concentrations of peptide were added and after 6 h at 37°C, β-galactosidase activity was measured. Each data point and error bar represents the mean ± standard error of duplicate or triplicate samples for all panels.
Discussion
Cell-penetrating peptides and peptides derived from them are effective antivirals with broad-spectrum activity. These characteristics raise the possibility that such peptides might be useful therapeutic agents. The TAT-C peptides have not been tested for antiviral efficacy in animal models, and this is a necessary step if these peptides are to be useful in treating or preventing infections. Previous work with these peptides used Vero cells, fibroblasts, and MDCK cells to test antiviral activity, which is not necessarily representative of ocular cells that might be targeted in vivo.8–11,13 Before testing TAT-Cd0 peptide in vivo, it was necessary to show that the peptide was active in protecting corneal epithelial cells. The data presented here demonstrate that TAT-Cd0 inhibits infection of HCE cells and that the peptide acts in multiple ways early in infection, including inhibition of entry, protection of pretreated cells, interference with attachment, and killing virions in solution (virucidal activity). The peptide affects multiple activities, and this might be an advantage compared to other antivirals that have a single target.
Previous studies using a number of other cell types14 indicated that CC50 values for the TAT-C peptide were >400 μM. The CC50 value of ∼100 μM with HCE cells suggests that these cells may be more sensitive than other cell types for reasons that are not known. Nevertheless, the TAT-C peptide showed no evidence of toxicity when applied topically to mouse corneas at concentrations >1,000 times the EC50 value.14 Further, the activity of TAT-C peptides is not chiral11 and that the presence of a charged (TAT-Cd−) or neutral (TAT-Cd0) carboxy-terminus has little, if any, effect on the antiviral activity of the peptide.11 Thus, it is unlikely that differences in toxicity are due to the use of slightly different peptides.
In a previous study where TAT-Cd0 was tested using Vero cells, the EC50 values for entry inhibition, cellular resistance, and virucidal activity were 0.9, 0.4, and 34 μM, respectively.11 These values compare favorably with the EC50 values of 0.1, 0.4, and 9.5 μM obtained with HCE cells for these activities. The observation that TAT peptides appear to have multiple activities raises questions as to which activity is most important and whether common or different mechanisms are involved. In both Vero and HCE cells, the EC50 values for entry inhibition and cellular protection are very similar; thus, both of these activities may be equally important. The EC50 values for virucidal activity are substantially higher, suggesting that this activity may be less critical, but the EC50 values suggest that all of these activities would occur at therapeutically relevant concentrations.
In terms of mechanism of action, preliminary data suggest that TAT-Cd0 binds to sialic acid residues on the virus (unpublished data) and cellular uptake (cell penetration) involves binding to heparan sulfate on the cell surface.21,22 It is not surprising that the cationic TAT-Cd0 peptide would bind to negatively charged carbohydrates, but it is not clear how this might be relevant to the antiviral activities or whether this represents a common mechanism. Clearly, binding of TAT-Cd0 to one or more of the HSV-1 fusion complex proteins could affect its assembly or function, but additional studies are needed to address this. Such an activity could be responsible for both the entry inhibition and virucidal activity. Cellular proteins could also involve TAT-Cd0 biding to sialic acids on surface proteins such as gD, gB, and gH co-receptors,23 but, again, further studies are needed. The binding of TAT-Cd0 to heparan sulfate is probably not involved, because we previously showed that TAT-C peptides do not interfere with HSV-1 binding.11
The virucidal activity of TAT-Cd0 raises the possibility that the peptide could be used to prepare an inactivated virus vaccine. However, we previously showed that inactivation was reversible to a level of 1% when TAT-Cd0-treated virions were incubated overnight in the peptide-free medium. This might allow for some level of replication, which might not be acceptable. Whether TAT-Cd0 peptides are immunogenic has not been rigorously addressed, but TAT-coupled moieties have been used for delivery in vivo and there are no reports of TAT-specific immune responses to our knowledge.24
Numerous antiviral peptides have been described in the literature, but few, if any, have been tested for efficacy in animal models. In a previous study, we tested a modified cationic θ-defensin (RC-2) in our murine model of HSV-1 keratitis.15 The peptide showed modest efficacy when preincubated with peptide, but was not effective in reducing clinical scores when treatment was started 4 h postinfection, even though reductions in titer were seen in this treatment paradigm.15 The activity, or lack thereof, could be affected by the formulation of the test article or pharmacokinetic properties of the peptide in the cornea. We used 2% methylcellulose in PBS, which likely increased the residence time on the corneal surface, but may not have enhanced penetration into the cornea. Because the TAT-Cd0 peptide is also cationic, proceeding with in vivo efficacy testing might yield the same result seen with RC-2. Before animal work, studies, such as this one, as well as formulation and pharmacokinetic studies may be necessary to design the optimum test of efficacy.
Acknowledgments
The authors would like to thank Dr. Araki-Sasaki for providing the HCE cell line, and Dr. Hermann Bultmann for technical advice and comments on the article. These studies were supported by grants from the NIH (R01 EY018597) to C.R.B., Vision Research Core grant P30 EY016665, a Research to Prevent Blindness Senior Scientist Award to C.R.B., and by an unrestricted grant from Research to Prevent Blindness, Inc., to the Department of Ophthalmology and Visual Sciences.
Author Disclosure Statement
No competing financial interests exist for Dr. Brandt or Ms. Larsen
References
- 1.Whitley R.J. Herpes simplex viruses. In: Fields B.N., editor; Knipe D.M., editor; Howley P.M., editor. Fields Virology. 3rd. Vol. 2. Philadelphia: Lippincott-Raven; 1996. pp. 2297–2342. [Google Scholar]
- 2.Liesegang T.J. Epidemiology and natural history of ocular herpes simplex virus infection in Rochester, Minnesota, 1950–1982. Trans. Am. Ophthalmol. Soc. 1998;86:688–724. [PMC free article] [PubMed] [Google Scholar]
- 3.Liesegang T.J. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelmus K.R. Gee L. Hauck W.W., et al. Herpetic Eye Disease Study. A controlled trial of topical corticosteroids for herpes simplex stromal keratitis. Ophthalmology. 1994;101:1883–1895. doi: 10.1016/s0161-6420(94)31087-6. [DOI] [PubMed] [Google Scholar]
- 5.Herpetic Eye Disease Study Group. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N. Engl. J. Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 6.Brandt C.R. Coakley L.M. Grau D.R. A murine model of herpes simplex virus-induced ocular disease for antiviral drug testing. J. Virol. Methods. 1992;36:209–222. doi: 10.1016/0166-0934(92)90052-f. [DOI] [PubMed] [Google Scholar]
- 7.Brandt C.R. Spencer B. Imesch P. Garneau M. Deziel R. Evaluation of a peptidomimetic ribonucleotide reductase inhibitor with a murine model of herpes simplex virus type 1 ocular disease. Antimicrob. Agents Chemother. 1996;40:1078–1084. doi: 10.1128/aac.40.5.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bultmann H. Busse J.S. Brandt C.R. Modified FGF4 signal peptide inhibits entry of herpes simplex virus type 1. J. Virol. 2001;75:2634–2645. doi: 10.1128/JVI.75.6.2634-2645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bultmann H. Brandt C.R. Peptides containing membrane-transiting motifs inhibit virus entry. J. Biol. Chem. 2002;277:36018–36023. doi: 10.1074/jbc.M204849200. [DOI] [PubMed] [Google Scholar]
- 10.Jones J.C. Turpin E.A. Bultmann H. Brandt C.R. Schultz-Cherry S. Inhibition of influenza virus infection by a novel antiviral peptide that targets viral attachment to cells. J. Virol. 2006;80:11960–11967. doi: 10.1128/JVI.01678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bultmann H. Teuton J. Brandt C.R. Addition of a C-terminal cysteine improves the anti-herpes simplex virus activity of a peptide containing the human immunodeficiency virus type 1 TAT protein transduction domain. Antimicrob. Agents Chemother. 2007;51:1596–1607. doi: 10.1128/AAC.01009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akkarawongsa R. Porcaro N.E. Case G. Kolb A.W. Brandt C.R. Multiple peptides homologous to herpes simplex virus type 1 glycoprotein B inhibit viral infection. Antimicrob. Agents Chemother. 2009;53:987–996. doi: 10.1128/AAC.00793-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altmann S.E. Jones J.C. Schultz-Cherry S. Brandt C.R. Inhibition of Vaccinia virus entry by a broad spectrum antiviral peptide. Virology. 2009;388:248–259. doi: 10.1016/j.virol.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akkarawongsa R. Cullinan A.E. Zinkel A. Clarin J. Brandt C.R. Corneal toxicity of cell-penetrating peptides that inhibit herpes simplex virus entry. J. Ocular Pharm. Ther. 2006;22:279–289. doi: 10.1089/jop.2006.22.279. [DOI] [PubMed] [Google Scholar]
- 15.Brandt C.R. Akkarawongsa R. Altmann S., et al. Evaluation of a θ-defensin in a murine model of herpes simplex virus type 1 keratitis. Invest. Ophthalmol. Vis. Sci. 2007;48:5118–5124. doi: 10.1167/iovs.07-0302. [DOI] [PubMed] [Google Scholar]
- 16.Araki-Sasaki K. Ohashi Y. Sasabe T., et al. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- 17.Goldstein D.J. Weller S.K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J. Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grau D.R. Visalli R.J. Brandt C.R. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Invest. Ophthalmol. Vis. Sci. 1989;30:2474–2480. [PubMed] [Google Scholar]
- 19.Visalli R.J. Brandt C.R. The HSV-1 UL45 18 kDa gene product is a true late protein and a component of the virion. Virus Res. 1993;29:167–178. doi: 10.1016/0168-1702(93)90057-t. [DOI] [PubMed] [Google Scholar]
- 20.Segel I.H. Biochemical Calculations. 2nd. New York: John Wiley & Sons; 1976. [Google Scholar]
- 21.Tyagi M. Rusnati M. Presta M. Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 2001;276:3254–3261. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 22.Ziegler A. Seelig J. Interaction of the protein transduction domain of HIV-1 TAT with heparan sulfate: binding mechanism and thermodynamic parameters. Biophys. J. 2004;86:252–263. doi: 10.1016/S0006-3495(04)74101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spear P.G. Longnecker R. Herpesvirus entry: an update. J. Virol. 2003;77:10179–10185. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richard J.P. Melikov K. Vives E., et al. Cell penetrating peptides. J. Biol. Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]