Abstract
A novel group of O2-acetoxymethyl-protected diazeniumdiolate-based non-steroidal anti-inflammatory prodrugs (NONO-NSAIDs) were synthesized by esterifying the carboxylate group of aspirin, ibuprofen, or indomethacin with O2-acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazeniumdiolate. The resulting nitric oxide (•NO)-releasing prodrugs (7–9) did not exhibit in vitro cyclooxygenase (COX) inhibitory activity against the COX-1 and COX-2 isozymes (IC50s > 100 μM). In contrast, prodrugs 7 and 8 significantly decreased carrageenan-induced rat paw edema showing enhanced in vivo anti-inflammatory activities (ID50’s = 552 and 174 μmol/kg, respectively) relative to those of the parent NSAIDs aspirin (ID50 = 714 μmol/kg), and ibuprofen (ID50 = 326 μmol/kg).. The rate of porcine liver esterase-mediated •NO release from prodrugs 7–9 (2 moles of •NO/mol of test compound in 0.6–6.5 min) was substantially higher compared to that observed without enzymatic catalysis (about 1 mol of •NO/mol of test compound in 40–48 h). These incubation studies suggest that both •NO and the parent NSAID would be released upon in vivo activation (hydrolysis) by esterases. Data acquired in an in vivo ulcer index (UI) assay showed that NONO-aspirin (UI = 0.8), NONO-indomethacin (UI = 1.3), and particularly NONO-ibuprofen (UI = 0), were significantly less ulcerogenic compared to the parent drugs aspirin (UI = 57), ibuprofen (UI = 46) or indomethacin (UI = 34) at equimolar doses. The release of aspirin and •NO from the NONO-aspirin (7) prodrug constitutes a potentially beneficial property for the prophylactic prevention of thrombus formation and adverse cardiovascular events such as stroke and myocardial infarction.
Keywords: Nitric oxide donors, Diazeniumdiolates, Non-ulcerogenic NSAIDs, Anti-inflammatories, Cyclooxygenase inhibition
1. Introduction
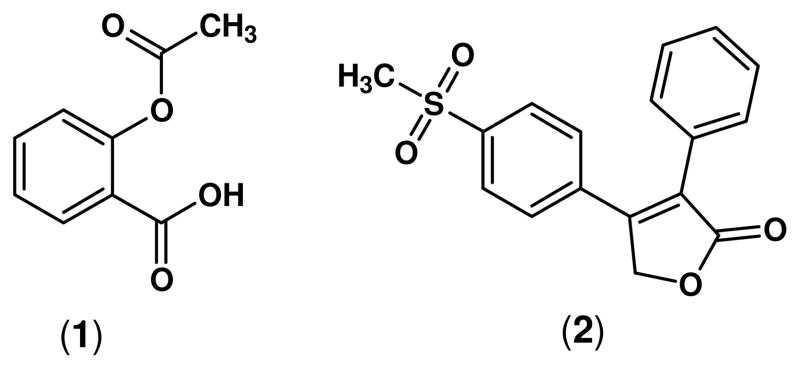
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most useful clinical therapies for the treatment of pain, fever and inflammation. It is estimated that more than 30 million people take NSAIDs every day.1 However, the major mechanism by which NSAIDs exert their anti-inflammatory activity, inhibition of cyclooxygenase-derived prostaglandin synthesis, is also responsible for the gastrointestinal,2–6 renal7–9 and hepatic10 side effects observed mainly in patients undergoing long-term treatment of chronic conditions. The most common side effects associated with NSAID administration are gastroduodenal erosions and ulcerations affecting around 15% of chronic NSAID users,11 and it has been recently proposed that the short and long term damage of NSAIDs on the small bowel (NSAID enteropathy) is even more frequent than NSAID gastropathy.10 While many of these clinical manifestations of NSAID-induced toxicity are mild, they may potentially develop into serious events such as bleeding, perforation, obstruction, and sudden death. Therefore, it is necessary to consider NSAID-induced toxicity as a serious public health problem contributing significantly to the morbidity and mortality of patients receiving these drugs. Furthermore, the gastric irritant effect of aspirin (1) can be a deterrent to its long-term use for the prophylactic prevention of adverse cardiovascular events such as stroke and myocardial infarction,12;13 or as a safe chemopreventive agent to avoid the recurrence of colorectal cancer (CRC).14
Two different strategies have emerged to improve the safety profile of NSAIDs: (a) the development of selective cyclooxygenase-2 (COX-2) inhibitors; and (b) the linkage of a nitric oxide (•NO)-releasing moiety to classical NSAIDs (NO-NSAIDs). The role of selective COX-2 inhibitors with respect to the adverse cardiovascular effects reported in some patients undergoing chronic treatment of pain and inflammation has attracted considerable recent attention.15 In this regard, the adverse hypertensive effect induced by rofecoxib (2) was the primary factor that prompted its withdrawal from the market.16
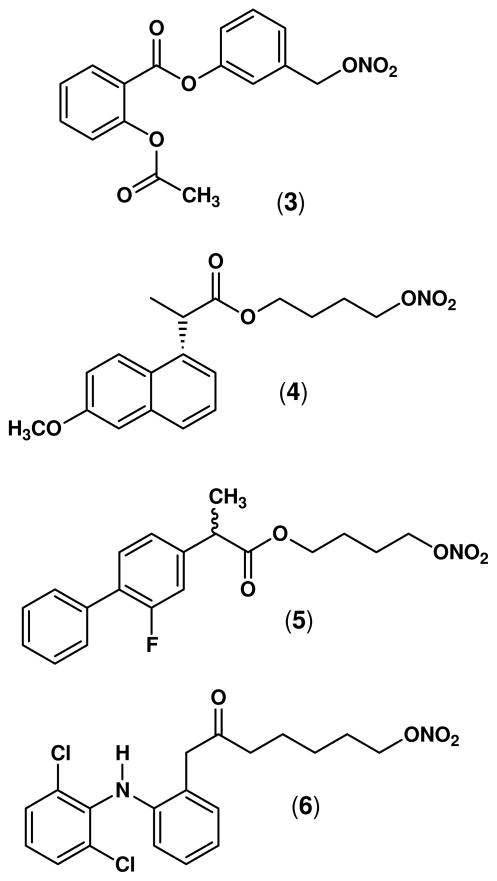
In animal studies, nitrate-based NO-NSAIDs (Figure 2) including the NO-aspirin (3),17 NO-naproxen (4),18 NO-flurbiprofen (5),19;20 and NO-diclofenac (6),21 have been shown to spare the gastrointestinal tract, even though they suppressed prostaglandin synthesis as effectively as the parent drugs.22–24 However, an important drawback to this design is the fact that production of •NO from nitrate esters requires a three-electron reduction, and this metabolic activation can decrease in efficiency on continued use of the drugs contributing to “nitrate tolerance”.25–27
Figure 2.
Structures of some representative NO-NSAIDs (organic nitrates): NO-aspirin (3, NCX-4016), NO-naproxen (4, AZD 3585), racemic NO-flurbiprofen (5, NCX-2216), and NO-diclofenac (6).
O2-Unsubstitued N-diazen-1-ium-1,2-diolates (NONOates) have the potential to release •NO without metabolic activation (first-order kinetics). They possess structural diversity, dependable rates of •NO-release, and rich derivatization chemistry that facilitates targeting of •NO to specific organ and/or tissue sites.28 These features distinguish NONOates from currently available nitrate-based clinical vasodilators that require redox activation before •NO is released. We recently reported the synthesis, •NO-release profile, and biological evaluation of a novel group of nitric oxide-releasing non-steroidal anti-inflammatory prodrugs possessing a NONOate29 moiety attached via a one-carbon methylene spacer to the carboxylic acid group of the traditional NSAIDs aspirin, ibuprofen, and indomethacin, the first in a series of NONO-NSAIDs. These prodrugs did not inhibit the catalytic activity of COX-1/COX-2 isozymes in vitro, but showed equipotent anti-inflammatory properties compared to their NSAID counterparts in a carrageenan-induced rat paw edema assay in vivo, without significant gastric toxicity when administered orally. As part of our ongoing research program targeted toward the development of improved anti-inflammatory agents with a greater safety profile, we now report the synthesis, in vitro COX-1/COX-2 inhibitory activity, in vivo anti-inflammatory activity, nitric oxide release data, and results from ulcerogenicity studies for a group of ester prodrugs of aspirin, ibuprofen and indomethacin possessing an O2-acetoxymethyl-protected diazen-1-ium-1,2-diolate as the •NOdonor moiety.
2. Chemistry
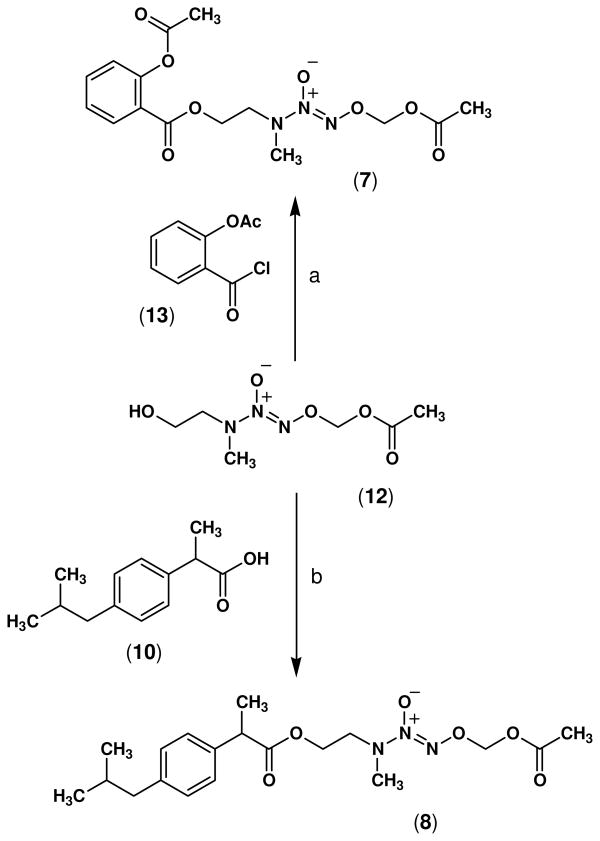
The synthesis of NONO-NSAIDs 7–9 was accomplished by esterification of the carboxylic acid group of conventional NSAIDs, namely aspirin (1), ibuprofen (10), and indomethacin (11) using the alcohol O2-acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (12) reported previously.30 In this regard, reaction of commercially available acetylsalicyloyl chloride (13) with the alcohol 12 in dry THF, and triethylamine as non-nucleophilic base, yielded compound 7 in 70 % yield. Unlike aspirin, the acid chloride derivative of ibuprofen is not commercially available so its esterification using the alcohol 12 was carried out using the well known dehydrating agent dicyclohexylcarbodiimide (DCC) in the presence of 4-dimethylaminopyridine (DMAP) which furnished the ester product 8 in 60% yield (Scheme 1).
Scheme 1.
Reagents and conditions: a) TEA, THF, 25 °C, 19 h; b) DCC, DMAP, CH2Cl2, 25 °C, 4 h.
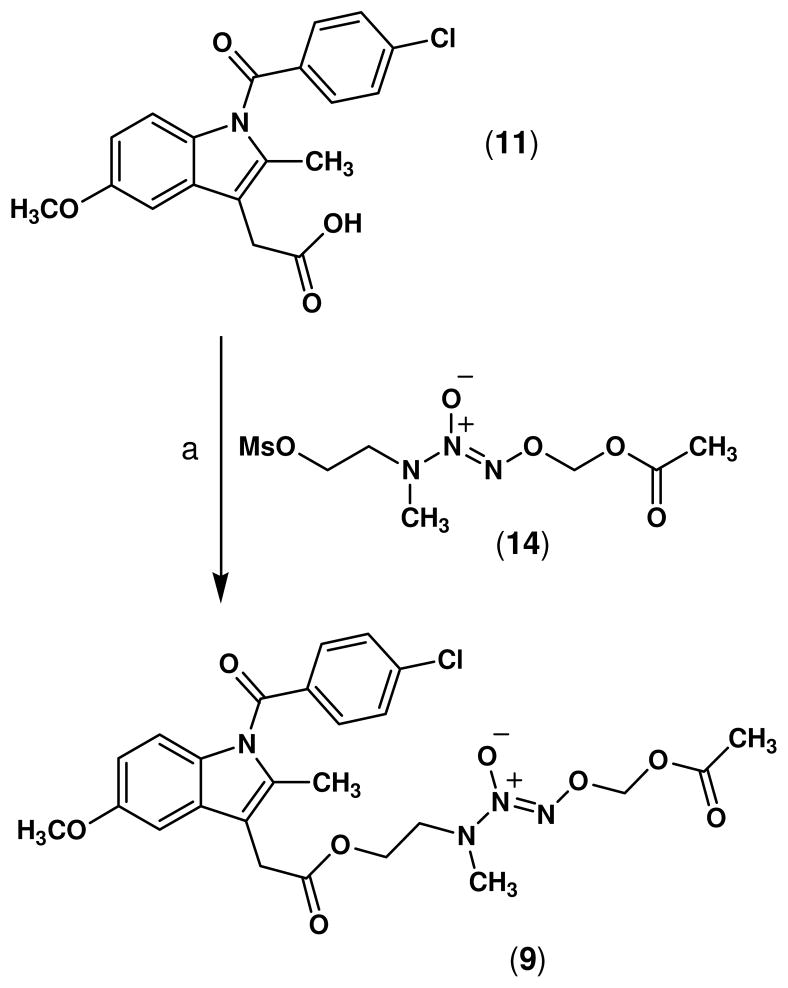
Although the indomethacin prodrug ester 9 could also be synthesized by direct coupling of indomethacin with the alcohol 12 using DCC, the yield was significantly lower (< 10%). Therefore, an alternative strategy was used which involved derivatization of the alcohol 12 to form the mesylate O2-acetoxymethyl 1-[N-(2-methylsulfonyloxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (14).30 Subsequent nucleophilic displacement of the mesylate group upon reaction with the potassium salt of indomethacin in HMPA afforded the ester prodrug 9 in 70% yield (Scheme 2).
Scheme 2.
Reagents and conditions: a) K2CO3, HMPA, 25 °C, 60 h.
3. Results and Discussion
A group of new •NO-releasing non-steroidal anti-inflammatory prodrugs (7–9), possessing an O2-acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate moiety (NONO-NSAIDs), were synthesized. In vitro COX enzyme inhibition studies (Table 1) showed that none of these compounds inhibited either the COX-1 or COX-2 isozyme at the highest test compound concentration used (100 μM). Thus, as it was previously reported for ester prodrugs possessing a 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate or 1-(N,N-dimethylamino)diazen-1-ium-1,2-diolate,29 the attachment of an ester group (the •NO-releasing diazeniumdiolate moiety) to the parent NSAID completely abolished the in vitro enzyme inhibitory activity of aspirin, ibuprofen and indomethacin. However, when administered orally to rats, the carrageenaninduced rat paw edema assay data (Table 1) showed improved ID50 values for prodrugs 7 (ID50 = 552.9 μmol/kg) and 8 (ID50 = 174.8 μmol/kg) compared with the reference drugs aspirin (ID50 = 714.3 μmol/kg) and ibuprofen (ID50 = 326.7 μmol/kg). NONO-indomethacin 9 (ID50 = 20.3 μmol/kg) was about 1.7-fold less potent relative to indomethacin (ID50 = 11.7 μmol/kg). The observation that ester prodrugs 7–9 were inactive in vitro inhibitors of COX-1 and COX-2 (IC50 > 100 μM), but are active anti-inflammatory agents in vivo, strongly suggests that NONO-NSAIDs 7–9 act as classical prodrugs that require metabolic activation by esterase-mediated hydrolysis. This interpretation is consistent with previous observations reported by our group,29 describing the anti-inflammatory properties of diazeniumdiolate-based NO-NSAIDs (NONO-NSAIDs) possessing either a 1-(pyrrolidin-1-yl)diazen-1-ium-1,2-diolate (PYRRO/NO) or 1-(dimethylamino)diazen-1-ium-1,2-diolate (DMA/NO) moiety attached via a one-carbon methylene spacer to the carboxylic acid group of traditional NSAIDs.
Table 1.
In vitro COX-1/COX-2 enzyme inhibition and in vivo anti-inflammatory (AI) activity data for NONO-NSAIDs 7–9.
| Compound | COX-1 IC50 (μM)a | COX-2 IC50 (μM)a | COX-2 S.I.b | AI activityc ID50 (μmol/kg) |
|---|---|---|---|---|
| 7 | > 100 | > 100 | - | 552.9 |
| 8 | > 100 | > 100 | - | 174.8 |
| 9 | > 100 | > 100 | - | 20.3 |
| Aspirin | 0.3 | 2.4 | 0.14 | 714.3 |
| Ibuprofen | 2.9 | 1.1 | 2.63 | 326.7 |
| Indomethacin | 0.1 | 5.7 | 0.01 | 11.7 |
The in vitro test compound concentration required to produce 50% inhibition of COX-1 or COX-2. The result (IC50, μM) is the mean of two determinations acquired using an ovine COX-1/COX-2 assay kit (Catalog No. 560101, Cayman Chemicals Inc., Ann Arbor, MI). The deviation from the mean was <10% of the mean value.
Selectivity index (SI) = COX-1 IC50/COX-2 IC50.
Inhibitory activity in a carrageenan-induced rat paw edema assay. The results are expressed as the ID50 value in μmol/kg, at 3 h after oral administration of the test compound.
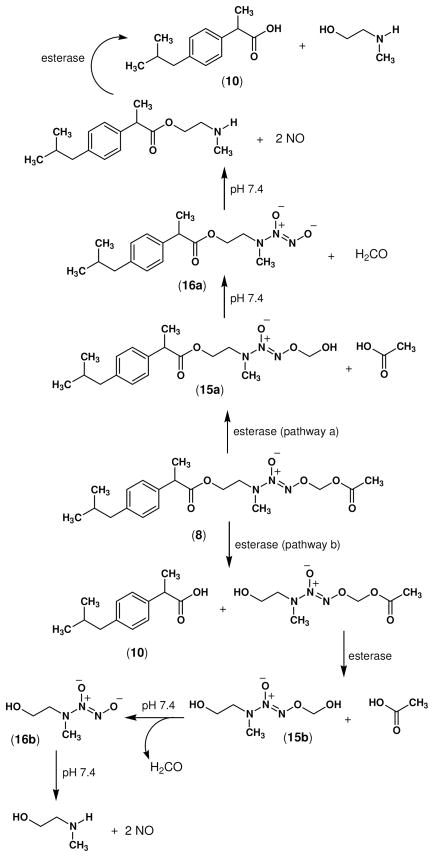
O2-Acetoxymethyl diazen-1-ium-1,2-diolates are stable compounds that hydrolyze slowly at pH 7.4.31 Consistent with these observations, when compounds 7–9 were incubated in phosphate buffer at pH 7.4, the time required to detect about 1 mol of •NO/mol of test compound (50 % of the total amount of •NO typically obtained from diazeniumdiolate ions) was 40–48 h, which is indicative of slow •NO release under these conditions. However, ester prodrugs 7–9 are hydrolyzed more extensively (2.0 moles of •NO/mol of prodrug) in the presence of porcine liver esterase (PLE) with a considerable increase in their corresponding rates of hydrolysis (100% of •NO release in 0.6 to 6.5 min, see Table 2). NONO-NSAID ester prodrugs 7–9 were designed that possess two different ester groups (three in the case of NONO-aspirin). One ester group links the carboxy group of the NSAID to the N-(2-hydroxyethyl)-N-methylamino-diazeniumdiolate (2-HEMA/NO), whereas the function of the second ester group is to protect the 2-HEMA/NO from releasing •NO spontaneously due to the presence of a one-carbon methylene spacer between the acetoxy group (protecting group) and the diazen-1-ium-1,2-diolate O2-atom. Following enzymatic hydrolysis of the acetate moiety, the O2-(hydroxymethyl)diazen-1-ium-1,2-diolate (15a or 15b) intermediate produced would spontaneously eliminate formaldehyde to form the free NONOate moiety (16a or 16b) which would subsequently fragment to release two molecules of •NO (Scheme 3). It is not currently known whether hydrolysis of the terminal O2-acetoxymethyl ester, that ultimately leads to •NO release, occurs before, or after, hydrolysis of the NSAID aminoethyl ester moiety. Future pharmacokinetic studies will be necessary to resolve this question.
Table 2.
Nitric oxide release studies for NONO-NSAIDs 7–9.
| Compound | Nitric oxide releaseda |
|||
|---|---|---|---|---|
| PBS (pH 7.4)b | t (h)c | PLEd | t (min)e | |
| 7 | 1.0f | 40.0 | 2.0f | 6.5 |
| 8 | 1.1f | 45.3 | 2.0f | 0.6 |
| 9 | 0.9g | 48.0 | 2.0g | 1.4 |
| 12 | 1.0f | 30.7 | 2.0f | 2.4 |
Moles of •NO/mol of test compound.
Incubated in phosphate buffer solution pH 7.4 at 37 °C.
Time required to detect about 50 % of the theoretical total amount of •NO/mol of test compound. Calculated graphically from the corresponding curves plotting time vs. mol •NO/min.
Moles of •NO released in the presence of porcine liver esterase (10 μL of a suspension in 3.2 M (NH4)2SO4, Sigma).
Time required to detect 100 % of the theoretical total amount of •NO/mol of test compound. Calculated graphically from the corresponding curves plotting time vs mol •NO/min.
10 μL of a 46 mM solution in 3 mL of phosphate buffer pH 7.4.
10 μL of a 77 mM solution in 3 mL of phosphate buffer pH 7.4.
Scheme 3.
Theoretical enzyme-mediated activation pathways of NONO-ibuprofen (8). The two ester groups must be hydrolyzed to release both •NO and the NSAID.
Although conventional •NO donors can protect the stomach against NSAID-induced gastric damage, they are less effective than NSAIDs that are chemically linked to an •NO-releasing moiety.24 Since the most common side effect of NSAID therapy is gastrointestinal irritation and bleeding, it was important to evaluate the potential in vivo ulcerogenicity of prodrugs 7–9 in comparison to the corresponding parent drugs. The severity of gastric damage, assessed using an ulcerogenicity assay, is expressed as an ulcer index (UI), and the results are presented in Table 3. There was a remarkable difference between the UI values for prodrugs 7–9 (UI = 0.84, 0, and 1.3 respectively), and the reference drugs aspirin (UI = 57.4, 1.38 mmol/kg po dose), ibuprofen (UI = 45.8, 1.21 mmol/kg po dose) and indomethacin (34.4, 0.08 mmol/kg po dose) at equimolar doses. NONO-aspirin (7) and NONO-indomethacin (9) caused minimal ulcerogenicity, whereas no evidence of gastric bleeding was observed for NONO-ibuprofen (8). These data are consistent with previous reports showing a safer pharmacological profile for hybrid NONO-NSAIDs containing PYRRO/NO or DMA/NO.29 The decreased gastric toxicity of prodrugs 7–9, relative to the parent NSAIDs, could be due to release of •NO that increases mucosal blood flow resulting in enhanced mucosal resistance to ulceration32–34 and/or an enhanced ability of the intact prodrug to cross the gastric mucosal lining prior to the subsequent release of •NO and the NSAID.
Table 3.
Gastric ulcer index produced by an acute administration of the test compounds 7–9 and the reference drugs aspirin, ibuprofen and indomethacin.
| Compound | Dose (mmol/kg) | Ulcer indexa |
|---|---|---|
| aspirin | 1.38 | 57.4 ± 3.1 |
| 7 | 1.38 | 0.84 ± 0.1 |
| ibuprofen | 1.21 | 45.8 ± 2.9 |
| 8 | 1.21 | 0 |
| indomethacin | 0.08 | 34.4 ± 4.2 |
| 9 | 0.08 | 1.3 ± 0.1 |
| control group | - | 0b |
Calculated by adding the total length (in mm) of individual ulcers in each stomach and dividing by the number of animals (n = 4) in each group. Data are presented as mean total length ± SEM at 6 h after oral administration of the test compound.
1.0% methylcellulose solution.
4. Conclusions
Hybrid NO-NSAID ester prodrugs possessing an O2-acetoxymethyl-protected diazeniumdiolate (2-HEMA/NO) moiety attached via a two-carbon ethyl spacer to the carboxylic acid of traditional NSAIDs, constitute a useful alternative for the rational design of improved anti-inflammatory prodrugs with reduced gastric toxicity (ulcerogenicity). Considering the large number of commercially available secondary dialkylamines having an alcohol group in one (or both) alkyl groups, and the fact that virtually every NSAID possessing a free carboxylic acid is suitable for application of this methodology, the number of possible combinations leading to new NONO-NSAIDs is enormous. Accordingly, this concept offers an approach to design hybrid NSAID/•NO donor agents having clinically beneficial physicochemical properties and pharmacological profiles. In vivo activation (esterase-mediated hydrolysis) of the NONO-NSAIDs described herein constitutes a more flexible method to regulate •NO release compared to that for organic nitrates which require a metabolically demanding three-electron reduction for the release of •NO. Unlike nitrate-based NONSAIDs, tolerance is not expected to be an issue for hybrid NONO-NSAIDs having a diazen-1-ium-1,2-diolate moiety. Since NONO-NSAIDs 7–9 are practically devoid of gastric toxicity, their use may constitute a promising alternative for patients taking classical NSAIDs but diagnosed with gastropathy, or for patients at high risk for coronary artery disease taking selective COX-2 inhibitors. NONO-aspirins may also provide a promising alternative to the use of aspirin as an anti-thrombotic agent in the long-term prophylactic prevention of stroke and myocardial infarction, or as a safer chemopreventive agent for colorectal cancer.
5. Experimental
1H NMR spectra were acquired using a Bruker AM-300 spectrometer (300 MHz), or a Varian Unity Inova spectrometer (400 MHz). UV spectra were recorded using an Agilent 8453 spectrophotometer (Agilent Technologies). Infrared spectra were recorded using a M500 IR spectrometer (Buck Scientific). Microanalyses were performed by Midwest Analytic (Indianapolis, IN) and were within ± 0.4% of theoretical values for all elements listed. Flash column chromatography was performed using Versapak® 23 × 53 mm or 23 × 110 mm cartridges (silica gel 20–45 μm). Nitric oxide gas was purchased from Matheson Gas Products (Montgomeryville, PA). Quantification of •NO by chemiluminescence was determined using a Sievers nitric oxide analyzer (NOA) model 280 or 280i, as previously described.35 O2-Acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (12), and O2-acetoxymethyl 1-[N-(2-methylsulfonyloxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (14) were prepared according to a reported procedure.30 All other reagents (including the porcine liver esterase, used as a 3.2 M ammonium sulfate suspension) were purchased from Aldrich Chemical (Milwaukee, WI) and used without further purification. The in vivo anti-inflammatory36 and ulcer index assays37 were carried out using protocols approved by the Health Sciences Animal Welfare Committee at the University of Alberta.
5.1 O2-Acetoxymethyl 1-[N-(2-(acetylsalicyloyloxy)ethyl)-N-methylamino]diazen-1-ium-1,2-diolate (7)
O2-Acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (12, 1.0 g, 4.8 mmol) in dry THF (5 mL) was added dropwise to a solution of acetylsalicyloyl chloride (13, 1.1 g, 5.7 mmol), and triethylamine (0.8 mL, 5.7 mmol) with stirring and the reaction was allowed to proceed at 25 °C for 19 h. Addition of ethyl acetate (100 mL) to dilute the reaction, washing the organic phase with water (3 × 30 mL), drying the organic fraction (Na2SO4), and removal of the organic solvent in vacuo gave a light brown liquid residue which was purified by flash column chromatography (hexane-EtOAc, 2:1, v/v) to afford 7 as a pale yellow liquid (1.2 g, 70% yield); UV (PBS pH 7.4) λmax (ε) 230 nm (17.7 mM−1 cm−1); IR (NaCl) 3026 (C-H aromatic), 2956 (C-H aliphatic), 1763 (CO2), 1726 (CO2), 1223, 1168 (N=N-O) cm−1; 1H NMR (CDCl3) δ 7.99 (dd, J = 7.9, 1.8 Hz, 1H, phenyl H-6), 7.58 (td, J = 7.6, 1.8 Hz, 1H, phenyl H-4), 7.32 (td, J = 7.3, 1.8 Hz, 1H, phenyl H-5), 7.11 (dd, J = 8.2, 1.8 Hz, 1H, phenyl H-3), 5.75 (s, 2H, OCH2O), 4.46 (t, J = 5.1 Hz, 2H, CO2CH2), 3.78 (t, J = 5.1, 2H, CH2N), 3.14 (s, 3H, NCH3), 2.35 (s, 3H, PhOCOCH3), 2.06 (s, 3H, COCH3). Anal. calcd. for C15H19N3O8: C, 48.78; H, 5.19; N, 11.38. Found: C, 48.39; H, 5.10; N, 11.09.
5.2 O2-Acetoxymethyl 1-{N-[2-(2-[4-(isobutyl)phenyl]propanoyloxy)ethyl]-N-methylamino}diazen-1-ium-1,2-diolate (8)
O2-Acetoxymethyl 1-[N-(2-hydroxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (12, 0.5 g, 2.6 mmol) in dichloromethane (5 mL) was added dropwise to a solution of ibuprofen (10, 0.5 g, 2.4 mmol), 1,3-dicyclohexylcarbodiimide (0.5 g, 2.6 mmol), and 4-(dimethylamino)pyridine (0.05 g, 0.4 mmol) with stirring, and the reaction was allowed to continue at 25 °C for 4 h. After standing at 4 °C for 24 h, the solid precipitate was filtered off and the solvent was removed in vacuo. The residual pale yellow liquid obtained was purified by flash column chromatography (hexane-EtOAc, 4:1, v/v) to furnish 8 as a pale yellow oil (0.6 g, 60% yield); UV (PBS pH 7.4) λmax (ε) 227 nm (10.2 mM−1 cm−1); IR (NaCl) 2989 (C-H aromatic), 2814 (C-H aliphatic), 1760 (CO2), 1722 (CO2), 1286, 1129 (N=N-O) cm−1; 1H NMR (CDCl3) δ 7.19 (d, J = 8.2 Hz, 2H, phenyl H-2, H-6), 7.09 (d, J = 8.2 Hz, 2H, phenyl H-3 and H-5), 5.75 (s, 2H, OCH2O), 4.26 (t, J = 4.8 Hz, 2H, CO2CH2), 3.67 (quartet, J = 7.3 Hz, 1H, PhCHCH3), 3.59 (t, J = 4.8 Hz, 2H, NCH2), 2.90 (s, 3H, NCH3), 2.44 (d, J = 7.0 Hz, 2H, PhCH2CH), 2.11 (s, 3H, COCH3), 1.88-1.81 [m, 1H, CH(CH3)2], 1.49 (d, J = 7.3 Hz, 3H, PhCHCH3), 0.89 [d, J = 6.4 Hz, 6H, CH(CH3)2]. Anal. calcd. for C19H29N3O6: C, 57.71; H, 7.39; N, 10.63. Found: C, 57.67; H, 7.29; N, 10.38.
5.3 O2-Acetoxymethyl 1-{N-[2-(2-(1-(4-chlorobenzoyl)-5-methoxy-2-methyl-1H-indol-3-yl)acetoxy)ethyl]-N-methylamino}diazen-1-ium-1,2-diolate (9)
O2-Acetoxymethyl 1-[N-(2-methylsulfonyloxyethyl)-N-methylamino]diazen-1-ium-1,2-diolate (14, 1.2 g, 4.2 mmol) in HMPA (5 mL) was added dropwise to a solution of indomethacin (11, 1.0 g, 2.8 mmol), and potassium carbonate (0.4 g, 2.9 mmol) in HMPA (5 mL), and the mixture was stirred at 25 °C for 60 h. Addition of ethyl acetate (100 mL) to dilute the reaction, washing the organic phase with water (5 × 30 mL), drying the organic fraction (Na2SO4), and removal of the solvent in vacuo gave a pale yellow liquid which was purified by flash column chromatography (hexane-EtOAc, 1:1, v/v) to afford 9 as a pale yellow oil (1.1 g, 70% yield); UV (PBS pH 7.4) λmax (ε) 227 nm (14.8 mM−1 cm−1), λmax (ε) 267 nm (14.4 mM−1 cm−1); IR (NaCl) 3011 (C-H aromatic), 2969 (C-H aliphatic), 1754 (CON), 1698 (CO2), 1289, 1162 (N=N-O) cm−1; 1H NMR (CDCl3) δ 7.67 (d, J = 8.5 Hz, 2H, benzoyl H-2, H-6), 7.48 (d, J = 8.5 Hz, 2H, benzoyl H-3, H-5), 6.96 (d, J = 2.4 Hz, 1H, indolyl H-4), 6.86 (d, J = 9.1 Hz, 1H, indolyl H-7), 6.67 (dd, J = 9.1, 2.4 Hz, 1H, indolyl H-6), 5.76 (s, 2H, OCH2O), 4.30 (t, J = 5.5 Hz, 2H, CO2CH2), 3.84 (s, 3H, OCH3), 3.68 (s, 2H, CH2CO2), 3.64 (t, J = 5.5 Hz, 2H, NCH2), 3.00 (s, 3H, NCH3), 2.39 (s, 3H, C-2 CH3), 2.10 (s, 3H, COCH3). Anal. calcd. for C25H27ClN4O8: C, 54.90; H, 4.98; N, 10.24. Found: C, 54.70; H, 4.82; N, 9.98.
6. In vitro cyclooxygenase (COX) inhibition assays
The ability of the test compounds listed in Table 1 to inhibit ovine COX-1 and COX-2 (IC50 value, μM) was determined using an enzyme immuno assay (EIA) kit (catalog number 560101, Cayman Chemical, Ann Arbor, MI, USA) according to our previously reported method.38
7. Anti-inflammatory assay
Anti-inflammatory activity was measured using a method described by Winter et al.36
8. Acute ulcerogenesis assay
The ability to produce gastric damage was evaluated according to a reported procedure.37 Ulcerogenic activity was evaluated after oral administration of aspirin (1.38 mmol/kg), ibuprofen (1.21 mmol/kg), indomethacin (0.08 mmol/kg) or an equivalent amount of the correspondent test compound (7–9). All drugs were suspended and administered in 1.7 mL of a 1% methylcellulose solution. Control rats received oral administration of vehicle (1.7 mL of 1.0% methylcellulose solution). Food, but not water, was removed 24 h before administration of test compounds. Six hours after oral administration of the drug, rats were euthanized in a CO2 chamber and their stomachs were removed, cut out along the greater curvature of the stomach, gently rinsed with water and placed on ice. The number and the length of ulcers observed in each stomach were determined using a magnifier lense. The severity of each gastric lesion was measured along its greatest length (1 mm = rating of 1, 1–2 mm = rating of 2, >2 mm = rating according to their length in mm). The “ulcer index” (UI) for each test compound was calculated by adding the total length (L, in mm) of individual ulcers in each stomach, divided by the number of animals in each group (n=4): UI = (L1+L2+L3+L4)/4
9. Nitric oxide release assay
•NO gas measurements were performed using a Sievers nitric oxide analyzer (NOA), model 280 or 280i using a method described earlier.35 The instruments were calibrated before each experiment with nitrogen as the zero gas. Mixtures of •NO/He (certified standards, MG Industries, Morrisville, PA) at different concentrations were injected into the reaction chamber, recording the area under the curve (AUC) for each peak, and plotting μmol of •NO vs. peak area. Linear regression analysis of resultant graphs showed correlation coefficients of 0.999 or better. Measurement of •NO released from the test compounds was performed by injecting the prodrug (10 μL) dissolved in DMSO (46 mM for 7, 8 and 12, or 77 mM for 9) into a clean, dry, NOA measurement cell (sealed with a rubber septum) containing deoxygenated phosphate buffer solution (3 mL, pH 7.4). The •NO generated from the samples was carried from the phosphate buffer solution to the NOA via a constant nitrogen purge. Integration of the resulting AUC was used to calculate the amount of •NO released from each test compound, based on the calibration curve. The experiments with porcine liver esterase (10 μL of a suspension in 3.2 M ammonium sulfate) were carried out in phosphate buffer solution (3 mL) pH 8.0.
Figure 1.
Structures of aspirin (1), and rofecoxib (2).
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Grant No. MOP-14712), and in part with federal funds from the National Cancer Institute, National Institutes of Health (NIH) under contract N01-CO-12400 with SAIC-Frederick Inc., as well as the Intramural Research Program of the NIH, Center for Cancer Research. We are also grateful to the National Council of Science and Technology (CONACYT, México) for a graduate scholarship to C.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Singh G, Triadafilopoulos G. J Rheumatol Suppl. 1999;56:18. [PubMed] [Google Scholar]
- 2.Go MF. Gastrointest Endosc Clin N Am. 2006;16:83. doi: 10.1016/j.giec.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Naesdal J, Brown K. Drug Saf. 2006;29:119. doi: 10.2165/00002018-200629020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Cryer B. Am J Gastroenterol. 2005;100:1694. doi: 10.1111/j.1572-0241.2005.50565.x. [DOI] [PubMed] [Google Scholar]
- 5.Lazzaroni M, Bianchi PG. Aliment Pharmacol Ther. 2004;20 Suppl 2:48. doi: 10.1111/j.1365-2036.2004.02037.x. [DOI] [PubMed] [Google Scholar]
- 6.James MW, Hawkey CJ. Br J Clin Pharmacol. 2003;56:146. doi: 10.1046/j.1365-2125.2003.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider V, Levesque LE, Zhang B, Hutchinson T, Brophy JM. Am J Epidemiol. 2006;164:881. doi: 10.1093/aje/kwj331. [DOI] [PubMed] [Google Scholar]
- 8.Mounier G, Guy C, Berthoux F, Beyens MN, Ratrema M, Ollagnier M. Therapie. 2006;61:255. [PubMed] [Google Scholar]
- 9.Zadrazil J. Vnitr Lek. 2006;52:686. [PubMed] [Google Scholar]
- 10.Adebayo D, Bjarnason I. Postgrad Med J. 2006;82:186. doi: 10.1136/pgmj.2005.039586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorucci S, Antonelli E. Inflamm Allergy Drug Targets. 2006;5:121. doi: 10.2174/187152806776383161. [DOI] [PubMed] [Google Scholar]
- 12.Sanmuganathan PS, Ghahramani P, Jackson PR, Wallis EJ, Ramsay LE. Heart. 2001;85:265. doi: 10.1136/heart.85.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collaborative overview of randomised trials of antiplatelet therapy-I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists’ Collaboration. BMJ. 1994;308:81. [PMC free article] [PubMed] [Google Scholar]
- 14.Arber N, Levin B. Curr Top Med Chem. 2005;5:517. doi: 10.2174/1568026054201659. [DOI] [PubMed] [Google Scholar]
- 15.Dogne JM, Supuran CT, Pratico D. J Med Chem. 2005;48:2251. doi: 10.1021/jm0402059. [DOI] [PubMed] [Google Scholar]
- 16.Scheen AJ. Rev Med Liege. 2004;59:565. [PubMed] [Google Scholar]
- 17.Chiroli V, Benedini F, Ongini E, Del SP. Eur J Med Chem. 2003;38:441. doi: 10.1016/s0223-5234(03)00055-2. [DOI] [PubMed] [Google Scholar]
- 18.Nitronaproxen: AZD 3582, HCT 3012, Naproxen Nitroxybutylester, NO-Naproxen. Drugs R D. 2006;7:262. doi: 10.2165/00126839-200607040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Holm L, Phillipson M, Perry MA. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1090. doi: 10.1152/ajpgi.00480.2001. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL, Muscara MN, De Nucci G, Zamuner S, Cirino G, Del Soldato P, Ongini E. J Pharmacol Exp Ther. 2004;309:626. doi: 10.1124/jpet.103.063453. [DOI] [PubMed] [Google Scholar]
- 21.Wallace JL, Reuter B, Cicala C, McKnight W, Grisham M, Cirino G. Eur J Pharmacol. 1994;257:249. doi: 10.1016/0014-2999(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 22.Rigas B, Kashfi K. Trends Mol Med. 2004;10:324. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Wallace JL, McKnight W, Reuter B, Cicala C, Grisham M, Cirino G. Gastroenterology. 1994;106:A208. doi: 10.1016/0016-5085(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 24.Wallace JL, Reuter BK, Cirino G. J Gastroenterol Hepatol. 1994;9 Suppl 1:S40. doi: 10.1111/j.1440-1746.1994.tb01300.x. [DOI] [PubMed] [Google Scholar]
- 25.Csont T, Ferdinandy P. Pharmacol Ther. 2005;105:57. doi: 10.1016/j.pharmthera.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hu R, Siu CW, Lau E-O, Wang WQ, Lau C-P, Tse H-F. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2006.10.011. (In Press) [DOI] [PubMed] [Google Scholar]
- 27.Fung HL, Bauer JA. Cardiovasc Drugs Ther. 1994;8:489. doi: 10.1007/BF00877927. [DOI] [PubMed] [Google Scholar]
- 28.Keefer LK. Annu Rev Pharmacol Toxicol. 2003;43:585. doi: 10.1146/annurev.pharmtox.43.100901.135831. [DOI] [PubMed] [Google Scholar]
- 29.Velazquez C, Praveen Rao PN, Knaus EE. J Med Chem. 2005;48:4061. doi: 10.1021/jm050211k. [DOI] [PubMed] [Google Scholar]
- 30.Velazquez C, Knaus EE. Bioorg Med Chem. 2004;12:3831. doi: 10.1016/j.bmc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Saavedra JE, Shami PJ, Wang LY, Davies KM, Booth MN, Citro ML, Keefer LK. J Med Chem. 2000;43:261. doi: 10.1021/jm9903850. [DOI] [PubMed] [Google Scholar]
- 32.Perini R, Fiorucci S, Wallace JL. Can J Gastroenterol. 2004;18:229. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 33.Bastaki SM, Wallace JL. Can J Gastroenterol. 1999;13:123. doi: 10.1155/1999/738968. [DOI] [PubMed] [Google Scholar]
- 34.Wallace JL. Scand J Gastroenterol Suppl. 1992;192:3. doi: 10.3109/00365529209095973. [DOI] [PubMed] [Google Scholar]
- 35.Keefer LK, Nims RW, Davies KM, Wink DA. Methods Enzymol. 1996;268:281. doi: 10.1016/s0076-6879(96)68030-6. [DOI] [PubMed] [Google Scholar]
- 36.Winter CA, Risley EA, Nuss GW. Proc Soc Exp Biol Med. 1962;111:544. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 37.Cocco MT, Congiu C, Onnis V, Morelli M, Felipo V, Cauli O. Bioorg Med Chem. 2004;12:4169. doi: 10.1016/j.bmc.2004.05.025. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 38.Uddin MJ, Rao PN, Knaus EE. Bioorg Med Chem. 2004;12:5929. doi: 10.1016/j.bmc.2004.08.021. [DOI] [PubMed] [Google Scholar]