Abstract
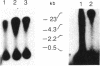
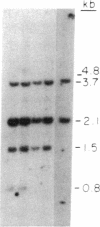
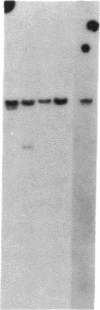
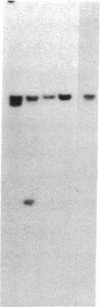
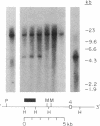
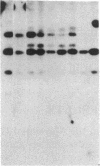
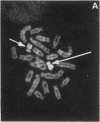
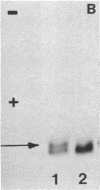
In marsupials and eutherian mammals, X chromosome dosage compensation is achieved by inactivating one X chromosome in female cells; however, in marsupials, the inactive X chromosomes is always paternal, and some genes on the chromosome are partially expressed. To define the role of DNA methylation in maintenance of X chromosome inactivity, we examined loci for glucose-6-phosphate dehydrogenase and hypoxanthine phosphoribosyltransferase in a North American marsupial, the opossum Didelphis virginiana, by using genomic hybridization probes cloned from this species. We find that these marsupial genes are like their eutherian counterparts, with respect to sex differences in methylation of nuclease-insensitive (nonregulatory) chromatin. However, with respect to methylation of the nuclease-hypersensitive (regulatory) chromatin of the glucose-6-phosphate dehydrogenase locus, the opossum gene differs from those of eutherians, as the 5' cluster of CpG dinucleotides is hypomethylated in the paternal as well as the maternal gene. Despite hypomethylation of the 5' CpG cluster, the paternal allele, identified by an enzyme variant, is at best partially expressed; therefore, factors other than methylation are responsible for repression. In light of these results, it seems that the role of DNA methylation in eutherian X dosage compensation is to "lock in" the process initiated by such factors. Because of similarities between dosage compensation in marsupials and trophectoderm derivatives of eutherians, we propose that differences in timing of developmental events--rather than differences in the basic mechanisms of X inactivation--account for features of dosage compensation that differ among mammals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs A. H., Axelman J., Migeon B. R. Reactivation of X-linked genes in human fibroblasts transformed by origin-defective SV40. Somat Cell Mol Genet. 1986 Nov;12(6):585–594. doi: 10.1007/BF01671944. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Taggart M. H., Bird A. P. Unmethylated domains in vertebrate DNA. Nucleic Acids Res. 1983 Feb 11;11(3):647–658. doi: 10.1093/nar/11.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. W., VandeBerg J. L., Sharman G. B., Poole W. E. Phosphoglycerate kinase polymorphism in kangaroos provides further evidence for paternal X inactivation. Nat New Biol. 1971 Mar 31;230(13):155–157. doi: 10.1038/newbio230155a0. [DOI] [PubMed] [Google Scholar]
- Cullen C. R., Hubberman P., Kaslow D. C., Migeon B. R. Comparison of factor IX methylation on human active and inactive X chromosomes: implications for X inactivation and transcription of tissue-specific genes. EMBO J. 1986 Sep;5(9):2223–2229. doi: 10.1002/j.1460-2075.1986.tb04488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartler S. M., Riggs A. D. Mammalian X-chromosome inactivation. Annu Rev Genet. 1983;17:155–190. doi: 10.1146/annurev.ge.17.120183.001103. [DOI] [PubMed] [Google Scholar]
- Johnston P. G., Sharman G. B. Studies on metatherian sex chromosomes. I. Inheritance and inactivation of sex-linked allelic genes determining glucose-6-phosphate dehydrogenase variation in kangaroos. Aust J Biol Sci. 1975 Dec;28(5-6):567–574. doi: 10.1071/bi9750567. [DOI] [PubMed] [Google Scholar]
- Keith D. H., Singer-Sam J., Riggs A. D. Active X chromosome DNA is unmethylated at eight CCGG sites clustered in a guanine-plus-cytosine-rich island at the 5' end of the gene for phosphoglycerate kinase. Mol Cell Biol. 1986 Nov;6(11):4122–4125. doi: 10.1128/mcb.6.11.4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M., Lambert H., Evans R. E., Liskay R. M. Differences in the DNA of the inactive X chromosomes of fetal and extraembryonic tissues of mice. Cell. 1983 May;33(1):37–42. doi: 10.1016/0092-8674(83)90332-x. [DOI] [PubMed] [Google Scholar]
- Kratzer P. G., Chapman V. M. X chromosome reactivation in oocytes of Mus caroli. Proc Natl Acad Sci U S A. 1981 May;78(5):3093–3097. doi: 10.1073/pnas.78.5.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer P. G., Gartler S. M. HGPRT activity changes in preimplantation mouse embryos. Nature. 1978 Aug 3;274(5670):503–504. doi: 10.1038/274503a0. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Chapman V. M., Hammer R. E., Brinster R., Tilghman S. M. Differential expression of alpha-fetoprotein genes on the inactive X chromosome in extraembryonic and somatic tissues of a transgenic mouse line. Nature. 1986 Jan 16;319(6050):224–226. doi: 10.1038/319224a0. [DOI] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Melton D. W., Caskey C. T., Martin G. R. Methylation of the mouse hprt gene differs on the active and inactive X chromosomes. Mol Cell Biol. 1986 Mar;6(3):914–924. doi: 10.1128/mcb.6.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock L. F., Takagi N., Martin G. R. Methylation of the Hprt gene on the inactive X occurs after chromosome inactivation. Cell. 1987 Jan 16;48(1):39–46. doi: 10.1016/0092-8674(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Lyon M. F. X-chromosome inactivation and developmental patterns in mammals. Biol Rev Camb Philos Soc. 1972 Jan;47(1):1–35. doi: 10.1111/j.1469-185x.1972.tb00969.x. [DOI] [PubMed] [Google Scholar]
- Martini G., Toniolo D., Vulliamy T., Luzzatto L., Dono R., Viglietto G., Paonessa G., D'Urso M., Persico M. G. Structural analysis of the X-linked gene encoding human glucose 6-phosphate dehydrogenase. EMBO J. 1986 Aug;5(8):1849–1855. doi: 10.1002/j.1460-2075.1986.tb04436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Do T. T. In search of non-random X inactivation: studies of fetal membranes heterozygous for glucose-6-phosphate dehydrogenase. Am J Hum Genet. 1979 Sep;31(5):581–585. [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R., Schmidt M., Axelman J., Cullen C. R. Complete reactivation of X chromosomes from human chorionic villi with a switch to early DNA replication. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2182–2186. doi: 10.1073/pnas.83.7.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeon B. R. Stability of X chromosomal inactivation in human somatic cells. Nature. 1972 Sep 8;239(5367):87–89. doi: 10.1038/239087a0. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Wolf S. F., Axelman J., Kaslow D. C., Schmidt M. Incomplete X chromosome dosage compensation in chorionic villi of human placenta. Proc Natl Acad Sci U S A. 1985 May;82(10):3390–3394. doi: 10.1073/pnas.82.10.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohandas T., Sparkes R. S., Shapiro L. J. Reactivation of an inactive human X chromosome: evidence for X inactivation by DNA methylation. Science. 1981 Jan 23;211(4480):393–396. doi: 10.1126/science.6164095. [DOI] [PubMed] [Google Scholar]
- Monk M., Harper M. I. Sequential X chromosome inactivation coupled with cellular differentiation in early mouse embryos. Nature. 1979 Sep 27;281(5729):311–313. doi: 10.1038/281311a0. [DOI] [PubMed] [Google Scholar]
- Richardson B. J., Czuppon A. B., Sharman G. B. Inheritance of glucose-6-phosphate dehydrogenase variation in kangaroos. Nat New Biol. 1971 Mar 31;230(13):154–155. doi: 10.1038/newbio230154a0. [DOI] [PubMed] [Google Scholar]
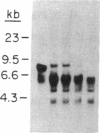
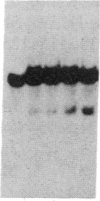
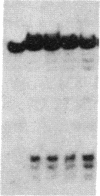
- Samollow P. B., Ford A. L., VandeBerg J. L. X-linked gene expression in the Virginia opossum: differences between the paternally derived Gpd and Pgk-A loci. Genetics. 1987 Jan;115(1):185–195. doi: 10.1093/genetics/115.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi N. Differentiation of X chromosomes in early female mouse embryos. Exp Cell Res. 1974 May;86(1):127–135. doi: 10.1016/0014-4827(74)90657-0. [DOI] [PubMed] [Google Scholar]
- Toniolo D., D'Urso M., Martini G., Persico M., Tufano V., Battistuzzi G., Luzzatto L. Specific methylation pattern at the 3' end of the human housekeeping gene for glucose 6-phosphate dehydrogenase. EMBO J. 1984 Sep;3(9):1987–1995. doi: 10.1002/j.1460-2075.1984.tb02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VandeBerg J. L., Cooper D. W., Sharman G. B., Poole W. E. Studies on metatherian sex chromosomes. IV. X linkage of PGK-A with paternal X inactivation confirmed in erythrocytes of grey kangaroos by pedigree analysis. Aust J Biol Sci. 1977 Apr;30(1-2):115–125. [PubMed] [Google Scholar]
- VandeBerg J. L., Johnston P. G., Cooper D. W., Robinson E. S. X-chromosome inactivation and evolution in marsupials and other mammals. Isozymes Curr Top Biol Med Res. 1983;9:201–218. [PubMed] [Google Scholar]
- West J. D., Frels W. I., Chapman V. M., Papaioannou V. E. Preferential expression of the maternally derived X chromosome in the mouse yolk sac. Cell. 1977 Dec;12(4):873–882. doi: 10.1016/0092-8674(77)90151-9. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Dintzis S., Toniolo D., Persico G., Lunnen K. D., Axelman J., Migeon B. R. Complete concordance between glucose-6-phosphate dehydrogenase activity and hypomethylation of 3' CpG clusters: implications for X chromosome dosage compensation. Nucleic Acids Res. 1984 Dec 21;12(24):9333–9348. doi: 10.1093/nar/12.24.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Jolly D. J., Lunnen K. D., Friedmann T., Migeon B. R. Methylation of the hypoxanthine phosphoribosyltransferase locus on the human X chromosome: implications for X-chromosome inactivation. Proc Natl Acad Sci U S A. 1984 May;81(9):2806–2810. doi: 10.1073/pnas.81.9.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S. F., Mareni C. E., Migeon B. R. Isolation and characterization of cloned DNA sequences that hybridize to the human X chromosome. Cell. 1980 Aug;21(1):95–102. doi: 10.1016/0092-8674(80)90117-8. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Migeon B. R. Clusters of CpG dinucleotides implicated by nuclease hypersensitivity as control elements of housekeeping genes. Nature. 1985 Apr 4;314(6010):467–469. doi: 10.1038/314467a0. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Patel P., Chinault A. C., Mohandas T., Shapiro L. J. Differential methylation of hypoxanthine phosphoribosyltransferase genes on active and inactive human X chromosomes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1759–1763. doi: 10.1073/pnas.81.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]