Abstract
This article describes a parallel and distributed processing model (PDPM) of joint attention, self-referenced processing and autism. According to this model, autism involves early impairments in the capacity for rapid, integrated processing of self-referenced (proprioceptive and interoceptive) and other-referenced (exteroceptive) information. Measures of joint attention have proven useful in research on autism because they are sensitive to the early development of the ‘parallel’ and integrated processing of self- and other-referenced stimuli. Moreover, joint attention behaviors are a consequence, but also an organizer of the functional development of a distal distributed cortical system involving anterior networks including the prefrontal and insula cortices, as well as posterior neural networks including the temporal and parietal cortices. Measures of joint attention provide early behavioral indicators of atypical development in this parallel and distributed processing system in autism. In addition it is proposed that an early, chronic disturbance in the capacity for integrating self- and other-referenced information may have cascading effects on the development of self awareness in autism. The assumptions, empirical support and future research implications of this model are discussed.
Keywords: joint attention, neurodevelopment, distributed cortical processes, self referenced information processing, other referenced information processing, self referenced memory
One of the cardinal social communication symptoms of autism involves a marked reduction in the tendency to spontaneously share enjoyment, interests, or achievements with other people, as indicated by a lack of showing, bringing, or pointing out objects of interest to others (American Psychiatric Association, 2000). In the first years of life this symptom is often evident on measures of joint attention (Charman, 2004; Mundy and Crowson, 1997; Zwaigenbaum et al., 2005). Joint attention (JA) development begins as early as the 3rd to 9th month of postnatal life (Mundy et al., 2007; Mundy and Newell, 2007). JA reflects the degree to which a child or adult coordinates their attention with the attention of a social partner, such as engaging in a common line of visual regard, in order to share a common perceptual experience of objects or events (Bruner, 1975; Mundy and Newell, 2007; see Figure 1). Three decades of research have led to the following observations about JA and autism: a) children with autism tend to display fewer JA behaviors relative to peers with and without developmental disabilities; b) as early as 15–18 months infants at risk for autism often display fewer acts of both responding to and initiating JA; c) over the course of development deficits in the tendency to initiate JA (spontaneously sharing attention) remain more robust than deficits in responding to the JA bids of others; and d) early interventions that increase JA have cascading effects on subsequent social-learning, supporting the hypothesis that JA impairment plays a pivotal role in the developmental course of autism (e.g., Charman, 2004; Cassel et al., 2006; Jones et al., 2006; Kasari et al., 2007, 2008; Mundy and Crowson, 1997; Mundy et al., 1994; Nation and Penny, 2008; Sigman and McGovern, 2005; Sigman and Ruskin, 1999; Whalen et al., 2006).
Figure 1.
Illustrations of different types of infant social attention coordination behaviors
Note. a) Responding to Joint Attention-RJA involving following another person’s gaze and pointing gesture; b) Initiating Joint Attention-IJA involving a conventional gesture ‘pointing’ to share attention regarding a poster on the wall, c1,2,3) IJA involving alternating eye contact to share attention with respect to a toy, d) Initiating Behavior Request involving pointing to elicit aid in obtaining an out of reach object, and e) Responding to Behavior Requests involving following an adult’s open-palm ‘give it to me’ gesture.
To shed new light on this symptom domain of autism we have proposed a model wherein joint attention is presumed to involve the interactive processing of self-referenced information, and information about another person’s attention, and information about a commonly referenced object or event (e.g., Mundy and Hogan, 1994; Mundy et al., 1992; Mundy et al., 2009). In this model, self-referenced processing does not connote self awareness; rather it is a mode of processing information that is necessary, but not sufficient for the emergence of self awareness. Instead, self-referenced processing refers to processing of information about the physiological status of the body (interoception), and processing information about the actions, postures and specific positions of the body (proprioception). Practice with interactive, parallel self-, other-, and object/event-referenced information processing during joint attention in infancy is affected by, and has an effect on the development of a distributed neural processing network that involves distal frontal, temporal and parietal cortical systems. With development and experience, this distributed neural network serves a social-executive function that enables relatively effortless coordination of attention to external objects/events during social interactions. This, in turn, provides the basis for the development of coordinated attention to internal, cognitive representations of self and other which contributes to the foundation for symbolic thought and social cognition. Hence, we refer to this as a parallel and distributed processing model (PDPM) of joint attention (Mundy et al., 2009).
A postulate of this model is that problems with the parallel and interactive processing of self-referenced and other-referenced domains of information, rather than problems with processing information from either domain alone, may be at the root of joint attention impairments in autism. Moreover, practice with parallel self- and other-referenced information is thought to be integral to a dynamic cognitive synthesis of information that is necessary for the emergence of self awareness and social cognition (Mundy et al., 1992, 2009). A corollary of this assumption is that, while theory and research often emphasize other-referenced processing problems in the study of autism, joint attention impairments may be associated with atypical self-referenced processing and ultimately problems of self awareness in autism (Mundy, 2003). In the following sections, we review theory and empirical evidence relevant to this model and offer recommendations about future research designs to directly test these assumptions.
An alternative paradigm for joint attention research
Contemporary research on joint attention frequently highlights what Bruner (1995) described as the ‘epistemological question’ or studies about the types of knowledge of others’ intentions that may be involved in joint attention development and its impairments in autism (e.g., Baron-Cohen, 1995; Tomasello et al., 2005). Still other approaches have emphasized the possibility that joint attention deficits in children may be secondary to impairments in primary psychological processes, including identification, attention regulation, or social motivation (e.g., Hobson and Hobson, 2007; Landry and Bryson, 2004; Reddy, 2003). These perspectives have employed to descriptions of typical and atypical development in terms of the emergence of discrete stages of social knowledge (Tomasello et al., 2005), cognitive modules (Baron-Cohen, 1995), or stages of self awareness that are requisite to joint attention development (Reddy, 2003). Alternatively, we have adopted five guiding assumptions that led to a different paradigm for the study of joint attention and autism. These are described as follows.
First we argue that joint attention is not simply a precursor to social cognition, nor merely an outcome of the development of one or another more primary psychological process (Mundy, 1995; Mundy et al., 2009). Rather, joint attention is an early emerging psychological process unto itself that retains a critical functional presence throughout the life span. The development of this psychological process is conceptualized, in part, in terms of increasing facility with the processing of multiple streams of information in real time during social interactions. A maxim of this perspective is that typical and atypical human development can be studied in terms of continuous changes in the speed, efficiency, and coordination of information processing that gives rise to knowledge or psychological phenomenon (Hunt, 1999). Specifically, we propose that joint attention is a unique form of information processing that makes a significant contribution to the development of human levels of social cognition defined in terms of knowledge, or ‘mentalizing’ the intentions of self and others.
Second, the PDPM also adopts a constructivist view of cognitive and psychological development. That is to say that cognitive development originates as much or more from infants’ continual practice of actions and the processing of information that results from those actions as it does from the passive perception of information available in the environment (Piaget, 1952). Infants generate knowledge through their own self-initiated actions. Concomitantly, impairments of autism are viewed as potentially arising from developmental disturbances in systems involved in the self-initiation of actions and the resulting impoverishment of information processing that arises from the attenuation of self-initiated actions (Mundy, 2003).
Third, the parallel and distributed processing assumption of this model is related to connectionist perspectives in neuroscience which caution us that, because of the massively parallel nature of human brain networks, it is likely that the development of cognitive functions ‘emerge[s] from the flow of information between brain areas’ rather than from brain regions or modules that are singularly dedicated to a specific cognitive domain (Ramnani et al., 2004, p. 613). This notion fits with empirical evidence of the distributed brain systems involved in joint attention; hence we describe its typical development, as well as its impairments in autism, in terms of a constructivist parallel and distributed neurocognitive information processing model (Mundy et al., 2009; Mundy and Vaughan Van Hecke, 2008). A major premise of the model is that psychological development is most appropriately described in terms of continuous, incremental changes in the speed, efficiency and coordination of information processing networks that give rise to changes in knowledge and cognitive structures (e.g., Case, 1985; Mareschal et al., 2007; Posner and Rothbart, 2007a).
A fourth and more methodological assumption of our model is that while there may well be social, cognitive, and attention precursors of true joint attention, the limitations of psychological measurement in very young infants may hinder empirical investigation of this possibility. Research on infant assessment over the last 40 years indicates that two of the bedrocks of scientific measurement, test-retest reliability and predictive validity estimates associated with large effect sizes, often prove elusive in the assessment of young infants (Singer, 2001). Consequently, it should not be surprising that research with infants at risk for autism has, to date, yielded little behavioral evidence of the manifest onset of the disorder prior to 6 months of age (Rogers, 2009). This does not mean that psychological phenomenon associated with autism do not exist in the first 6 months. However, the empirical verification of their nature is difficult because of unrecognized, if not intractable measurement limitations. This issue may partially explain why a review of 11 tests of precursor hypotheses revealed little evidence that measures of earlier developing psychological processes mediate the relations between joint attention and diagnostic or developmental outcomes measures in young children with autism (Mundy et al., 2009).
Compounding the psychometric challenges of identifying developmental precursors of joint attention is the fact that joint attention itself begins rapidly developing by 3 to 4 months of age (e.g., D’Entremont et al., 1997). Logically then, precursors of joint attention may best be sought in the identification of strong neonatal predictors of development, which is a challenging empirical task to say the least. Our focus, then, has been on the development of psychological processes after 3 to 4 months of age when maturation of frontal networks begins to allow for the level of voluntary visual attention control necessary for joint attention (Mundy and Vaughan Van Hecke, 2008; Mundy et al., 2009). Moreover, to our knowledge, it is only after this period, and really the 6- to 9- month period, that there is psychometric data to support the assumption that joint attention or other infant assessments begin to have the measurement characteristics sufficient for sound, clinically applicable developmental research (Morales et al., 2000; Mundy et al., 2007; Singer, 2001).
Finally, we recognize and emphasize that the nature and meaning of joint attention development described within the PDPM framework need not claim to be the one and only valid perspective. In our opinion it is unlikely that any one paradigm can provide a comprehensive explanation of the complex facilities of the developing human mind. Instead, the generation, comparison, and ultimate synthesis of information from alternative viewpoints is the tried and true path for uncovering the processes underlying any complex phenomenon. In order compare alternate models, each must result in testable hypotheses. To this end, we now turn to a more thorough description of the role of self- referenced processing offered by the PDPM of joint attention and autism and the testable predictions that may be derived from this description.
Joint attention and self-referenced processing
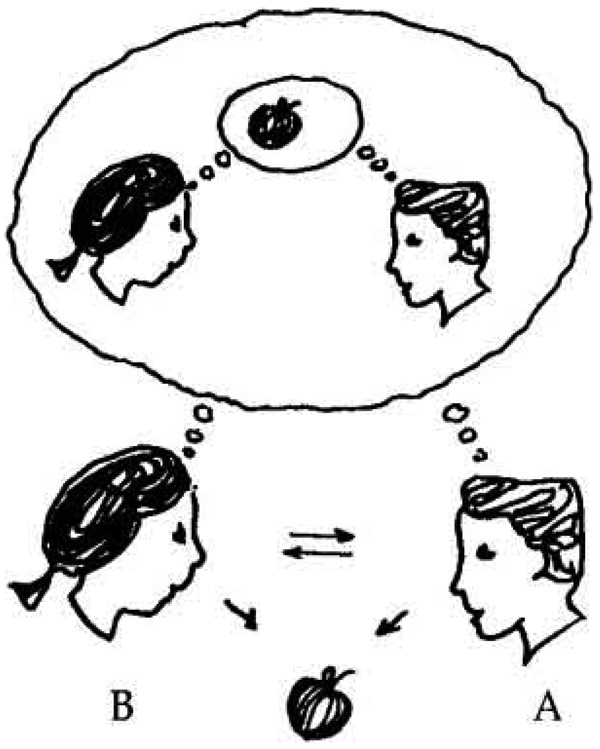
As illustrated in Figure 2, joint attention involves a tripartite deployment of attention between the self, another person, and an object or event. In addition to attention deployment it also involves triadic information processing. When we engage in joint attention we attend to and process a) information about an object, b) information about another person’s attention and behavior related to the object, and c) self-referenced information about our own attention to, and experience of, the object and the situation. In this context, self-referenced processing refers to implicit, subjective, and pre-reflective processing and integration of interoceptive and proprioceptive information from one’s own body (e.g., heart rate, volitional muscle movement) with information about perceptual and cognitive activity including representations in working or long term memory (Northoff et al., 2006; Zahavi, 2003).
Figure 2.
A schematic representation of the triadic processing of self-referenced, other-referenced and object reference information involved in joint attention.
Interoception describes sensitivity to physiological information originating within the body, such as heart rate, respiration, autonomic arousal, respiration, and emotional states. Proprioception involves sensitivity to the position, location, orientation, and movement of the body. Research suggests that specific, distributed neural systems may be involved in processing interoceptive information (anterior insula) and proprioceptive information about movement and orientation (anterior cingulate and parietal cortices). These associative cortical systems also may be primary in integrating these two dimensions of self-referenced information (Craig, 2009; Balslev and Miall, 2008; Uddin and Menon, 2009). In particular comparative research suggests that interoception and its integration with proprioception may be more elaborated in humans via Von Economo neurons of the anterior cingulate than in other primates and mammals (Allman et al., 2005; Craig, 2003).
In contrast to self-referenced information processing, exteroception describes the processing of information about the environment, including social partners, that is separate from the body. In addition to the primary sensory cortex, associative cortical networks in the parietal, temporal and frontal lobes are thought to support a specific sub-domain of exteroception that involves processing of spatial/postural, behavioral, vocal and affect information arising from other people (Decety and Sommerville, 2003; Emery, 2000; Northoff et al., 2006). Although partially overlapping, the self- and other-referenced information processing networks are distinct. One hypothesis is that developmental articulation of these systems aids the psychological differentiation of self from other in early development (cf. Decety and Sommerville, 2003; Northoff et al., 2006).
In past discussions we primarily focused our descriptions of self-referenced information processing in joint attention on the proprioceptive functions of medial frontal structures including the anterior cingulate, with only implicit reference to elements of interoception (Mundy, 2003). Therefore, one goal of this paper is to more explicitly elaborate and describe the dual nature of self-referenced information processing in joint attention in terms of a) proprioception, such as feedback from ocular muscle control and the vestibular system related to the spatial direction of one’s own visual attention and head posture (see Butterworth and Jarrett, 1991, for related discussion), and b) interoception including information about arousal and the positive (rewarding), neutral or negative valence of each participant’s perceptions of the object or event, as well as the valence attributed to sharing attention with a social partner during joint attention. We propose that joint attention episodes involve at least as much self-referenced processing of internal sensory and affective experiences as they do processing of information about other people’s behavior or intentions. With this in mind it is possible that joint attention impairments in autism may originate from problems with self-referenced processing (interoception and/or proprioception) or the coordinated, parallel processing of self- and other-referenced information during episodes of shared attention.
Joint attention, symbols and self awareness
Although effortless for many adults and older children, the capacity to meet the demands of multi-source attention deployment and information processing in joint attention is only gradually acquired. Social attention coordination improves rapidly over infancy as a function of both frequent practice with joint attention as well as neurocognitive maturation beginning by 3 months of age (Carpenter et al., 1998; D’Entremont et al., 1997; Morales et al., 2000). With practice, the process of coordinating attention to external objects or events with a social partner becomes internalized and provides cognitive operations that enable us to socially coordinate mental attention to representations. This ability to socially coordinate mental attention in order to share the experience of mental representations is essential for social cognition, social learning, and symbolic development (Mundy et al., 2009; Werner and Kaplan, 1963). Indeed, Tomasello et al. (2005) proposed that the capacity of an abstract stimulus to elicit attention to shared representations across people is the defining feature of symbolic cognition.
The process whereby active overt joint visual attention becomes internalized into covert, representational operations follows the same general mechanisms described in constructivist cognitive and neurocognitive developmental theory (e.g., Mareschal et al., 2007; Piaget, 1952). However, neurodevelopmental impairments may limit the extent to which children with autism engage in a sufficiently active social constructivist role during their own development. Theoretically, limited practice with overt joint visual attention in infancy leads to poorly developed cognitive operations that support the coordination of attention to mental representations with social partners. In turn, this contributes to the varying levels of impairment in social cognition, abstract symbolic thinking, and self awareness characteristic of children with autism (Mundy and Burnette, 2005; Mundy, 2003; Mundy et al., 1993, 2009).
The latter hypothesis stems from the school of thought that human self awareness does not simply involve advances in proprioception and interoception. Rather, self awareness is an incrementally achieved property of social relational processing (Chen et al., 2006; Piaget, 1952; Vygotsky, 1962) that involves the parallel processing of self- and other-referenced information. That is, the development of self awareness is a socially-embedded process that requires a deep and prolonged synthesis of self-(proprioceptive/interoceptive) and other-referenced information processing (e.g., Piaget, 1952). Neurocognitive research suggests that this synthesis of perspectives occurs by way of repeated social experiences in combination with the maturation of a distributed frontal, temporal and parietal cortical system (Decety and Sommerville, 2003; Keysers and Perrett, 2006). Keysers and Perrett (2006), for example, suggest that the relational processing of self- and other-referenced information in infancy facilitates social-cognitive development through Hebbian learning principles. Neural networks that are repeatedly co-actived become associated, such that activity in one network triggers activity in the other (Hebb, 1949). Keysers and Perrett (2006) suggest that co-activation of neural networks for processing self-generated information and information about people is fundamental to the development of representations and knowledge about self and others.
We have proposed that over the course of infancy and many practice trials, the co-activation of self- and other-processing systems becomes more efficient and effortless and provides the basis for social executive functions. One definition of executive functions is that they involve the neural transmission of bias signals to selectively inhibit relatively automatic behavioral responses in favor of more volitional, planned and goal-directed responses (Miller and Cohen, 2001). The aggregate effect of these bias signals is to guide the flow of neural activity along pathways that establish the proper mappings between inputs, internal states, and outputs needed to perform a given task more efficiently (Miller and Cohen, 2001).
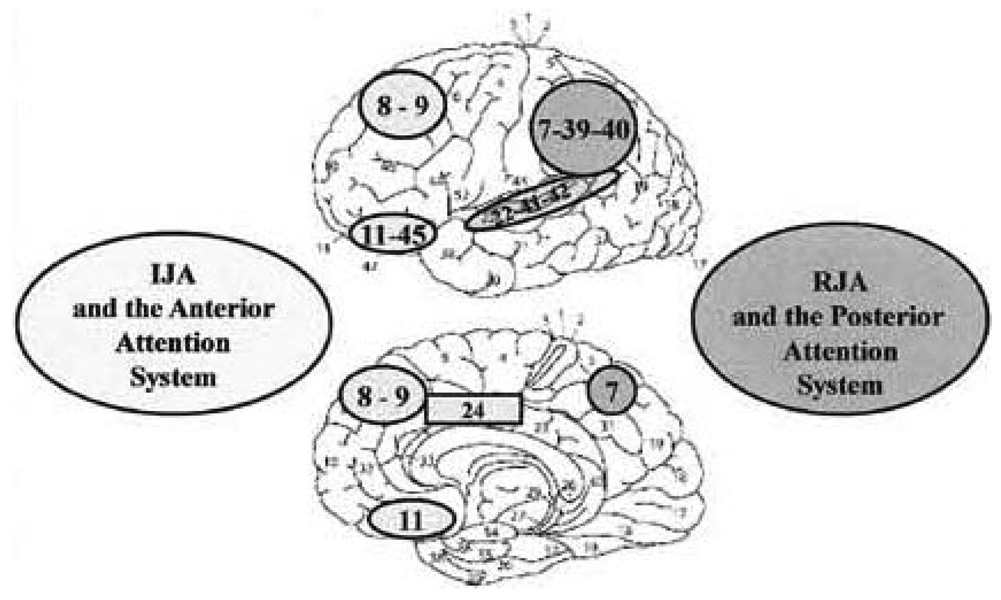
In this regard, we conceptualize the repeated practice of joint attention behaviors in infancy as both a cause and consequence of frontal bias signals that establish the proper mappings of exteroceptive, or outside-in, information about other people’s attention with the interoceptive and/or proprioceptive, or inside-out, processing of internal states and information related to the intentional control of visual attention. This mapping results in the development of a distributed anterior and posterior cortical joint attention system (Figure 3; Mundy, 2003; Mundy et al., 2009). In our view, the early establishment of this cortical mapping of a joint processing system is formative with respect to the development of the shared neural network of representations of self and others that have been described as driving the progression of self awareness across development (e.g., Decety and Grezes, 2006; Keysers and Perrett, 2006; Vanderwal et al., 2008). That is to say, the Hebbian mapping process underlying social cognition described by Keysers and Perrett (2006) begins with the integrated anterior cortical processing of information about self-produced visual attention and posterior cortical processing of the visual attention of others (Mundy et al., 2009). With sufficient practice and experience, the parallel processing of self- and other-attention becomes more coordinated and requires less mental effort. As basic joint attention processes are mastered and require less effort, they serve a social executive function that fosters social cognitive skills and competence.
Figure 3.
Illustration from Mundy and Newell (2007) depicting the lateral (top) and medial (bottom) illustrations of the distributed system of Brodmann’s cytoarchitectonic areas of the cerebral cortex associated with Initiating Joint Attention and the anterior attention system, as well as RJA and the posterior attention systems. The former include areas 8 (frontal eye fields), 9 (prefrontal association cortex), 24 (anterior cingulate), 47 and 43 (orbital prefrontal and insula associative cortices). The latter include areas 7 (precuneous, posterior parietal association area), 22, 41, and 42 (superior temporal cortex), and 39 and 40 (parietal, temporal, occipital association cortex).
The developmental process we envision has much in common with dynamic systems developmental theory. Thelen and Smith’s (1994) theory of dynamic systems in development suggests that the cumulative experience of interactions of multiple sources of information, over time and practice, coalesce into higher order processes that yield the complex structures of human thought and consciousness. Thus, psychological development may be viewed as arising from the interactions between cognitive processes, rather than as the output of any one isolated cognitive system or module (Karmiloff-Smith, 1996; Thelen and Smith, 1994). To us, this seems highly analogous to the neural connectionist notion that cognitive functions and their development ‘emerge from the flow of information between brain areas’ rather than from brain regions or modules that are singularly dedicated to one or another cognitive domain (Ramnani et al., 2004, p. 613). In our view, the development of joint attention is a behavioral expression of such a dynamic, connectionist system in human development. Practice with the rapid, conjunctive processing of self-referenced information and other-referenced information in joint attention contributes to the development of a parallel and distributed frontal, parietal and temporal cortical information processing system that is pivotal with respect to the emergence of self awareness in humans. This assumption leads to the expectation that there should be evidence of a connection between self-related information processing and joint attention in development. If atypical self-referenced processing contributes to joint attention problems in children with autism then individual differences in self-referenced information processing should predict symptom severity in children and adults with autism. In the following sections we consider research relevant to these predictions and offer suggestions for future research directions.
Research on self-referenced processing, joint attention, and autism
An earlier paper suggested that joint attention and social impairments in autism may be associated with proprioceptive self-monitoring and regulation via functions of the anterior cingulate and supervisory attention system (Mundy, 2003). This conjecture and earlier related theory (Mundy et al., 1993) motivated several studies.
Self-recognition is often viewed as a valid indicator of self-referenced processing, if not self awareness (Gallup et al., 2003). In one study, Nichols, Fox and Mundy (2005) observed that infants who displayed mirror self-recognition between 14- and 18-months of age displayed higher rates of initiating joint attention at 18 months compared to same-age infants who did not display evidence of mirror self-recognition. Importantly, other measures of cognitive development such as performance on a delay non-match to sample task or a means-ends task did not mediate the relations between self-recognition and joint attention. Thus, self-recognition appeared to have a specific association with initiating joint attention that could not be explained in terms of other cognitive factors.
Another line of research follows from the work of Posner and Rothbart (2007a, 2007b), who have long argued that infants’ use of intentional shifts of attention to manage arousal and conflict between goal-directed actions is a primary step in the development of self awareness and self-regulation. In this regard we have observed that infants’ abilities to shift attention in response to bids for JA at 6 months predicted the tendency of 2-year-olds to utilize shifts of attention to self-regulate and resist temptation in a delay of gratification task (Morales et al., 2005). In addition, more optimal JA in the first 18 months of life is associated with reduced risk for self-regulatory problems at 30 and 36 months of age in at-risk and typically developing children (Sheinkopf et al., 2004; Vaughan Van Hecke, 2007).
Related to these findings, the RUPP Autism Network recently reported a significant positive effect of methylphenidate on rates of initiating and responding to JA bids in 5- to 13-year-old children with autism (Jahromi et al., 2009). One possible explanation for this finding is that methylphenidate impacted JA through dopaminergic adjustment of the anterior cingulate’s top-down regulation of attention via enhanced self-referenced proprioceptive processing of information about reward (Groen et al., 2008; Haes et al., 2007; Kronenberg et al., 2008). Although often hypothesized (e.g., Mundy, 2003), the role of the anterior cingulate and associated self-monitoring and self-regulation processes in joint attention and its impairment in autism has yet to be directly tested. However, just as variability in joint attention has been observed to be associated with the intensity of symptom presentation in autism (Kasari et al., 2008; Mundy et al., 1994), several studies indicate that variability in anterior cingulate functioning is also associated with symptom intensity in autism (e.g., Haznedar et al., 2000; Onishi et al., 2000). Moreover, functional analyses of differences within samples of children with autism and between autism and comparisons groups suggests that the anterior cingulate’s role in self-monitoring and resolution of errors in goal-directed behaviors is associated with both social symptom and repetitive behavior symptom presentation in autism (Henderson et al., 2006; Thakkar et al., 2008). Furthermore, in a recent intriguing and innovative study using a complex multiple participant imaging method, Chiu et al. (2008) reported that a measure of parallel self- and other-processing in an interpersonal exchange game is associated with cingulate activity in children with autism. Importantly, diminished cingulate activity during the game was associated with more intense social symptom presentation among children with autism.
Of course, not all studies of self-related action monitoring yield effects specific to autism. In a paradigm that did not involve conflict resolution, or self-other information exchange, Williams and Happé (2009) observed no evidence of self awareness impairments in a sample of children with autism. Understanding the differences between paradigms that do and do not reveal differences in self-referenced processing will likely prove essential to a more precise definition of the nature of self development in this syndrome. Indeed, information from many convergent and divergent paradigms will be necessary to fully explore the complexities of the role of self-referenced processing in autism.
Our own interest in indentifying convergent methods led to research on self-referenced memory (SRM). In one SRM paradigm, participants read lists of adjectives under three conditions: the self-referenced condition where they decide whether the words describe something about themselves; the other-referenced condition in which they are asked if the words describe something about a familiar character (e.g., Harry Potter); or the word/letter-referenced condition in which they decide whether the words contain specific letter features (e.g., a “th” blend, more than five letters, et cetera). Adults and children tend to display a significant recognition memory advantage for words processed under ‘self’ versus ‘other’ conditions and ‘other’ versus ‘letter’ conditions. However, observations reported by Toichi et al. (2002) raised the possibility that higher functioning adults with autism fail to display the expected advantage for self- versus other-referenced words.
Henderson et al. (2009) expanded this paradigm to study SRM in higher functioning children with autism. Similar to the findings of Toichi et al. (2002), children with HFA did not display the expected advantage for self-referenced processing that was evident among a comparison sample of age-, gender-, and IQ-matched typically developing children. HFA participants did, however, recognize significantly more self- and other-referenced words relative to words processed in the letter count condition. Importantly, the magnitude of the advantage for self- versus other-referenced words was inversely associated with symptom severity for children with HFA. Other studies of children (Klein et al., 2009) and adults (Lombardo et al., 2007) with autism have reported comparable effects.
The Lombardo study is especially interesting in that it includes observations that self-referenced memory was associated with, and perhaps explained by, social cognition. Lombardo et al. (2007) suggested that self-referenced memory taps into the same general ‘mentalizing about minds’ processes involved in theory of mind deficits in autism. In contrast, although Henderson et al. (2009) reported a significant relation between social-cognitive mentalizing and SRM task performance, social-cognitive mentalizing did not mediate the association between SRM and symptom presentation in HFA children. Hence, these data raise the possibility that SRM tasks tap some unique vulnerability in self-referenced processing that contributes to symptom presentation over and above more general social cognitive impairments characteristic of autism.
Neurocognitive research has long suggested that self-monitoring functions associated with the anterior cingulate play a role in SRM task performance (Craik et al., 1999). More recent studies have suggested that SRM task performance is associated with a distributed cortical processing system that involves functions of the medial frontal cortex and more dorsal and ventral frontal cortical regions, as well as parietal cortex (Macrae et al., 2004). Two extremely insightful studies suggest that the anterior cingulate is equipotent in processing self- and other-referenced information, but the more rostral medial frontal cortical nodes of this network become specialized for self-referenced processing of internal arousal, cognition and temperamental tendencies (Kelley et al., 2002; Kennedy and Courschesne, 2008). The latter study expressly suggests that diminished anterior cingulate activity is associated with the processing of many aspects of associated self–other information, but that diminished activity in more medial networks is distinctively associated with problems in self-referenced processing of internal (psychological) rather than external (physical) self-related attributes.
This conclusion is consistent with a review by Northoff et al. (2006) that describes a distributed neural system of frontal, temporal and partial networks that support the processing of self- and other-referenced information. However, there is also a distributed frontal network that appears to be singularly active in self-referential processing and ideation. We interpret our data to suggest that depth of information processing in this frontal network may be diminished relative to controls in children with autism (Henderson et al., 2009). Theoretically, depth of processing in memory is related to the distribution and number of neural clusters (nodes) activated in a cortical processing network (McClelland and Rogers, 2003; Otten et al., 2001). If so, the diminished advantage for self-processing in SRM tasks suggests that the distribution, number or frontal neural nodes, and/or functional connectivity involved in frontal, internal self-referenced processing may be attenuated in autism. Before higher-order self-related attributes and ideations develop, we propose that this impoverishment may begin with problems in self-referenced proprioception and interoception early in life.
Future research directions
The hypothesis that difficulty with some aspects of self-referenced processing plays a role in atypical joint attention and social development in autism is viable and useful, but requires much more research to be validated. There are at least four plausible directions for future research that could usefully expand this literature.
First, effective early intervention for autism impairments in children with autism is possible and yields collateral effects on other domains of development (Jones et al., 2006; Kasari et al., 2008; Whalen et al., 2006). These collateral outcomes have been measured in terms of cognitive, language and symptom effects. However, if joint attention development is linked to self-referenced processing autism then joint attention intervention may be expected to yield positive outcomes on measures of self-monitoring, self-recognition and self-regulation. Thus, targeting self-referenced processing in intervention studies could provide experimental tests of some of the hypotheses raised in this paper.
Advances in joint attention measurement have also begun to make it possible to provide more direct tests of our hypotheses with older children with autism. Jahromi et al. (2009) described such a method (called JAMES) in their study of 5- to 13-year-olds with autism. Mosconi et al. (2009) described a method called the Social Orienting Continuum and Response Scale (SOC-RS), which can be applied to video records of Autism Diagnostic Observation Schedule (ADOS) assessments to provide continuous measurements of IJA and RJA. Hobson and Hobson (2007) developed a ‘sharing looks’ measurement method, which captures behaviors in school-age children that are associated with the construct of initiating joint attention. These methods make it increasingly possible to directly examine the relations between joint attention development in older children with autism and behavioral or neurocognitive measures of self-regulation and self-referenced information processing, including SRM.
A third direction for future research is offered by advances in the application of EEG coherence measures to investigations of autism (Murias, Webb, et al., 2007), as well other forms of child and adult psychopathology (Murias, Swanson, et al., 2007; Schutter et al., 2005). EEG coherence measures provide a method for directly examining parallel and distributed processing hypotheses about JA (Mundy et al., 2000, 2003, 2009). In terms of brain-behavior methods, EEG coherence has two critical advantages over comparable fMRI measures in this regard. First, the temporal sensitivity of EEG is extremely important for the real time measurement of parallel and coordinated (e.g., sequential or simultaneous) processing across distributed neural networks. Second, it is more practical to collect data on relatively large samples of children using EEG rather than functional imaging measures. This enables increased power for the types of within-group individual difference analyses, as well as between-group analyses, that are critical to the accurate interpretation of data from heterogeneous populations, such as people affected by autism spectrum disorders.
Finally, the neurodevelopmental emphasis of our model motivates us to stay abreast of and incorporate new theory and research on neurocognitive functions in autism. One new area of research focuses on the brain systems involved in interoceptive self-referenced processing. Craig (2009) has reviewed a variety of evidence to suggest that the anterior insula cortex (medial to the anterior temporal lobes) is primary in interoceptive self-referenced processing and contains an anatomical substrate that significantly contributes to the phylogenetic and ontogenetic emergence of human levels of self awareness. Furthermore, DiMartino et al. (2009) conducted a meta-analysis of 24 studies examining social information processing and 15 studies examining non-social information processing in autism. Their meta-analytic results suggest that a parallel and distributed system involving the anterior cingulate cortex and anterior insula cortex was specifically hypoactive in social information processing among individuals with autism. In non-social studies, there was an effect for hyper-activation of a region of the rostral anterior cingulate that was typically suppressed during attention tasks among controls. Uddin and Menon (2009) have subsequently suggested that the anterior insula, most likely in conjunction with the anterior cingulate, serves as a hub mediating interactions between large scale brain networks that are ‘involved in externally oriented attention and internally oriented cognitive processes’. Uddin and Menon go on to suggest that in ‘ASD this critical system is impaired leading to social dysfunctions characteristic of the disorder’ (pp. 4–5).
We heartily agree that this is an intriguing possibility and our model leads us to suggest that joint attention impairment in autism is associated with disturbance of the typical development of this system. The reviews by Graig (2009) and Uddin and Menon (2009) also point to ways and means to test related hypotheses. For example Graig (2009) notes there is a specific role of the anterior insula in a prototypical form of interoception – heartbeat awareness (Critchley et al., 2004) – and that heartbeat awareness correlates with a measure of individual subjective emotional awareness (Barrett et al., 2004). As of this writing we could find no studies of heartbeat awareness in people with autism. Nevertheless, the synthesis we have presented here suggests that examining heartbeat awareness in conjunction with measure of joint attention, social cognition, and subjective emotional awareness may be informative with regard to the role of self-referenced information processing in the social deficits of autism.
Summary
Much of the current research on the social deficits of autism, from studies of imitation to face processing to social cognition, emphasizes the ‘responsive’ measurement of the degree to which people with autism can act on or process information about the behavior of other people. In addition, though, the facile development of social competence involves learning from self-generated behaviors, and learning how to integrate self-generated social behavior in real time with the behavior of other people. Therefore, we can expect theory and research on autism to advance as we become increasingly capable of the study of the dynamic interplay between self- and other-referenced action and cognition in social development. Without this we may fail to consider valid alternatives to current interpretations of data on the social deficits of autism. Fortunately, the development of theory that leads to testable hypotheses about the role of self-referenced processing is increasingly possible based on the abundance of new informative data arising from basic studies of social development and cognitive neuroscience. In this paper we hope to have provided one constructive example of such theory development in our explication of the role of self-referenced processing in the parallel and distributed processing model of joint attention impairment in autism.
Acknowledgements
The research and theory development reported in this paper were supported by NIH Grants HD 38052, MH 071273 and UCEDD 90DD0596, as well as the Lisa Capps Chair Endowment Fund to the UC Davis M.I.N.D. Institute and School of Education.
Contributor Information
Peter Mundy, The M.I.N.D. Institute and School of Education, University of California at Davis.
Mary Gwaltney, The M.I.N.D. Institute and School of Education, University of California at Davis.
Heather Henderson, University of Miami, Florida.
References
- Allman J, Watson K, Tetreault N, Hakeem A. Intuition and Autism: A Possible Role of Von Economo Neurons. Trends in Cognitive Sciences. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual on Mental Disorders. 4th ed., TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Balslev D, Miall RC. Eye Position Representation in Human Anterior Parietal Cortex. The Journal of Neuroscience. 2008;28:8968–8972. doi: 10.1523/JNEUROSCI.1513-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. Mindblindness. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Barrett L, Quigley K, Bliss-Moreau E, Aronson K. Interoceptive Sensitivity and Self-Reports of Emotional Experience. Journal of Personality and Social Psychology. 2004;87:684–697. doi: 10.1037/0022-3514.87.5.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner JS. From Joint Attention to the Meeting of Minds: An Introduction. In: Moore C, Dunham PJ, editors. Joint Attention: Its Origins and Role in Development. Hillsdale, NJ: Lawrence Erlbaum; 1995. pp. 1–14. [Google Scholar]
- Bruner JS. From Communication to Language: A Psychological Perspective. Cognition. 1975;3:255–287. [Google Scholar]
- Butterworth G, Jarrett N. What Minds Have in Common is Space: Spatial Mechanisms in Serving Joint Visual Attention in Infancy. British Journal of Developmental Psychology. 1991;9:55–72. [Google Scholar]
- Carpenter M, Nagell K, Tomasello M. Social Cognition, Joint Attention, and Communicative Competence from 9 to 15 Months of Age. Monographs of the Society for Research in Child Development. 1998;63((4, Serial No. 255)) [PubMed] [Google Scholar]
- Case R. Intellectual Development: Birth to Adulthood. New York: Academic Press; 1985. [Google Scholar]
- Cassel T, Messinger D, Ibanez L, Haltigan JD, Acosta S, Buchman A. Early Social and Communication in Infant Siblings of Children with Autism Spectrum Disorders: An Examination of the Broad Phenotype. Journal of Autism and Developmental Disorders. 2006;37:122–132. doi: 10.1007/s10803-006-0337-1. [DOI] [PubMed] [Google Scholar]
- Charman T. Why is Joint Attention a Pivotal Skill in Autism? Philosophical Transactions of the Royal Society of London. 2004;358:315–324. doi: 10.1098/rstb.2002.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Boucher H, Tapias M. The Relational Self: Integrative Conceptualization and Implication for Interpersonal Life. Psychological Bulletin. 2006;132:151–179. doi: 10.1037/0033-2909.132.2.151. [DOI] [PubMed] [Google Scholar]
- Chiu P, Kayali MA, Kishida K, Tomlin D, Klinger L, Klinger M, Montague PR. Self Responses along the Cingulate Cortex Reveal Quantitative Neural Phenotype for High-Functioning Autism. Neuron. 2008;57:463–473. doi: 10.1016/j.neuron.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Interoception: The Sense of the Physiological Condition of the Body. Current Opinion in Neurobiology. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Craig AD. How Do You Feel – Now? The Anterior Insula and Human Awareness. Nature Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, Kapur S. In Search of the Self: A Positron Emission Tomography Study. Psychological Science. 1999;10:26–34. [Google Scholar]
- Critchley H, Wiens S, Rotshtein P, Oham A, Dolon R. Neural Systems Supporting Interoceptive Awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Decety J, Grezes J. The Power of Simulation: Imagining One’s Own and Other’s Behavior. Cognitive Brain Research. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- Decety J, Sommerville J. Shared Representations between Self and Other: A Social Cognitive Neuroscience View. Trends in Cognitive Sciences. 2003;7:527–533. doi: 10.1016/j.tics.2003.10.004. [DOI] [PubMed] [Google Scholar]
- D’Entremont B, Hains S, Muir D. A Demonstration of Gaze Following in 3- to 6-Month-Olds. Infant Behavior and Development. 1997;20:569–572. [Google Scholar]
- DiMartino A, Ross K, Uddin L, Sklar A, Costellanos FX. Functional Brain Correlates of Social and Nonsocial Processes in Autism Spectrum Disorders: An Activation Likelihood Estimation Meta-Analysis. Biological Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallup G, Povinelli D, Suarez S, Anderson J, Lethmate J, Menzel E. Further Reflections on Self Recognition in Primates. Animal Behaviour. 1995;50:1525–1532. [Google Scholar]
- Groen W, Zwien M, van der Gaag R, Buitelaar J. The Phenotype and Neural Correlates of Language in Autism: An Integrative Review. Neuroscience & Biobehavioral Reviews. 2009;32:1416–1425. doi: 10.1016/j.neubiorev.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Haes U, Maguire R, Jager P, Paans A, de Boer J. Methylphenidate-Induced Activation of the Anterior Cingulate but Not the Striatum. Human Brain Mapping. 2007;28:625–635. doi: 10.1002/hbm.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar M, Buchsbaum M, Wei T, Hof P, Cartwright C, Bienstock C, Hollander E. Limbic Circuitry in Patients with Autism Spectrum Disorders Studied with Positron Emission Tomography and Magnetic Resonance Imaging. American Journal of Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Hebb D. The Organization of Behaviour. New York: Wiley; 1949. [Google Scholar]
- Henderson HA, Schwartz CB, Mundy P, Burnette C, Sutton SK, Zahka N, Pradella A. Response Monitoring, the Error-Related Negativity, and Differences in Social Behavior in Autism. Brain and Cognition. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson H, Zahka N, Kojkowski N, Inge A, Schwarz C, Hileman C, et al. Self Referenced Memory, Social Cognition and Symptom Presentation in Autism. Journal of Child Psychology & Psychiatry. 2009;50:853–861. doi: 10.1111/j.1469-7610.2008.02059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson J, Hobson P. Identification: The Missing Link between Joint Attention and Imitation? Development and Psychopathology. 2007;19:411–431. doi: 10.1017/S0954579407070204. [DOI] [PubMed] [Google Scholar]
- Hunt E. Intelligence and Human Resources: Past, Present and Future. In: Ackerman P, Kyllonen P, Roberts R, editors. Learning and Individual Differences. Washington, DC: American Psychological Association; 1999. pp. 3–30. [Google Scholar]
- Jahromi L, Kasari C, McCraken J, Lee L, Aman M, et al. Positive Effects of Methylphenidate on Social Communication and Self-Regulation in Children with Pervasive Developmental Disorders and Hyperactivity. Journal of Autism and Developmental Disorders. 2009;39:395–404. doi: 10.1007/s10803-008-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E, Carr E, Feeley K. Multiple Effects of Joint Attention Intervention for Children with Autism. Behavior Modification. 2006;30:782–834. doi: 10.1177/0145445506289392. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Beyond Modularity: A Developmental Perspective on Cognitive Science. Cambridge, MA: MIT Press; 1996. [Google Scholar]
- Kasari C, Freeman S, Paparella T. The UCLA RCT on Play and Joint Attention; Paper presented at the Biennial Conference of the Society for Research on Child Development; Boston, MA. 2007. Apr, [Google Scholar]
- Kasari C, Paparella T, Freeman S, Jahromi L. Language Outcomes in Autism: Randomized Comparison of Joint Attention and Play Interventions. Journal of Consulting and Clinical Psychology. 2008;76:125–137. doi: 10.1037/0022-006X.76.1.125. [DOI] [PubMed] [Google Scholar]
- Kelley W, McCrae CN, Wyland C, Cagler S, Inati S, Heatherton T. Finding the Self? An Event Related fMRI Study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Courchesne E. Functional Abnormalities of the Default Network during Self- and Other-Reflection in Autism. Social Cognitive Affective Neuroscience (SCAN) 2008;3:177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Perrett D. Demystifying Social-Cognition: A Hebbian Perspective. Trends in Cognitive Science. 2006;8:501–507. doi: 10.1016/j.tics.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Klein C, Travers B, Klinger L, Klinger M, Kana R. ‘Neural Bases of Self Representation in Higher Functioning Autism. Paper presented at the International Meeting of Autism Research; May 7th; Chicago. 2009. [Google Scholar]
- Kronenberg G, Ende G, Alm B, Deuschle M, Heuser I, Colla M. Increase in NAA and Reduced Choline Levels in the Anterior Cingulate following Chronic Methylphenidate. European Archives of Psychiatry and Clinical Neuroscience. 2008;7 doi: 10.1007/s00406-008-0810-2. 446–45. [DOI] [PubMed] [Google Scholar]
- Landry R, Bryson S. Impaired Disengagement of Attention in Young Children with Autism. Journal of Child Psychology and Psychiatry. 2004;45:115–1122. doi: 10.1111/j.1469-7610.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S. Self-Referential Cognition and Empathy in Autism. PLoS ONE. 2007;9(e883):1–11. doi: 10.1371/journal.pone.0000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran J, Heatherton T, Banfield J, Kelley W. Medial Prefrontal Activity Predicts Memory for Self. Cerebral Cortex. 2004;14:647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Johnson M, Sirois S, Spratling S, Thomas M, Wasserman G. Neuroconstructivism I: How the Brain Constructs Cognition. New York: Oxford University Press; 2007. [Google Scholar]
- McClelland J, Rogers T. The Parallel Distributed Processing Approach to Semantic Cognition. Nature Reviews: Neuroscience. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen J. An Integrative Theory of Prefrontal Cortex Functioning. Annual Review of Neurosciences. 2001;24:167–2002. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morales M, Mundy P, Crowson M, Neal R, Delgado C. Individual Differences in Infant Attention Skills, Joint Attention, and Emotion Regulation Behavior. International Journal of Behavioral Development. 2005;29:259–263. [Google Scholar]
- Morales M, Mundy P, Delgado CEF, Yale M, Messinger D, Neal R, Schwartz HK. Responding to Joint Attention across the 6- to 24-Month Age Period and Early Language Acquisition. Journal of Applied Developmental Psychology. 2000;21:283–298. [Google Scholar]
- Mosconi M, Cody-Hazlett H, Poe M, Gerig G, Gimpel-Smith R, Piven J. Longitudinal Study of Amygdale Volume and Joint Attention in 2- to 4-Year-Old Children with Autism. Archive of General Psychiatry. 2009;66:509–516. doi: 10.1001/archgenpsychiatry.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P. Joint Attention and Social-Emotional Approach Behavior in Children with Autism. Development and Psychopathology. 1995;7:63–82. [Google Scholar]
- Mundy P. The Neural Basis of Social Impairments in Autism: The Role of the Dorsal Medial-Frontal Cortex and Anterior Cingulate System. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2003;44:793–809. doi: 10.1111/1469-7610.00165. [DOI] [PubMed] [Google Scholar]
- Mundy P, Block J, Vaughan Van Hecke A, Delgadoa C, Venezia Parlade M, Pomares Y. Individual Differences and the Development of Infant Joint Attention. Child Development. 2007;78:938–954. doi: 10.1111/j.1467-8624.2007.01042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Burnette C. Joint Attention and Neurodevelopment. In: Volkmar F, Klin A, Paul R, editors. Handbook of Autism and Pervasive Developmental Disorders. 3rd ed. Hoboken, NJ: John Wiley; 2005. pp. 650–681. [Google Scholar]
- Mundy P, Card J, Fox N. EEG Correlates of the Development of Infant Joint Attention Skills. Developmental Psychobiology. 2000;36:325–338. [PubMed] [Google Scholar]
- Mundy P, Crowson M. Joint Attention and Early Social Communication: Implications for Research on Intervention with Autism. Journal of Autism and Developmental Disorders. 1997;27:653–676. doi: 10.1023/a:1025802832021. [DOI] [PubMed] [Google Scholar]
- Mundy P, Fox N, Card Joint Attention, EEG Coherence and Early Vocabulary Development. Developmental Science. 2003;6:48–54. [Google Scholar]
- Mundy P, Hogan A. Intersubjectivity, Joint Attention and Autistic Developmental Pathology. In: Cicchetti D, Toth S, editors. Rochester Symposium of Developmental Psychopathology, Vol. 5, A Developmental Perspective on the Self and its Disorders; Hillsdale, NJ. Lawrence Erlbaum; 1994. pp. 1–30. [Google Scholar]
- Mundy P, Kasari C, Sigman M. Nonverbal Communication, Affective Sharing, and Intersubjectivity. Infant Behavior and Development. 1992;15:377–381. [Google Scholar]
- Mundy P, Newell L. Attention, Joint Attention and Social Cognition. Current Directions in Psychological Science. 2007;16:269–274. doi: 10.1111/j.1467-8721.2007.00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Kasari C. The Autistic Person’s Theory of Mind and Early Nonverbal Joint Attention Deficits. In: Baron-Cohen S, Tager-Flusberg H, Cohen D, Volkmar F, editors. Understanding Other Minds: Perspectives from Autism. Oxford: Oxford University Press; 1993. pp. 181–201. [Google Scholar]
- Mundy P, Sigman M, Kasari C. Joint Attention, Developmental Level, and Symptom Presentation in Children with Autism. Development and Psychopathology. 1994;6:389–401. [Google Scholar]
- Mundy P, Sullivan L, Mastergeorge A. A Parallel and Distributed Processing Model of Joint Attention and Autism. Autism Research. 2009;2:2–21. doi: 10.1002/aur.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Vaughan Van Hecke A. Neural Systems, Gaze Following, and the Development of Joint Attention. In: Nelson C, Luciana M, editors. Handbook of Developmental Neuroscience. Cambridge, MA: MIT Press; 2008. pp. 819–838. [Google Scholar]
- Murias M, Swanson J, Srinivasen R. Functional Connectivity of Frontal Cortex in Health and ADHD Children Reflected in EEG Coherence. Cerebral Cortex. 2007;17:1788–1799. doi: 10.1093/cercor/bhl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murias M, Webb S, Greenson J, Dawson G. Resting State Cortical Connectivity in EEG Coherence in Individuals with Autism. Biological Psychiatry. 2007;62:270–273. doi: 10.1016/j.biopsych.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nation K, Penny S. Sensitivity to Eye Gaze in Autism: Is it Normal? Is it Automatic? Is it Social? Development and Psychopathology. 2008;20:79–97. doi: 10.1017/S0954579408000047. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Fox N, Mundy P. Joint Attention, Self-Recognition and Neurocognitive Functioning. Infancy. 2005;7:35–51. doi: 10.1207/s15327078in0701_4. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzell A, Greck M, Bermpohl F, Debrowolny H, Panksepp J. Self Referenced Processing in Our Brain – A Meta Analysis of Imaging Studies of the Self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal Regional Cerebral Blood Flow in Childhood Autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Otten L, Henson R, Rugg M. Depth of Processing Effects on Neural Correlates of Memory Encoding. Brain. 2001;125:399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Origins of Intelligence in Children. New York: Norton; 1952. [Google Scholar]
- Posner M, Rothbart M. Research on Attention Networks as a Model for the Integration of Psychological Science. Annual Review of Psychology. 2007a;58:1–23. doi: 10.1146/annurev.psych.58.110405.085516. [DOI] [PubMed] [Google Scholar]
- Posner M, Rothbart M. Educating the Human Brain. Washington DC: American Psychological Association; 2007b. [Google Scholar]
- Ramnani N, Behrens T, Penny W, Matthews P. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Reddy V. On Being the Object of Attention: Implications for Self-Other Consciousness. Trends in Cognitive Sciences. 2003;7:397–402. doi: 10.1016/s1364-6613(03)00191-8. [DOI] [PubMed] [Google Scholar]
- Rogers S. What Are Infant Siblings Teaching Us about Autism in Infancy? Autism Research. 2009;2:126–137. doi: 10.1002/aur.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutter D, Peper J, Koppleschaar H, Kahn R, van Hank J. Administration of Testosterone Increases Frontal Connectivity in Cortico-Cortical Depression Circuits. Journal of Neuropsychiatry. 2005;17:372–377. doi: 10.1176/jnp.17.3.372. [DOI] [PubMed] [Google Scholar]
- Sheinkopf S, Mundy P, Claussen A, Willoughby J. Infant Joint Attention Skill and Preschool Behavioral Outcomes in At-Risk Children. Development and Psychopathology. 2004;16:273–293. doi: 10.1017/s0954579404044517. [DOI] [PubMed] [Google Scholar]
- Sigman M, McGovern C. Improvements in Cognitive and Language Skills from Preschool to Adolescence in Autism. Journal of Autism and Developmental Disorders. 2005;35:15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Sigman M, Ruskin E. Continuity and Change in the Social Competence of Children with Autism, Down Syndrome, and Developmental Delays. Monographs of the Society for Research in Child Development. 1999;64 doi: 10.1111/1540-5834.00002. (1, Serial No. 256) [DOI] [PubMed] [Google Scholar]
- Singer L. General Issues in Infant Assessment and Development. In: Singer L, Zeskind P, editors. Biobehavioral Assessment of the Infant. New York: Guilford Press; 2001. pp. 3–17. [Google Scholar]
- Smith L, Thelen E. Development as a Dynamic System. Trends in Cognitive Science. 2003;7:343–348. doi: 10.1016/s1364-6613(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Striano T, Chen X, Cleveland A, Bradshaw S. Joint Attention Social Cues Influence Infant Learning. European Journal of Developmental Psychology. 2006;3:289–299. [Google Scholar]
- Thakkar K, Polli F, Joseph R, Tuch D, Hadjikhani N, et al. Response Monitoring, Repetitive Behaviours and Anterior Cingulate Activity in Autism Spectrum Disorders. Brain. 2008;7:1–15. doi: 10.1093/brain/awn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelen E, Smith L. A Dynamic Systems Approach to the Development of Cognition and Action. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding Sharing Intentions:The Origins of Cultural Cognition. Brain and Behavior Sciences. 2005;28:675–690. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Toichi M, Kamio Y, Okada T, Sakihama M, Youngstrom EA, Findling RL, Yamamoto K. A Lack of Self-Consciousness in Autism. American Journal of Psychiatry. 2002;159:1422–1424. doi: 10.1176/appi.ajp.159.8.1422. [DOI] [PubMed] [Google Scholar]
- Uddin L, Menon V. The Anterior Insula and Autism: Under-connected and Under-examined. Neuroscience and Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.06.002. doi: 1016/neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwal T, Hunyadi E, Grupe D, Conners C, Schultz R. Self, Mother and Abstract Other: An fMRI Study of Reflective Social Processing. Neuroimage. 2008;41:1437–1446. doi: 10.1016/j.neuroimage.2008.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan (Van Hencke) A, Mundy P, Acra CF, Block J, Delgado C, Parlade M, Meyer J, Neal R, Pomares Y. Infant Joint Attention, Temperament, and Social Competence in Preschool Children. Child Development. 2007;78:53–69. doi: 10.1111/j.1467-8624.2007.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vygotsky L. Thought and Language. Cambridge, MA: M.I.T. Press; 1962. [Google Scholar]
- Werner H, Kaplan B. Symbol Formation. Oxford: Wiley; 1963. [Google Scholar]
- Whalen C, Schreibman L, Ingersol B. The Collateral Effects of Joint Attention Training on Social Initiations, Positive Affect, Imitation and Spontaneous Speech in Young Children with Autism. Journal of Autism and Developmental Disorders. 2006;36:665–664. doi: 10.1007/s10803-006-0108-z. [DOI] [PubMed] [Google Scholar]
- Williams D, Happe F. Pre Conceptual Self Awareness in Autism Spectrum Disorders: The Case of Action Monitoring. Journal of Autism and Developmental Disorders. 2008;39:251–259. doi: 10.1007/s10803-008-0619-x. [DOI] [PubMed] [Google Scholar]
- Zahavi D. Subjectivity and Selfhood: Intersubjectivity and the First Person Perspective. Cambridge, MA: MIT Press; 2005. [Google Scholar]