Abstract
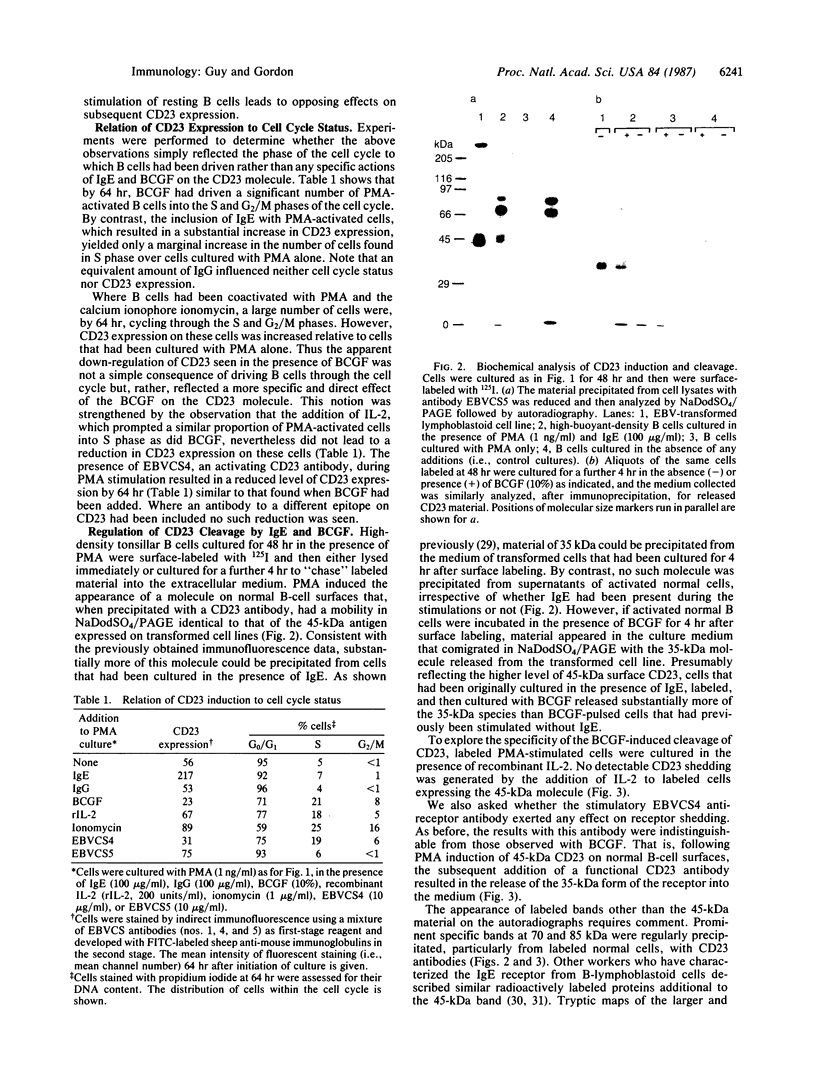
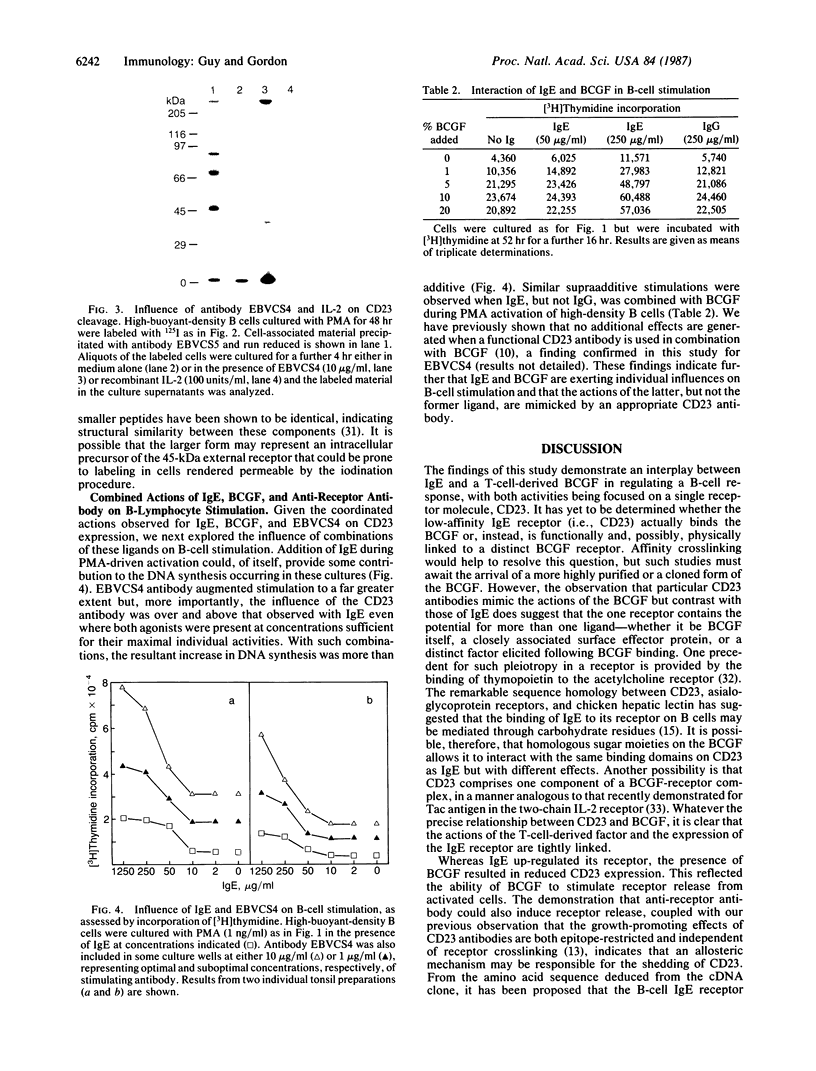
The CD23 (BLAST-2) antigen, recently identified as the low-affinity IgE receptor of B lymphocytes, has also been implicated as the focus for growth-promoting signals delivered to activated B cells by a low molecular weight B-cell growth factor (BCGF). Here we show that IgE and BCGF can coordinate B-lymphocyte growth through their opposing effects on the CD23 molecule. While the activation of purified quiescent B cells with phorbol 12-myristate 13-acetate led to the induction of 45-kDa CD23 at the surface membrane, the inclusion of IgE increased CD23 expression by a factor of approximately equal to 5. The addition of BCGF resulted in the rapid release of a 35-kDa form of CD23 from the cell surface. This shed molecule is associated with autocrine growth factor activity. Substantially more of this material was generated by BCGF acting on cells that had been stimulated in the presence of IgE. The combined effects of IgE and BCGF on DNA synthesis in activated B cells were more than additive. IgE similarly augmented the stimulatory capacity of a CD23 antibody that mimics the biological actions of BCGF. Binding of the anti-receptor antibody to its 45-kDa target at the B-cell surface also prompted the release of the 35-kDa soluble species. These results demonstrate a pleiotropy in the CD23 molecule with regard to both ligand binding and the subsequent behavior of the receptor. The ability of this single receptor to orchestrate a B-lymphocyte response through a variety of ligands and its role in normal and transformed autocrine growth are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capron M., Jouault T., Prin L., Joseph M., Ameisen J. C., Butterworth A. E., Papin J. P., Kusnierz J. P., Capron A. Functional study of a monoclonal antibody to IgE Fc receptor (Fc epsilon R2) of eosinophils, platelets, and macrophages. J Exp Med. 1986 Jul 1;164(1):72–89. doi: 10.1084/jem.164.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark E. A., Ledbetter J. A. Activation of human B cells mediated through two distinct cell surface differentiation antigens, Bp35 and Bp50. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4494–4498. doi: 10.1073/pnas.83.12.4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Ohara J., Bond M. W., Carty J., Zlotnik A., Paul W. E. B cell stimulatory factor-1 enhances the IgE response of lipopolysaccharide-activated B cells. J Immunol. 1986 Jun 15;136(12):4538–4541. [PubMed] [Google Scholar]
- Fujimoto J., Stewart S. J., Levy R. Immunochemical analysis of the released Leu-2 (T8) molecule. J Exp Med. 1984 Jul 1;160(1):116–124. doi: 10.1084/jem.160.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Ley S. C., Melamed M. D., English L. S., Hughes-Jones N. C. Immortalized B lymphocytes produce B-cell growth factor. Nature. 1984 Jul 12;310(5973):145–147. doi: 10.1038/310145a0. [DOI] [PubMed] [Google Scholar]
- Gordon J., Rowe M., Walker L., Guy G. Ligation of the CD23,p45 (BLAST-2,EBVCS) antigen triggers the cell-cycle progression of activated B lymphocytes. Eur J Immunol. 1986 Sep;16(9):1075–1080. doi: 10.1002/eji.1830160908. [DOI] [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Guy G. R., Walker L. Triggering of B lymphocytes through CD23: epitope mapping and studies using antibody derivatives indicate an allosteric mechanism of signalling. Immunology. 1987 Apr;60(4):517–521. [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Walker L., Guy G. R., Rowe M. Evidence for an association between CD23 and the receptor for a low molecular weight B cell growth factor. Eur J Immunol. 1986 Dec;16(12):1627–1630. doi: 10.1002/eji.1830161225. [DOI] [PubMed] [Google Scholar]
- Ishizaka K. Twenty years with IgE: from the identification of IgE to regulatory factors for the IgE response. J Immunol. 1985 Jul;135(1):i–x. [PubMed] [Google Scholar]
- Joseph M., Capron A., Ameisen J. C., Capron M., Vorng H., Pancré V., Kusnierz J. P., Auriault C. The receptor for IgE on blood platelets. Eur J Immunol. 1986 Mar;16(3):306–312. doi: 10.1002/eji.1830160318. [DOI] [PubMed] [Google Scholar]
- Jurgensen C. H., Ambrus J. L., Jr, Fauci A. S. Production of B cell growth factor by normal human B cells. J Immunol. 1986 Jun 15;136(12):4542–4547. [PubMed] [Google Scholar]
- Kikutani H., Inui S., Sato R., Barsumian E. L., Owaki H., Yamasaki K., Kaisho T., Uchibayashi N., Hardy R. R., Hirano T. Molecular structure of human lymphocyte receptor for immunoglobulin E. Cell. 1986 Dec 5;47(5):657–665. doi: 10.1016/0092-8674(86)90508-8. [DOI] [PubMed] [Google Scholar]
- Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975 Jul;66(1):188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee F., Yokota T., Otsuka T., Meyerson P., Villaret D., Coffman R., Mosmann T., Rennick D., Roehm N., Smith C. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. T., Conrad D. H. Murine B cell hybridomas bearing ligand-inducible Fc receptors for IgE. J Immunol. 1986 Jun 15;136(12):4573–4580. [PubMed] [Google Scholar]
- Lüdin C., Hofstetter H., Sarfati M., Levy C. A., Suter U., Alaimo D., Kilchherr E., Frost H., Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. 1987 Jan;6(1):109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Cone R. E., Santer V. Enzymic iodination. A probe for accessible surface proteins of normal and neoplastic lymphocytes. Biochem J. 1971 Oct;124(5):921–927. doi: 10.1042/bj1240921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S. R., Conrad D., Sandler R., Morgan J., Montagna R., Maizel A. L. Purification of human B cell growth factor. J Immunol. 1985 Nov;135(5):3298–3302. [PubMed] [Google Scholar]
- Meinke G. C., Magro A. M., Lawrence D. A., Spiegelberg H. L. Characterization of an IgE receptor isolated from cultured B-type lymphoblastoid cells. J Immunol. 1978 Oct;121(4):1321–1328. [PubMed] [Google Scholar]
- Muraguchi A., Nishimoto H., Kawamura N., Hori A., Kishimoto T. B cell-derived BCGF functions as autocrine growth factor(s) in normal and transformed B lymphocytes. J Immunol. 1986 Jul 1;137(1):179–186. [PubMed] [Google Scholar]
- Neckers L. M., Cossman J. Transferrin receptor induction in mitogen-stimulated human T lymphocytes is required for DNA synthesis and cell division and is regulated by interleukin 2. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3494–3498. doi: 10.1073/pnas.80.11.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Peterson L. H., Conrad D. H. Fine specificity, structure, and proteolytic susceptibility of the human lymphocyte receptor for IgE. J Immunol. 1985 Oct;135(4):2654–2660. [PubMed] [Google Scholar]
- Pezzutto A., Dörken B., Moldenhauer G., Clark E. A. Amplification of human B cell activation by a monoclonal antibody to the B cell-specific antigen CD22, Bp 130/140. J Immunol. 1987 Jan 1;138(1):98–103. [PubMed] [Google Scholar]
- Puca G. A., Abbondanza C., Nigro V., Armetta I., Medici N., Molinari A. M. Estradiol receptor has proteolytic activity that is responsible for its own transformation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5367–5371. doi: 10.1073/pnas.83.15.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin E. M., Ohara J., Paul W. E. B-cell stimulatory factor 1 activates resting B cells. Proc Natl Acad Sci U S A. 1985 May;82(9):2935–2939. doi: 10.1073/pnas.82.9.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin L. A., Jay G., Nelson D. L. The released interleukin 2 receptor binds interleukin 2 efficiently. J Immunol. 1986 Dec 15;137(12):3841–3844. [PubMed] [Google Scholar]
- Sanderson C. J., O'Garra A., Warren D. J., Klaus G. G. Eosinophil differentiation factor also has B-cell growth factor activity: proposed name interleukin 4. Proc Natl Acad Sci U S A. 1986 Jan;83(2):437–440. doi: 10.1073/pnas.83.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendeman S., Thorley-Lawson D. A. The activation antigen BLAST-2, when shed, is an autocrine BCGF for normal and transformed B cells. EMBO J. 1987 Jun;6(6):1637–1642. doi: 10.1002/j.1460-2075.1987.tb02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshigawara K., Wang H. M., Kato K., Smith K. A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J Exp Med. 1987 Jan 1;165(1):223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Swendeman S. L., Edson C. M. Biochemical analysis suggests distinct functional roles for the BLAST-1 and BLAST-2 antigens. J Immunol. 1986 Mar 1;136(5):1745–1751. [PubMed] [Google Scholar]
- Venkatasubramanian K., Audhya T., Goldstein G. Binding of thymopoietin to the acetylcholine receptor. Proc Natl Acad Sci U S A. 1986 May;83(10):3171–3174. doi: 10.1073/pnas.83.10.3171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Otsuka T., Mosmann T., Banchereau J., DeFrance T., Blanchard D., De Vries J. E., Lee F., Arai K. Isolation and characterization of a human interleukin cDNA clone, homologous to mouse B-cell stimulatory factor 1, that expresses B-cell- and T-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5894–5898. doi: 10.1073/pnas.83.16.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukawa K., Kikutani H., Owaki H., Yamasaki K., Yokota A., Nakamura H., Barsumian E. L., Hardy R. R., Suemura M., Kishimoto T. A B cell-specific differentiation antigen, CD23, is a receptor for IgE (Fc epsilon R) on lymphocytes. J Immunol. 1987 Apr 15;138(8):2576–2580. [PubMed] [Google Scholar]