Abstract
Heliothis virescens larval plasma contains high levels of an antiviral activity against the budded form of the Helicoverpa zea single nucleopolyhedrovirus (HzSNPV) in vitro. Preliminary results indicated that phenoloxidase is primarily responsible for this virucidal effect. However it is known that other enzymes that generate antimicrobial reactive oxygen intermediates and reactive nitrogen intermediates are present in hemolymph that could contribute to the observed virucidal activity. To elucidate the contributions of phenoloxidase and other candidate activities to plasma innate immune response against baculovirus infection specific metabolic inhibitors were used. In vitro the general inhibitors of melanization (N-acetyl cysteine, ascorbate and glutathione), and specific inhibitors of phenoloxidase (phenylthiourea and Kojic acid), completely blocked virucidal activity up to the level seen in controls. Addition of the enzyme superoxide dismutase to plasma did not affect virucidal activity; however addition of catalase had an inhibitory effect. Inhibitors of nitric oxide synthase activity did not affect virucidal activity. Our results confirm that phenoloxidase is the predominate activity in larval plasma accounting for inactivation of Hz SNPV in vitro, and that phenoloxidase-dependent H2O2 production may contribute to this virucidal activity.
Keywords: catalase, hydrogen peroxide, HzAM-1 cells, HzSNPV, Kojic acid, nitric oxide synthase, superoxide, superoxide dismutase
Abbreviations: HzSNPV, Helicoverpa zea single nucleopolyhedrosis virus; HvPPO, Heliothis virescens prophenoloxidase; HvPO, Heliothis virescens phenoloxidase; PTIO, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3 oxide
INTRODUCTION
Insects possess some of the innate antiviral immune responses homologous to those present in vertebrates. For example, Drosophila melanogaster may utilize the mechanism of RNA interference to resist infection by the flock house virus (Li et al. 2002). Recently it has been reported that recognition of an infection caused by Drosophila X virus in Drosophila larvae may be mediated by a toll receptor (Zambon et al. 2005). The plasma protein hemolin is induced by baculovirus infection of the Chinese oak silkmoth, Antheraea pernyi, and other lepidoptera (Hirai et al. 2004). Infection with the tomato spotted wilt virus of its vector insect, the western flower thrip, Frankliella occidentalis, resulted in upregulation of a suite of antimicrobial response genes homologous to those identified in other insects (Medeiros et al. 2004). The primary mechanism of lepidopteran larval resistance to per os baculovirus infection is clearing of infected midgut cells by apoptosis (Clarke and Clem 2003). Cell-mediated antiviral immune mechanisms utilizing melanization of infective foci have also been observed in the hemocoel of infected larvae, i.e. melanotic encapsulation of AcMNPV infected midgut and tracheoblast cells by corn earworm,Helicoverpa zea, hemocytes (Washburn et al. 1996;Washburnet al. 2000;Trudeau et al. 2001).
Beyond the infective foci of the midgut and tracheoblasts, levels of the plasma enzyme phenoloxidase [PO: L-DOPA: oxygen oxidoreductase; EC 1.14.18.1] in larval lepidoptera are correlated with resistance to baculovirus infection (Wilson et al. 2001). Plasma phenoloxidase (HvPO) of the tobacco budworm, Heliothis virescens (F.), has been demonstrated to exhibit in vitro a virucidal activity against several vertebrate viruses (Ourth and Renis 1993; Ourth 2004) and against the budded form of the baculovirus H. zea single nucleopolyhedrosis virus (HzSNPV) (Popham et al. 2004). In this report further evidence is provided for inactivation of baculovirus budded virus particles by phenoloxidase in isolated plasma via phenoloxidase-dependent production of reactive oxygen species.
MATERIALS AND METHODS
Chemicals
Chemicals, enzymes and inhibitors were purchased from Sigma Chemical Co. (www.sigmaaldrich.com).
Insects, and insect diets
H. virescens eggs were received from the North Carolina State University Dept. of Entomology Insectary from a colony established from field insects in July of 2002. Larvae were reared individually on an artificial wheat germ based diet (Catalog # F9781B, BioServe, www.bio-serv.com) under a photoperiod of 14:10 L:D at 55% relative humidity at 28°C to the appropriate assay instar. This diet contained a basal level of ascorbic acid of 2.4 g/L (personal communication, BioServe technical staff). Diets with elevated levels of ascorbic acid were prepared by adding ascorbic acid in multiples of 2.4 g/L to the basal amount during mixing. Pupation and emergence times were determined by the ViStat 2.1 program (Hughes 1990).
Insect cells and virus
An H. zea cell line, HzAM-1, was maintained as monolayers at 28°C in Excel 401 medium (JRH Biosciences, www.jrhbio.com) supplemented with 10% fetal bovine serum (Integen Co., www.intergenco.com). Wild type HzSNPV isolate was used and amplified in Hz AM-1 cells (Popham et al. 2004).
Collection and Processing of Plasma
Staged larvae were surface sterilized in ethanol, rinsed with sterile water, and anesthetized on ice before bleeding. Hemolymph was gently extruded from an anterior proleg, through a small puncture wound made with a sterile 26 gauge needle, and collected directly into a chilled 1.5 ml microcentrifuge tube containing ice cold, sterile PBS (50 mM NaHPO4, pH 6.8) (Popham et al. 2004). Hemolymph was adjusted to a final dilution of 1:10 by addition of cold PBS after which hemocytes were removed by centrifugation at 8,000 rpm for 3 minutes. The plasma supernatant was sterilized by centrifugation through a 0.65 micron Millipore Ultrafree™-MC centrifugal filter (Millipore, Inc., www.waters.com). Aliquots of each filter sterilized plasma collection were allowed to stand at room temperature for two hours to individually monitor the extent of melanization. When used, inhibitors were added to buffer before hemolymph collection at the concentrations indicated.
Plasma in vitro virucidal assay
Virucidal activity in larval H. virescens plasma was quantified by endpoint dilution assay as detailed (Popham et al. 2004). In brief, plasma dilutions were combined with Hz SNPV at a ratio of 3:1 (v/v) and allowed to incubate at 20°C for 1 h. PBS was used as a control in the absence of plasma. Viral titers of these incubations were determined by end-point dilution assay (Slavicek et al. 2001). HzAM-1 cells were seeded at 5 x 104 cells/ml in P96 well plates (BD Falcon, www.bdbiosciences.com) and allowed to attach for 1 h. The cells were infected with dilutions of virus/plasma or virus/PBS at dilutions of 10−1 to 10−6 and plates were incubated for one week at 28°C. The plate wells were then scored positive, if polyhedra were visible within two or more cells, or negative for viral infection, and the results were used to calculate the viral titer as the tissue culture infectious dose per ml (TCID50/ml) of inoculum. Plasma samples were assayed in quadruplicate. Statistical comparisons were done using SigmaStat (SPSS Inc.,www.spss.com).
Phenoloxidase activity assay
HvPO activity was measured according to a modification of Jiang et al. 2003 using dopamine as a substrate. Twenty μl of diluted plasma was mixed with 800 μl of 2 mM dopamine in 50 mM sodium phosphate, pH 6.5, and the change in absorbance at 472 nm was then monitored using a BioMate-3 spectrophotometer (ThermoSpectronic, Rochester, NY) in triplicate.
RESULTS
Virucidal activity in H. virescens larval plasma was measured throughout development from the late 3rd instar until the late 5th instar (Table 1). High activity, two orders of magnitude reduction in viral survival, was evident from the late 3rd until the early 5th instar, but declined almost to control PBS buffer levels as 5th instar development proceeded (data not shown). For this reason, and because larger volumes of plasma could be more easily collected, early 5th instar larvae were bled for all experiments shown below.
Table 1.
Plasma antiviral activity against HzSNPV over the course of the 3rd, 4th, and 5th larval instars of H. virescens.
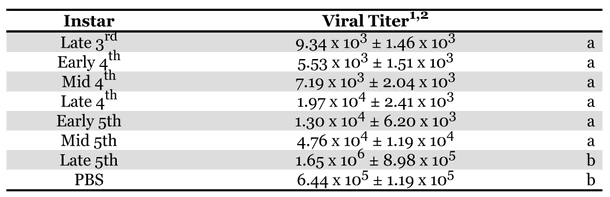
If virucidal activity were dependent upon the activity of HvPO, then the two activities should be strongly correlated. We therefore measured plasma HvPO activity according to Jiang et al 2003, and virucidal activity in the same plasma fractions (Figs. 1A and 1B). High HvPO activity was observed in all 1/10 diluted plasma samples; the optimum dilution empirically determined for virucidal activity assays (Popham et al., 2004). HvPO enzymatic activity was rapidly diluted by the addition of PBS (n = 4, p < 0.02, Student-Newman-Keuls multiple comparison test; Fig. 1A). HvPO activity was not evident at dilutions below 1/30 during the 10 minute incubations (Fig 1A). Virucidal activity in the same fractions also was rapidly diluted, becoming undetectable at the 1/40 dilution in the standard one hour incubation period (n = 4, p < 0.001, Tukey multiple comparison test; Fig. 1B), c.f.Popham et al. 2004.
Figure 1.
Correlations between phenoloxidase and virucidal activity in early 5th instar larval Heliothis virescens plasma dilutions. (A) Spectrophotometric determination of phenoloxidase activity in serial dilutions of plasma. PBS, phosphate buffered saline. (B) Virucidal activity determined from the same fractions (n = 3, mean ± SEM).
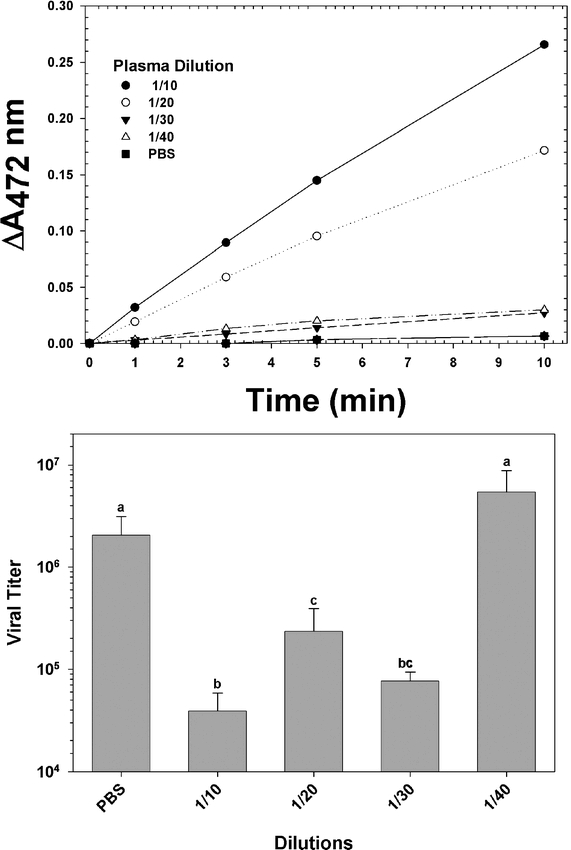
Addition of serine proteinase inhibitors, that prevent the activation of HvPPO to HvPO, and of the inhibitor phenylthiourea, that chelates copper from the active site of the enzyme, was previously demonstrated to inhibit plasma virucidal activity in larval H. virescens plasma (Popham et al. 2004). To further elucidate the enzymatic nature of the virucidal activity, additional specific inhibitors of HvPO activity were sought. Kojic acid (5-hydroxy-2-hydroxymethyl-γ-pyrone) is a specific competitive inhibitor of insect phenoloxidases (Dowd 1988; Chen et al. 1991a; Chen et al. 1991b; Li and Kubo 2004). Preincubation of diluted early 5th instar H. virescens plasma with 1 mM Kojic acid completely abolished virucidal activity (n = 4, p < 0.013, Student-Newman-Keuls multiple comparison test; Fig. 2). Additionally, the copper chelator diethyldithiocarbamic acid when fed to larvae has been reported to suppress resistance to infection with the baculovirus AcMNPV (Washburn et al. 1996). This inhibitor proved to be too toxic to HzAM-1 cells and thus could not used in further experiments (data not shown).
Figure 2.
The effect of specific phenoloxidase inhibitors on larval Heliothis virescens plasma virucidal activity. The inhibitors were added to diluted plasma samples before the addition of HzSNPV. PBS, No plasma control; Kojic, 1 mM Kojic acid control; Kojic + Plasma, 1 mM Kojic acid added to plasma (letters indicate significant differences between treatments; n = 4, mean ± SEM).
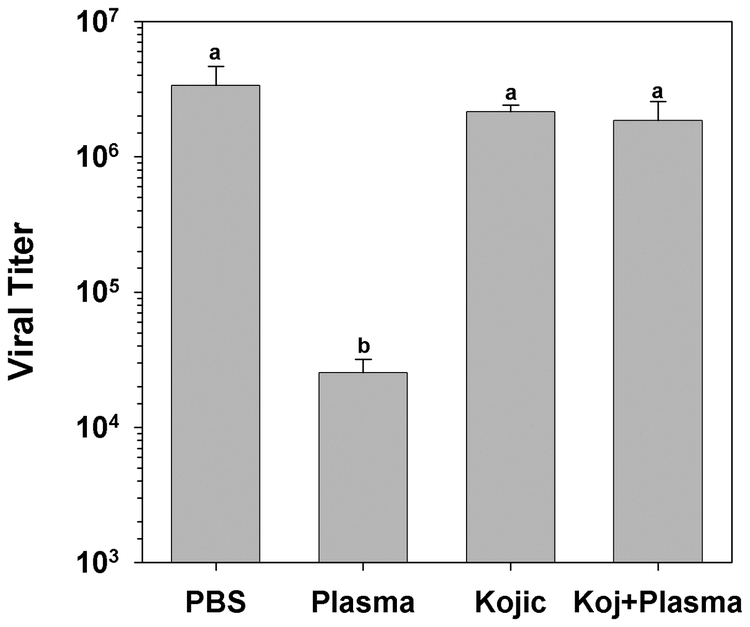
Several reactive free radicals have been detected in the hemolymph of insects in addition to those generated by phenoloxidase (Nappi et al. 2004). Activated phenoloxidase generates a toxic cloud of free radicals including cytotoxic quinones and reactive oxygen species (Nappi et al. 2004). In order to rule out a contribution of other free radical generating enzymes from consideration, we examined the effect of several free radical scavengers upon plasma virucidal activity (Fig. 3). The free radical scavenger N-acetyl-cysteine inhibited virucidal activity, as did reduced glutathione. Addition of the nitric oxide scavenger PTIO to plasma before addition of HzSNPV had no detectable effect upon virus survival (n = 4, p < 0.011, Student-Newman-Keuls multiple comparison test; Fig. 3).
Figure 3.
The effect of the free radical scavengers N-acetyl cysteine (NAC), reduced glutathione (GSH) and 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3 oxide (PTIO) on larval early 5th instar Heliothis virescens plasma virucidal activity. NAC control (40 mM); NAC+Plasma, NAC (40 mM) mixed with plasma; GSH (10 mM) control; GSH+Plasma, GSH (10 mM) mixed with plasma; PTIO, PTIO control (100 μM); PTIO+Plasma, plasma mixed with 100 μM PTIO. The inhibitors were added to diluted plasma samples before the addition of HzSNPV (letters indicate significant differences between treatments; n = 4, mean ± SEM).
In separate experiments we had observed that inclusion of excess dietary ascorbic acid inhibited the melanization activity of larval H. virescens plasma. High plasma levels of ascorbic acid might then be expected to alter the melanization-dependent virucidal activity. Larvae were reared on artificial diet supplemented with increasing amounts of ascorbic acid, and pupation, adult emergence and mortality data were collected. The time required for larvae to progress to pupation and adult emergence increased with the concentration of added dietary ascorbic acid (Fig. 4A). Larvae fed basal diet (2.4 mg/ml) attained 50% pupation after 12.8 ± 0.1 days and 50% had emerged as adults by 24.8 ± 0.2 days. When dietary ascorbic acid levels were raised to 12.0 to 48.0 mg/ml (5 to 20 times the basal level) pupation was delayed until 13.2 ± 0.2, 14.8 ± 0.2, and 17.6 ± 0.4 days, while emergence was delayed until 25.3 ± 0.3, 27.3 ± 0.3, and 29.5 ± 0.5 days, with highest mortality at the highest concentration of ascorbic acid (Fig. 4A). Pupal weights did not differ significantly between those reared on diets containing from basal to 24.0 mg/ml ascorbate (246.5 ± 26.5, 247.8 ± 33.4, and 250.7 ± 26.6 mg/pupa) (Fig. 4B). However, the pupal weight attained by larvae reared on diet containing 48.0 mg/ml ascorbic acid was significantly lower than the other groups (220.8 ± 28.2 mg, n = 30, p < 0.018, Dunn’s pairwise multiple comparison test; Fig. 4B) which correlated with the very high mortality of this group (62%).
Figure 4A.
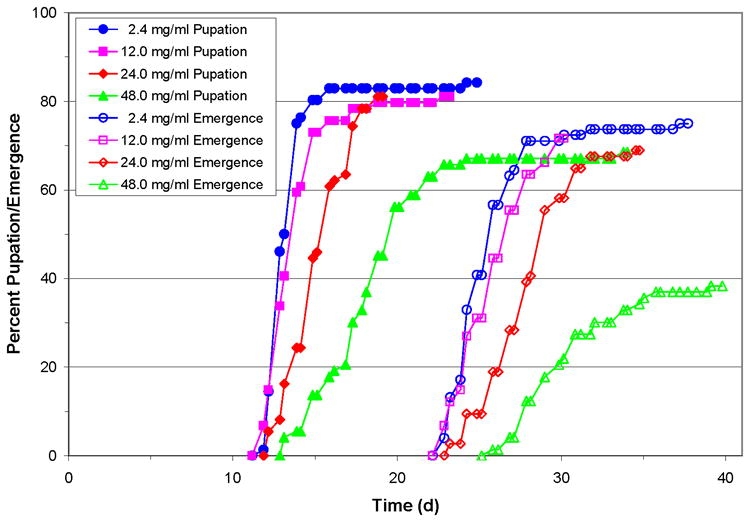
Elevated levels of dietary ascorbic acid resulted in delayed pupation and adult emergence times of Heliothis virescens larvae compared to those reared on basal diet. Diets were supplemented with 12.0, 24.0, and 48.0 mg/ml ascorbic acid (5 to 20 times the basal level (2.4 mg/ml) of ascorbic acid. Larvae were placed on the diets as neonates (n = 75).
Figure 4B.
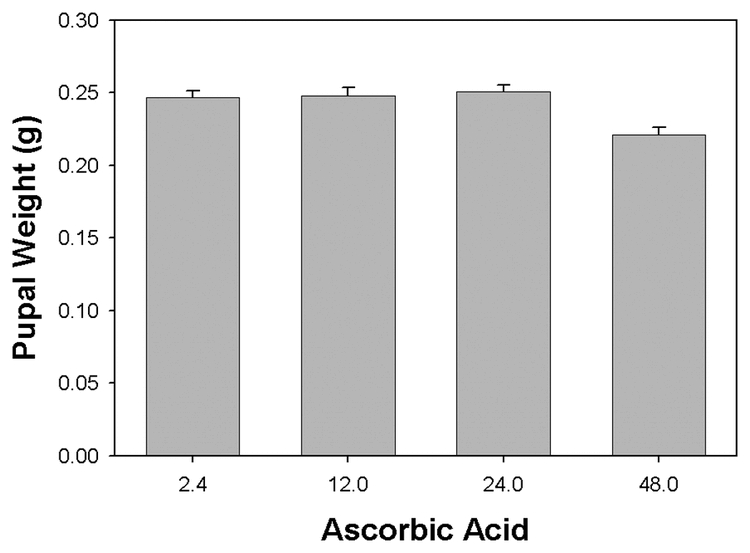
Elevated levels of dietary ascorbic acid resulted in reduced pupal weights attained by Heliothis virescens larvae compared to those reared on basal diet. Basal diet was supplemented with 12.0, 24.0 and 48.0 mg/ml ascorbic acid (basal level 2.4 mg/ml). Larvae were placed on the diets as neonates (letters indicate significant differences between treatments; n = 30, mean ± SEM).
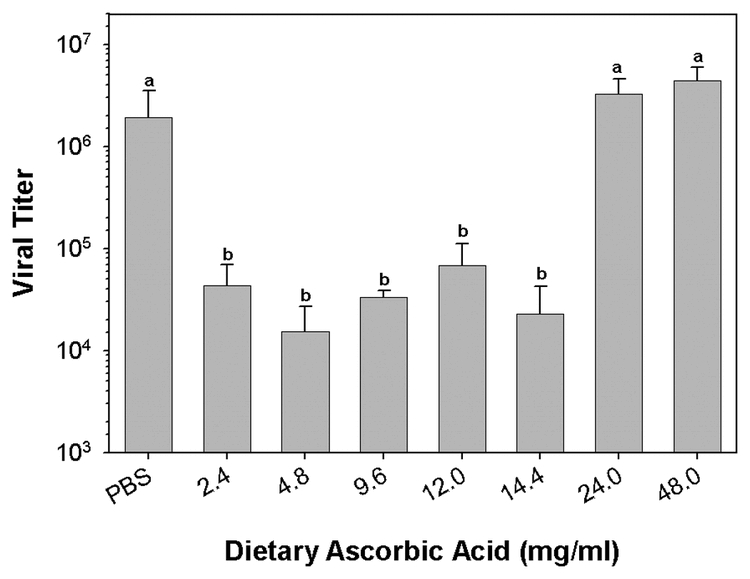
Plasma collected from early 5th instar H. virescens larvae reared on basal diet, and from larvae reared on diets supplemented with from 4.8 to 14.4 mg/ml ascorbic acid, did not exhibit significant inhibition of virucidal activity (n = 4, p < 0.001, Dunn’s method multiple comparison test; Fig. 5A). However, plasma collected from larvae reared on diets with from 24.0 to 48.0 mg/ml ascorbic acid contained no residual virucidal activity (Fig. 5A). Because high dietary levels of ascorbic acid may result in higher plasma concentrations of ascorbic acid it is probable that HvPO activity was reduced in these larvae. We did not measure plasma ascorbic acid concentrations following dietary supplementation. However, in support of the hypothesis, aliquots of the diluted plasma collected from larvae reared on the 24.0 and 48.0 mg/ml ascorbic acid diets failed to melanize. Aliquots of plasma collected from basal diet fed larvae, and 4.8 to 14.4 mg/ml ascorbic acid fed larvae did visibly melanize. Visible melanization of plasma aliquots correlated very strongly with plasma virucidal activity. Addition of increasing concentrations of ascorbic acid directly to plasma from larvae reared on basal diet exhibited a similar pattern of inhibition of virucidal activity (Fig. 5B). In vitro, virucidal activity was completely inhibited at concentrations of 10 and 50 mg/ml (n = 4, p < 0.002, Student-Newman-Keuls multiple comparison test; Fig. 5B).
Figure 5A.
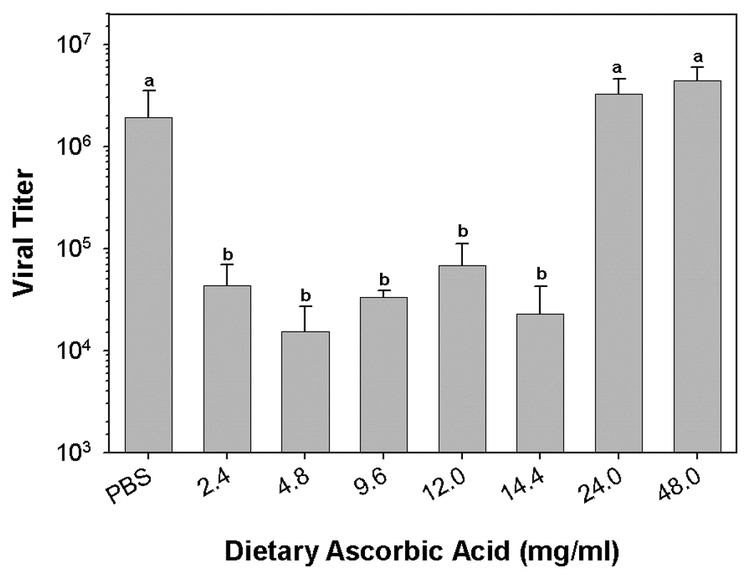
Elevated levels of dietary ascorbic acid inhibit 5th instar larvalHeliothis virescens plasma virucidal activity. Larvae were reared on basal diet from the neonate stage, and on diets supplemented with 4.8 to 48.0 mg/ml ascorbic acid (basal level 2.4 mg/ml). Plasma was collected from early 5th instars for assay of virucidal activity (letters indicate significant differences between treatments; n = 4, mean ± SEM).
Figure 5B.
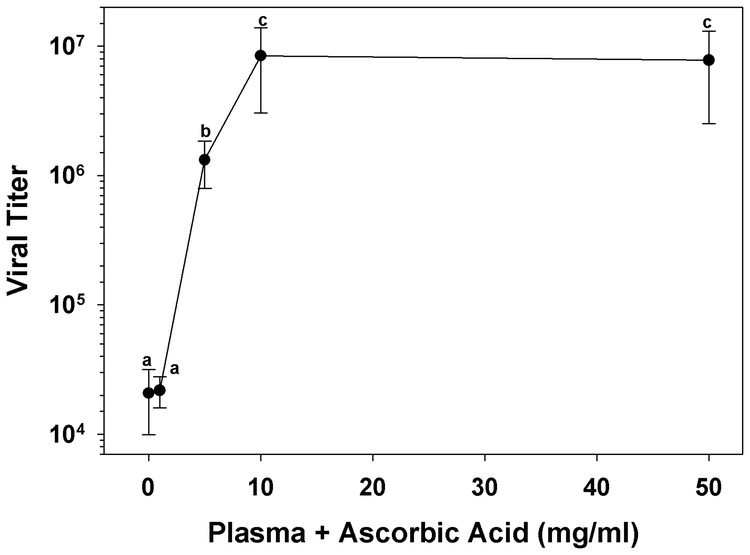
Ascorbic acid inhibits the in vitro plasma virucidal activity present in 5th instar larval Heliothis virescens HzSNPV. Following a 1 hr incubation with HzSNPV plasma was assayed for virucidal activity (letters indicate significant differences between treatments; n = 4, mean ± SEM).
Addition of the enzyme catalase, which inactivates H2O2, to diluted H. virescens plasma partially inhibited the virucidal activity (n = 4, p < 0.013, Student-Newman-Keuls multiple comparison test; Fig. 6). Addition of the same amount of catalase to HzAM-1 cells had no effect on the survival of added HzSNPV. Addition of the enzyme superoxide dismutase, which inactivates superoxide, had no effect on virucidal activity. Addition of superoxide dismutase to HzAM-1 also had no effect on virus survival. This suggests that at least some of the virucidal enzymatic free radicals generated by HvPO may be H2O2.
Figure 6.
Enzymatic scavenging of the reactive oxygen species H2O2 from early 5th instar larvalHeliothis virescens plasma reduces virucidal activity. PBS, No plasma control; Cat, catalase (100 U/ml) control; Cat+Plasma, catalase incubated with plasma; superoxide dismutase (100 U/ml) control; superoxide dismutase +Plasma, incubation of plasma with the enzyme superoxide dismutase (100 U/ml). The enzymes were added to diluted plasma samples before the addition ofHzSNPV (letters indicate significant differences between treatments; n = 4, mean ± SEM).
DISCUSSION
A number of observations converge upon phenoloxidase activity as an important determinant of resistance to entomopathogens. Density-dependent prophylaxis against a wide variety of microbial and viral pathogens observed in lepidopteran larvae is directly correlated with elevated cuticular and plasma melanizing capability,i.e. phenoloxidase activity (Wilson et al. 2001). Conversely, reduction of plasma melanizing activity, by targeting prophenoloxidase expression in the mosquito Armigeres subalbatus with a Sindbis virus encoding an antisense transcript, eliminated melanization ofDirofilaria immitis microfilaria (Shiao et al. 2001). Inhibition of the host melanization response by parasitoids, filarial parasites, bacteria, as well as by entomopathogenic fungi appears to be abettedvia reduced phenoloxidase activity (Beerntsen et al. 2000; Shelby et al. 2000). Plasma of larval H. virescens, has been demonstrated to exhibit antiviral activity against several vertebrate viruses in vitro (herpes simplex virus types 1 and 2, vesicular stomatitis virus, parainfluenza-3, coxsackie B3, Sindbis virus, and HIV-1) (Ourth and Renis 1993;Ourth 2004) and in addition inactivates the baculovirus HzSNPV (Popham et al. 2004). Susceptibility of Helicoverpa zea larvae to AcMNPV infection was elevated by addition of the phenoloxidase-inhibiting copper chelators phenylthiourea and diethyldithiocarbamic to the diet (Washburn et al. 1996).
Susceptibility of larval lepidoptera to fatal baculovirus infection generally decreases during development, a phenomenon known as developmental resistance (Hoover et al. 2002; Kirkpatrick et al. 1998). Instar specific and intrastadial differences in the sloughing of infected midgut cells appears to be the primary mode of resistance to AcMNPV infection of H. virescens larvae (Kirkpatricket al. 1998; Engelhard and Volkman 1995). Intrastadial resistance or variable susceptibility within instars may occur. Mortality following LdMNPV infection of gypsy moth, Lymantria dispar, 4th instar larvae exhibited a biphasic pattern with mortality greatest at the beginning and at the end of the instar (Hoover et al. 2002). This pattern was obtained both for per os infection and intrahaemocoelic injection indicating that resistance factors operating beyond the midgut barrier (i.e., viral clearing from the hemolymph, or cells becoming refractory to infection) were present (Hoover et al. 2002; and references therein). Developmental resistance of H. virescens larvae to AcMNPV infection increased during the first 18 hrs of the 4th instar, but decreased thereafter, while intrahaemocoelic injections of virus exhibited no evidence of developmental resistance (Kirkpatrick et al. 1998).
We have previously reported the inhibitory effects of phenylthiourea and serine proteinase inhibitors on the in vitro virucidal activity ofH. virescens larval plasma against HzSNPV (Popham et al. 2004). Using the fungal metabolite Kojic acid as a specific competitive inhibitor of insect phenoloxidases (Dowd 1988; Chen et al. 1991a; Chen et al. 1991b; Li and Kubo 2004) we found that the virucidal activity present in early 5th instar H. virescens larval plasma is dependent upon HvPO activity. These data in combination provide strong evidence that HvPO has a strong virucidal activity against the budded form of baculoviruses.
Generation of cytotoxic free radicals (e.g., semiquinones, superoxide, hydrogen peroxide, nitric oxide) in the hemolymph of insects by soluble enzymatic activities and by lipopolysaccharide-stimulated hemocytes are known responses to microbial, filarial and parasitoid infection (Nappi et al. 2004; Nappi and Christenson 2005). In the larval waxworm, Galleria mellonella, phenoloxidase-dependent production of DOPA-semiquinone free radicals is inhibited by fungal infection (Slepnevaet al. 2003). The possible virucidal activity of these free radicals generated in the plasma of infected insects has yet to be adequately explored. Inhibition of H2O2 generation by inclusion of the enzyme catalase in the in vitro reaction substantially reduced activity. H2O2 is a known byproduct of phenoloxidase activity (Komarov et al. 2005), and this suggests that free radicals produced by phenoloxidase cause viral inactivation. A superoxide- and H2O2-generating antimicrobial adduct of glutathione and β-alanyl-DOPA (N-β-Alanyl-5-S-glutathonyl-3,4-dihydroxyphenylalanine) was isolated from immunized flesh flies, Sarcophaga peregrina, and also is a byproduct of melanization (Leemet al. 1996). Plasma ascorbic acid acts as a free radical scavenger. Excess dietary ascorbic acid suppressed hemolymph melanization, and reduced the melanization of Sephadex beads or Plasmodium berghei by Anopheles gambiae (Kumar et al. 2003). WhenH. virescens larvae were reared on diet containing excess dietary ascorbic acid plasma melanization and the virucidal activity were completely inhibited. Thus, the free radical scavengers ascorbic acid, N-acetyl cysteine, and reduced glutathione, all of which block larval H. virescens plasma melanization, also block plasma virucidal activity against HzSNPV.
Nitric oxide inhibits replication of viruses within infected vertebrate cells, and acts against a number of parasitic infestations in a variety of vertebrate and invertebrate model systems in vitro and in vivo (Nappi et al. 2004; Foley and O’Farrell 2003; Ascenzi and Gradoni 2002; Luckhart et al. 1998). By including the efficient nitric oxide scavenger, PTIO, in the virucidal activity assay we found that nitric oxide generation does not play a detectable role against HzSNPV in vitro. Although our initial results indicate that nitric oxide generation does not account for any significant virucidal activity in larval plasma, the possibility that generation of nitric oxide by other tissues such as hemocytes or fat body, which were excluded from our analysis, are involved in immune reactions cannot be excluded (Nappi et al. 2004).
In summary, specific chemical inhibitors of phenoloxidase, or of prophenoloxidase activation, and addition of the enzyme substrate dopamine, provide strong evidence that a constitutive humoral innate antiviral immune response attributable to the activity of phenoloxidase is capable of limiting baculovirus infection beyond the midgut barrier in lepidopteran larvae. Taken together these data establish that products of the plasma enzyme phenoloxidase, including superoxide, contribute to the observed virucidal activity against baculoviruses, and against other classes of viruses. If plasma phenoloxidase constitutes an innate immune response against viral infection in vivo then foliar compounds which inhibit phenoloxidase activity may also alter observed mortality to microbial infections (Dowd 1988; Dowd 1999; Kubo et al., 2003). For example, foliar phenolics such as tannic, chlorogenic, and caffeic acids have been demonstrated to inhibit melanization and to influence susceptibility to baculovirus infection (Feldman et al. 1999; Ali et al. 1999; Hoover et al. 1998;Hoover et al. 2000). Although one mode of action of these inhibitors appears to be within the lumen of the midgut, our data indicate that inhibitory compounds that diffuse from the lumen into the hemolymph may inhibit phenoloxidase activity in plasma, and may thereby alter susceptibility to viral infection. The importance of these results should be weighed in relation to other insect-transmitted viruses that often must traverse the hemocoelic immune barriers of the vector. Therefore plasma phenoloxidase may be involved in vector competence of arthropods that transmit viruses to vertebrates or plants (Medeiros et al. 2004;Beerntsen et al. 2000).
Acknowledgments
The authors thank Larry Brown, John Willenberg, and Darrell Davis for technical assistance.
Names are necessary to report factually on available data: however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by the USDA implies no approval of the product to the exclusion of others that may also be suitable. All programs and services of the U.S. Department of Agriculture are offered on a non-discriminatory basis without regard to race, color, national origin, religion, sex, age, marital status, or handicap.
REFERENCES
- Ali M. I., Bi J. L., Young S. Y., Felton G. W. Do foliar phenolics provide protection to Heliothis virescens from a baculovirus. Journal of Chemical Ecology. 1999;25:2193–2204. [Google Scholar]
- Ascenzi P., Gradoni L. NO limits parasite development in vectors and in invertebrate intermediate hosts. IUBMB Life. 2002;53:121–123. doi: 10.1080/15216540211472. [DOI] [PubMed] [Google Scholar]
- Beerntsen B. T., James A. A., Christensen B. M. Genetics of mosquito vector competence. Microbiology and Molecular Biology Reviews. 2000;64:115–137. doi: 10.1128/mmbr.64.1.115-137.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. S., Wei C., Marshall M. R. Inhibition mechanism of Kojic acid on polyphenol oxidase. Journal of Agricultural Food Chemistry. 1991a;39:1897–1901. [Google Scholar]
- Chen J. S., Wei C., Rolle R. S., Otwell W. S., Balban M. O., Marshall M. R. Inhibitory effect of Kojic acid on some plant and crustacean polyphenol oxidases. Journal of Agricultural Food Chemistry. 1991b;39:1396–1401. [Google Scholar]
- Clarke T. E., Clem R. J. Insect defenses against virus infection: the role of apoptosis. International Review of Immunology. 2003;22:401–424. doi: 10.1080/08830180305215. [DOI] [PubMed] [Google Scholar]
- Dowd P. F. Toxicological and biochemical interactions of the fungal metabolites fusaric acid and Kojic acid with xenobiotics in Heliothis zea (F.) and Spodoptera frugiperda (J.E. Smith) Pesticide Biochemistry and Physiology. 1988;32:123–134. [Google Scholar]
- Dowd P. F. Relative inhibition of insect phenoloxidase by cyclic fungal metabolites from insect and plant pathogens. Nat Toxins. 1999;7:337–341. doi: 10.1002/1522-7189(199911/12)7:6<337::aid-nt69>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Engelhard E. K., Volkman L. E. Developmental resistance in fourth instar Trichoplusia ni orally inoculated with Autographa californica M nuclear polyhedrosis virus. Virology. 1995;209:384–389. doi: 10.1006/viro.1995.1270. [DOI] [PubMed] [Google Scholar]
- Feldman K. S., Sambandam A., Bowers K. E., Appel H. M. Probing the role of polyphenol oxidation in mediating insect-pathogen interactions. Galloyl-derived electrophilic traps forLymantria dispar nuclear polyhedrosis virus matrix protein polyhedrin. Journal of Organic Chemistry. 1999;64:5794–5803. [Google Scholar]
- Foley E., O’Farrell P. H. Nitric oxide contributes to induction of innate immune responses to gram-negative bacteria in Drosophila. Genes and Development. 2003;17:115–125. doi: 10.1101/gad.1018503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M., Terenius O., Li W., Faye I. Baculovirus and dsRNA induce Hemolin, but no antibacterial activity in Antheraea pernyi. Insect Molecular Biology. 2004;13:399–405. doi: 10.1111/j.0962-1075.2004.00497.x. [DOI] [PubMed] [Google Scholar]
- Hoover K., Alaniz S. A., Yee J. L., Rocke D. M., Hammock B. D., Duffey S. S. Dietary protein and chlorogenic acid effect on baculoviral disease of noctuid larvae. Environmental Entomology. 1998;27:1264–1272. [Google Scholar]
- Hoover K., Grove M. J., Su S. Systemic component to intrastadial developmental resistance inLymantria dispar to its baculovirus. Biological Control. 2002;25:92–98. [Google Scholar]
- Hoover K., Washburn J. O., Volkman L. E. Midgut-based resistance of Heliothis virescens to baculovirus infection mediated by phytochemicals in cotton. Journal of Insect Physiology. 2000;46:999–1007. doi: 10.1016/s0022-1910(99)00211-5. [DOI] [PubMed] [Google Scholar]
- Hughes P. R. ViStat. Statistical Package for the Analysis of Baculovirus Bioassay Data. Ithaca, NY: Cornell Univ. Press; 1990. [Google Scholar]
- Jiang H., Wang Y., Yu X-Q., Kanost M. R. Prophenoloxidase-activating proteinase-2 from hemolymph ofManduca sexta. A bacteria-inducible serine proteinase containing two clip domains. Journal of Biological Chemistry. 2003;278:3552–3561. doi: 10.1074/jbc.M205743200. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. A., Washburn J. O., Volkman E. AcMNPV pathogenesis and developmental resistance in fifth instarHeliothis virescens. Journal of Invertebrate Pathology. 1998;72:63–72. doi: 10.1006/jipa.1997.4752. [DOI] [PubMed] [Google Scholar]
- Komarov D. A., Slepneva I. A., Glupov V. V., Khramtsov V. V. Superoxide and hydrogen peroxide formation during enzymatic oxidation of DOPA by phenoloxidase. Free Radical Research. 2005;39:853–858. doi: 10.1080/10715760500166693. [DOI] [PubMed] [Google Scholar]
- Kubo I., Kinst-Hori I., Nihei K., Soria F., Takasaki M., Calderon J. S., Cespedes C. L. Tyrosinase inhibitors from galls of Rhus javanica leaves and their effects on insects. Zeitschrift für Naturforschung. 2003;58C:719–725. doi: 10.1515/znc-2003-9-1022. [DOI] [PubMed] [Google Scholar]
- Kumar S., Christophides K., Cantera R., Charles B., Han Y. S., Meister S., Dimopoulos G., Kafatos F. C., Barillas-Mury C. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proceedings of the National Academy of Sciences U S A. 2003;100:14139–14144. doi: 10.1073/pnas.2036262100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem J. Y., Nishimura C., Kurata S., Shimada I., Kobayashi A., Natori S. Purification and characterization of N-B-alanyl-5-S-glutathionyl-3,4-dihydroxyphenyl-alanine, a novel antibacterial substance of Sarcophaga peregrina (Flesh fly) Journal of Biological Chemistry. 1996;271:13573–13577. doi: 10.1074/jbc.271.23.13573. [DOI] [PubMed] [Google Scholar]
- Li H., Li W. X., Ding S. W. Induction and Suppression of RNA Silencing by an Animal Virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Li W., Kubo I. QSAR and kinetics of the inhibition of benzaldehyde derivatives against Sarcophaga neobelliaria phenoloxidase. Bioorganic and Medicinal Chemistry. 2004;12:701–713. doi: 10.1016/j.bmc.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Luckhart S., Vodovotz Y., Cui L., Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of NO. Proceedings of the National Academy of Sciences U S A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R. B., Resende Rde-O., de Avila A. C. The plant virus Tomato Spotted Wilt Tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. Journal of Virology. 2004;78:4976–4982. doi: 10.1128/JVI.78.10.4976-4982.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi A. J., Kohler L., Mastore M. Signaling pathways implicated in the cellular innate immune responses of Drosophila. Invertebrate Survival Journal. 2004;1:5–33. [Google Scholar]
- Nappi A. J., Christenson B. M. Melanogenesis and associated cytotoxic reactions: Applications to insect innate immunity. Insect Biochemistry and Molecular Biology. 2005;35:443–449. doi: 10.1016/j.ibmb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Ourth D. D. Antiviral activity against human immunodeficiency virus-1in vitro by myristoylated-peptide fromHeliothis virescens. Biochemical and Biophysical Research Communications. 2004;320:190–196. doi: 10.1016/j.bbrc.2004.05.137. [DOI] [PubMed] [Google Scholar]
- Ourth D. D., Renis H. E. Antiviral melanization reaction of Heliothis virescens hemolymph against DNA and RNA viruses in vitro. Comparative Biochemistry and Physiology. 1993;105B:719–723. doi: 10.1016/0305-0491(93)90111-h. [DOI] [PubMed] [Google Scholar]
- Popham H. J. R., Shelby K. S., Brandt S. L., Coudron T. A. Potent virucidal activity against HzSNPV in larvalHeliothis virescens plasma. Journal of General Virology. 2004;85:2225–2261. doi: 10.1099/vir.0.79965-0. [DOI] [PubMed] [Google Scholar]
- Shelby K. S., Adeyeye O. A., Okot-Kotber B. M., Webb B. A. Parasitism-linked block of host plasma melanization. Journal of Invertebrate Pathology. 2000;75:218–225. doi: 10.1006/jipa.2000.4925. [DOI] [PubMed] [Google Scholar]
- Shiao S. H., Higgs S., Adelman Z., Christensen B. M., Liu S. H., Chen C. C. Effect of prophenoloxidase expression knockout on the melanization of microfilariae in the mosquito Armigeres subalbatus. Insect Molecular Biology. 2001;10:315–321. doi: 10.1046/j.0962-1075.2001.00268.x. [DOI] [PubMed] [Google Scholar]
- Slavicek J. M., Hayes-Plazolles N., Kelly M. E. Identification of a Lymantria dispar Nucleopolyhedrovirus Isolate that does not accumulate few-polyhedra mutants during extended serial passage in cell culture. Biological Control. 2001;22:159–168. [Google Scholar]
- Slepneva I. A., Komarov D. A., Glupov V. V., Serebrov V. V., Khramtsov V. V. Influence of fungal infection on the DOPA-semiquinone and DOPA-quinone production in haemolymph of Galleria mellonella larvae. Biochemical and Biophysical Research Communications. 2003;300:188–191. doi: 10.1016/s0006-291x(02)02766-3. [DOI] [PubMed] [Google Scholar]
- Trudeau D., Washburn J. O., Volkman L. E. Central Role of Hemocytes in Autographa californica M Nucleopolyhedrovirus Pathogenesis inHeliothis virescens and Helicoverpa zea. Journal of Virology. 2001;75:996–1003. doi: 10.1128/JVI.75.2.996-1003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn J. O., Kirkpatrick B. A., Volkman L. E. Insect protection against viruses. Nature. 1996;383:767. [Google Scholar]
- Washburn J. O., Haas-Stapleton J. A., Tan F. F., Beckage N. E., Volkman L. E. Co-infection of Manduca sexta larvae with polydnavirus from Cotesia congregata increases susceptibility to fatal infection by Autographa californica M nucleopolyhedrovirus. Journal of Insect Physiology. 2000;46:179–190. doi: 10.1016/s0022-1910(99)00115-8. [DOI] [PubMed] [Google Scholar]
- Wilson K., Cotter S. C., Reeson A. F., Pell J. K. Melanism and disease resistance in insects. Ecology Letters. 2001;4:637–649. [Google Scholar]
- Zambon R. A., Nandakumar M., Vakharia V. N., Wu L. P. The Toll pathway is important for an antiviral response inDrosophila. Proceedings of the National Academy of Sciences USA. 2005;102:7257–7262. doi: 10.1073/pnas.0409181102. [DOI] [PMC free article] [PubMed] [Google Scholar]


