Abstract
We compared neonatal outcomes in twin pregnancies following moderately preterm birth (MPTB), late preterm birth (LPTB) and term birth. A secondary analysis of a multi-center, randomized controlled trial of multiple gestations was conducted. MPTB was defined as delivery between 320/7 and 336/7 weeks and LPTB between 340/7 and 366/7 weeks. Primary outcome was a neonatal outcome composite consisting of one or more of the following: neonatal death, respiratory distress syndrome, early onset culture-proven sepsis, stage 2 or 3 necrotizing enterocolitis, bronchopulmonray dysplasia, grade 3 or 4 intraventricular hemorrhage, periventricular leukomalacia, pneumonia, or severe retinopathy of prematurity. Among 552 twin pregnancies, the MPTB rate was 14.5%, LPTB 49.8% and term birth rate 35.7%. The rate of the primary outcome was different between groups: 30.0% for MPTB, 12.8% for LPTB, 0.5% for term (p< 0.001). Compared with term neonates, the primary neonatal outcome composite was increased following MPTB (relative risk [RR] 58.5; 95% confidence interval [CI] 11.3 to 1693.0) and LPTB (RR 24.9; 95% CI 4.8 to 732.2). Twin pregnancies born moderately and late preterm encounter higher rates of neonatal morbidities compared to twins born at term.
Keywords: twin pregnancy, late preterm birth, neonatal morbidities
Introduction
In 2005, a workshop sponsored by the National Institute of Child Health and Human Development (NICHD) developed definitions to replace the common obstetrical phrase, “near term.” It was felt that “near term” portrayed the impression that these infants are almost term and safe to deliver. To emphasize that these infants are in fact preterm and often behave prematurely, the term “late preterm birth” (LPTB) was preferred.1 Moderately preterm birth (MPTB) commonly includes infants born between 320/7 and 336/7 weeks of pregnancy, whereas LPTB is typically defined as infants born between 340/7 and 366/7 weeks of pregnancy.2 Although LPTB constitutes 9% of all deliveries, it accounts for 75% of all preterm births. 3–4 Both neonatal mortality and morbidity including respiratory distress syndrome (RDS), sepsis, intraventricular hemorrhage (IVH), phototherapy, and intubation in the delivery room in those born late preterm are increased compared to those born after 37 weeks. 3
The rate of twins has increased 67% since 1980 mainly due to the increase in late childbearing age and assisted reproductive techniques.2 Over the last few decades, the rate of preterm birth among twin pregnancies has risen in parallel from 48% to 60%, with the largest group comprised of LPTB between 34 to 36 weeks of pregnancy.5 In a recent randomized placebo-controlled trial to determine whether 17 α-hydroxyprogesterone caproate decreased the rate of preterm birth in twin pregnancies, the overall rate of preterm birth was 69.9%.6 Our objective was to analyze and compare neonatal mortality and morbidity rates of MPTB and LPTB compared with births at term in twin pregnancies to estimate the magnitude of increased risk associated with these preterm births.
Materials and Methods
This is a secondary analysis of a multicenter, randomized controlled trial of multiple gestations. Of the 655 twin pregnancies included in the primary study, 325 received 17 α-hydroxyprogesterone caproate (17P) weekly and 330 received placebo at 16 to 20 weeks until the end of 35 weeks or delivery. Only twin gestations were included; triplets and higher-order pregnancies were excluded. There were similar rates in delivery or fetal death before 35 weeks between groups: 41.5% of pregnancies in women who received 17P and 37.3% in those who received placebo. Our study was approved by the Committee for the Protection of Human Subjects Institutional Review Board at the University of Texas Health Science Center at Houston.
In this study, only pregnancies with two liveborn infants and those who delivered after 320/7 weeks were included; those with an intrauterine fetal death were excluded. MPTB was defined as delivery between 320/7 and 336/7 weeks and LPTB between 340/7 and 366/7 weeks.1 Term delivery was defined as delivery 370/7 weeks or greater of pregnancy. Neonatal outcomes were compared between groups based on gestational age categories (i.e., MPTB versus LPTB versus term birth).
The primary outcome was a composite outcome of serious adverse events defined as one or more of the following outcomes per pregnancy: neonatal death, RDS, early onset culture-proven sepsis, stage 2 or 3 necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), grade 3 or 4 IVH, periventricular leukomalacia (PVL), and severe retinopathy of prematurity (ROP). Secondary outcomes included a respiratory composite consisting of one or more of the following outcomes per pregnancy: RDS, transient tachypnea of the newborn (TTN), need for mechanical ventilation (MV), or need for oxygen supplementation. Other secondary outcomes included 5-minute Apgar score <7, pneumonia, seizures, patent ductus arteriosus, neonatal intensive care unit (NICU) admission, length of longest NICU stay, TTN, MV, duration of MV, duration of supplemental oxygen. Maternal demographics including maternal age, race and ethnicity, rate of indicated preterm birth prior to 35 weeks of pregnancy, rate of nullitparity, and mode of delivery of twins with MPTB and LPTB were compared with term births. Confounding factors including rate of antenatal corticosteroid use (ACS), rate of preterm premature rupture of membranes (PPROM), and rate of preeclampsia or gestational hypertension were also evaluated.
For the primary study, strict definitions were used for the diagnosis of neonatal outcomes.5 An infant was considered to have RDS if the infant was diagnosed with type 1 RDS and required oxygen therapy (FiO2≥0.40) for greater than or equal to 24 hours or if the infant died before 24 hours of age and received a clinical diagnosis of RDS type 1 and oxygen therapy (FiO2≥0.40). The clinical diagnosis of RDS type 1 included hyaline membrane disease and respiratory insufficiency of prematurity, but not TTN. The clinical diagnosis of RDS Type II included TTN. TTN was defined as an infant weighing > 1000 grams and requiring oxygen therapy and/or MV during the first 24 hours of life but not demonstrating evidence of other causes of respiratory distress such as hyaline membrane disease. The duration of MV was the total days on the mechanical ventilator, including multiple periods on the ventilator. This did not include continuous positive airway pressure (CPAP) or cycled CPAP. The duration of oxygen supplementation was the total days that the infant received supplemental oxygen (FiO2>0.21). This did not include “blow by” oxygen administration. A pregnancy was considered for NICU admission if either of twins was admitted to the NICU. The length of stay for the NICU was defined as the longer length of stay for either twin. Likewise, the length of MV or supplemental oxygen was defined as the longer length required for either twin. All outcomes used for primary composite outcome, secondary respiratory composite, and other secondary outcomes were collected in the primary trial for all subjects.5
The analyses were conducted by twin-pair using the worse outcome for each pair as the outcome of interest. Continuous variables were compared using the Kruskal-Wallis test. Categorical variables were compared using the Cochran-Armitage trend test. The relative risk (RR) for the primary outcome composite and the respiratory composite was compared between twins with MPTB to term birth, and between twins with LPTB to term birth, and adjusted for ACS exposure and cesarean delivery. A p value of <0.05 was considered significant.
Results
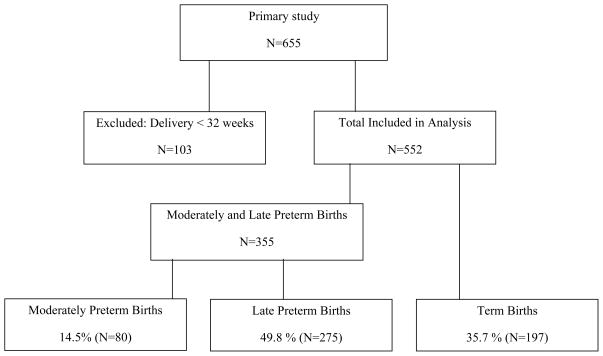
In the primary study, there were 655 twin pregnancies analyzed. Preterm birth less than 32 weeks of pregnancy occurred in 15.7% (n=103) and were thus excluded from this secondary analysis. Among the 552 remaining twin pregnancies included in this analysis, the MPTB rate was 14.5% (n=80), the LPTB rate was 49.8% (n=275), and the term birth rate 35.7% (n=197; Fig. 1).
Figure 1.
Proportion of twin pregnancies delivering moderately preterm, late preterm and at term.
Women in all groups had similar maternal age, race and ethnicity, and rate of nulliparity as described in Table 1. The rate of the primary outcome was progressively higher in those twins with MPTB and LPTB compared to term, (MPTB 30.0% versus LPTB 12.8% versus term births 0.5%, p<0.001; Table 2). Compared to term neonates, the primary neonatal outcome composite was increased following MPTB (RR 58.5; 95% confidence interval [CI] 11.3 to 1693.0) and LPTB (RR 24.9; 95% CI 4.8 to 732.2). The rate of the secondary respiratory composite was also progressively higher in twins with MPTB and LPTB compared with term births (MPTB 67.5% versus LPTB 33.8% versus term births 8.1%, p < 0.001; Table 3). The respiratory outcome composite was increased following MPTB (RR 8.3; 95% CI 5.1 to 13.6) and LPTB (RR 4.2; 95% CI 2.5 to 6.9) compared to term neonates.
Table 1.
Maternal characteristics
| MPTB (n=80) | LPTB (n=275) | Term (n=197) | p Value | |
|---|---|---|---|---|
| Maternal age (years) | 29.0 ± 6.7 | 29.9 ± 6.8 | 30.3 ± 6.7 | 0.31 |
| Nulliparity | 38 (47.5) | 114 (41.5) | 89 (45.2) | 0.55 |
| Race | 0.36 | |||
| African American | 20 (25.0) | 62 (22.6) | 34 (17.3) | |
| Caucasian | 53 (66.3) | 182 (66.2) | 146 (74.1) | |
| Other | 7 (8.8) | 31 (11.3) | 17 (8.6) | |
| Hispanic ethnicity | 6 (7.5) | 47 (17.1) | 27 (13.7) | 0.09 |
| Indicated delivery at < 35 weeks | 19 (23.8) | 19 (6.9) | 0 (0) |
Results are mean ± standard deviation or n (%). LPTB, late preterm birth; MPTB, moderately preterm birth.
Table 2.
Primary neonatal outcomes
| Neonatal outcome | MPTB | LPTB | Term | Cochran-Armitage Trend p value |
|---|---|---|---|---|
| Primary Composite | 24 (30.0) | 35 (12.8) | 1 (0.5) | <0.001 |
| RDS | 23 (28.8) | 32 (11.7) | 0 (0) | <0.001 |
| Sepsis | 4 (5.0) | 6 (2.2) | 0 (0) | 0.004 |
Results are n (%). There were no cases of severe retinopathy of prematurity, stage 3 or 4 intraventricular hemorrhage, or periventricular leukomalacia. There was one case of stage 2 or 3 necrotizing enterocolitis in the MPTB group, one case of bronchopulmonary dysplasia in the LPTB group, and one neonatal death in the LPTB group. LPTB, later preterm birth; MPTB, moderately preterm birth; RDS, respiratory distress syndrome; Sepsis, early onset, culture-proven sepsis.
Table 3.
Secondary neonatal outcomes
| Neonatal outcome | MPTB | LPTB | Term | p value* |
|---|---|---|---|---|
| Respiratory Composite | 54 (67.5) | 93 (33.8) | 16 (8.1) | <0.001 |
| RDS | 23 (28.8) | 32 (11.7) | 0 (0) | <0.001 |
| TTN | 31 (38.8) | 57 (20.8) | 13 (6.7) | <0.001 |
| MV | 21 (26.3) | 17 (6.2) | 2 (1.0) | <0.001 |
| Oxygen supplementation | 47 (58.8) | 82 (30.2) | 11 (5.7) | <0.001 |
| Length of MV (d) | 0.5 ± 1.0 | 0.3 ± 2.1 | 0.02 ± 0.23 | <0.001† |
| Length of oxygen (d) | 2.9 ± 4.5 | 1.2 ± 7.1 | 0.16 ± 1.15 | <0.001† |
| Length of NICU (d) | 22.7 ± 9.4 | 12.4 ± 14.7 | 6.8 ± 6.5 | <0.001† |
| NICU admission | 78 (97.5) | 148 (54.0) | 33 (16.9) | <0.001 |
| PDA | 0 (0) | 2 (0.7) | 5 (2.6) | 0.05 |
| Pneumonia | 2 (2.5) | 4 (1.5) | 1 (0.5) | 0.17 |
| 5 Minute Apgar Score <7 | 6 (7.5) | 10 (3.7) | 5 (2.6) | 0.08 |
p value is from the Cohran-Armitage test for trend except where indicated
p value is from the Kruskal-Wallis test
Results are mean ± standard deviation or n (%). TThere was one case of seizure in the LPTB group. LPTB, late preterm birth; MPTB, moderately preterm birth; MV, mechanical ventilation; NICU, neonatal intensive care unit; PDA, patent ductus arteriosus; RDS, respiratory distress syndrome; TTN, transient tachypnea of the newborn.
Most of the differences in neonatal outcomes were respiratory morbidities. RDS contributed the greatest effect to the primary outcome. Both MPTB and LPTB twins had significantly higher rates of RDS, neonatal sepsis, TTN, MV, and NICU admissions compared with term births. Additionally, the lengths of NICU stay, MV, and supplemental oxygen were significantly longer in those twins with MPTB and LPTB compared with term births.
Confounding factors such as the rates of exposure to ACS and preeclampsia or gestational hypertension were significantly higher the earlier the gestation (Table 4). The highest rate of PPROM occurred in the LPTB group. There were no differences between groups in the rate of cesarean delivery.
Table 4.
Clinical characteristics
| MPTB | LPTB | Term | Cochran-Armitage Trend p value | |
|---|---|---|---|---|
| ACS | 42 (52.5) | 71 (25.9) | 16 (8.1) | <0.001 |
| PPROM | 10 (12.5) | 47 (17.2) | 11 (5.6) | 0.01 |
| Cesarean delivery | 46 (57.5) | 156 (56.7) | 131 (66.5) | 0.06 |
| Preeclampsia or GH | 21 (26.3) | 63 (22.9) | 25 (12.7) | 0.003 |
Results are n (%). ACS, antenatal corticosteroid; GH, gestational hypertension; LPTB, late preterm birth; MPTB, moderately preterm birth; PPROM, preterm premature rupture of membranes.
There were no cases of severe ROP, stage 3 or 4 IVH, or PVL. There was only one case of BPD, stage 2 or 3 NEC, seizures, and neonatal death within the preterm groups. There were no differences between groups in the rate of pneumonia.
Discussion
In this secondary analysis, twin pregnancies with MPTB and LPTB encountered significantly higher rates of neonatal morbidities than those born at term. In our study, the rate of MPTB was 14.5% and LPTB 49.8%. Our study confirms that there is an increased rate of morbidity among these preterm twin infants compared to term infants. But the emphasis in morbidity is primarily respiratory, with a fourfold increase risk among LPTB twins and an eightfold increase risk among MPTB twins. Even so, these respiratory complications appear temporary, as demonstrated by the short length of MV and supplemental oxygen needed by the preterm infants.
The morbidities seen among twins delivering prematurely in our study are similar to other studies involving only singleton pregnancies. Prior reports show that singleton infants born moderately and late preterm have higher rates of both short-term and long-term morbidities compared to those born at term.7–9 In addition, these infants are at significantly higher risk of re-hospitalization even when discharged home in healthy condition. 8, 9 The rate of RDS in singleton pregnancies ranged from 7.4 to 28.9% at 34 weeks compared to 0.6 to 4.2% at term.1, 10–14 MV was required more often in those with LPTB (3.3 to 19.8%) compared to term births (0.5 to 4%).3, 11,12, 15
Over the last few decades, the rate of preterm birth among twin pregnancies has risen from 48 to 60%, with the largest group comprised of LPTB between 34 and 36 weeks of pregnancy.5 From 1990 to 2005, the rate of LPTB has steadily increased 3% per year from 22.6 to 32.2 per 1000 births in the United States.5 The rate of twins has increased 67% since 1980 mainly due to the increase in late childbearing age and assisted reproductive techniques.2 Like singletons with MPTB and LPTB, twins born moderately or late preterm have an increase in morbidity including respiratory complications, NICU admissions, temperature instability, hypoglycemia, and feeding difficulties.2
From an epidemiological perspective, there has been a trend toward delivering infants earlier in gestation leading to the alarming increase in rate of LPTB.4 Our study confirms that twins with MPTB and LPTB have an increased rate of neonatal morbidity. The greatest magnitude in risk among these preterm infants is primarily pulmonary in etiology. The respiratory composite was designed to assess frequency in pulmonary complications but has a wide range in severity. Both RDS and TTN are included in this composite. Despite the strict definition used to define RDS and TTN, the diagnosis may still be subject to clinical opinion. Although RDS and TTN are typically mutually exclusive, it is possible that such cases can be explained by progressive improvement of an infant from RDS to TTN, in which case the infant would count for each diagnosis. Although this could falsely influence the respiratory complication rates, such perceived discrepancies should be resolved by the inherent nature of the respiratory composite. Even so, the respiratory complications seen in our study appear temporary, as demonstrated by the short length of MV and supplemental oxygen needed by the preterm infants.
Overall, the fetal mortality rate, late fetal deaths (>28 weeks), infant deaths, and neonatal deaths (all per 1000 births) have all decreased over the past 25 years.5, 16 Importantly, the fetal mortality rate has declined slowly and steadily. The majority of this decline has been in late fetal mortality with little change in the early fetal mortality rate.5 Our study indicates an increase in respiratory morbidity in MPTB and LPTB compared with term births. Although it is possible that changes in obstetric practice patterns regarding timing of delivery can reduce the stillbirth rate at the expense of increasing the LPTB rate and neonatal respiratory complications, our study was not designed to answer this question.
There are both strengths and weaknesses to this secondary analysis. Strengths included the large sample size within this multicenter diverse study. Our study did find low rates of major morbidities. This could be reflected by the pregnancies participating in large tertiary care institutions that have the capability of monitoring the pregnancies closely and are equipped with skilled neonatal expertise. Thus, our findings may not be generalizable to all twin gestations delivering within these preterm groups in other clinical settings.
In summary, there is a high rate of MPTB and LPTB among twin pregnancies. These preterm infants have up to an eightfold increase risk of respiratory morbidities compared with term infants. However, these pulmonary complications appear to be mild and temporary. Such information may be valuable for health care providers in counseling women at risk of delivering moderately or late preterm. Future studies are needed to investigate further the impact of MPTB and LPTB in twin pregnancies.
Acknowledgments
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD27869, HD21410, HD40512, HD34136, HD34208, HD40485, HD27915, HD40544, HD40560, HD27917, HD40500, HD34116, HD40545, HD27860, HD36801).
The authors would like to acknowledge subcommittee members who contributed as follows: Susan M. Ramin, M.D. and Sean Blackwell, M.D. (protocol development and oversight), Elizabeth Thom, Ph.D. (protocol/data management and statistical analysis), Margaret Cotroneo, R.N. and Allison Northen, R.N. M.S.N. (protocol development and coordination between clinical research centers).
In addition to the authors, other members of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network are as follows:
University of Alabama at Birmingham — W. Andrews, J. Sheppard, A. Northen
University of Pittsburgh — E. Daugherty, M. Cotroneo, H. Simhan
Northwestern University — M. Dinsmoor (Evanston Hospital), G. Mallett, P. Simon, M. Huntley, M. Ramos
Drexel University — M. Hoffman, S. Wilson, C. Tocci, M. Lake, M. Talucci
University of Utah — K. Anderson, F. Porter (LDS Hospital), A. Guzman (McKay-Dee Hospital Center), K. Jolley (Utah Valley Regional Medical Center), S. Quinn (LDS Hospital)
Columbia University — R. Berkowitz, S. South, L. Paley, S. Bousleiman, V. Carmona
The Ohio State University — F. Johnson, C. Latimer
Case Western Reserve University — C. Milluzzi, C. Heggie, H. Ehrenberg, B. Stetzer, A. Merlino
University of North Carolina at Chapel Hill — K. Boggess, K. Dorman, S. Timlin
Wayne State University — G. Norman, C. Sudz, S. Blackwell
Brown University — D. Allard
University of Texas Southwestern Medical Center, Dallas — K. Leveno, L. Moseley
University of Texas Health Science Center at Houston — D. Soebbing-Cross, J. Martinez, B. Glenn-Cole, L. Gilstrap
Wake Forest University Health Sciences — P. Meis, M. Swain, K. Johnson, K. Lanier, C. Leftwich
The George Washington UniversityBiostatistics Center — E. Thom, A. Braga, E. Cardenas, L. Leuchtenburg
Eunice Kennedy Shriver National Institute of Child Health and Human Development — S. Pagliaro MFMU Network Steering Committee Chair (University of Texas Medical Center, Galveston, TX) — G. Anderson, M.D.
Footnotes
Presented at the Society for Maternal Fetal Medicine Annual Meeting, January 26-31, 2009.
References
- 1.Raju TN, Higgins RD, Stark AR, Leveno KJ. Optimizing care and outcome for late-preterm (near-term) infants: A summary of the Workshop Sponsored by the National Institute of Child Health and Human Development. Pediatrics. 2006;118:1207–1214. doi: 10.1542/peds.2006-0018. [DOI] [PubMed] [Google Scholar]
- 2.Lee YM, Cleary-Goldman J, D’Alton ME. The impact of multiple gestations on late preter (near-term) births. Clin Perinatol. 2006;33:777–792. doi: 10.1016/j.clp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 3.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111:35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 4.Davidoff MJ, Dias T, Damus K, Russell R, Bettegowda VR, et al. Changes in the gestational age distribution among U.S singleton births: Impact on rates of late preterm birth, 1992 to 2002. Semin Perinatol. 2006;30:8–15. doi: 10.1053/j.semperi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Martin JA, Kung HC, Mathews TJ, Hoyert DL, Strobino DM, Guyer B, Sutton SR. Annual summary of vital statistics: 2006. Pediatrics. 2007;121:788–801. doi: 10.1542/peds.2007-3753. [DOI] [PubMed] [Google Scholar]
- 6.Rouse DJ, Caritis SN, Peaceman AM, Sciscione A, Thom EA, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–61. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 7.Bastek JA, Sammel MD, Pare E, Srinivas SK, Posencheg MA, Elovitz MA. Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. Am J Obstet Gynecol. 2008;199:367–369. doi: 10.1016/j.ajog.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Escobar GJ, McCormick MC, Supancic JA, Coleman-Phox K, Armstrong MA, Greene JD, Eichenwald EC, Richardson DK. Unstudied infants: outcomes of moderately premature infants in the neonatal intensive care unit. Arch Dis Child Fetal Neonatal Ed. 2006;91:F238–F244. doi: 10.1136/adc.2005.087031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shapiro-Mendoza CK, Tomasheck KM, Kotelchuck M, Barfield W, Weiss J, Evans S. Risk factors for neonatal morbidity and mortality among “healthy” late preterm newborns. Semin Perinatol. 2006;30:54–60. doi: 10.1053/j.semperi.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 11.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30:28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert WM, Nesbitt TS, Danielsen B. The cost of prematurity: Quantification by gestational age and birth weight. Obstet Gynecol. 2003;102:488–92. doi: 10.1016/s0029-7844(03)00617-3. [DOI] [PubMed] [Google Scholar]
- 13.Engle WA, Tomashek KM, Wallman C, et al. “Late-preterm” infants: A population at risk. Am Academy of Pediatrics. 2007;120:1390–1400. doi: 10.1542/peds.2007-2952. [DOI] [PubMed] [Google Scholar]
- 14.Yoder BA, Gordon MC, Barth WH. Late-preterm birth. Does the changing obstetric paradigm alter the epidemiology of the respiratory complications? Obstet Gynecol. 2008;111:814–22. doi: 10.1097/AOG.0b013e31816499f4. [DOI] [PubMed] [Google Scholar]
- 15.Gladstone IM, Katz VL. The morbidity of the 34 to 35 week gestation: Should we reexamine the paradigm? Am J Perinatol. 2004;21:9–13. doi: 10.1055/s-2004-820503. [DOI] [PubMed] [Google Scholar]
- 16.Ananth CV, Gyamfi C, Jain L. Characterizing risk profiles of infants who are delivered at late preterm gestations: does it matter? Am J Obstet Gynecol. 2008;199:329–331. doi: 10.1016/j.ajog.2008.08.040. [DOI] [PubMed] [Google Scholar]