Abstract
Objective
The present study investigates the role of Src and Syk tyrosine kinases in signaling by G-protein coupled and platelet adhesion receptors.
Methods and Results
Using Syk−/− platelets or the Src kinase inhibitor PP2, we demonstrate a critical role for Src and Syk kinases in mediating lamellipodia formation on VWF, collagen, CRP, fibrinogen, and fibronectin. In all cases, the spreading defect was overcome by addition of thrombin. Conversely, platelet aggregation and αIIbβ3 activation induced by thrombin was similar to controls, arguing against a functional role for Src and Syk in αIIbβ3 activation. Unexpectedly, CRP potentiated integrin αIIbβ3 activation and platelet aggregation induced by subthreshold concentrations of thrombin in Syk−/− platelets or in the presence of the Src kinase inhibitor PP2. Potentiation in the presence of PP2 was lost in the absence of FcRγ-chain or GPVI confirming that it was mediated through the immunoglobulin receptor. Further delineation of this PP2-resistant synergy revealed that PAR4 could trigger the enhanced response in combination with CRP.
Conclusions
We show that Syk is critical for lamellipodia formation on a range of immobilized proteins but that this can be overcome by addition of thrombin. Further, we reveal a novel role for GPVI in supporting thrombin-induced activation, independent of Syk and Src kinases.
Keywords: platelets, GPVI signaling, Src kinases, Syk, thrombin
The tyrosine kinase Syk contains two SH2 domains and a C-terminal kinase domain and is expressed in cells of the hematopoietic lineage. The absence of Syk results in the abrogated development of B cells, functional defects in immune receptors, and perinatal lethality.1,2 Interestingly, mice deficient in Syk, or the Syk substrate SLP-76, also show a failure to separate emerging blood vessels from those of the emerging lymphatic system, thereby triggering embryonic hemorrhaging.3
In platelets, Syk is recognized for its role in mediating signaling of the collagen receptor complex, GPVI-FcRγ chain, by binding to the immunoreceptor tyrosine-based activation motif (ITAM) within the intracellular portion of the FcRγ chain. Signaling by this receptor complex parallels that by immune receptors, such as the T- and B-cell antigen receptors and Fc receptors. Reviewed by Watson et al,4,5 the two Src kinases Fyn and Lyn, are believed to initially phosphorylate the ITAM of the FcRγ chain. Syk then binds to the dually phosphorylated tyrosine residues via its SH2 domains, resulting in autophosphorylation of Syk and sequential activation and phosphorylation of several adapter proteins, including LAT, SLP-76, and a number of signaling proteins, including Tec kinases and PI-3 kinase. This ultimately leads to phosphorylation of phospholipase C (PLC) γ isoforms and a rise in intracellular calcium.
Syk is also implicated in signaling by other classes of adhesion and G protein–coupled receptors in platelets. For example, Syk plays a critical role in signaling by the major platelet integrin, αIIbβ3. This has been shown by a series of elegant studies in Chinese hamster ovary (CHO) cell models for integrin αIIbβ3 and biochemical analyses of human and murine platelets.6–8 Syk undergoes tyrosine phosphorylation and activation during adhesion of αIIbβ3-expressing CHO cells to the matrix protein fibrinogen, implicating Syk in the proximal signaling events of αIIbβ3.6 Phosphotyrosine-independent binding of Syk to the cytoplasmic tail of the β3 subunit of αIIbβ3 9 initiates a downstream cascade that leads to tyrosine phosphorylation of Vav1, Vav3, SLP-76, ADAP, and PLCγ2.7,8
More recently, the interaction of GPIb/V/IX with VWF in the presence of the snake venom toxin botrocetin10 and the interaction of collagen with integrin α2β 1 11 have been shown to trigger Syk autophosphorylation and kinase activity. In both cases, the interactions of Syk with GPIb/V/IX or α2β1 are implicated in mediating spreading, but definitive evidence for this is not available. Recent studies show that Syk plays a significant role in mediating signaling downstream of the novel platelet receptor CLEC-2, after platelet stimulation by the snake venom toxin, rhodocytin.12 Lastly, Syk becomes activated and phosphorylated in platelets by G protein–coupled receptors agonists, including thrombin, ADP, and TxA2.13,14 However, despite all that is recognized on the phosphorylation and activation of Syk, the functional role for Syk in signaling by many of these receptors is unclear.
The present study was designed to determine more clearly the role of Syk in signaling by G protein–coupled receptors and to extend our understanding of the contribution of Syk to signaling by platelet adhesion receptors, including integrin αIIbβ3, GPIb/V/IX, and GPVI. Using platelets obtained from radiation chimeric mice transplanted with wild-type or Syk−/− embryonic liver cells, we demonstrate a critical role for Syk in mediating spreading, but not adhesion, on a wide variety of extracellular matrix proteins, although it does not appear to play a role in supporting platelet activation by thrombin. Unexpectedly, we reveal a novel synergy between GPVI and thrombin that exists in the absence of Src kinases and Syk. Given the central role of Syk in numerous signaling cascades, it is increasingly viewed as a target for the development of novel antithrombotic therapeutics. This study furthers this notion and may suggest that Syk presents a worthwhile antithrombotic target when used concurrently with antithrombin therapies.
Materials and Methods
For detailed descriptions of the materials and methods, please see supplemental material (available online at http://atvb.ahajournals.org).
Mouse Strains
Syk−/− and wild-type radiation chimeras were generated as described. 15 Hemopoietic progenitor cells were obtained from fetal liver from wild-type B10.D2 or Syk-deficient 15 to 16 day embryos, and injected intravenously into lethally-irradiated wild-type mice. FcRγ chain−/− mice and GPVI−/− mice have been described previously.16,17
Preparation of Washed Platelets
Murine platelets were obtained and prepared as described,18 in accordance with Animals (Scientific Procedures) Act 1986, United Kingdom. Human platelets were prepared as described,19 and all donors provided informed written consent, according to the Declaration of Helsinki.
Platelet Aggregation and Stimulation Studies
Murine platelets were stimulated with thrombin or collagen related peptide (CRP), in the presence/absence of an integrin αIIbβ3 inhibitor or PP2, and aggregation determined. Platelets were lysed and tyrosine phosphorylation determined after protein separation by SDS-PAGE and Western blotting, followed by immunoblotting with an antiphosphotyrosine antibody. Human platelets were aggregated with thrombin, protease activation receptors (PAR) activation peptides (TRAP and PAR4AP), and/or CRP, in the presence/absence of vehicle, PP2 and/or ADP receptor antagonists plus the TxA2 inhibitor, indomethacin.
Platelet Spreading and Imaging
Static adhesion assays were performed as described11,20 with the additional incubation of some samples with thrombin, ADP, or PP2. Adherent platelets were fixed and imaged using differential interference contrast (DIC) microscopy.21
Measurement of Integrin αIIbβ3 Activation
Murine platelets were stimulated with thrombin, CRP, ADP, thrombin/CRP, or ADP/CRP. The PE-conjugated JON/A antibody or Oregon-green fibrinogen (OG-FGN) was added and allowed to incubate. The reaction was terminated and samples were read in FL2/FL1. Control samples containing resting platelets labeled with JON/A or PE-labeled Rat IgG were tested. Human platelets were stimulated with thrombin, CRP, or thrombin/CRP, plus/minus PP2, before addition of OG-FGN. Samples were read using a FACs machine.
Statistical Analysis
Statistical significance of results was determined using one-way ANOVA, using the Prism Software program.
Results
The Requirement for Syk in Mouse Platelet Spreading Is Bypassed by Thrombin
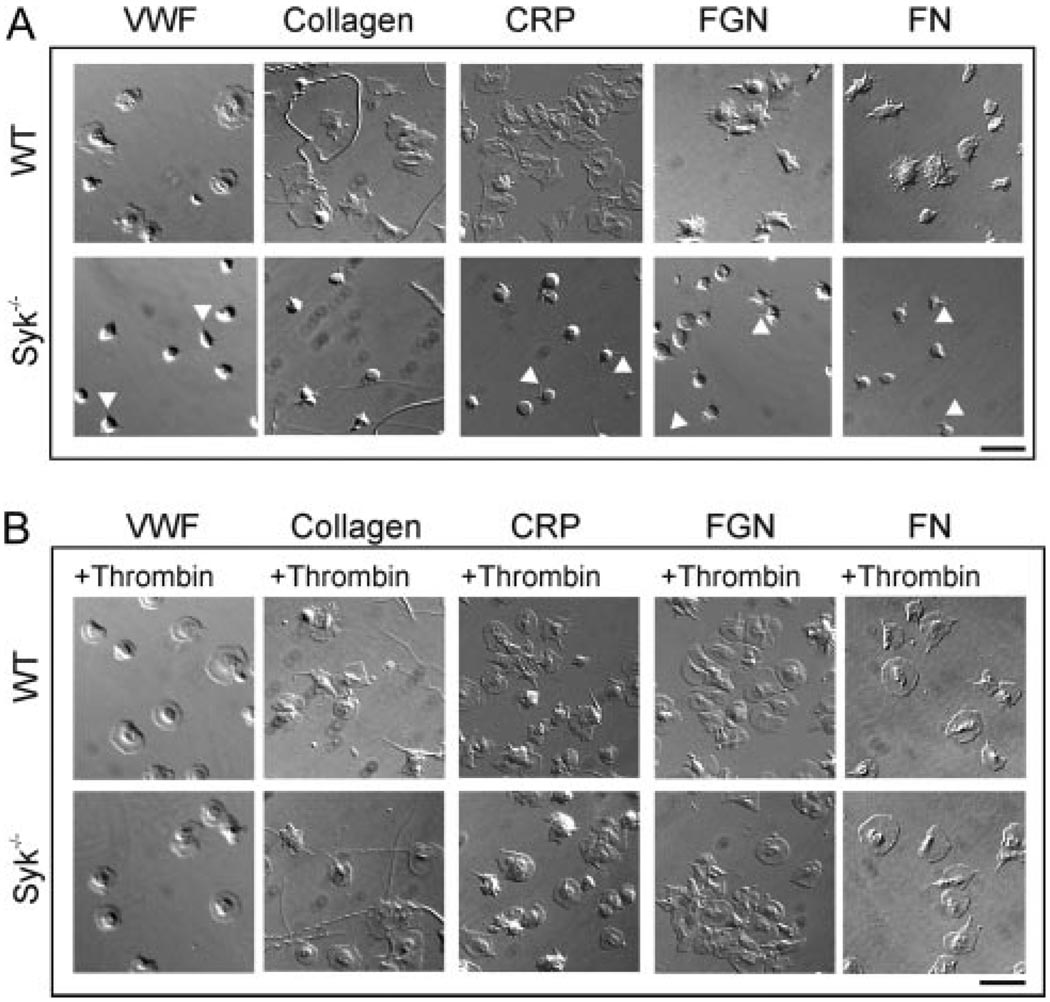
We examined the role for Syk in platelet spreading on a number of matrix proteins under static conditions both in the absence and presence of G protein receptor activation. Platelets obtained from radiation chimeric mice reconstituted with wild-type or Syk−/− fetal liver cells2 were allowed to adhere and spread on immobilized Von Willebrand Factor (VWF), collagen, CRP, fibronectin, or fibrinogen. As demonstrated in Figure 1A, wild-type platelets extend filopodia and lamellipodia when plated on all adhesive surfaces. In contrast, Syk−/− platelets were unable to generate lamellipodia on any of the matrices tested, although there was evidence of filopodia formation (Figure 1A, arrow heads). The inclusion of the G protein agonist thrombin restored maximal platelet spreading in Syk−/− murine platelets on all surfaces, as measured by the percentage of platelets with lamellipodia (Figure 1B and supplemental Table I). Spreading was also restored by ADP but to a limited extent (supplemental Table I). These data demonstrate that the defect in platelet spreading observed in the absence of Syk is overcome through activation of G protein coupled receptors.
Figure 1.
The requirement for Syk in spreading is by-passed by thrombin. Glass coverslips were coated with VWF plus botrocetin (10 µg/mL), collagen (100 µg/mL), CRP (100 µg/mL), fibrinogen (FGN; 200 µg/mL), or fibronectin (FN; 100 µg/mL). Nonimmobilized protein was removed and coverslips were blocked with BSA (5 mg/mL). Platelets from wild-type (WT) or Syk−/− mice were incubated on the coverslips for 45 minutes in the absence (A) or presence (B) of thrombin (1.0U/mL). Nonadherent platelets were removed and adherent platelets fixed then imaged using DIC microscopy. Scale bar=10 µm. Representative images are shown from 2 to 3 experiments performed in duplicate, with 5 fields taken per image.
It is widely accepted that Syk phosphorylation occurs distally to Src kinase activation in platelet activation pathways. 4,6,8 Therefore, to examine whether the compensatory mechanism triggered by G protein coupled receptor agonists occurred proximally or distally to Src kinases, we examined whether human platelet spreading on fibrinogen, VWF, and collagen in the presence of a Src kinase inhibitor (PP2) could be restored by thrombin. PP2-treatment blocked lamellipodia formation (see supplemental data) and reduced adhesion on all matrices tested. However, thrombin was able to fully compensate for blockade of Src kinases with respect to lamellipodia formation, with 96.0±1.6%, 97.2±0.6%, and 92.5±2.3% of PP2-treated platelets undergoing spreading on VWF, fibrinogen, and collagen, respectively (supplemental Table II). As observed for the Syk−/− platelets, spreading of PP2-treated platelets was also restored by ADP, but to a limited extent (supplemental Table II).
Thrombin Stimulates Tyrosine Phosphorylation Downstream of Src Kinases
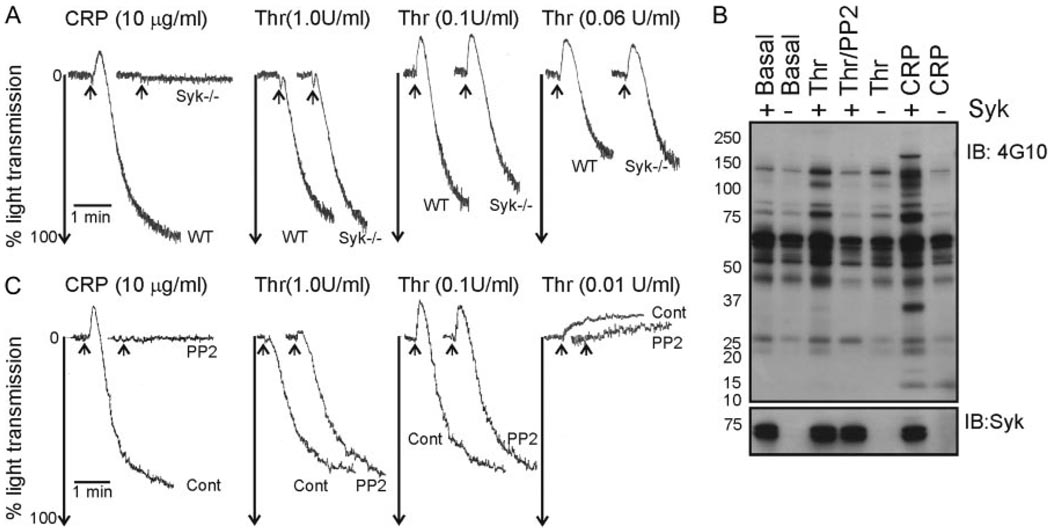
Having shown that thrombin and ADP could compensate for the absence of Syk and Src kinases in platelet spreading, we examined the underlying signaling processes. First we determined the contribution of Syk to general signaling triggered by thrombin-induced activation of platelets. There was no marked alteration in aggregation to a range of concentrations of thrombin in Syk−/− murine platelets (Figure 2A). There was, however, a partial reduction in thrombin-mediated whole cell tyrosine phosphorylation, measured under nonaggregating conditions, in the absence of Syk, in contrast to the complete abolition in the presence of the Src kinase inhibitor, PP2 (Figure 2B). These data demonstrate that although Syk contributes to thrombin-induced tyrosine phosphorylation in platelets, it is neither required for aggregation nor for thrombin-induced platelet spreading. These findings were also true for inhibition of Src kinases. We observed that PP2 had no deleterious effect on thrombin-induced aggregation of human platelets, despite inhibition of tyrosine phosphorylation (Figure 2B through 2C). This suggested that Src kinases, like Syk, contribute to thrombin-induced tyrosine phosphorylation in platelets but are not required for aggregation.
Figure 2.
Src and Syk kinases are required for optimal tyrosine phosphorylation in thrombin-stimulated platelets, but not aggregation. A and B, Platelets from wild-type (WT) or Syk-deficient (Syk −/−) mice were aggregated to thrombin (0.06 to 1.0U/mL, Thr) or CRP (10 µg/mL). The extent of aggregation was determined by change in light transmission. B, Washed platelets were treated with lotrafiban (10 µmol/L) then stirred in the absence (Basal), or presence of thrombin (1.0U/mL), thrombin/PP2 (20 µmol/L), or CRP (3 µg/mL). Stimulations were terminated and whole cell lysates were separated by SDS-PAGE, transferred and immunoblotted with antiphosphotyrosine (4G10) and anti-Syk antibodies. C, Washed human platelets were aggregated with thrombin (0.01 to 1.0U/mL, Cont), ± PP2 (10 µmol/L). Arrows mark the addition of agonist. These results are representative of at least 2 experiments using pooled murine platelets.
Potentiation of Integrin αIIbβ3 Inside-Out Signaling in Syk−/− Murine Platelets
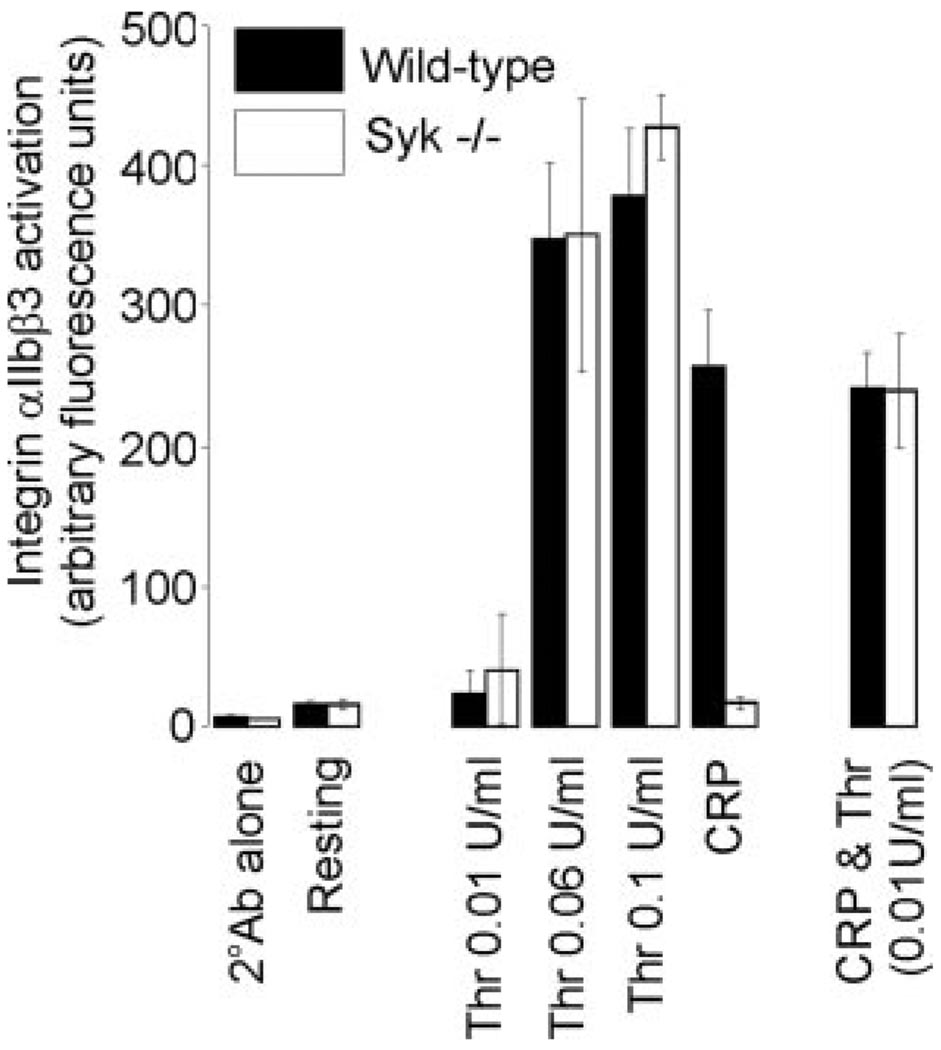
Having demonstrated that Syk is not required for thrombin-induced platelet aggregation, we chose to use a more sensitive measure of integrin αIIbβ3 activation in platelets, namely the αIIbβ3 activation-specific antibody, JON/A. Under the conditions used, JON/A binds specifically to the activated conformation of αIIbβ3.22 As shown in Figure 3, platelets stimulated with increasing thrombin concentrations showed a concomitant increase in JON/A binding, which was not altered in the absence of Syk. In contrast, the response to CRP was completely blocked in Syk−/− platelets. Strikingly, however, a concentration of thrombin (0.01 U/mL) that was unable to induce significant activation of αIIbβ3, was able to restore JON/A binding observed following CRP stimulation in Syk−/− platelets (Figure 3), thereby revealing a novel and unexpected synergy in integrin activation.
Figure 3.
CRP potentiates thrombin-induced integrin αIIbβ3 activation in Syk−/− platelets. Platelet suspensions from wild-type (black) or Syk−/− (white) mice were allowed to rest in the presence of vehicle (Resting) or the PE-labeled Rat IgG negative control (2°Ab alone). Alternatively, platelets were stimulated with thrombin (0.01 to 0.1U/mL), CRP (10 µg/mL), or CRP/thrombin (10 µg/mL and 0.01U/mL, respectively). Suspensions were incubated with JON/A antibody and samples read in FL2. Results show integrin αIIbβ3 activation, measured in arbitrary fluorescence units (mean±SEM); 3 mice per genotype.
CRP Potentiates Thrombin Aggregation of Human Platelets Through Src Kinase-Dependent and Independent Pathways
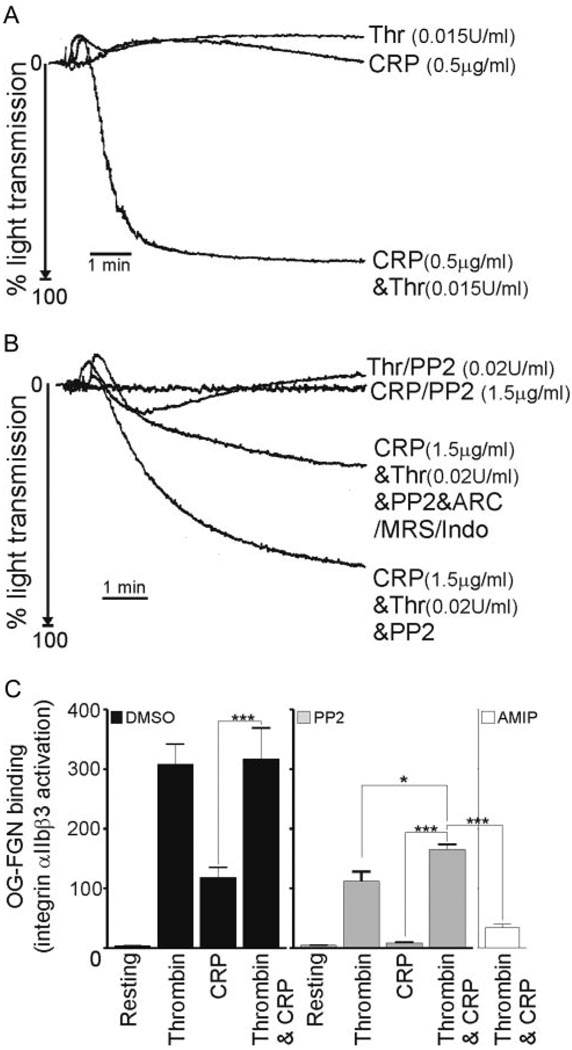
To determine the dependency of the observed synergy between GPVI and thrombin on Src kinases, we first examined the ability of control human platelets to undergo aggregation to subthreshold concentrations of CRP and thrombin, either alone or in combination. As demonstrated in Figure 4A, we observed a significant level of synergy between CRP and thrombin under these assay conditions. When we treated platelets with PP2, the observed synergy remained, although to a lesser extent (Figure 4B), demonstrating a novel Src-kinase independent synergy between CRP and thrombin.
Figure 4.
Novel synergy between CRP and thrombin involves Src kinases and secondary mediators. A and B, Human platelets were aggregated with thrombin (0.015 to 0.02U/mL), CRP (0.5 to 1.5 µg/mL), thrombin/CRP in combination, in the absence or presence of PP2 (10 µmol/L) and/or ARC66931MX (10 µmol/L), MRS2176 (100 µmol/L), and indomethacin (10 µmol/L). Platelet aggregation was plotted as the maximal extent of aggregation. C, Washed human platelets were treated with DMSO (black bars), PP2 (gray bars), or ARC69931MX, MRS2176, Indomethacin, and PP2 (AMIP) (white bars) before stimulation with thrombin (0.04U/mL), CRP (2.0 µg/mL), or CRP/thrombin (2.0 µg/mL and 0.04U/mL, respectively). Platelets bound OG-FGN for 5 minutes before samples were read using FL1. Results are arbitrary units (mean±SEM), n=3; *P<0.05; ***P<0.001.
The contribution of secondary mediators, ADP and thromboxane A2 (TxA2), to the PP2-independent synergy was investigated. PP2-treated platelets were incubated with two ADP receptor antagonists, ARC69931MX and MRS2179, in combination with indomethacin to inhibit Thromboxane A2 (TxA2), before aggregations with subthreshold concentrations of thrombin and CRP, alone or in combination. A combination of the inhibitors caused a marked reduction in aggregation to thrombin and CRP from 61.8±13.7% (+PP2) to 43.4±5.5% (+PP2/ARC/MRS/Indo; Figure 4B). The partial nature of the inhibition indicates the existence of an ADP/TxA2- and Src kinase-independent signaling process occurring in response to thrombin/CRP.
To extend these observations, we examined Oregon green-fibrinogen (OG-FGN) binding by flow cytometry in human platelets. OG-FGN was used in these studies instead of JON/A as a marker for integrin inside-out activation, as the latter does not recognize human αIIbβ3. We confirmed that OG-FGN binding to activated integrin αIIbβ3 was specific, as it was blocked by the integrin inhibitor, 7E3 (data not shown). The results demonstrated that in control platelets, thrombin and CRP in combination produced no additional effect on integrin inside-out activation leading to OG-FGN binding (Figure 4C). Conversely, in the presence of PP2, the combined effect of thrombin and CRP on OG-FGN binding was synergistic, when compared with the levels triggered by either thrombin/PP2 (P<0.05) or CRP/PP2 (P<0.001) alone. The synergism observed in the presence of PP2 was completely lost in the presence of ADP receptor antagonists and indomethacin (P<0.001), thereby revealing a critical role for secondary mediators in this response. It should be noted that we observed a reduction in thrombin-induced OG-FGN binding in the presence of PP2 (Figure 4C), which was not apparent when aggregation was measured turbidometrically (Figure 4B). This inhibition may reflect the use of a very low concentration of thrombin and the absence of integrin αIIbβ3 outside-in signaling.
CRP and Thrombin Potentiate Integrin αIIbβ3 Activation Through GPVI
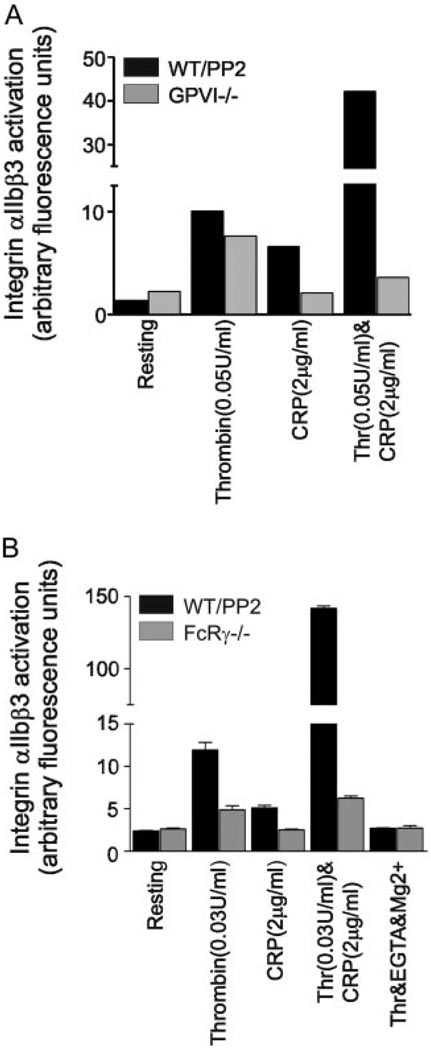
To confirm that the PP2-independent synergy was mediated through CRP acting on GPVI, we investigated responses to CRP and thrombin in mice deficient in GPVI17 or the FcRγ chain.16 Fluorescence-activated-cell sorter analysis of OG-FGN (Figure 5) binding of age and/or litter-matched murine platelets treated with PP2 demonstrated synergistic activation of integrin αIIbβ3 by CRP and thrombin (Figure 5A and 5B). Importantly, this Src-kinase independent synergy was completely lost in the GPVI−/− (Figure 5A) and FcRγ chain−/− (Figure 5B) platelets, thereby confirming that the action of CRP is mediated through GPVI. We also confirmed that OG-FGN binding to activated integrin αIIbβ3 was specific, as it was blocked by the presence of EGTA/Mg2+ (Figure 5B).
Figure 5.
CRP potentiates thrombin-induced integrin αIIbβ3 activation through GPVI. Platelet suspensions from wild-type mice were treated with PP2 (10 µmol/L, black), and compared with murine suspensions of (A) GPVI−/− or (B) FcRγ chain−/− platelets (gray). Platelets were allowed to rest in the presence of vehicle (Resting), or were stimulated with thrombin (0.03 or 0.05U/mL), CRP (2 µg/mL), or CRP/thrombin (2 µg/mL and 0.03 or 0.05U/mL, respectively). Some samples were treated with EGTA/Mg2+ and stimulated with thrombin (0.04 U/mL) to block integrin activation. Suspensions were incubated with OG-FGN and samples read in FL1. Results show integrin αIIbβ3 activation, measured in arbitrary fluorescence units. A, Data are from single data points from one experiment, representative of 2 to 3 experiments (WT=3, GPVI−/−=2). B, Data are pooled from triplicate samples from 3 individual experiments (mean ± SEM).
PAR4 Synergizes With CRP to Promote Integrin αIIbβ3 Activation in Human Platelets Independent of Src Kinases
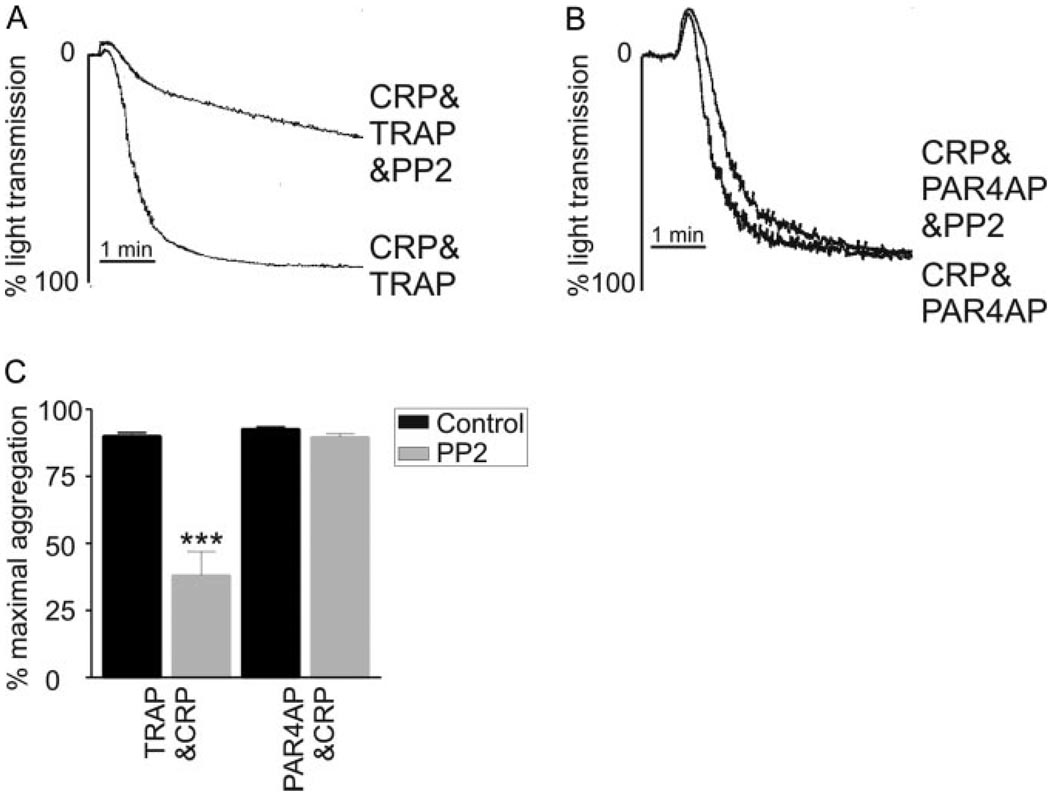
To examine the potential mechanism behind the thrombin-induced synergy with CRP, we determined the involvement of two main thrombin receptors, protease activated receptor (PAR)1 and PAR4, in this response in human platelets. Using specific PAR1 and PAR4 agonist peptides, SLLRN (TRAP) and AYPGKF (PAR4AP), respectively, we first determined that PAR1 and PAR4 could reproducibly synergize with CRP to promote maximal platelet aggregation (data not shown). Interestingly, when platelets were treated with PP2, the synergistic response evoked by subthreshold PAR4AP/CRP addition could be maintained (Figure 6B and 6C); however, the aggregation response to subthreshold TRAP/CRP was significantly reduced (Figure 6A and 6C). These data therefore suggested that PAR4, and not PAR1, is important for mediating the Src kinase-independent signaling by GPVI in combination with thrombin.
Figure 6.
PAR4, but not PAR1, synergizes with GPVI completely independently of Src kinases. A and B, washed human platelets were aggregated to subthreshold concentrations of PAR1 activation peptide, (A) TRAP (0.75 µmol/L), or (B) PAR4 activation peptide, AYPGKF (37.5 µmol/L), in combination with subthreshold CRP (0.15 µg/mL), in the presence of PP2 (10 µmol/L) for 5 minutes. Aggregation was determined as the change in light transmission and shown in the histograms (mean±SEM) (black bars=control, gray bars=PP2) (C). Representative aggregation traces are shown; ***P<0.001; n=5.
Discussion
The studies presented here provide further insight into the role of Syk and Src tyrosine kinases in platelet signaling cascades emanating from the adhesion receptors GPVI, GPIb/V/IX, and integrin αIIbβ3. Specifically, the data confirm and extend previous observations8 for an important role for Syk in mediating lamellipodia formation on fibrinogen and collagen. In addition, we have now characterized a role for Syk in lamellipodia formation on VWF, CRP, and fibronectin, and have shown that the spreading defect can be overcome through the addition of G protein receptor agonists, thrombin, and, to a lesser extent, ADP. Significantly, these studies also reveal that the GPVI-agonist CRP potentiates signaling by G protein–coupled receptors, notably thrombin through PAR4, independent of Syk and Src kinases. This novel finding was evident in studies measuring inside-out activation of αIIbβ3 and in platelet aggregation studies. Moreover, there appears to be a critical contribution of ADP and TxA2 to this synergy.
A major finding from the present study is the observation that subthreshold concentrations of thrombin synergize with CRP in promoting integrin αIIbβ3 activation in the absence of Syk and Src kinases. The observed synergy between thrombin and collagen is not unique, as a number of other studies have made similar findings23,24; however, our study is the first to show a synergistic effect in the absence of Syk or Src kinases. Further studies on GPVI and FcR γ-chain–deficient platelets demonstrate that the Src kinase- and Syk-independent signals are mediated downstream of the collagen receptor GPVI. Moreover, it would appear that secondary mediators, ADP and TxA2, play a significant role in this event.
Further delineation of the synergy suggested that PAR4, one of two main thrombin receptors in human and murine platelets, mediates the effect independent of Src kinases. Although it is recognized that PAR1 and PAR4 both contribute to thrombin signaling in human platelets, they have been shown to elicit their effects through different temporal profiles linked to calcium release and receptor internalization.25 It would therefore appear that the characteristics defining PAR4 signaling, notably slower internalization and a prolonged calcium release,25 synergize with GPVI signaling to promote integrin activation. This finding is consistent with Graff et al,23 who established that PAR4 acts synergistically with collagen to promote CD62 and Pac-1 expression,23 in a manner that depended on integrin αIIbβ3, the P2Y12 ADP receptor and TxA2. In addition, Keuren et al24 claimed that PAR1 and collagen could synergize to promote procoagulant platelet activity. Taken together, these data suggest that both PARs can participate in synergistic signaling with collagen. Our data has extended this by first demonstrating that GPVI alone can synergize with thrombin, as compared with studies using collagen which activates platelet receptors additional to GPVI. Second, we suggest that PAR4 and GPVI synergize in vitro, independently of Src kinases, which may affect thrombosis growth in vivo.
One possible mechanism for the Src kinase- and Syk-independent signaling downstream of GPVI and thrombin relates to the recognized interactions GPVI has with other signaling and adapter molecules, most notably calmodulin. Calmodulin is a calcium-binding protein that is involved in a wide variety of cellular processes, including cellular growth, movement, and proliferation (reviewed26). Recent studies in platelets have shown that calmodulin interacts with the GPIb/V/IX complex27 and a basic region within the cytoplasmic tail of GPVI,28–30 and dissociates from the latter following collagen-, CRP-, but not ionophore-mediated platelet activation.28 In addition, calmodulin dissociation from GPVI follows GPVI-mediated tyrosine phosphorylation and is blocked by the Src kinase inhibitor, PP1,28 suggesting that there is an activation-dependent mechanism of dissociation. Although the role for calmodulin in platelets remain unclear at this stage, mostly because of the contradictory findings within the literature concerning the use of “selective” calmodulin inhibitors, there is some evidence suggesting a role for the protein in platelet aggregation,31–33 T cell aggregation, 34 and protection of GPVI from proteolytic cleavage.35 It therefore remains an interesting possibility, that in the studies presented herein, CRP binding to GPVI may trigger calmodulin activation, which when combined with thrombin signaling, may trigger platelet aggregation. This therefore establishes a possible means for GPVI to potentiate signaling by thrombin, in the absence of critical components of the GPVI-FcRγ chain pathway.
The present findings have important implications for the clinical management of thrombosis. Specifically, these studies support recent findings by Mangin et al,36 who have observed that thrombin plays an important role in overcoming the hemostatic and thrombotic defect linked to a deficiency in the GPVI/FcRγ signaling cascade. Importantly, these studies raise the interesting scenario that complete prevention of thrombosis will require administration of both anti-GPVI agents and antithrombin agents (anticoagulants).36 This notion is supported by in vivo studies that have highlighted that the relative importance of signaling proteins in in vivo thrombosis models varies with a function of lesion severity.37 Nonne et al37 show that the major PLC isoform which signals downstream of GPVI receptor engagement, PLCγ2, plays an important role in platelet thrombosis under conditions of superficial injury, whereas inhibitors of thrombin significantly compromise thrombosis after severe injury.37 As such, we could predict that: first, concurrent anti-GPVI/antithrombin therapy would prevent thrombosis in vivo after both superficial and severe injury; second, both the compensation elicited by thrombin in the absence of Syk and Src kinases, and the synergistic signaling cascade triggered by the combination of sub-threshold GPVI agonists with thrombin-(PAR4), both described in this article, would be blocked under these therapeutic conditions.
In summary, this study further dissects the critical role of Syk in platelet activation downstream of adhesion and G protein–coupled platelet receptors. Specifically we show that platelet spreading on a variety of adhesion molecules requires Src and Syk kinases, yet their deficit can be overcome by G protein–coupled receptor agonists. In addition, we demonstrated that thrombin stimulates tyrosine phosphorylation partly through Syk and entirely downstream of Src kinases, yet neither kinase is critical for thrombin-induced platelet aggregation. Lastly and most interestingly, our studies reveal the existence of Syk- and Src kinase-independent signals generated by the GPVI in combination with thrombin(PAR4), which raises interesting issues for the clinical management of thrombosis.
Supplementary Material
Acknowledgments
The authors thank Prof Shaun Jackson (ACBD, Monash University, Australia) for valuable discussions.
Sources of Funding
S.C.H. holds a National Health and Medical Research Council (Aust.) CJ Martin Fellowship. S.P.W. holds a British Heart Foundation Chair. This work was supported by the Wellcome Trust. V.L.J.T. and E.S. were funded by the Medical Research Council, and V.L.J.T. has received a Seminar Honoraria. J.W. is funded by the National Institute of Health (HL50545). O.J.T.M. is currently funded by the American Heart Association Beginning Grant-in-Aid.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/static/html/reprints.html
Disclosures
None.
References
- 1.Cheng AM, Rowley B, Pao W, Hayday A, Bolen JB, Pawson T. Syk tyrosine kinase required for mouse viability and B-cell development. Nature. 1995;378:303–306. doi: 10.1038/378303a0. [DOI] [PubMed] [Google Scholar]
- 2.Turner M, Mee PJ, Costello PS, Williams O, Price AA, Duddy LP, Furlong MT, Geahlen RL, Tybulewicz VL. Perinatal lethality and blocked B-cell development in mice lacking the tyrosine kinase Syk. Nature. 1995;378:298–302. doi: 10.1038/378298a0. [DOI] [PubMed] [Google Scholar]
- 3.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson SP, Asazuma N, Atkinson B, Berlanga O, Best D, Bobe R, Jarvis G, Marshall S, Snell D, Stafford M, Tulasne D, Wilde J, Wonerow P, Frampton J. The role of ITAM- and ITIM-coupled receptors in platelet activation by collagen. Thromb Haemost. 2001;86:276–288. [PubMed] [Google Scholar]
- 5.Watson SP, Auger JM, McCarty OJT, Pearce AC. GPVI and integrin αIIbβ3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 6.Gao J, Zoller KE, Ginsberg MH, Brugge JS, Shattil SJ. Regulation of the pp72syk protein tyrosine kinase by platelet integrin alpha IIb beta 3. EMBO J. 1997;16:6414–6425. doi: 10.1093/emboj/16.21.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obergfell A, Judd BA, del Pozo MA, Schwartz MA, Koretzky GA, Shattil SJ. The molecular adapter SLP-76 relays signals from platelet integrin alphaIIbbeta3 to the actin cytoskeleton. J Biol Chem. 2001;276:5916–5923. doi: 10.1074/jbc.M010639200. [DOI] [PubMed] [Google Scholar]
- 8.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, Lowell CA, Shattil SJ. Coordinate interactions of Csk, Src, and Syk kinases with [alpha]IIb[beta]3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–275. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodside DG, Obergfell A, Talapatra A, Calderwood DA, Shattil SJ, Ginsberg MH. The N-terminal SH2 Domains of Syk and ZAP-70 Mediate Phosphotyrosine-independent Binding to Integrin beta Cytoplasmic Domains. J Biol Chem. 2002;277:39401–39408. doi: 10.1074/jbc.M207657200. [DOI] [PubMed] [Google Scholar]
- 10.Asazuma N, Ozaki Y, Satoh K, Yatomi Y, Handa M, Fujimura Y, Miura S, Kume S. Glycoprotein Ib-von Willebrand factor interactions activate tyrosine kinases in human platelets. Blood. 1997;90:4789–4798. [PubMed] [Google Scholar]
- 11.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SP, Schoenwaelder SM, Yuan Y, Salem HH, Cooray P. Non-receptor protein tyrosine kinases and phosphatases in human platelets. Thromb Haemost. 1996;76:640–650. [PubMed] [Google Scholar]
- 14.Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72syk by platelet agonists and the integrin alpha IIb beta 3. J Biol Chem. 1994;269:28859–28864. [PubMed] [Google Scholar]
- 15.Mocsai A, Zhou M, Meng F, Tybulewicz VL, Lowell CA. Syk is required for integrin signaling in neutrophils. Immunity. 2002;16:547–558. doi: 10.1016/s1074-7613(02)00303-5. [DOI] [PubMed] [Google Scholar]
- 16.Takai T, Li M, Sylvestre D, Clynes R, Ravetch JV. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 1994;76:519–529. doi: 10.1016/0092-8674(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 17.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein VI to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 2003;102:3592–3599. doi: 10.1182/blood-2003-04-1142. [DOI] [PubMed] [Google Scholar]
- 19.Goncalves I, Nesbitt WS, Yuan Y, Jackson SP. Importance of temporal flow gradients and integrin alphaIIbbeta3 mechanotransduction for shear activation of platelets. J Biol Chem. 2005;280:15430–15437. doi: 10.1074/jbc.M410235200. [DOI] [PubMed] [Google Scholar]
- 20.Yuan Y, Kulkarni S, Ulsemer P, Cranmer SL, Yap CL, Nesbitt WS, Harper I, Mistry N, Dopheide SM, Hughan SC, Williamson D, de la Salle C, Salem HH, Lanza F, Jackson SP. The von Willebrand factor-glyco-protein Ib/V/IX interaction induces actin polymerization and cytoskeletal reorganization in rolling platelets and glycoprotein Ib/V/IX-transfected cells. J Biol Chem. 1999;274:36241–36251. doi: 10.1074/jbc.274.51.36241. [DOI] [PubMed] [Google Scholar]
- 21.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergmeier W, Schulte V, Brockhoff G, Bier U, Zirngibl H, Nieswandt B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry. 2002;48:80–86. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- 23.Graff J, Klinkhardt U, Harder S. Pharmacodynamic profile of antiplatelet agents: marked differences between single versus costimulation with platelet activators. Thromb Res. 2004;113:295–302. doi: 10.1016/j.thromres.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Keuren JF, Wielders SJ, Ulrichts H, Hackeng T, Heemskerk JW, Deckmyn H, Bevers EM, Lindhout T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler Thromb Vasc Biol. 2005;25:1499–1505. doi: 10.1161/01.ATV.0000167526.31611.f6. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro MJ, Weiss EJ, Faruqi TR, Coughlin SR. Protease-activated receptors 1 and 4 are shut off with distinct kinetics after activation by thrombin. J Biol Chem. 2000;275:25216–25221. doi: 10.1074/jbc.M004589200. [DOI] [PubMed] [Google Scholar]
- 26.Chin D, Means AR. Calmodulin: a prototypical calcium sensor. Trends Cell Biol. 2000;10:322–328. doi: 10.1016/s0962-8924(00)01800-6. [DOI] [PubMed] [Google Scholar]
- 27.Andrews RK, Munday AD, Mitchell CA, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of the platelet membrane glycoprotein Ib-IX-V complex. Blood. 2001;98:681–687. doi: 10.1182/blood.v98.3.681. [DOI] [PubMed] [Google Scholar]
- 28.Andrews RK, Suzuki-Inoue K, Shen Y, Tulasne D, Watson SP, Berndt MC. Interaction of calmodulin with the cytoplasmic domain of platelet glycoprotein VI. Blood. 2002;99:4219–4221. doi: 10.1182/blood-2001-11-0008. [DOI] [PubMed] [Google Scholar]
- 29.Bori-Sanz T, Inoue KS, Berndt MC, Watson SP, Tulasne D. Delineation of the region in the glycoprotein VI tail required for association with the Fc receptor gamma-chain. J Biol Chem. 2003;278:35914–35922. doi: 10.1074/jbc.M301826200. [DOI] [PubMed] [Google Scholar]
- 30.Locke D, Liu C, Peng X, Chen H, Kahn ML. Fc Rgamma -independent signaling by the platelet collagen receptor glycoprotein VI. J Biol Chem. 2003;278:15441–15448. doi: 10.1074/jbc.M212338200. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthi S, Kakkar VV. Studies on the effect of platelet inhibitors on platelet adhesion to collagen and collagen-induced human platelet activation. Thromb Haemost. 1985;53:337–342. [PubMed] [Google Scholar]
- 32.Nishikawa M, Hidaka H. Role of calmodulin in platelet aggregation. Structure-activity relationship of calmodulin antagonists. J Clin Invest. 1982;69:1348–1355. doi: 10.1172/JCI110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishikawa M, Tanaka T, Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287:863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 34.Fagerholm SC, Prescott A, Cohen P, Gahmberg CG. An essential role for calmodulin in regulating human T cell aggregation. FEBS Lett. 2001;491:131–136. doi: 10.1016/s0014-5793(01)02182-2. [DOI] [PubMed] [Google Scholar]
- 35.Gardiner EE, Arthur JF, Kahn ML, Berndt MC, Andrews RK. Regulation of platelet membrane levels of glycoprotein VI by a platelet-derived metalloproteinase. Blood. 2004;104:3611–3617. doi: 10.1182/blood-2004-04-1549. [DOI] [PubMed] [Google Scholar]
- 36.Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, Schoenwaelder SM, Wright CE, Lanza F, Jackson SP. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcR{gamma} deficiency. Blood. 2006;107:4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 37.Nonne C, Lenain N, Hechler B, Mangin P, Cazenave JP, Gachet C, Lanza F. Importance of platelet phospholipase Cgamma2 signaling in arterial thrombosis as a function of lesion severity. Arterioscler Thromb Vasc Biol. 2005;25:1293–1298. doi: 10.1161/01.ATV.0000163184.02484.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.