Abstract
A common single nucleotide polymorphism (SNP) in the gene encoding catechol-O-methyltransferase (COMT), Val158Met, is thought to influence cognitive performance due to differences in prefrontal dopaminergic neurotransmission. Previous studies lend support for the hypothesis that the “at risk” genotype comprising two Val-alleles (low dopamine) might benefit more from plasticity-enhancing interventions than carriers of one or two Met-alleles. This study aimed to determine whether the response to dietary interventions, known to modulate cognition, is dependent on COMT genotype. Blood samples of 35 healthy elderly subjects (61.3 years ±8 SD; 19 women, 16 men, BMI: 28.2 kg/m2 ±4 SD) were genotyped for COMT Val158Met by standard procedures (Val/Val = 6; Val/Met = 20; Met/Met = 9). Subjects had previously completed a randomized controlled trial investigating the effects of caloric restriction (CR) or enhancement of unsaturated fatty acids (UFA) on immediate and delayed verbal recognition memory. Homozygous Val/Val-carriers had significantly lower memory scores than Met-carriers at baseline (p < 0.001). Significant interactions of genotype and dietary intervention with regard to cognition were found: CR- and UFA enhancement-induced memory improvements of Val/Val-carriers were considerably greater than those of Met-carriers (ANOVA p's < 0.02). The current study shows for the first time that cognitive effects of dietary interventions are dependent on COMT Val158Met genotype. Our findings lend further support to the hypothesis that an “at risk” genotype might benefit more from plasticity-enhancing interventions than the “not at risk” genotype. This might help to develop individualized therapies in future research based on genetic background.
Keywords: COMT, diet, aging, genetic variation, cognition, memory
Introduction
Previous studies reported genotype-associated variations in both brain functions and physiology for a common single nucleotide polymorphism (SNP) in the catechol-O-methyltransferase (COMT) gene, leading to a substitution of valine (Val) to methionine (Met) at the codon 158 on chromosome 22q11 (Val158Met; Goldberg and Weinberger, 2004). For example, homozygous carriers of the Val-allele performed worse in executive and memory tasks than carriers of the Met-allele (Egan et al., 2001; Meyer-Lindenberg et al., 2006; Raz et al., 2009).
This differential cognitive performance might depend on a higher activity of the Val-encoded COMT enzyme (Lotta et al., 1995), playing a unique role for degradation of dopamine in the prefrontal cortex (PFC) (Mattay et al., 2003). Due to higher dopamine turnover in Val/Val-homozygotes, these individuals may exhibit less dopamine availability and therefore less efficient DA-dependent signaling in the PFC (Diaz-Asper et al., 2006; Lindenberger et al., 2008). Moreover, the impact of COMT genotype on cognition is supposed to magnify during late adulthood (Diaz-Asper et al., 2006; Lindenberger et al., 2008). For example, Nagel et al. (2008) reported a clear advantage in cognitive performance of Met-carriers in old, but not in young participants. However, negative results have been reported as well (Potter et al., 2009), and the impact of COMT genotype on cognition is still a matter of debate (Barnett et al., 2008; Goldman et al., 2009).
Potential interactions between genetic phenotype and environmental factors have been suggested that render neurons more or less vulnerable to aging processes and neurodegenerative changes (Mattson et al., 2004b; Lindenberger et al., 2008). For example, COMT genotype has been linked with the inter-individual cognitive response to extrinsic manipulation: Cognitive performance, in this case working memory, was found to be improved in Val/Val-carriers after pharmacologically induced dopamine release by oral amphetamine administration, whereas performance in Met/Met-carriers was decreased at high working memory load (Mattay et al., 2003). This genotype–drug interaction was also evident in functional magnetic resonance imaging (fMRI) during working memory tasks in the same study, where Val/Val-carriers showed a more efficient activation after amphetamine administration, which was similar to the activation seen in Met/Met-carriers after placebo, and vice versa (Mattay et al., 2003). Similarly, when using a behavioral manipulation, Loughead et al. (2009) could demonstrate that Val/Val-carriers were more sensitive to a smoking abstinence challenge than Met-carriers, with respect to working memory performance and PFC activation.
In a previous interventional study of our group, we showed that caloric restriction (CR) improved memory performance in healthy elderly subjects (Witte et al., 2009). In that study, CR-associated increases in memory performance appeared to be correlated with changes in insulin signaling and inflammatory activity. Potential interactions with COMT genotype were not assessed.
The aim of the current study was to determine whether the response to the dietary intervention with regard to cognition was modulated by COMT Val/Met-allele carrier status. To address this question, we post hoc assessed COMT Val158Met genotype of subjects of the two intervention groups of the preceding study (Witte et al., 2009).
Materials and Methods
Subjects
Thirty-nine healthy normal-to-overweight elderly subjects who completed a dietary intervention period in a previous study (Witte et al., 2009) were considered for genotyping.
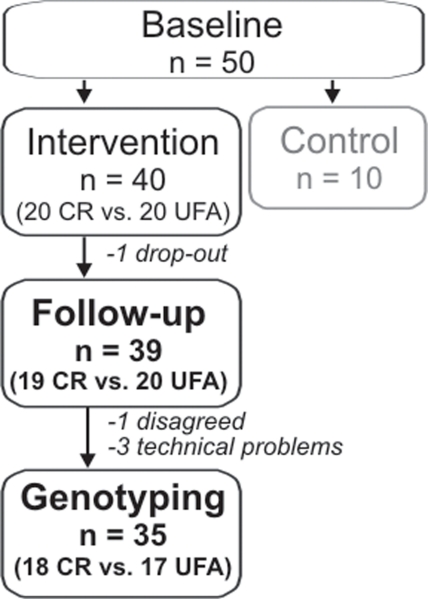
Briefly, subjects included in the original study were stratified into three groups. One group was instructed to reduce caloric intake aiming at a reduction of 30% compared to previous habits (CR, n = 20), over a period of 3 months. Minimal caloric intake was set to 1.200 kcals/day to avoid cognitive problems due to malnutrition. Another group was instructed to increase the relative amount of unsaturated fatty acid (UFA, n = 20) intake about 20% compared to previous habits, over a period of 3 months. The quantity of total fat intake was intended to remain unchanged. Participants of the two intervention groups were guided by experienced dieticians. The third group was instructed not to change dietary habits (control, n = 10), those subjects were not genotyped in the present study that aimed at examining gene–environment interactions. Demographic and anthropometric data, as well as details on dietary compliance have been reported elsewhere (see Witte et al., 2009). One subject of the CR group did not complete the interventional study (drop-out). Three subjects had to be excluded due to technical problems during genotyping (2 female and 1 male); 1 additional female did not consent to genotyping, leaving 35 subjects (CR: n = 18, UFA: n = 19) for further analysis, see also Figure 1.
Figure 1.
Number of subjects included in the interventional study and genetic analyses. Subjects of the intervention groups underwent either Caloric restriction (CR) or enhancement of unsaturated fatty acids (UFA).
Before and after the intervention, subjects were tested on cognitive performance using parallel German versions of the Rey Auditory Verbal Learning Task (AVLT; Helmstaedter and Kurthen, 2001; Strauss et al., 2006). In short, subjects were asked to learn and recall as many words as possible out of a list of 15 words. After a delay of 30 min, subjects had to recognize these words in a recognition trial, resulting in raw memory scores, as described elsewhere (Witte et al., 2009). The primary outcome measure “memory score” was the number of retrieved words after adjusting for false-positives (Hochhaus, 1972; Helmstaedter and Kurthen, 2001; Strauss et al., 2006). Secondary outcome measures were total number of retrieved words without adjustment, and total number of false-positive errors.
Subjects did also complete trail making tests (TMT) A and B (Reitan and Herring, 1985), and forward/backward digit span (WMS-R, Markowitsch and Härting, 1996). In addition, all subjects filled in a 7 day custom-made dietary record to assess dietary habits. Fasting blood samples were collected in the morning to determine levels of insulin, glucose, lipid profile (triglycerides, cholesterol, LDL, HDL), inflammatory markers [high sensitive C-reactive protein (hsCRP) and tumor-necrosis factor (TNF)-alpha], and neurotrophins [brain-derived neurotrophic factor (BDNF) and insulin-like growth factor 1 (IGF-1)]. Memory scores of subjects of the CR group were significantly higher after the intervention, whereas scores of the other two group did not change significantly [ANOVARM F(2, 46) = 5.42, P = 0.008; post hoc paired t-test t(18) = 4.73, P = 0.0002; published in Witte et al., 2009].
All participants provided written informed consent and received reimbursement after participation. The research protocol was approved by the Ethics Committee of the University Hospital of Münster.
Genotyping
Genomic DNA was extracted from whole blood using a DNA blood mini-kit (Quiagen) for sequencing of COMT Val158Met genotype. We used genebank sequences1 to identify the common coding variant in the COMT gene, a G→A polymorphism responsible for the Val158Met substitution on chromosome 22q11 (Nagel et al., 2008). We designed sequencing primers (primer3)2 (COMT-F GGGGCCTACTGTGGCTACTC; COMT-R GGTTTTCAGTGAACGTGGTG) resulting in a 174 bp polymerase chain reaction (PCR) product. Total volume of amplification reactions was 50 μl, containing 4 μl of DNA material (DNA concentration 34 ng/μl), 5 μl of each primer, 1 μl dNTP (Desoxyribonukleosidtriphosphate), 5 μl PCR-buffer (Quiagen) and 0.2 μl of Hot Start Taq Polymerase (Quiagen GmbH, Hilden, Germany). PCR cycling conditions comprised an initial denaturation for 15 min at 95°C, followed by 30 cycles of 94°C for 45 s, 59°C for 45 s, 72°C for 45 s and a final extension at 72°C for 10 min. PCR products were electrophoresed on a 2.5% agarose gel stain (Biozym LE Agarose; Biozym Scientific GmbH) and sequencing was completed using the Big Dye Terminator 3.1 chemistry on a 3730 DNA-Analyzer (Applied Biosystems, Darmstadt, Germany).
Based on their Val–Met-allele carrier status, participants were stratified into two genotype groups: homozygous Val/Val-carriers (n = 6) vs. one or two Met-allele carriers (n = 29), similar to previous studies (Mattay et al., 2003; Loughead et al., 2009).
Statistical analysis
To detect significant differences in the AVLT memory score with regard to genotype at baseline, unpaired t-tests were calculated with dependent variable “memory score” and between-subjects factor “COMT Val158Met genotype” (Val/Val-carriers vs. Val/Met- or Met/Met-carriers). Secondary outcome measures (additionally AVLT measures, TMT A and B, digit span forward and backward), as well as demographic parameters and peripheral blood measures were compared between “genotype” groups by unpaired t-tests or non-parametric tests as appropriate.
To assess significant gene × diet interactions, ANOVARM were conducted with repeated factor “time” (AVLT memory scores at baseline vs. after intervention) and between-subjects factor “COMT Val158Met genotype” (Val/Val-carriers vs. Val/Met- or Met/Met-carriers) in the two dietary interventions group separately (CR, UFA). Unpaired t-tests (two-tailed) were calculated post hoc to detect significant differences in diet-induced memory changes. Additionally, ANOVARM or non-parametric tests were calculated with secondary outcome parameters as appropriate.
Significance was set at p = 0.05, all data are presented as mean with standard error of the mean, unless indicated otherwise.
Results
Genetic analysis of COMT Val158Met genotype revealed a distribution of 6 Val/Val-carriers (CR: n = 3, UFA: n = 3) and 29 Met-carriers (CR: n = 13, UFA: n = 14). Demographic characteristics (sex, age, BMI, education) did not differ significantly with regard to genotype (p > 0.05); see Table 1 for details.
Table 1.
Demographic characteristics and fasting serum levels of COMT Val158Met genotype groups, dependent on intervention group and allelic variant.
| COMT Val158Met genotype | ||||||
|---|---|---|---|---|---|---|
| Val/Val | Val/Met or Met/Met | |||||
| CR | UFA | Total | CR | UFA | Total | |
| CHARACTERISTICS | ||||||
| n | 3 | 3 | 6 | 15 | 14 | 29 |
| Women, no. | 1 | 2 | 3 | 8 | 8 | 14 |
| Age, years ± SD (minimum/maximum) | 56.3 ± 6 (53–63) | 61 ± 9 (52–70) | 58.7 ± 7 (52–70) | 60.3 ± 8 (50–72) | 62.4 ± 8 (51–79) | 61.8 ± 8 (50–79) |
| Body mass index, kg/m2 ±SD (minimum/maximum) | 30.8 ± 2 (28.7–32.8) | 23.9 ± 2 (23.9–26.4) | 27.3 ± 4 (23.9–32.8) | 29.7 ± 4 (23.7–39.3) | 27 ± 2 (23.7–30.6) | 28.4 ± 4 (23.7–39.3) |
| Education, years ±SD (minimum/maximum) | 14 ± 2 (13–16) | 14 ± 2 (13–16) | 14 ± 2 (13–16) | 16.3 ± 6 (10–30) | 15.7 ± 5 (10–23) | 15.8 ± 5 (10–30) |
| FASTING SERUM LEVELS, MEAN ± SD | ||||||
| Triglyceride, mg/dL | 183 ±80 | 110.7 ±38 | 146.8 ±69 | 188.2 ± 116 | 140.2 ±60 | 167.9 ±98 |
| Cholesterol, mg/dL | 218.3 ±34 | 231.7 ± 14 | 225 ±25 | 257.3 ±60 | 244.9 ±34 | 252.1 ±51 |
| HDL cholesterol, mg/dL | 54.3 ± 14 | 73.3 ± 18 | 63.8 ± 18 | 57.3 ± 15 | 58.7 ± 16 | 57.9 ± 15 |
| LDL cholesterol, mg/dL | 127.4 ±23 | 136.2 ±23 | 131.8 ±21 | 162.4 ±49 | 158.1 ±32 | 160.6 ±42 |
| Insulin, mU/L | 16.6 ± 6.1 | 6 ± 3.6 | 11.3 ±7.3 | 15.2 ± 15 | 10.7 ± 4.4 | 13.3 ± 12.1 |
| Glucose, mg/dL | 101.3 ±1.2 | 91 ±9 | 96.2 ±8 | 104.7 ±26 | 101.5 ± 10 | 103.4 ±20 |
| BDNF, pg/mL | 1220 ± 277 | 1183 ± 136 | 1202 ± 196 | 1102 ± 351 | 1251 ± 333 | 1165 ± 345 |
| IGF-1, ng/mL* | 107.3 ±34 | 89.3 ±7 | 98.3 ±24 | 141.4 ±34 | 123.5 ±55 | 133.1 ±45 |
| hs nbCRP, pg/mL | 0.13 ± 0.06 | 0.38 ± 0.3 | 0.26 ± 0.2 | 0.42 ± 0.8 | 0.21 ± 0.2 | 0.33 ± 0.6 |
| TNF-alpha, pg/mL | 1.14 ±1.5 | 2.8 ± 4.3 | 1.99 ±3 | 1.8 ±1.6 | 3.9 ± 2.7 | 2.7 ± 2.4 |
CR, caloric restriction; UFA, unsaturated fatty acids.
*Only IGF-1 concentrations were significantly different between genotype groups at baseline (Val/Val vs. Met; p = 0.02).
Effects of genotype at baseline
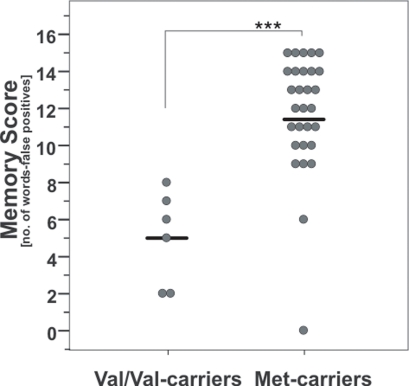
There was a significant difference between memory scores of the AVLT at baseline when comparing COMT Val158Met genotypes (t-test; t(1, 33) = 4.8, p < 0.001; Figure 2), namely that Val/Val-carriers performed significantly poorer compared to Met carriers.
Figure 2.
Baseline memory scores according to COMT Val158Met genotype. Note that Val/Val-carriers had significant lower values than carriers of at least one Met-allele. ***, p = 0.001; according to unpaired t-test. Circles indicate individual values, solid lines indicate the mean.
In contrast, no significant genotype-dependent differences were found regarding executive functions and working memory (Trail Making Test A and B, digit span forward and backward; t-tests, all p > 0.05). Note that the two dietary interventions did not influence these tests in the initial study (Witte et al., 2009).
There were no differences between genotype groups in anthropometric data (age, education, BMI) or fasting serum parameters (insulin, glucose, triglycerides, cholesterol, HDL, LDL, hsCRP, BDNF, TNF-alpha; Table 1), except for IGF-1: Val/Val-carriers had significantly lower concentrations at baseline than Met-carriers (Table 1, Mann–Whitney test, p = 0.02). This difference persisted after the intervention, namely that Val/Val-carriers again showed lower IGF-1 concentrations than Met-carriers (Mann–Whitney test, p = 0.02).
Gene × diet interactions
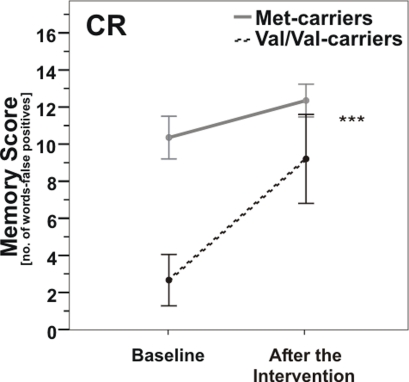
In the CR group, there was a significant COMT genotype × time interaction [repeated measures analysis of variance (ANOVARM), F(1, 16) = 14.9, p = 0.001; Figure 3]. The CR-induced improvement of memory score was considerably greater in COMT Val/Val-carriers than in Met-carriers, so that memory scores of both genotype groups were no longer significantly different from each other after the intervention (post hoc t-test, p > 0.05); however t-test of changes in memory score failed to reach significance between groups (p = 0.15).
Figure 3.
Memory scores according to COMT Val158Met genotype pre- vs. post-caloric restriction (CR). Note that Val/Val-carriers (dashed line) nearly reached baseline levels of carriers of at least one Met-allele (gray line) after CR. ***, p = 0.001; ***, according to ANOVARM. Error bars indicate standard error (SE).
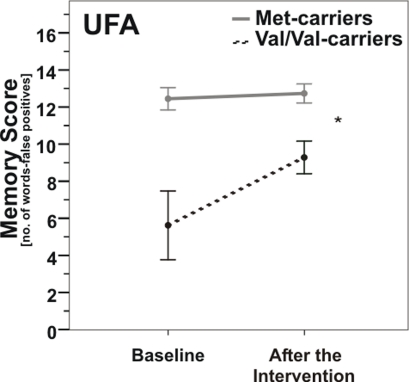
Likewise in the UFA-group, ANOVARM detected a significant interaction effect of COMT genotype × time (F(1, 15) = 6.9, p = 0.019; Figure 4). Post hoc t-tests of changes in memory score showed that Val/Val-carriers experienced significantly greater improvements due to the intervention than Met-carriers (t(1, 15) = −2.6, p = 0.019).
Figure 4.
Memory scores according to COMT Val158Met genotype pre- vs. post-enhancement of unsaturated fatty acids (UFA). Note that Val/Val-carriers (dashed line) nearly reached baseline levels of carriers of at least one Met-allele (gray line) after UFA enhancement. *, p = 0.019; *, according to ANOVARM. Error bars indicate standard error (SE).
No significant differences were detected for genotype groups with regard to changes in anthropometric data or fasting serum parameters (see above for details) due to the intervention (all p's > 0.05).
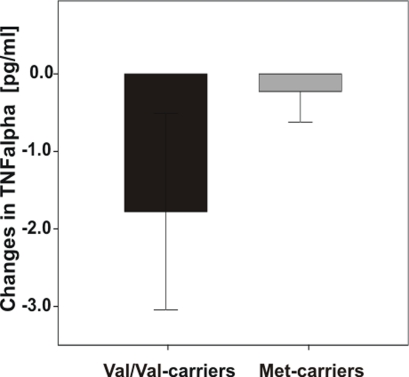
However, an interesting trend emerged for markers of inflammation: Diet-associated reductions in TNF-alpha levels after the intervention were more pronounced in Val/Val-carriers (Figure 5), however ANOVARM failed to reach significance.
Figure 5.
Differences in TNF-alpha levels pre- vs. post-dietary intervention according to COMT Val158Met genotype. Note that Val/Val-carriers exhibited greater reductions of TNF-alpha than Met-carriers, however analysis failed to reach significance. Error bars indicate standard error (SE).
Considering IGF-1, there was an overall significant increase after the intervention over the entire study group (Wilcoxon-Ranks test, p = 0.021), which was not significantly different with regard to genotype group (p > 0.05).
Discussion
Our study provides first-time evidence that the cognitive response to dietary interventions is dependent on COMT Val158Met genotype. At baseline, Val/Val-carriers had significantly lower memory scores compared to Val/Met-carriers and Met/Met-carriers, which is in line with previous studies on cognitive performance (e.g., de Frias et al., 2004; Bertolino et al., 2006). Overall improvements in memory score due to dietary intervention were then significantly greater in Val/Val-carriers compared to Met-carriers, leading to comparable memory scores in the genetic groups after the intervention.
Comt genotype and cognition
Our findings of lower memory performance in Val/Val-carriers at baseline are consistent with several previous reports, summarized in a recent meta-analysis (Raz et al., 2009). For example, in a longitudinal sample of 286 men, Met/Met-carrier status was associated with better episodic and semantic memory (de Frias et al., 2004). Similarly using an fMRI recognition memory paradigm, Bertolino et al. (2006) found poorer performance and differential activation in memory-related brain areas such as the hippocampus and ventrolateral PFC in Val-carriers compared to Met/Met-carriers. Notably, both studies distinguished carriers of one or two Val-allele vs. homozygous Met/Met-carriers; but brain activation patterns rather depict a dose-dependent relationship of Met-alleles in these studies (see, e.g., Figures 2 and 3 in Bertolino et al., 2006). Several other behavioral and fMRI studies found, similar to our results, a clear distinction between Val/Val vs. Met-allele carriers (Mattay et al., 2003; Enoch et al., 2009; Loughead et al., 2009; Krach et al., 2010).
Considering tasks probing executive functions, working memory, and attention, we did not find significant differences dependent on COMT genotype, similar to most (see, e.g., meta-analysis by Barnett et al., 2008), but not all studies (Egan et al., 2001; Mier et al., 2009). Thus, there is still considerable debate about the impact of COMT genotype on specific cognitive functions, and future studies using larger number of subjects will have to resolve this issue.
The COMT gene is widely distributed in the brain (Hong et al., 1998), including prefrontal and mesotemporal/hippocampal areas which are strongly linked with memory performance (Shing et al., 2010). Notably, COMT is thought to be crucially involved in the regulation of dopamine flux in the PFC, due to the minimal role of other degrading pathways as, for example, the dopamine transporter in this area (Lewis et al., 2001; Mazei et al., 2002; Morón et al., 2002). Therefore, the higher enzymatic activity of the COMT enzyme encoded by the Val-allele (Lotta et al., 1995) induces lower brain dopamine levels compared to Met-carriers especially in prefrontal areas (Diaz-Asper et al., 2006), which could account for differences in prefrontal neuronal processing. Notably, decreases in memory performance during late adulthood are thought to depend on impairments in both prefrontal and mesotemporal/hippocampal processing (Shing et al., 2010), therefore COMT-dependent, less efficient prefrontal dopamine signaling could lead to deficits in recall and delayed recognition in Val/Val-carriers.
Moreover, the effect of COMT genotype on cognitive processing per se is thought to be magnified with advancing age (Lindenberger et al., 2008; Nagel et al., 2008). Nagel and colleagues used a test of executive function, the WCST, in young and in elderly individuals to show that the effect of COMT Val/Val-carrier status is significantly more disadvantageous in old than in young age. Their findings support the hypothesis that optimal cognitive function is associated with optimal brain dopamine levels (Lindenberger et al., 2008; Nagel et al., 2008). As normal aging is linked with an overall decline in dopaminergic neuromodulation (Volkow et al., 1998; Backman et al., 2000; Erixon-Lindroth et al., 2005), individuals with low dopamine levels even at young age, such as Val/Val-carriers, might be more vulnerable to these adverse effects than others. At the same time, these individuals may benefit more from dopamine-enhancing strategies, a hypothesis that already received experimental support (e.g., Mattay et al., 2003): In that study, due to dopamine-enhancing amphetamine administration, Val/Val-carriers showed an improvement in working memory tasks as well as more efficient brain activation using fMRI, whereas carriers of at least one Met-allele performed worse after the same drug dosage (Mattay et al., 2003). Likewise, Val/Val-carriers showed a significant improvement of working memory and other cognitive tasks in response to the COMT-inhibitor tolcapone (Giakoumaki et al., 2008). Similar results were also found for a behavioral intervention testing the response to a smoking abstinence challenge in heavy smokers (Loughead et al., 2009). Here, Val/Val-carriers were more sensitive to an overnight smoking cessation than Met-carriers, demonstrated by stronger prefrontal blood oxygen level-dependent (BOLD)-signals and by slower reaction time during n-back tasks at smoking abstinence in Val/Val-carriers only (Loughead et al., 2009).
Gene × diet interactions
In line with these studies, we found a better cognitive response to dietary interventions for Val/Val-carriers compared to carriers of one or two Met-alleles: The former experienced a considerably greater improvement of memory scores after CR and after UFA enhancement.
Both CR and UFA may improve neuronal plasticity and cognitive functions such as memory via several pathways, including on the one hand direct augmentation of prefrontal dopamine release, e.g., via glucoregulatory processes (Ritter et al., 2006), or via beneficial effects of diet-induced neurotrophic factor release on dopaminergic neurons (Mattson et al., 2004a).
Moreover, CR and UFA may activate other plasticity-enhancing mechanisms, such as increase in membrane fluidity, improvement of glucose/insulin-dependent second messenger cascades, and down-regulation of inflammatory activity (see, e.g., Mattson et al., 2004a; Zhao et al., 2004; Gomez-Pinilla, 2008; Stranahan and Mattson, 2008; Witte et al., 2009). These processes may then compensate for the genotype-related deficits in cognition due to less dopaminergic neurotransmission in Val/Val-carriers. Considering inflammatory markers, our results of stronger diet-associated reductions in TNF-alpha levels in Val/Val-carriers lend some experimental support to this hypothesis.
A consistent reduction of IGF-1 levels in Val/Val-carriers was noted compared to Met-carriers before and after the intervention, in line with previous studies (Hong et al., 2003). However we did not find genotype-dependent differences in the response of IGF-1 to the CR intervention, or regarding BDNF, glucose or insulin. These negative findings may be due to limited power because of the small sample size. Further studies with larger sample sizes are needed to explore these suggested mechanisms in more detail.
Taken together, we hypothesize that diet-associated beneficial processes were able to move the genotype-related lower memory scores in our Val/Val-carriers toward the performance of Met-carriers, underlining that the “at risk” genotype with regard to brain function takes greater advantage of a behavioral intervention. Data from another common learning-relevant SNP which has been linked to memory performance and hippocampal processing, BDNF Val66Met (Egan et al., 2003), support this idea: Carriers of the Met-allele, associated with lower BDNF-levels, experienced greater advantages due to exercise than carriers of the Val-allele (Mata et al., 2010). A larger gene–lifestyle interaction for the “at risk” carriers with regard to cognition have also been observed for other learning-relevant genes, e.g., apo-lipoprotein E (APOE) (Dore et al., 2009), and “kidney and brain” (KIBRA) (Zhang et al., 2009). For example, the negative effect of diabetes on cognitive performance was found to be enlarged in carriers of the “at risk” APOE epsilon 4-alleles, compared to non-APOE epsilon 4 carriers (Dore et al., 2009). Smoking status has been shown to modulate cognitive functions in KIBRA T-allele carriers, considered as “at risk” genotype because of worse performance on executive tasks, but not in non-T-allele carriers (Zhang et al., 2009). Taken together, there is some evidence that homozygous “at risk”-allele carriers of specific plasticity-related polymorphisms (such as COMT Val158Met, or BDNF Val66Met) tend to better respond to lifestyle or pharmacological interventions.
Limitations
Several limitations have to be considered when interpreting our results. First, genotyping of the study was retrospective, therefore sample size was low, and not all subjects originally included in the study could be genotyped. Also, we did not assess other gene × gene interactions, which could have exerted influence on dopaminergic neurotransmission. For example, the impact of genetic variations in the genes encoding the dopamine transporter, the dopamine D2 receptor, as well as additional COMT locations (Meyer-Lindenberg et al., 2006), warrant to be determined in future research.
Conclusions
In this retrospective assessment of COMT Val158Met genotype in healthy elderly subjects, we found first-time evidence that the “at risk” genotype Val/Val might benefit more from dietary modifications with regard to cognitive functions than Met-allele carriers. Our findings may help to explain inter-individual differences in the response to cognition-enhancing interventions, and may constitute a first step toward the development of individualized therapies in future research (Mayor, 2007). Further studies are now needed to help clarifying the mechanisms underlying these effects, which will allow to specifically develop new agents and/or lifestyle interventions targeting the identified mechanisms, with the ultimate aim to preserve cognitive functions until old age (Corneveaux et al., 2010).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work has been supported by grants of the German Research Foundation (DFG; to Agnes Flöel: Fl 379-4/2, DFG-Exc 257; to Agnes Flöel and Anja Veronica Witte: FL 379-8/1), by grants of the German Ministry of Education and Research (BMBF, to Agnes Flöel: 01EO0801, to Agnes Flöel and Anja Veronica Witte: FKZ 0315673A), by grants of the “Interdisziplinäres Zentrum für klinische Forschung” to Agnes Flöel (IZKF, Floe3/004/08).
Footnotes
References
- Backman L., Ginovart N., Dixon R. A., Wahlin T. B., Wahlin A., Halldin C., Farde L. (2000). Age-related cognitive deficits mediated by changes in the striatal dopamine system. Am. J. Psychiatry 157, 635–637 10.1176/appi.ajp.157.4.635 [DOI] [PubMed] [Google Scholar]
- Barnett J. H., Scoriels L., Munafò M. R. (2008). Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108met polymorphism. Biol. Psychiatry 64, 137–144 10.1016/j.biopsych.2008.01.005 [DOI] [PubMed] [Google Scholar]
- Bertolino A., Rubino V., Sambataro F., Blasi G., Latorre V., Fazio L., Caforio G., Petruzzella V., Kolachana B., Hariri A., Meyer-Lindenberg A., Nardini M., Weinberger D. R., Scarabino T. (2006). Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 60, 1250–1258 10.1016/j.biopsych.2006.03.078 [DOI] [PubMed] [Google Scholar]
- Corneveaux J. J., Liang W. S., Reiman E. M., Webster J. A., Myers A. J., Zismann V. L., Joshipura K. D., Pearson J. V., Hu-Lince D., Craig D. W., Coon K. D., Dunckley T., Bandy D., Lee W., Chen K., Beach T. G., Mastroeni D., Grover A., Ravid R., Sando S. B., Aasly J. O., Heun R., Jessen F., Kölsch H., Rogers J., Hutton M. L., Melquist S., Petersen R. C., Alexander G. E., Caselli R. J., Papassotiropoulos A., Stephan D. A., Huentelman M. J. (2010). Evidence for an association between KIBRA and late-onset Alzheimer's disease. Neurobiol. Aging 31, 901–909 10.1016/j.neurobiolaging.2008.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias C. M., Annerbrink K., Westberg L., Eriksson E., Adolfsson R., Nilsson L.-G. (2004). COMT gene polymorphism is associated with declarative memory in adulthood and old age. Behav. Genet. 34, 533–539 10.1023/B:BEGE.0000038491.06972.8c [DOI] [PubMed] [Google Scholar]
- Diaz-Asper C. M., Weinberger D. R., Goldberg T. E. (2006). Catechol-O-methyltransferase polymorphisms and some implications for cognitive therapeutics. NeuroRx 3, 97–105 10.1016/j.nurx.2005.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore G. A., Elias M. F., Robbins M. A., Elias P. K., Nagy Z. (2009). Presence of the APOE epsilon4 allele modifies the relationship between type 2 diabetes and cognitive performance: the Maine–Syracuse Study. Diabetologia 52, 2551–2560 10.1007/s00125-009-1497-2 [DOI] [PubMed] [Google Scholar]
- Egan M. F., Goldberg T. E., Kolachana B. S., Callicott J. H., Mazzanti C. M., Straub R. E., Goldman D., Weinberger D. R. (2001). Effect of COMT val108/158 met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 98, 6917–6922 10.1073/pnas.111134598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F., Kojima M., Callicott J. H., Goldberg T. E., Kolachana B. S., Bertolino A., Zaitsev E., Gold B., Goldman D., Dean M., Lu B., Weinberger D. R. (2003). The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112, 257–269 [DOI] [PubMed] [Google Scholar]
- Enoch M. A., Waheed J. F., Harris C. R., Albaugh B., Goldman D. (2009). COMT val158met and cognition: main effects and interaction with educational attainment. Genes Brain Behav. 8, 36–42 10.1111/j.1601-183X.2008.00441.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erixon-Lindroth N., Farde L., Wahlin T. B., Sovago J., Halldin C., Backman L. (2005). The role of the striatal dopamine transporter in cognitive aging. Psychiatry Res. 138, 1–12 10.1016/j.pscychresns.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Giakoumaki S. G., Roussos P., Bitsios P. (2008). Improvement of prepulse inhibition and executive function by the COMT inhibitor tolcapone depends on COMT Val158Met polymorphism. Neuropsychopharmacology 33, 3058–3068 10.1038/npp.2008.82 [DOI] [PubMed] [Google Scholar]
- Goldberg T. E., Weinberger D. R. (2004). Genes and the parsing of cognitive processes. Trends Cogn. Sci. (Regul. Ed.) 8, 325–335 [DOI] [PubMed] [Google Scholar]
- Goldman D., Weinberger D. R., Malhotra A. K., Goldberg T. E. (2009). The role of COMT val158met in cognition. Biol. Psychiatry 65, e3–e4 10.1016/j.biopsych.2008.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F. (2008). The influences of diet and exercise on mental health through hormesis. Ageing Res. Rev. 7, 49–62 10.1016/j.arr.2007.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C., Kurthen M. (2001). Memory and epilepsy: characteristics, course, and influence of drugs and surgery. Curr. Opin. Neurol. 14, 211–216 10.1097/00019052-200104000-00013 [DOI] [PubMed] [Google Scholar]
- Hochhaus L. (1972). A table for the calculation of d’ and beta. Psychol. Bull. 77, 375–376 10.1037/h0032459 [DOI] [Google Scholar]
- Hong C. C., Thompson H. J., Jiang C., Hammond G. L., Tritchler D., Yaffe M., Boyd N. F. (2003). Val158met Polymorphism in catechol-O-methyltransferase gene associated with risk factors for breast cancer. Cancer Epidemiol. Biomarkers Prev. 12, 838–847 [PubMed] [Google Scholar]
- Hong J., Shu-Leong H., Tao X., Lap-Ping Y. (1998). Distribution of catechol-O-methyltransferase expression in human central nervous system. Neuroreport 9, 2861–2864 10.1097/00001756-199808240-00033 [DOI] [PubMed] [Google Scholar]
- Krach S., Jansen A., Krug A., Markov V., Thimm M., Sheldrick A. J., Eggermann T., Zerres K., Stöcker T., Shah N. J., Kircher T. (2010). COMT genotype and its role on hippocampal-prefrontal regions in declarative memory. Neuroimage 53, 978–984 10.1016/j.neuroimage.2009.12.090 [DOI] [PubMed] [Google Scholar]
- Lewis D. A., Melchitzky D. S., Sesack S. R., Whitehead R. E., Auh S., Sampson A. (2001). Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J. Comp. Neurol. 432, 119–136 10.1002/cne.1092 [DOI] [PubMed] [Google Scholar]
- Lindenberger U., Nagel I. E., Chicherio C., Li S. C., Heekeren H. R., Backman L. (2008). Age-related decline in brain resources modulates genetic effects on cognitive functioning. Front. Neurosci. 2, 234–244 10.3389/neuro.01.039.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T., Vidgren J., Tilgmann C., Ulmanen I., Melén K., Julkunen I., Taskinen J. (1995). Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 34, 4202–4210 10.1021/bi00013a008 [DOI] [PubMed] [Google Scholar]
- Loughead J., Wileyto E. P., Valdez J. N., Sanborn P., Tang K., Strasser A. A., Ruparel K., Ray R., Gur R. C., Lerman C. (2009). Effect of abstinence challenge on brain function and cognition in smokers differs by COMT genotype. Mol. Psychiatry 14, 820–826 10.1038/mp.2008.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch H. J., Härting C. (1996). Interdependence of priming performance and brain-damage. Int. J. Neurosci. 85, 291–300 10.3109/00207459608986690 [DOI] [PubMed] [Google Scholar]
- Mata J., Thompson R. J., Gotlib I. H. (2010). BDNF genotype moderates the relation between physical activity and depressive symptoms. Health Psychol. 29, 130–133 10.1037/a0017261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattay V. S., Goldberg T. E., Fera F., Hariri A. R., Tessitore A., Egan M. F., Kolachana B., Callicott J. H., Weinberger D. R. (2003). Catechol O-methyltransferase val158-Met genotype and individual variation in the brain response to amphetamine. Proc. Natl. Acad. Sci. U.S.A. 100, 6186–6191 10.1073/pnas.0931309100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Maudsley S., Martin B. (2004a). A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev. 3, 445–464 10.1016/j.arr.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Duan W., Wan R., Guo Z. (2004b). Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx 1, 111–116 10.1602/neurorx.1.1.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S. (2007). Fitting the drug to the patient. BMJ 334, 452–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei M. S., Pluto C. P., Kirkbride B., Pehek E. A. (2002). Effects of catecholamine uptake blockers in the caudate-putamen and subregions of the medial prefrontal cortex of the rat. Brain Res. 936, 58–67 10.1016/S0006-8993(02)02542-8 [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Nichols T., Callicott J. H., Ding J., Kolachana B., Buckholtz J., Mattay V. S., Egan M., Weinberger D. R. (2006). Impact of complex genetic variation in COMT on human brain function. Mol. Psychiatry 11, 867–877 10.1038/sj.mp.4001860 [DOI] [PubMed] [Google Scholar]
- Mier D., Kirsch P., Meyer-Lindenberg A. (2009). Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry. 15, 918–927 10.1038/mp.2009.36 [DOI] [PubMed] [Google Scholar]
- Morón J. A., Brockington A., Wise R. A., Rocha B. A., Hope B. T. (2002). Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J. Neurosci. 22, 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel I. E., Chicherio C., Li S.-C., von Oertzen T., Sander T., Villringer A., Heekeren H. R., Bäckman L., Lindenberger U. (2008). Human aging magnifies genetic effects on executive functioning and working memory. Front. Hum. Neurosci. 2, 1. 10.3389/neuro.09.001.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter G. G., Taylor W. D., McQuoid D. R., Steffens D. C., Welsh-Bohmer K. A., Krishnan K. R. R. (2009). The COMT Val158Met polymorphism and cognition in depressed and non-depressed older adults. Int. J. Ger. Psychiatry 24, 1127–1133 10.1002/gps.2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Rodrigue K. M., Kennedy K. M., Land S. (2009). Genetic and vascular modifiers of age-sensitive cognitive skills: effects of COMT, BDNF, ApoE, and hypertension. Neuropsychology 23, 105–116 10.1037/a0013487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. M., Herring S. (1985). A short screening device for identification of cerebral dysfunction in children. J. Clin. Psychol. 41, 643–650 [DOI] [PubMed] [Google Scholar]
- Ritter S., Dinh T. T., Li A. J. (2006). Hindbrain catecholamine neurons control multiple glucoregulatory responses. Physiol. Behav. 89, 490–500 10.1016/j.physbeh.2006.05.036 [DOI] [PubMed] [Google Scholar]
- Shing Y. L., Werkle-Bergner M., Brehmer Y., Muller V., Li S. C., Lindenberger U. (2010). Episodic memory across the lifespan: the contributions of associative and strategic components. Neurosci. Biobehav. Rev. 34, 1080–1091 10.1016/j.neubiorev.2009.11.002 [DOI] [PubMed] [Google Scholar]
- Stranahan A. M., Mattson M. P. (2008). Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Med. 10, 209–218 10.1007/s12017-008-8043-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E., Sherman E. M., Spreen O. (2006). A Compendium of Neuropsychological Tests. Administration, Norms, and Commentary. Oxford: Oxford University Press [Google Scholar]
- Volkow N. D., Gur R. C., Wang G. J., Fowler J. S., Moberg P. J., Ding Y. S., Hitzemann R., Smith G., Logan J. (1998). Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am. J. Psychiatry 155, 344–349 [DOI] [PubMed] [Google Scholar]
- Witte A. V., Fobker M., Gellner R., Knecht S., Floel A. (2009). Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. U.S.A. 106, 1255–1260 10.1073/pnas.0808587106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Kranzler H. R., Poling J., Gruen J. R., Gelernter J. (2009). Cognitive flexibility is associated with KIBRA variant and modulated by recent tobacco use. Neuropsychopharmacology 34, 2508–2516 10.1038/npp.2009.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W. Q., Chen H., Quon M. J., Alkon D. L. (2004). Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 490, 71–81 10.1016/j.ejphar.2004.02.045 [DOI] [PubMed] [Google Scholar]