Abstract
The superior temporal gyrus has been implicated in language processing and social perception. Therefore, anatomical abnormalities of this structure may underlie some of the deficits observed in autism, a severe neurodevelopmental disorder characterized by impairments in social interaction and communication. In this study, volumes of the left and right superior temporal gyri were measured using magnetic resonance imaging obtained from 18 boys with high-functioning autism (mean age = 13.5 ±3.4 years; full-scale IQ = 103.6 ±13.4) and 19 healthy controls (mean age = 13.7 ±3.0 years; full-scale IQ = 103.9 ±10.5), group-matched on age, gender, and handedness. When compared to the control group, right superior temporal gyral volumes were significantly increased in the autism group after controlling for age and total brain volume. There was no significant difference in the volume of the left superior temporal gyrus. Post-hoc analysis revealed a significant increase of the right posterior superior temporal gyral volume in the autism group, before and after controlling for age and total brain volume. Examination of the symmetry index for the superior temporal gyral volumes did not yield statistically significant between-group differences. Findings from this preliminary investigation suggest the existence of volumetric alterations in the right superior temporal gyrus in children and adolescents with autism, providing support for a neuroanatomical basis of the social perceptual deficits characterizing this severe neurodevelopmental disorder.
Keywords: Autism, biological motion, language, social brain, structural MRI, and superior temporal gyrus
1. Introduction
Autism is a pervasive developmental disorder characterized by impairments in reciprocal social interaction, verbal and nonverbal language and communication, and a restricted range of interests and repetitive behavior (APA, 2000). Sensory and motor signs and symptoms, inattention with hyperactivity, emotion dysregulation, and intellectual disability are also integral aspects of this syndrome in many, though not all, affected individuals (Rogers and Dawson, 2009). The myriad social, language, cognitive, emotional, and behavioral problems observed in autism suggest that the syndrome affects a functionally diverse and widely distributed set of neural systems as evidenced by the wide range of structural abnormalities that have been reported (Brambilla et al., 2003; Palmen and van Engeland, 2004). Several brain regions have been examined extensively; however, the superior temporal gyrus (STG), an established node in the “social brain” network (Baron-Cohen et al., 1999; Bigler et al., 2007; Brothers and Ring, 1992), has received relatively little attention. In fact, a very limited number of morphometric investigations have been conducted to examine the size of the STG, a key structure that has been implicated in several neuropsychological and physiological functions thought to be abnormal in this severe neurodevelopmental disorder (Pelphrey et al., 2004).
Investigating STG abnormalities in autism is a logical endeavor given its important roles in language processing and social perception. The STG is perhaps best known for the former as it consists of the primary auditory cortex and Wernicke’s area. Abnormalities in these regions can result in profound language difficulties as illustrated in the extreme cases of cortical deafness and receptive aphasia (Eggert, 1977; Wernicke, 1874). Since the description of cortical deafness, it has been known that the STG is bilaterally involved in the initial stages of auditory perception (Zilbovicius et al., 1995). While the STG’s important role in language processing has been known since the 19th century, its role in social perception has a much more recent history. There exists now an extensive body of literature demonstrating the STG’s role in social perception. The STG’s importance in social perception was spawn by the use of functional magnetic resonance imaging (fMRI) in cognitive neuroscience research. Numerous tasks tapping social perception have been used in conjunction with fMRI to demonstrate the involvement of the superior temporal region such as the STG and superior temporal sulcus, and these studies have been reviewed extensively elsewhere (Pelphrey and Carter, 2008b; Redcay, 2008). Furthermore, the STG is highly connected to other key regions of the brain such as the superior temporal sulcus, frontal and parietal lobes, and the limbic and associated sensory systems (Gloor, 1997; Pandya and Yeterian, 1985; Seltzer and Pandya, 1978). Therefore, the STG may play a critical role in processing and integrating different types of information in order to give proper meaning to the surrounding world, and it has been suggested that temporal region dysfunction is implicated in almost all deficits observed in autism (Boddaert and Zilbovicius, 2002).
Numerous studies implementing a wide range of research modalities have reported different types of STG abnormalities in autism. In an influential postmortem study of the cytoarchitecture of the cerebral cortex, Casanova and colleagues reported abnormal cortical minicolumns (a basic functional neuronal unit) in the STG of patients with autism when compared to healthy controls (Casanova et al., 2002). In voxel-based MRI investigations, Waiter et al. and Salmond et al. also reported STG abnormalities such as increased gray matter volume (Salmond et al., 2003; Waiter et al., 2004). Moreover, a number of positron emission tomography (PET) (Boddaert et al., 2003; Castelli et al., 2002; Muller et al., 1999; Zilbovicius et al., 1995), single photon emission computed tomography (SPECT) (Mountz et al., 1995; Ohnishi et al., 2000), fMRI (Baron-Cohen et al., 1999; Boddaert et al., 2003; Gomot et al., 2006; Pelphrey and Carter, 2008a; Pelphrey et al., 2005), event-related potential (ERP) (Bruneau et al., 2003; Bruneau et al., 1999), and magnetoencephalography (MEG) (Gage et al., 2003; Roberts et al., 2010; Rojas et al., 2008) studies have also revealed STG abnormalities in autism, reporting abnormal STG activation/activity both during rest and while performing various tasks.
Despite this broad array of evidence supporting STG abnormalities in autism, a limited number of studies have been conducted specifically examining the size of this structure. Of these limited number of studies, most looked exclusively at asymmetry rather than group comparison of STG volume (De Fosse et al., 2004; Gage et al., 2009; Herbert et al., 2002; Herbert et al., 2005). There are, however, at least two studies directly comparing STG volume between autism and control groups. One volumetric study examined total STG volume in autism and found no significant volumetric differences when compared to a matched control group (Bigler et al., 2007). In contrast, the voxel-based MRI study mentioned previously, found increased STG gray matter volume when compared to controls (Waiter et al., 2004). In light of these inconsistent findings, this study was carried out to examine the total left and right STG size in high-functioning male children and adolescents with autism. Given that overgrowth has been associated with dysfunction at least during younger ages (Courchesne et al., 2004), the replicable finding of increased brain volume (Piven et al., 1995), and evidence suggesting the frontal/temporal lobes are most affected (Courchesne et al., 2004), it is hypothesized that STG volumes will be increased in subjects with autism when compared to healthy controls.
2. Results
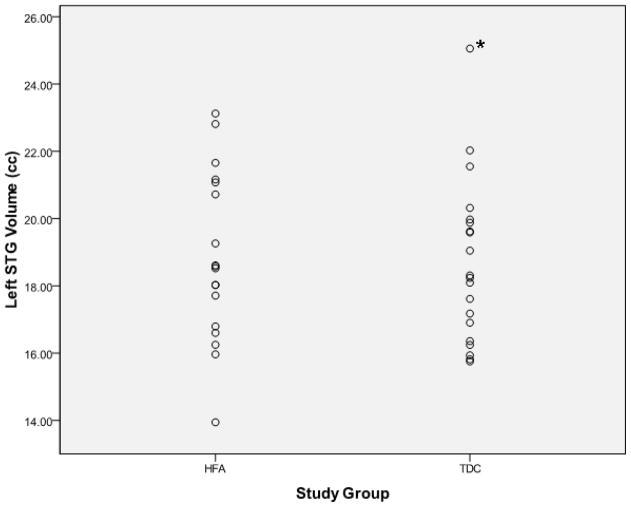
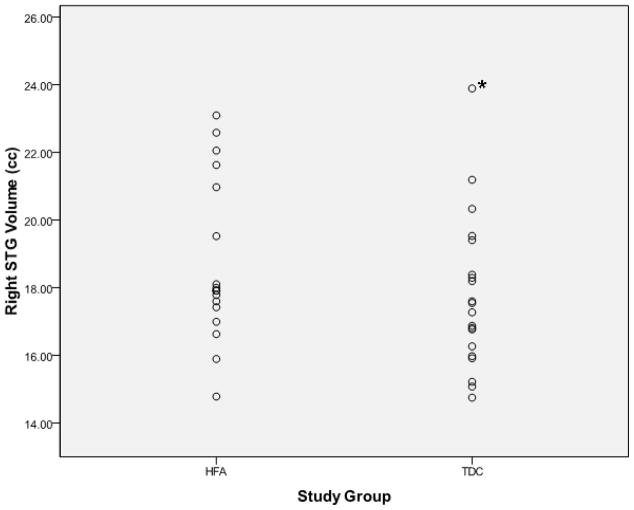
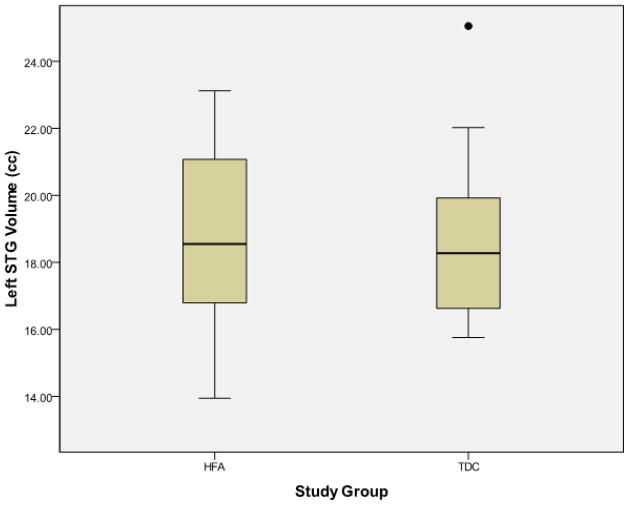
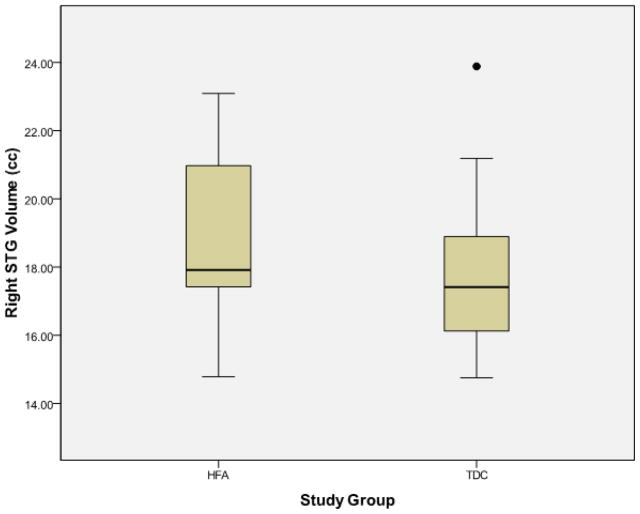
Examination of the scatter plots depicting all participants’ STG volumetric data by study group (Figure 1) revealed one typically developing control (TDC) subject who was an outlier in all volumetric measures, including left STG (TDC mean = 18.67±2.41 cc, outlier value = 25.06 cc) and right STG (TDC mean = 17.76±2.26 cc, outlier value = 23.89 cc). No outliers were present in the high-functioning autism (HFA) group. This outlier is clearly identified using box plots as depicted in Figure 2.
Figure 1.
Scatter plots depicting superior temporal gyral volumes (y-axis) for individual participants separated by study group membership (x-axis). The asterisk indentifies the outlier excluded from the final analysis. HFA = high-functioning autism, TDC = typically developing controls, STG = superior temporal gyrus, cc = cubic centimeters
Figure 2.
Box plots representing superior temporal gyral volumes (y-axis) separated by study group membership (x-axis). The outlier excluded from the final analysis is clearly identified by the solid dot. HFA = high-functioning autism, TDC = typically developing controls, STG = superior temporal gyrus, cc = cubic centimeters
In light of the potential problem of a single oultier in a small sample, final analysis was conducted after excluding this subject (N = 37; HFA = 18, TDC = 19). The resultant HFA and TDC groups did not differ in mean age, full-scale IQ (FSIQ), verbal IQ, and performance IQ (Table 1). Right STG volume was increased in the HFA group with a trend toward statistical significance (p = 0.075). After controlling for age and total brain volume (TBV), right STG volume was significantly higher in the HFA group (p = 0.040). There were no significant group differences in left STG volume before and after controlling for age and TBV. Post-hoc comparison of right posterior STG volume revealed significant increase in the HFA group (p = 0.038, uncorrected). After controlling for age and TBV, right posterior STG volume remained significantly higher in the HFA group (p = 0.028, uncorrected). The analyses for volumetric data are summarized in Table 2.
Table 1.
Group comparison in age and cognitive functioning
| HFA (N = 18) | TDC (N = 19) | Independent-samples t test (df = 35) | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | t | p | |
| Age | 13.5 | 3.4 | 8.8 – 18.3 | 13.7 | 3.0 | 9.4 – 18.4 | −0.193 | 0.848 |
| Height (cm) | 161.5 | 19.1 | 127 – 196 | 162.1 | 17.1 | 137 – 185 | −0.099 | 0.922 |
| FSIQ | 103.6 | 13.4 | 83 – 130 | 103.9 | 10.5 | 86 – 118 | −0.086 | 0.932 |
| VIQ | 106.8 | 13.4 | 84 – 133 | 104.8 | 9.9 | 88 – 119 | 0.517 | 0.608 |
| PIQ | 99.1 | 13.5 | 79 – 133 | 102.5 | 10.9 | 83 – 116 | −0.850 | 0.401 |
| SES | 3.21 | 1.31 | 1 – 5 | 3.33 | 0.98 | 2 – 5 | −0.279 | 0.758 |
HFA = high-functioning autism, TDC = typically developing controls, SD = standard deviation, FSIQ = full-scale IQ, VIQ = verbal IQ, PIQ = performance IQ, SES = socioeconomic scale
Table 2.
Brain Volume Comparisons Between Autism and Control Groups
| HFA (N = 18) | TDC (N = 19) | Independent samples t test (df = 35) | Age and TBV as covariates (df = 1, 33) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | p | F | p | |
| Left STG | 18.82 | 2.51 | 18.34 | 1.94 | 0.660 | 0.513 | 0.743 | 0.395 |
| Left posterior STG† | 14.07 | 2.09 | 13.79 | 1.38 | 0.481 | 0.634 | 0.609 | 0.441 |
| Right STG | 18.71 | 2.39 | 17.44 | 1.79 | 1.836 | 0.075 | 4.577 | 0.040* |
| Right posterior STG† | 12.79 | 1.87 | 11.60 | 1.47 | 2.154 | 0.038* | 5.317 | 0.028* |
| Total Brain Volume | 1326 | 120 | 1300 | 135 | 0.604 | 0.549 | NA | NA |
STG = superior temporal gyrus, HFA = high-functioning autism, TDC = typically developing controls, SD = standard deviation, STG = superior temporal gyrus, TBV = total brain volume, NA = not applicable.
Statistically significant at p <0.05.
Post-hoc analysis
There were no age-related effects on STG volume for both the HFA group and TDC group. For the HFA group, Pearson correlation for the age and left STG (r = 0.070, p = 0.784) as well as age and right STG (r = 0.102, p =0.686) was not significant. For the TDC group, Pearson correlation for the age and left STG (r = 0.295, p =0.220) as well as age and right STG (r = 0.127, r = 0.603) was not significant.
Examination of the symmetry index did not yield statistically significant between-group differences. Specifically, there were no significant differences in the symmetry index between HFA and TDC groups when examining left and right STG volumes (HFA = 0.49±16.49, TDC = 4.94±15.46, t = −0.847, p = 0.403). In both groups, the positive symmetry index values indicate that the region is larger in the left hemisphere than in the right, though this appears to be more so for the TDC group.
3. Discussion
Given the STG’s important roles in language processing (left hemisphere) and social perception (right hemisphere), the main goal of this study was to examine STG volumes in a sample of children and adolescents with autism. It was hypothesized that STG volumes would be greater in subjects with autism compared to healthy controls based on the replicable finding of brain overgrowth (which is associated with dysfunction) which is more prominent in the frontal/temporal lobes. The results of this study provide support to this hypothesis. Enlarged right STG in the autism group was the only statistically significant finding observed in the current study, but only after controlling for age and TBV. Notably, in the present study, there were no significant differences in the right-left asymmetry of the STG between the two groups, a finding which is also consistent with most, but not all studies investigating STG asymmetry in autism (De Fosse et al., 2004; Gage et al., 2009; Herbert et al., 2002; Herbert et al., 2005).
The STG volumetric findings reported in the current study differ from the only known study to date specifically examining whole STG volume in autism. Bigler and colleagues measured whole STG volumes in a sample of children and adolescent males with autism and compared them with matched typically developing subjects (Bigler et al., 2007). Their study consisted of 30 subjects with autism (mean age = 12.6±3.5 years, FSIQ = 100.1±22.2, TBV = 1398.0±137.2 cc) and 26 typically developing subjects (mean age = 11.8±3.7 years, FSIQ = 105.7±17.8, TBV = 1436.8±145.9 cc). They did not find any significant between-group differences in STG volumes. The reason for this inconsistency is unclear, but likely owing to differences in study procedures including site differences in subject samples, scanning parameters, morphometric measurements, and image-processing methods. Notable differences with the current study include subject characteristics. The subjects with autism in the present investigation have slighter larger TBV than control subjects; however, the opposite is true in the Bigler study. While the TBV differences are not significant in both studies, controlling for TBV in the current study draws out additional significant enlargements of STG volumes in the autism group. Group-wise comparison of STG volumes in the Bigler study were not performed controlling for TBV. Other clear differences that might contribute to lack of agreement include imaging protocol (i.e. magnet strength, resolution, etc) and method used to measure STG volumes (i.e. software package, tracing procedures, etc).
In contrast, the findings reported here are concordant with mounting evidence suggesting STG abnormalities in autism as supported by postmortem, neuroimaging, and electrophysiology studies. In a postmortem study of the cytoarchitecture of the cerebral cortex, Casanova and colleagues reported abnormal cortical minicolumns in the posterior STG of autistic patients compared to controls; cell columns in the brains of autistic patients were significantly smaller and less compact in their configuration, with less neuropil space in their periphery (Casanova et al., 2002). In a voxel-based investigation of brain structure in male adolescents with autism, Waiter and colleagues found the brains in the autism group to be associated with increased grey matter volume in the superior and middle temporal cortices when compared to controls (Waiter et al., 2004). Several PET studies have also reported temporal lobe abnormalities (Boddaert et al., 2003; Castelli et al., 2002; Muller et al., 1999; Zilbovicius et al., 1995). In their PET study, Boddaert and colleagues reported abnormal STG activation in autistic patients while listening to speech-like sounds (Boddaert et al., 2003). Castelli and colleagues showed diminished activation in Brodmann areas 21/22 (superior temporal sulcus) in a group with autism and Asperger’s syndrome while performing mentalizing tasks (Castelli et al., 2002). Muller and colleagues demonstrated reduced bilateral temporal activations in adults with autism while performing an auditory task when compared to a group of control subjects (Muller et al., 1999). Zilbovicius and colleagues reported significant hypoperfusion in the bilateral STG in a group of children with autism (Zilbovicius et al., 1995). SPECT studies have also shown a reduction in regional cerebral blood flow in the superior temporal region of children and adults with autism (Mountz et al., 1995; Ohnishi et al., 2000). In an fMRI study using a theory of mind task, Baron-Cohen and colleagues demonstrated greater STG activation in a group of individuals with autism when compared to controls (Baron-Cohen et al., 1999). In a more recent fMRI study, Gomot and colleagues reported reduced activation in the bilateral superior temporal region during a novelty detection task in children with autism (Gomot et al., 2006). Finally, ERP (Bruneau et al., 2003; Bruneau et al., 1999) and MEG (Gage et al., 2003; Roberts et al., 2010; Rojas et al., 2008) studies have also revealed STG abnormalities in autism. Although heterogeneous, these abnormal findings across multiple research modalities involving the STG strongly suggest a possible link between STG dysfunction/structure and autistic behavior.
At first glance, the finding of right-sided STG abnormalities appears counterintuitive given that language (one core impairment in autism) is usually left-lateralized. However, this finding is consistent with the right STG’s key role in social perception. Social perception refers to the processing of face expressions, eye gaze, body movements, and other type of biological motion with the overall goal of gauging the mental states of others (Allison et al., 2000). Abnormality in social interaction is the sine qua non of autism spectrum disorders, and is essential for the diagnosis of autistic disorder, Aspeger’s disorder, and pervasive developmental disorder not otherwise specified (APA, 2000). A large number of reports comparing groups with autism to healthy controls have reported abnormal activity in the superior temporal region (STG and the related superior temporal sulcus) using fMRI in conjunction with tasks tapping social perception skills (Redcay, 2008). Abnormal volume of this structure supports the possible existence of structural defects which may help explain the array of aforementioned functional abnormalities. Perhaps more intriguing, however, is the finding of increased right posterior STG volume in the post-hoc analysis. This region includes the well-known posterior superior temporal sulcus, an area of intense research which has been implicated in explaining the social perceptual deficits in autism (Pelphrey and Carter, 2008b).
To date, this is study is one of few specifically addressing whole STG volume in autism, reporting increased right-sided STG volume in children and adolescents with autism. However, the findings of this study must be interpreted in the context of several limitations. First, the sample size was relatively small; therefore, type I error cannot be excluded, especially in light of a previously reported negative study (Bigler et al., 2007). Second, the sample consisted of only high-functioning males with autism ages eight to 18 years. This narrow demographic profile limits the generalizability of the results. Moreover, it may not be the case that the STG alterations found in HFA are also found in lower-functioning autism because their underlying etiology and neurobiology may differ. Third, clinical characterization of the HFA group is limited and detailed assessments of social and language deficits are unavailable for correlation with imaging data. Fourth, the volumetric measures obtained in this study are for the whole STG only and do not separate gray and white matter volumes; therefore, it is not possible to know what type of brain tissue is driving this effect. Finally, significant results were apparent only after exclusion of an extreme outlier in the control group. This strategy, while not ideal, was necessary to avoid the risks of artificially skewing the statistical analysis by a single outlier in a small study sample.
The STG is a critical node of the social brain as evidenced by its role in language processing (left hemisphere) and social perception (right hemisphere), and anatomical interconnection to association cortices and the limbic system. Therefore, abnormalities in this structure could possibly explain some of the core and secondary symptoms of autism. While preliminary, this study provides evidence for structural abnormalities of the right STG, and its posterior subdivision, in a sample of children and adolescent males with autism. This finding is consistent with the numerous reports of superior temporal abnormalities using a multitude of research modalities. Most notable are right posterior STG (right superior temporal sulcus) abnormalities detected using fMRI during tasks tapping social perceptual skills. However, in light of the aforementioned limitations, additional studies are needed before any conclusions can be made regarding abnormal STG structure in individuals with autism. Additional cross-sectional and longitudinal studies using larger sample sizes are warranted. Future studies ideally should include subjects carefully matched for age and sex and should incorporate periodic longitudinal clinical evaluations to assess relationships between imaging and clinical data. Finally, combining multiple neuroimaging modalities such as high-resolution structural MRI, MR spectroscopy, fMRI, and diffusion tensor imaging would also be helpful in understanding the role of the STG and help clarify its altered development in individuals with autism.
4. Experimental Procedure
Participants
Subjects were 18 individuals with HFA between the ages of 8 and 18 years of age and 20 TDC individuals between 9 and 18 years of age. The study was confined to right-handed boys because the sample size was insufficient to accommodate for the structural variability associated with handedness and gender. While restricting the study to high-functioning individuals generally leads to greater success in completing MRI scanning procedures, it does exclude a larger number of individuals with autism who are lower functioning.
The diagnosis of autism was established through expert clinical evaluation in accordance with published clinical descriptions of high-functioning individuals with autism (Minshew, 1996) and two structured research diagnostic instruments, the Autism Diagnostic Interview-Revised (Lord et al., 1994) and Autism Diagnostic Observation Schedule (Lord et al., 1989). Subjects meeting these instruments’ criteria for autism, but without delayed or deviant language development, were considered to have Asperger’s Disorder and were excluded from this study. The TDC group consisted of children and adolescents recruited from the community through advertisements in areas socially and economically comparable to the subjects with autism. Potential control subjects were screened by questionnaire, telephone, face-to-face interview, and observation during screening psychometric tests. All subjects were medically healthy and had FSIQ scores of 70 or higher.
Potential subjects with autism were excluded if found to have evidence of an associated infectious, genetic, or metabolic disorder such as tuberous sclerosis and fragile X. Potential TDC and HFA subjects were excluded if found to have evidence of birth asphyxia, head injury, or seizure disorder. Exclusions were based on medical/neurological history, physical examination, chromosomal analyses, or metabolic testing if indicated. Potential TDC subjects were also screened to exclude those with family history of autism, developmental cognitive disorder, learning disability, affective disorder, anxiety disorder, schizophrenia, obsessive-compulsive disorder, or other neurological/psychiatric disorders thought to have a genetic component. The socioeconomic status of the family of origin was assessed using a modification of the Hollingshead method (Hollingshead, 1975). The age appropriate version of the Wechsler Intelligence Scales (Wechsler, 1991; Wechsler, 1997) was administered to measure FSIQ, performance IQ, and verbal IQ. Methodology of the study, including MRI of minors, was approved by the Institutional Review Board. Procedures were fully explained to all subjects and, when appropriate, to their parent or legal guardian. Written informed consent was obtained from subjects and/or their guardians.
Procedures
MRI scans
All scans were obtained using a General Electric (Milwaukee, WI, USA) 1.5 Tesla Signa scanner. The imaging protocol consisted of two T1-weighted (repetition time = 500 ms, echo time = 20 ms) series: a sagittal series of 3 mm slice thickness parallel to the midline structure, and an axial series of 5 mm slice thickness. An additional 1.5 mm SPGR (spoiled gradient recalled echo in steady state) coronal series (repetition time = 35 ms; echo time = 5 ms, number of excitations = 1, flip angle = 45 degrees) was acquired. Data were assigned an alphanumeric label to preserve patient anonymity and transferred from the acquisition facility to the image analysis laboratory via File Transfer Protocol and archived on compact disks.
Volumetric Measurements
Anatomical measurements of the left and right STG were conducted on a Linux workstation using the semi-automated software suite, BRAINS2 (Magnotta et al., 2002). The STG was identified bilaterally in reference to standard brain atlases (Jackson and Duncan, 1996; Matsumoto et al., 2001). STG tracing was done manually in the coronal plane by a trained evaluator, blind to group assignment and to the subjects’ identity. The intraclass correlation coefficients were established by tracing 10 random MRI scans. For right STG, intraclass correlation coefficient was 0.91. For the left STG, intraclass correlation coefficient was 0.90.
Superior Temporal Gyrus (STG)
The methodology for tracing the STG for volumetric measurement was based on previously published studies (De Bellis et al., 2002; Matsumoto et al., 2001; Rajarethinam et al., 2000) and will be briefly described here. Total left and total right STG volumes were calculated by first measuring anterior and posterior sections which are separated by the mammillary bodies. The anterior division extended from the first slice anterior to the appearance of the mammillary bodies to the point where the superior temporal sulcus dividing the STG and the middle temporal gyrus could no longer be clearly visualized in either coronal or sagittal views. The posterior division extended caudally from the first slice containing the mammillary bodies to the upward angulation of the Sylvian fissure, or to the caudal end of the splenium of the corpus callosum, whichever came first. Each coronal slice within this range containing the STG was manually traced along the gyral boundaries (Figure 3). An automated algorithm was used to multiply cross sectional area by slice thickness to obtain volume measurements for each division of the STG. Anterior and posterior subdivisions were later added to determine the total STG volumes separately for left and right hemispheres.
Figure 3.
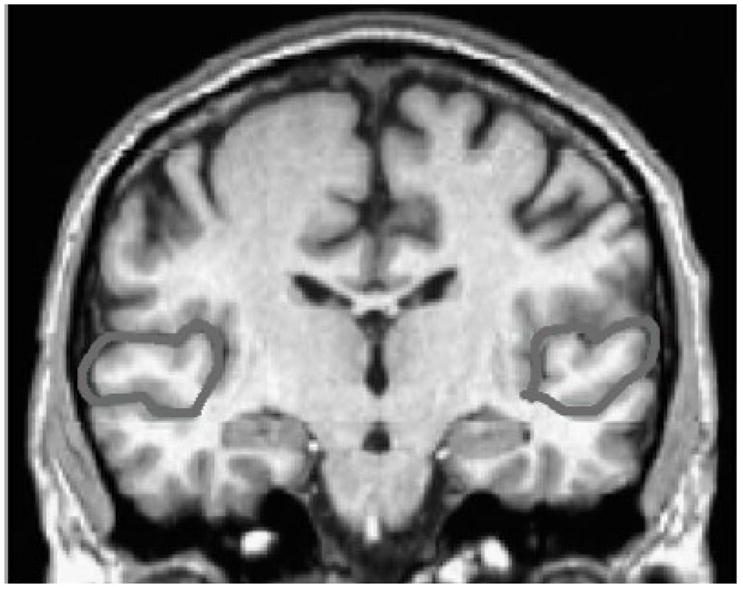
Manually tracing the outer boundaries of the superior temporal gyrus. The superior temporal gyrus was identified bilaterally in reference to standard brain atlases. Each coronal slice containing the STG was manually traced along the gyral boundaries. An automated algorithm was used to multiply cross sectional area by slice thickness to obtain volumetric measurements.
Asymmetry
Comparisons between STG volumes in the left and right hemisphere were expressed as the symmetry index (Galaburda et al., 1990). This was calculated for each region in each brain as 2*(left − right)/(left + right), and multiplied by 100 to provide a percentage value. Positive symmetry index values indicate that the region is larger in the left hemisphere than in the right. Negative symmetry index values indicate that the region is larger in the right hemisphere than in the left.
Total Brain Volume (TBV)
Measurements were made on a Gateway 2000 graphics workstation (Gateway Inc., Irvine, CA, USA) using locally developed custom graphics software. A semiautomated thresholding procedure was used for segmenting brain from cerebral spinal fluid and extracerebral tissue, as described elsewhere (Aylward et al., 1998). Measurements were performed blind to diagnosis. Intrarater reliability for obtaining brain volumes with this procedure yielded an intraclass correlation coefficient of 0.99 on 10 brains. Since two different programs were used to conduct the morphometric studies, TBV measurements were obtained from 10 scans using both software and revealed high reliability between the two programs (0.95) and acceptable intraclass correlation coefficient (0.85).
Data Analysis
All data analyses were performed using Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA). Scatter plots (Figure 1) and box plots (Figure 2) were generated for all variables to identify potential outliers. Descriptive statistics were performed for the data set. All volume measurements between the two groups were compared using Student’s t test (independent-samples t test). Data are expressed as mean ± standard deviation unless otherwise specified. An analysis of covariance was used to compare the two groups on STG measurements while controlling for age and TBV. A two-tailed statistical significance level was set at p <0.05 for all analyses. Post-hoc t tests of posterior STG volumes were conducted due to growing interest in its role in social perception (Pelphrey and Carter, 2008b). Given that the age of the children and adolescents in both groups span nearly ten years, during a time of major maturational change and development, age-related effects were examined with volume-age Pearson correlations for each group.
Acknowledgments
This work was supported by a National Institute of Mental Health (NIMH) grant MH 64027 (Dr. Hardan), National Institute of Child Health and Human Development (NICHD) grant HD 35469 (Dr. Minshew), and NICHD Collaborative Program of Excellence in Autism (CPEA). This work was also supported, in part, by the ANA/Pfizer Fellowships in Clinical Practice from Pfizer’s Medical and Academic Partnership program (Dr. Jou).
Abbreviations
- STG
superior temporal gyrus
- HFA
high-functioning autism
- TDC
typically-developing control
- TBV
total brain volume
- FSIQ
full-scale IQ
- fMRI
functional magnetic resonance imaging
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- ERP
event-related potential
- cc
cubic centimeter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- A.P.A. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision (DSM-IV-TR) [Google Scholar]
- Aylward EH, Anderson NB, Bylsma FW, Wagster MV, Barta PE, Sherr M, Feeney J, Davis A, Rosenblatt A, Pearlson GD, Ross CA. Frontal lobe volume in patients with Huntington’s disease. Neurology. 1998;50:252–258. doi: 10.1212/wnl.50.1.252. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Mortensen S, Neeley ES, Ozonoff S, Krasny L, Johnson M, Lu J, Provencal SL, McMahon W, Lainhart JE. Superior temporal gyrus, language function, and autism. Dev Neuropsychol. 2007;31:217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Belin P, Chabane N, Poline JB, Barthelemy C, Mouren-Simeoni MC, Brunelle F, Samson Y, Zilbovicius M. Perception of complex sounds: abnormal pattern of cortical activation in autism. Am J Psychiatry. 2003;160:2057–2060. doi: 10.1176/appi.ajp.160.11.2057. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Zilbovicius M. Functional neuroimaging and childhood autism. Pediatr Radiol. 2002;32:1–7. doi: 10.1007/s00247-001-0570-x. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Hardan A, di Nemi SU, Perez J, Soares JC, Barale F. Brain anatomy and development in autism: review of structural MRI studies. Brain Res Bull. 2003;61:557–569. doi: 10.1016/j.brainresbull.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Brothers L, Ring B. A neuroethological framework for the representation of minds. J Cogn Neurosci. 1992;4:107–118. doi: 10.1162/jocn.1992.4.2.107. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Bonnet-Brilhault F, Gomot M, Adrien JL, Barthelemy C. Cortical auditory processing and communication in children with autism: electrophysiological/behavioral relations. Int J Psychophysiol. 2003;51:17–25. doi: 10.1016/s0167-8760(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Adrien JL, Barthelemy C. Auditory associative cortex dysfunction in children with autism: evidence from late auditory evoked potentials (N1 wave-T complex) Clin Neurophysiol. 1999;110:1927–1934. doi: 10.1016/s1388-2457(99)00149-2. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58:428–432. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- Castelli F, Frith C, Happe F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125:1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Redcay E, Kennedy DP. The autistic brain: birth through adulthood. Curr Opin Neurol. 2004;17:489–496. doi: 10.1097/01.wco.0000137542.14610.b4. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Frustaci K, Shifflett H, Iyengar S, Beers SR, Hall J. Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry. 2002;51:544–552. doi: 10.1016/s0006-3223(01)01374-9. [DOI] [PubMed] [Google Scholar]
- De Fosse L, Hodge SM, Makris N, Kennedy DN, Caviness VS, McGrath L, Steele S, Ziegler DA, Herbert MR, Frazier JA, Tager-Flusberg H, Harris GJ. Language-association cortex asymmetry in autism and specific language impairment. Ann Neurol. 2004;56:757–766. doi: 10.1002/ana.20275. [DOI] [PubMed] [Google Scholar]
- Eggert GH. Wernicke’s Works on Aphasia: A Sourcebook and Review. Mouton; New York, NY: 1977. [Google Scholar]
- Gage NM, Juranek J, Filipek PA, Osann K, Flodman P, Isenberg AL, Spence MA. Rightward hemispheric asymmetries in auditory language cortex in children with autistic disorder: an MRI investigation. J Neurodev Disord. 2009;1:205–214. doi: 10.1007/s11689-009-9010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage NM, Siegel B, Roberts TP. Cortical auditory system maturational abnormalities in children with autism disorder: an MEG investigation. Brain Res Dev Brain Res. 2003;144:201–209. doi: 10.1016/s0165-3806(03)00172-x. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Rosen GD, Sherman GF. Individual variability in cortical organization: its relationship to brain laterality and implications to function. Neuropsychologia. 1990;28:529–546. doi: 10.1016/0028-3932(90)90032-j. [DOI] [PubMed] [Google Scholar]
- Gloor P. The Temporal Lobe and the Limbic System. Oxford University Press; New York, NY: 1997. [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, Baron-Cohen S. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–484. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, Lange NT, Chabris CF, Bakardjiev A, Hodgson J, Takeoka M, Tager-Flusberg H, Caviness VS. Abnormal asymmetry in language association cortex in autism. Ann Neurol. 2002;52:588–596. doi: 10.1002/ana.10349. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Kennedy DN, Filipek PA, Bakardjiev AI, Hodgson J, Takeoka M, Makris N, Caviness VS. Brain asymmetries in autism and developmental language disorder: a nested whole-brain analysis. Brain. 2005;128:213–226. doi: 10.1093/brain/awh330. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- Jackson GD, Duncan JS. MRI Anatomy: A New Angle on the Brain. Churchill Livingstone; New York, NY: 1996. [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Simmons A, Williams S, Hadjulis M, Pipe R, Murray R, Frangou S. Superior temporal gyrus abnormalities in early-onset schizophrenia: similarities and differences with adult-onset schizophrenia. Am J Psychiatry. 2001;158:1299–1304. doi: 10.1176/appi.ajp.158.8.1299. [DOI] [PubMed] [Google Scholar]
- Minshew NJ. Autism. In: Berg BO, editor. Principles of Child Neurology. McGraw-Hill; New York, NY: 1996. pp. 1713–1730. [Google Scholar]
- Mountz JM, Tolbert LC, Lill DW, Katholi CR, Liu HG. Functional deficits in autistic disorder: characterization by technetium-99m-HMPAO and SPECT. J Nucl Med. 1995;36:1156–1162. [PubMed] [Google Scholar]
- Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, Chugani HT. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, Sasaki M. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123:1838–1844. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, van Engeland H. Review on structural neuroimaging findings in autism. J Neural Transm. 2004;111:903–929. doi: 10.1007/s00702-003-0068-9. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 4. Plenum Press; New York, NY: 1985. pp. 3–61. [Google Scholar]
- Pelphrey K, Adolphs R, Morris JP. Neuroanatomical substrates of social cognition dysfunction in autism. Ment Retard Dev Disabil Res Rev. 2004;10:259–271. doi: 10.1002/mrdd.20040. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Brain mechanisms for social perception: lessons from autism and typical development. Ann N Y Acad Sci. 2008a;1145:283–299. doi: 10.1196/annals.1416.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Carter EJ. Charting the typical and atypical development of the social brain. Dev Psychopathol. 2008b;20:1081–1102. doi: 10.1017/S0954579408000515. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Neural basis of eye gaze processing deficits in autism. Brain. 2005;128:1038–1048. doi: 10.1093/brain/awh404. [DOI] [PubMed] [Google Scholar]
- Piven J, Arndt S, Bailey J, Havercamp S, Andreasen NC, Palmer P. An MRI study of brain size in autism. Am J Psychiatry. 1995;152:1145–1149. doi: 10.1176/ajp.152.8.1145. [DOI] [PubMed] [Google Scholar]
- Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R. Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res. 2000;41:303–312. doi: 10.1016/s0920-9964(99)00083-3. [DOI] [PubMed] [Google Scholar]
- Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008;32:123–142. doi: 10.1016/j.neubiorev.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Roberts TP, Khan SY, Rey M, Monroe JF, Cannon K, Blaskey L, Woldoff S, Qasmieh S, Gandal M, Schmidt GL, Zarnow DM, Levy SE, Edgar JC. MEG detection of delayed auditory evoked responses in autism spectrum disorders: towards an imaging biomarker for autism. Autism Res. 2010;3:8–18. doi: 10.1002/aur.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Dawson G. Early Start Denver Model for Young Children with Autism: Promoting Language, Learning, and Engagement. Guilford Press; New York, NY: 2009. [Google Scholar]
- Rojas DC, Maharajh K, Teale P, Rogers SJ. Reduced neural synchronization of gamma-band MEG oscillations in first-degree relatives of children with autism. BMC Psychiatry. 2008;8:66–74. doi: 10.1186/1471-244X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond CH, de Haan M, Friston KJ, Gadian DG, Vargha-Khadem F. Investigating individual differences in brain abnormalities in autism. Philos Trans R Soc Lond B Biol Sci. 2003;358:405–413. doi: 10.1098/rstb.2002.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Wernicke C. Der Aphatische Symptomencomplex: Eine psychologische Studie auf anatomischer Basis. Cohn and Weigert; Breslau, Germany: 1874. [Google Scholar]
- Zilbovicius M, Garreau B, Samson Y, Remy P, Barthelemy C, Syrota A, Lelord G. Delayed maturation of the frontal cortex in childhood autism. Am J Psychiatry. 1995;152:248–252. doi: 10.1176/ajp.152.2.248. [DOI] [PubMed] [Google Scholar]