Abstract
Extensive research during last two decades has revealed the mechanism by which continued oxidative stress can lead to chronic inflammation, which in turn could mediate most chronic diseases including cancer, diabetes, cardiovascular, neurological and pulmonary diseases. Oxidative stress can activate a variety of transcription factors including NF-κB, AP-1, p53, HIF-1α, PPAR-γ, β-catenin/Wnt, and Nrf2. Activation of these transcription factors can lead to the expression of over 500 different genes, including those for growth factors, inflammatory cytokines, chemokines, cell cycle regulatory molecules, and anti-inflammatory molecules. How oxidative stress activates inflammatory pathways leading to transformation of a normal cell to tumor cell, tumor cell survival, proliferation, chemoresistance, radioresistance, invasion, angiogenesis and stem cell survival is the focus of this review. Overall, observations to date suggest that oxidative stress, chronic inflammation, and cancer are closely linked.
Keywords: Oxidative stress, Inflammation, Cancer, Pro-oxidants, Anti-oxidants, NF-κB
1. Introduction
Oxidative stress is defined as an imbalance between production of free radicals and reactive metabolites, so-called oxidants or reactive oxygen species (ROS), and their elimination by protective mechanisms, referred to as antioxidants. This imbalance leads to damage of important biomolecules and cells, with potential impact on the whole organism [1]. ROS are products of a normal cellular metabolism and play vital roles in stimulation of signaling pathways in plant and animal cells in response to changes of intra- and extracellular environmental conditions [2]. Most ROS are generated in cells by the mitochondrial respiratory chain [3]. During endogenous metabolic reactions, aerobic cells produce ROS such as superoxide anion (O2-), hydrogen peroxide (H2O2), hydroxyl radical (OH•), and organic peroxides as normal products of the biological reduction of molecular oxygen [4]. The electron transfer to molecular oxygen occurs at the level of the respiratory chain, and the electron transport chains are located in membranes of the mitochondria [5, 6]. Under hypoxic conditions, the mitochondrial respiratory chain also produces nitric oxide (NO), which can generate other reactive nitrogen species (RNS) [3]. RNS can further generate other reactive species, e.g., reactive aldehydes-malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), by inducing excessive lipid peroxidation [7]. Proteins and lipids are also significant targets for oxidative attack, and modification of these molecules can increase the risk of mutagenesis [8].
Under a sustained environmental stress, ROS are produced over a long time, and thus significant damage may occur to cell structure and functions and may induce somatic mutations and neoplastic transformation [9, 10]. Indeed, cancer initiation and progression has been linked to oxidative stress by increasing DNA mutations or inducing DNA damage, genome instability, and cell proliferation [11].
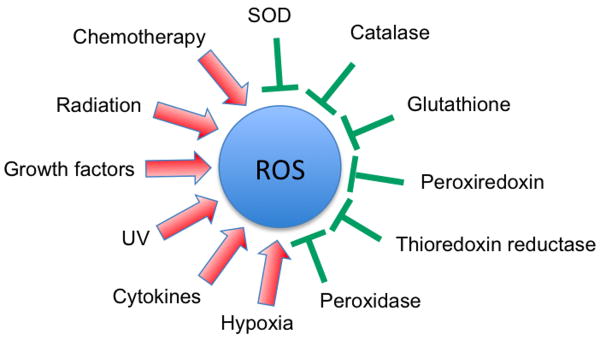
The skin, for example, is chronically exposed to both endogenous and environmental pro-oxidants due to its interface function between the body and the environment, and to protect the skin against this overload of oxidant species, it needs a well-organized system of both chemical and enzymatic antioxidants [12]. The lungs, which are directly exposed to oxygen concentrations higher than in most other tissues, are protected against these oxidants by a variety of antioxidant mechanisms [13]. Furthermore, aging, which is considered as an impairment of body functions over time, caused by the accumulation of molecular damage in DNA, proteins and lipids, is also characterized by an increase in intracellular oxidative stress due to the progressive decrease of the intracellular ROS scavenging [14]. Acting to protect the organism against these harmful pro-oxidants is a complex system of enzymatic antioxidants [e.g., superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase, catalase] and nonenzymatic antioxidants [e.g., glutathione (GSH), vitamins C and D] [15] (Figure 1).
Figure 1. Schematic representation of various activators and inhibitors of reactive oxygen species production.
ROS are involved in a wide spectrum of diseases, including chronic inflammation (Table 1), and in a wide variety of different cancers (Table 2).
Table 1. A partial list of diseases that have been linked to reactive oxygen species.
| Disease | Reference |
|---|---|
| Acute Respiratory Distress Syndrome | [16] |
| Aging | [17] |
| Alzheimer | [18, 19] |
| Atherosclerosis | [20] |
| Cancer | [21-23] |
| Cardiovascular Disease | [24, 25] |
| Diabetes | [26] |
| Inflammation | [27] |
| Inflammatory Joint Disease | [28] |
| Neurological Disease | [29] |
| Obesity | [30, 31] |
| Parkinson | [32, 33] |
| Pulmonary fibrosis | [34, 35] |
| Rheumatoid arthritis | [36] |
| Vascular Disease | [37, 38] |
Table 2. A partial list of cancers that have been linked to reactive oxygen species.
| Cancer | Reference |
|---|---|
| Bladder Cancer | [39] |
| Brain Tumor | [40] |
| Breast Cancer | [41] |
| Cervical Cancer | [42] |
| Gastric (Stomach) Cancer | [43] |
| Liver Cancer | [44] |
| Lung Cancer | [45] |
| Melanoma | [46] |
| Multiple Myeloma | [47] |
| Leukemia | [48] |
| Lymphoma | [49] |
| Oral Cancer | [50] |
| Ovarian Cancer | [51] |
| Pancreatic Cancer | [52] |
| Prostate Cancer | [10] |
| Sarcoma | [53] |
Chronic inflammation is induced by biological, chemical, and physical factors and is in turn associated with an increased risk of several human cancers [54]. The link between inflammation and cancer has been suggested by epidemiological and experimental data [55, 56] and confirmed by anti-inflammatory therapies that show efficacy in cancer prevention and treatment [57]. The fact that continuous irritation over long periods of time can lead to cancer had already been described in the traditional Ayurvedic (meaning, the science of long life) medical system, written as far back as 5000 years ago [58]. Whether this irritation is the same as what Rudolf Virchow referred to as inflammation in the nineteenth century is uncertain [59]. Virchow first noted that inflammatory cells are present within tumors and that tumors arise at sites of chronic inflammation [60]. This inflammation is now regarded as a “secret killer” for diseases such as cancer. For example, inflammatory bowel diseases such as Crohn's disease and ulcerative colitis are associated with increased risk of colon adenocarcinoma [61-63], and chronic pancreatitis is related to an increased rate of pancreatic cancer [64].
The exact mechanisms by which a wound-healing process turns into cancer are topics of intense research [57, 65], and possible mechanisms include induction of genomic instability, alterations in epigenetic events and subsequent inappropriate gene expression, enhanced proliferation of initiated cells, resistance to apoptosis, aggressive tumor neo-vascularization, invasion through tumor-associated basement membrane, and metastasis [66]. How oxidative stress modulates these different stages of inflammation-induced carcinogenesis is the focus of this review.
2. Inflammatory network
The sources of inflammation are widespread and include microbial and viral infections, exposure to allergens, radiation and toxic chemicals, autoimmune and chronic diseases, obesity, consumption of alcohol, tobacco use, and a high-calorie diet [60, 67]. In general, the longer the inflammation persists, the higher the risk of cancer. Two stages of inflammation exist, acute and chronic inflammation. Acute inflammation is an initial stage of inflammation (innate immunity), which is mediated through the activation of the immune system. This type of inflammation persists only for a short time and is usually beneficial for the host. If the inflammation lasts for a longer period of time, the second stage of inflammation, or chronic inflammation, sets in and may predispose the host to various chronic illnesses, including cancer [68]. During inflammation, mast cells and leukocytes are recruited to the site of damage, which leads to a ‘respiratory burst’ due to an increased uptake of oxygen, and thus, an increased release and accumulation of ROS at the site of damage [7, 65].
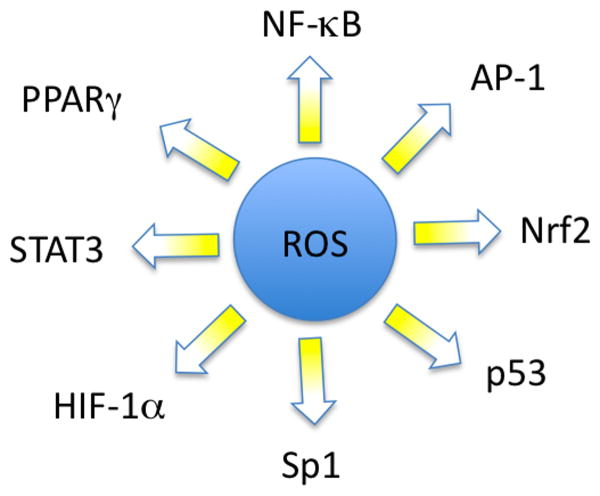
On the other hand, inflammatory cells also produce soluble mediators, such as metabolites of arachidonic acid, cytokines and chemokines, which act by further recruiting inflammatory cells to the site of damage and producing more reactive species. These key mediators can activate signal transduction cascades as well as induce changes in transcription factors, such as nuclear factor kappa B (NF-κB), signal transducer and activator of transcription 3 (STAT3), hypoxia-inducible factor-1α (HIF1-α), activator protein-1 (AP-1), nuclear factor of activated T cells (NFAT) and NF-E2 related factor-2 (Nrf2), which mediate immediate cellular stress responses (Figure 2). Induction of cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS), aberrant expression of inflammatory cytokines [tumor necrosis factor (TNF), interleukin-1 (IL-1), IL-6 and chemokines [IL-8; CXC chemokine receptor 4 (CXCR4)], as well as alterations in the expression of specific microRNAs, have also been reported to play a role in oxidative stress-induced inflammation [69]. This sustained inflammatory/oxidative environment leads to a vicious circle, which can damage healthy neighboring epithelial and stromal cells and over a long period of time may lead to carcinogenesis [70].
Figure 2. Schematic representation of various transcription factors that are modulated by reactive oxygen species.
As an example, mutations in the rat sarcoma viral oncogene (RAS) induce an inflammatory response. RAS, which is mutated in approximately 25% of all malignancies [71], promotes cell proliferation, tumor growth, and angiogenesis of malignant cells. During inflammatory stimuli, Ras induces the expression of various inflammatory gene products, including the pro-inflammatory cytokines IL-1, IL-6 and IL-11 and the chemokine IL-8 [72].
3. Pro-oxidant network
Following an inflammatory stimulus, initiation of carcinogenesis mediated by ROS may be direct (oxidation, nitration, halogenation of nuclear DNA, RNA, and lipids), or mediated by the signaling pathways activated by ROS. With the help of the mitochondrial respiratory chain, aerobic organisms are able to attain a far greater energy production efficiency compared with anaerobic organisms. However, one disadvantage of aerobic respiration is continuous electron leakage to O2 during mitochondrial ATP synthesis. In fact, 1–5% of total oxygen consumed in aerobic metabolism gives rise to the superoxide anion (O2-), an example of ROS. To protect against this free radical, the main enzyme for its degradation, the manganese-superoxide dismutase (Mn-SOD), dismutates it into H2O2 and water [73].
H2O2, another example of ROS, may be formed either by dismutation from superoxide anion or spontaneously in peroxisomes from molecular oxygen [74-76]. Despite its lesser reactivity compared with other ROS, H2O2 plays however an important role in carcinogenesis because it is capable of diffusing throughout the mitochondria and across cell membranes and producing many types of cellular injury [74, 75]. The main injurious effects of ROS in mammalian cells are however mediated by the hydroxyl radical (·OH). It has a very unstable electron structure and is therefore unable to diffuse more than one or two molecular diameters before it reacts in practice with any cellular component [76, 77]. The majority of ·OH in vivo is produced in the presence of reduced transition metals (ions of Fe, Cu, Co, or Ni), mainly via the Fenton reaction when Fe2+ contacts H2O2. The ·OH-derived DNA damage includes the generation of 8-hydroxyguanosine (8-OHG), the hydrolysis product of which is 8-hydroxydeoxyguanosine (8-OHdG). 8-OHdG is the most widely used fingerprint of radical attack towards DNA [77, 78]. 8-OHdG has been strongly implicated in carcinogenesis progression. For example, in breast carcinomas, 8-OHdG has been reported to be increased 8- to 17-fold in breast primary tumors compared with nonmalignant breast tissue [79-81].
NO·, another free radical implicated in carcinogenesis, is a short-lived free radical generated from L-arginine [82], that is effective against pathogens. The major part of NO· is synthesized by iNOS, usually after challenge by immunological or inflammatory stimuli [82, 83]. NO is synthesized from -arginine by the enzyme nitric oxide synthase (NOS). The constitutive (calcium-dependent) isoforms, neuronal NOS (nNOS or bNOS) and endothelial NOS (eNOS), produce small amounts of NO which act as a neurotransmittor and vasodilator, respectively [84]. The inducible (calcium-independent) isoform (iNOS) produces much larger amounts of NO and is only expressed during inflammation. Whereas iNOS can produce injurious amounts of RNS (check), eNOS and nNOS produce beneficial amounts under physiological conditions [85]. iNOS is induced by cytokines such as γ-interferon (γ-IFN), TNF-α, IL-1, and lipopolysaccharide (LPS). LPS activation induces the translocatation of NF-κB, from the cytoplasm to the nucleus, where it interacts with κB elements in the NOS2 (iNOS) 5′ flanking region, triggering NOS2 transcription [86].
Defective autophagy of old mitochondria (mitophagy) can also be a major source of ROS [87]. These ROS produced by damaged mitochondria, can promote tumor development, likely by perturbing the signal transduction adaptor function of p62-controlling pathways [88].
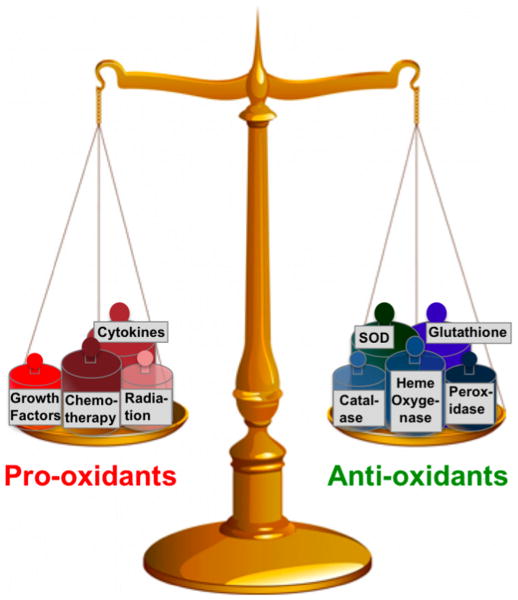
To control the balance between production and removal of ROS (Figure 3), a variety of DNA repair enzymes exist, although antioxidants are more specific and efficient in protecting cells from radicals. This antioxidant system includes both endogenous and exogenous and enzymatic and non-enzymatic antioxidants. Glutathione (GSH), is a tripeptide and the major endogenous antioxidant produced by the cells, which helps to protect cells from ROS such as free radicals and peroxides [89]. It is now well established that ROS and electrophilic chemicals can damage DNA, and that GSH can protect against this type of damage [90]. GSH can also directly detoxify carcinogens through phase II metabolism and subsequent export of these chemicals from the cell. On the other hand, elevated GSH levels are observed in various types of cancerous cells and solid tumors, and this tends to make these cells and tissues more resistant to chemotherapy [91-93].
Figure 3. Model of a balance between pro-oxidants and anti-oxidants.
Under normal conditions, anti-oxidants outbalance pro-oxidants, but under oxidative conditions, pro-oxidants prevail over anti-oxidants, which can lead to many inflammatory diseases including cancer.
SODs were the first characterized antioxidant enzymes [94]. Three different types of SOD are expressed in human cells, copper-zinc SOD (Cu-ZnSOD), Mn-SOD, and extracellular-SOD (EC-SOD), all of which are able to dismutate two O2·- anions to H2O2 and molecular oxygen. Catalase is then responsible for detoxification of H2O2 to water. GPx are another group of enzymes capable of reducing hydroperoxides, including lipid hydroperoxides, using GSH as substrate. The oxidized form of glutathione disulfide (GSSG) is again reduced by the specific enzyme glutathione reductase. Peroxiredoxins (Prx) were first described 20 years ago and as in catalase and GPx, the main function of peroxiredoxins is to reduce alkyl hydroperoxides and H2O2 to the corresponding alcohol or water.
Direct effects of ROS, generally attributed to high concentrations at the site of damage, include DNA strand breaks, point mutations, aberrant DNA cross-linking, and mutations in proto-oncogenes and tumor-suppressor genes, thus promoting neoplastic transformation [7, 95]. For example, ROS can reduce the expression and enzymatic activity of the DNA mismatch repair genes mutS homologue 2 and 6 and can increase the expression of DNA methyltransferases, leading to a global hypermethylation of the genome [60]. This leads to promoter silencing of several genes, such as adenomatous polyposis coli (APC), cyclin-dependent kinase inhibitor-2 (CDKN-2), breast cancer susceptibility gene 1 (BRCA1), retinoblastoma protein (Rb), and murine double minute 2 (MDM2), and the DNA mismatch repair gene, human mutL homolog 1 (hMLH1) [96, 97].
On the other hand, low or transient levels of ROS can activate cellular proliferation or survival signaling pathways, such as the NF-κB, AP1, extracellular signal-regulated kinase/mitogen-activated protein kinase (ERK/MAPK), and phosphoinositide 3- kinase/AKT8 virus oncogene cellular homolog (PI3K/Akt) pathways (Table 3).
Table 3. A partial list of signaling pathways linked to reactive oxygen species.
| Signaling intermediate | Reference |
|---|---|
| AHR | [98] |
| AP-1 | [99, 100] |
| ATM | [101] |
| cAMP | [102] |
| cAMP-dependent PKA | [103] |
| CDK5 | [104] |
| Chemokine | [70] |
| c-myc | [99] |
| CREB | [103] |
| Cyclins and Cell Cycle Regulation | [105] |
| Cytokine Network | [66] |
| DNA Methylation | [106] |
| DNA Repair Mechanism | [107] |
| EGF | [108] |
| eNOS | [109] |
| ERK | [110] |
| Fas | [111] |
| FOXO | [112] |
| HIF-1α | [113] |
| HO-1 | [114] |
| IL-10 | [115] |
| iNOS | [109] |
| Integrin | [116] |
| Interferon | [117] |
| JAK/STAT | [118] |
| JNK | [119] |
| MAPK | [110] |
| Mismatch Repair | [120] |
| mTor | [121] |
| NAD(P)H quinone oxidoreductase 1 | [122] |
| NF-κB | [123] |
| Nfr2 | [124] |
| PI3K/Akt | [125] |
| p38 | [126] |
| p53 | [127, 128] |
| PKC | [129] |
| PPARγ | [130] |
| PTEN | [131] |
| PTPs/PTKs | [132] |
| Sp1 | [133] |
| TNF | [5] |
| VEGF | [134] |
| WNT | [135, 136] |
For example, H2O2 is able to degrade IκBα, the inhibitory subunit of NF-κB [137]. Protein kinase C, which participates in a variety of pathways regulating transcription and cell cycle control, is also activated by H2O2 [137]. In addition, ROS induces both the activation and synthesis of AP-1, a regulator of cell growth, proliferation, and apoptosis [138, 139] and transcription factors such as STAT3, HIF-1α, and p53 [118, 140, 141].
4a. Cellular transformation
Chronic inflammation has been linked to various steps involved in carcinogenesis, including cellular transformation, promotion, survival, proliferation, invasion, angiogenesis, and metastasis [65, 142]. How oxidative stress is involved in these various steps is discussed in the following sections.
Cancer is a multistage process defined by at least three stages: initiation, promotion, and progression [143-145]. Oxidative stress interacts with all three stages of this process. During the initiation stage, ROS may produce DNA damage by introducing gene mutations and structural alterations of the DNA. In the promotion stage, ROS can contribute to abnormal gene expression, blockage of cell- to cell communication, and modification of second messenger systems, thus resulting in an increase of cell proliferation or a decrease in apoptosis of the initiated cell population. Finally, oxidative stress may also participate in the progression stage of the cancer process by adding further DNA alterations to the initiated cell population [146].
In recent years, considerable evidence has demonstrated that ROS are involved in the link between chronic inflammation and cancer [147-149]. Indeed, an important characteristic of tumor promoters is their ability to recruit inflammatory cells and to stimulate them to generate ROS [150, 151]. Tumor promotion, for example, can be inhibited in animal models by the use of agents, including certain antioxidants as well as steroids and retinoids, that can inhibit the phagocyte respiratory burst [148, 150]. Moreover, increased levels of oxidatively modified DNA bases (such as thymidine glycol, 5-hydroxymethyl-2′-deoxyuridine and 8-OHdG) have been induced in the skin of mice by topical phorbol 12-myristate 13- acetate (PMA) exposure [152]. 8-OHdG has also been identified in the epidermis of nude mice exposed to near-UV [153]. In addition, genetic damage and neoplastic transformation have been demonstrated in cells co-cultured in vitro with activated phagocytes [149] and the genotoxic effects observed include formation of DNA strand breaks [151], sister chromatid exchange [154] and mutations [155]. Furthermore, the DNA base modifications observed are characteristic of an attack by reactive oxygen species OH. [156]. Inflammatory cells may also increase DNA damage by activating procarcinogens to DNA-damaging species, for example neutrophils can activate aromatic amines, aflatoxins, estrogens, phenols, and polycyclic aromatic hydrocarbons by ROS-dependent mechanisms [148, 157]. On the other hand, both neutrophils and macrophages have themselves been shown to release large quantities of superoxide, hydrogen peroxide, and hydroxyl radical following activation of their redox metabolism [158].
In fact, initial experiments on the role of ROS in tumor initiation have assumed that oxidative stress acts as a DNA-damaging agent, effectively increasing the mutation rate within cells and thus promoting oncogenic transformation [159]. However, more recent studies have revealed that in addition to inducing genomic instability, ROS can specifically activate certain signaling pathways and thus contribute to tumor development through the regulation of cellular proliferation, angiogenesis, and metastasis [160]. For example, nitrosative stress has been shown to play a critical role in inflammation-associated carcinogenesis by activating AP-1, a representative redox-sensitive transcription factor [161], which is involved in cell transformation and proliferation [139, 162].
4b. Tumor cell survival
One of the key characteristics of tumor cells is their increased ability to survive compared with normal cells. ROS are reported to be tumorigenic by virtue of their ability to increase cell proliferation, survival, and cellular migration. ROS can induce DNA damage, leading to genetic lesions that initiate tumorigenicity and subsequent tumor progression. On the other hand, ROS can also induce cellular senescence and cell death and can therefore function as anti-tumorigenic agents. Whether ROS promote tumor cell survival or act as anti-tumorigenic agents depends on the cell and tissues, the location of ROS production, and the concentration of individual ROS.
ROS has been reported to play a major role in tumor initiation and survival induced by a variety of agents both in animal models and humans [158, 163, 164] by mediating cellular signal transduction pathways. These signaling pathways are involved in the transmission of inter or intracellular information and are critical for supporting tumor cell survival and establishing cell fate. The reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) family of enzymes, one of the potential sources of ROS production, has been reported to promote tumor cell survival and growth [165]. For example, Nox4 and Nox5 promote tumor cell survival in pancreatic and lung cancers, respectively [165]. The serine-threonine kinase Akt has been reported to down-regulate antioxidant defenses and promote tumor cell survival [166]. ROS has also been reported to activate Akt by inhibiting phosphatase and tensin homolog deleted from chromosome 10 (PTEN), the phosphatase counteracting PI3K-dependent Akt activation [167]. Akt may foster tumorigenesis by multiple means [168, 169], for example, by stabilizing cellular avian myeloblastosis virus oncogene (c-Myc) and cyclin D1 or by inducing degradation of the cyclin-dependent kinase (Cdk) inhibitor, p27 kinase inhibitor protein (p27Kip1). Akt is also a profound inhibitor of apoptosis due to its ability to inactivate pro-apoptotic molecules, including caspase-9 and the Bcl-2 homology3 (BH3)-only protein Bcl-XL/Bcl-2-associated death promoter (Bad), and by triggering the activity of the transcription factor NF-κB. In addition, Akt promotes nuclear translocation of the ubiquitin ligase MDM2, which counteracts p53-mediated apoptosis. An important aspect of Akt's promotion of cell survival involves alterations in cellular energy metabolism [168, 169]. Thus, by preventing apoptosis and increasing oxidative metabolism, Akt lies at the hub of complex signaling networks that integrate a multitude of potentially oncogenic signals.
4c. Tumor cell proliferation
Uncontrolled tumor cell proliferation requires the upregulation of multiple intracellular signaling pathways including cascades involved in survival, proliferation, and cell cycle progression. The most significant effects of oxidants on signaling pathways have been observed in the mitogen-activated protein (MAP) kinase/AP-1 and NF-κB pathways [170]. The induction of redox-sensitive pathways during tumor cell proliferation is necessary since cell division presents tremendous energy requirements and the production of metabolites from energy-generating reactions must be buffered to prevent oxidative damage and ultimately cell death [171].
Of the MAP kinase family, which modulates gene expression through phosphorylation of a wide array of transcription factors, the ERK pathway is the most commonly linked with the regulation of cell proliferation. Activation of the ERK, c-Jun N-terminal kinase (JNK), and p38 subfamilies has been observed in response to changes in the cellular redox balance [172]. The induction of AP-1 by H2O2, cytokines, and other stressors, for example, is mediated mainly by JNK and p38 MAP kinase cascades [173]. Once activated, JNK proteins translocate to the nucleus and phosphorylate c-Jun and activating transcription factor-2 (ATF-2), enhancing transcriptional activities [174, 175]. H2O2 can activate MAP kinases and thereby AP-1 in several manners.
Redox status has also been shown to have an impact on NF-κB regulation. NF-κB regulates several genes involved in cell transformation, proliferation, and angiogenesis [176]. Carcinogens and tumor promoters including UV radiation, phorbol esters, asbestos, alcohol, and benzo(a)pyrene are among the external stimuli that activate NF-κB [177, 178]. Expression of NF-κB has been shown to promote cell proliferation, whereas inhibition of NF-κB activation blocks cell proliferation [179]. Additionally, tumor cells from blood neoplasms, and cell lines from different cancers, including colon, breast, pancreas, and squamous cell carcinoma, have all been reported to constitutively express activated NF-κB [180]. The mechanism for activation of NF-κB by ROS is not clear, and the relationship between NF-κB and ROS is complex [123]. Although mild oxidative stress can lead to modest NF-κB activation, extensive oxidative stress can inhibit NF-κB [123]. Furthermore, NF-κB can protect cells from oxidative stress through induction of the ferritin heavy chain and SOD2 genes, which are both regulated by NF-κB [181, 182]. On the other hand, ROS are believed to be implicated as second messengers involved in activation of NF-κB via TNF and IL-1 [183] and indeed, suppression of TNF and IL-1 were shown to downregulate the expression of active NF-κB and inhibit proliferation of lymphoma and myelogenous leukemia cells [184]. The importance of ROS on NF-κB activation is further supported by studies demonstrating that activation of NF-κB by nearly all stimuli can be blocked by antioxidants, such as L-cysteine, N-acetylcysteine (NAC), thiols, green tea polyphenols, and vitamin E [185, 186], although this might be not very specific because antioxidants have multiple targets [187]. Likewise, NF-κB activity was increased in cells that overexpressed SOD and decreased in cells overexpressing catalase [188].
Kinases, such as protein kinase C (PKC) can also be activated by H2O2 and redox cycling quinones [189, 190]. Similarly, H2O2 leads to the activation of protein kinase B/Akt (PKB/Akt), which is associated with heat shock protein 27 (Hsp27) [191].
That ROS such as H2O2 and superoxide anion induce mitogenesis and cell proliferation has now been demonstrated in several mammalian cell types [192]; and a reduction in cellular oxidants via supplementation with antioxidants such as superoxide dismutase, catalase, β-carotene, and flavonoids inhibits cell proliferation in vitro [193]. However, paradoxically high concentrations of ROS can trigger apoptotic or necrotic cell death [194-196].
4d. Tumor cell invasion
Oxygen radicals may augment tumor invasion and metastasis by increasing the rates of cell migration. During transformation into invasive carcinoma, epithelial cells undergo profound alterations in morphology and adhesive mode, resulting in a loss of normal epithelial polarization and differentiation, and a switch to a more motile, invasive phenotype. For example, treatment of mammalian carcinoma cells with hydrogen peroxide prior to intravenous injection into mice enhances lung metastasis formation, indicating that an important function for ROS is the seeding of metastatic tumor cells [197]. This might be due to a decreased attachment of tumor cells to the basal lamina, or alternatively be due to the increased activity or expression of proteins that regulate cellular motility. For instance, oxidative stress regulates the expression of intercellular adhesion protein-1 (ICAM-1), a cell surface protein in endothelial and epithelial cells, most likely due to the activation of NF-κB. ICAM-1 together with IL-8 regulates the transendothelial migration of neutrophils and has a potential function in tumor metastasis [198].
On the other hand, it is believed that the matrix metalloproteinases (MMPs) play the central role, and their increased expression reportedly is associated with the invasion and metastasis of malignant tumors of different histogenetic origins [199]. For example, Mori et al. found that MMP-13, MMP-3, and MMP-10 were remarkably upregulated by the oxidant directly, and their activities were critically implicated in the invasive potential induced in NMuMG cells in the reconstituted model [200]. Another subgroup of MMPs, gelatinases (MMP-2 and -9), which are key enzymes for degrading type IV collagen and are thought to play a critical role in tumor invasion and metastasis [199], were also found to be activated post-transcriptionally by prolonged oxidative treatment. These effector molecules activated under prolonged oxidative stress relate chronic inflammation to malignant transformation, in particular to the invasive potential of cells, at least at a molecular level.
MMPs are capable of cleaving most components of the basement membrane and extracellular matrix [201]. The activation of MMPs, such as MMP-2, probably occurs by the reaction of ROS with thiol groups in the protease catalytic domain [202]. In additional to their role as key regulators of MMP activation, ROS have been implicated in MMP gene expression [203]. Both hydrogen peroxide and nitric oxide donors, as well as the increased expression of iNOS, stimulate the expression of several MMPs (MMP-1, MMP-3, MMP-9, MMP-10, MMP-13) [203]. In fibroblastic cells, the sustained production of H2O2 recently was shown to activate MMP-2 and to increase cell invasion [204]. Oxidative stress may also modulate MMP expression by activation of the rat sarcoma viral oncogene (RAS), or direct activation of the MAPK family members extracellular-signal regulated kinase 1/2 (ERK1/2), p38, and JNK, or inactivation of phosphatases that regulate these proteins [160].
In addition, several studies have reported the involvement of chemokines and chemokine receptors in the invasion and metastasis of different types of tumors [205-208]. The metastatic potential of chemokines is attributed to their ability to induce the expression of MMPs, which facilitate tumor invasion [208, 209]. Moreover, silencing of endogenous CXCR4 gene expression by CXCR4-shRNA inhibited the proliferation, adhesion, chemotaxis and invasion of mucoepidermoid carcinoma cells [210]. In addition, recent data point to a role for the small guanosine triphosphatase Rac1 (GTPase Rac1) in motility and invasion of tumor cells in vitro by altering cell-cell and cell-matrix adhesion. For example, Rac1 activity induces ROS production in endothelial cells. These ROS can mediate Rac1-induced loss of cell-cell adhesion in primary human endothelial cells and thus might loosen the integrity of the endothelium [211].
It is becoming clear that a number of steps in the metastatic cascade, such as invasion, intravasation and extravasation are regulated by redox signaling [212]. One such redox signalling molecule is the electrophilic cyclopentenone prostaglandin 15d-PGJ2 (15-deoxy-12,14 -prostaglandin J2), an inflammatory molecule [213], that can affect redox signalling through the post-translational modification of critical cysteine residues in proteins, such as actin, vimentin and tubulin [214, 215]. The fact that 15d-PGJ2 can alter the cytoskeleton [212], may coincides with decreased migration and increased focal-adhesion disassembly, that might have important implications in the inhibition of metastatic processes such as invasion, intravasation and extravasation. These results suggest a role for redox signalling pathways, rather than direct cytoskeletal disruption, in the mechanism of 15d-PGJ2 in cancer cells.
Finally, Cheng et al demonstrated that ROS enhance the transendothelial migration (TEM) of melanoma cells during intravasation, and that this mechanism could potentially be triggered by ultraviolet radiation through the increased expression of thioredoxin interacting protein (Txnip) and inhibition of thioredoxin (Trx) [216].
4e. Tumor cell angiogenesis
Solid tumors induce an angiogenic response by the host blood vessels to form a new vascular network for the supply of nutrients and oxygen [217]. This neovascular response is partly responsible for tumor growth and metastatic spread [218, 219]. Angiogenesis in tumors is controlled by the so-called ‘angiogenic switch,’ which allows the transition from low invasive and poorly vascularized tumors to highly invasive and angiogenic tumors. To further increase in size, tumor cells express a set of molecules that initiate tumor vascularization.
A number of cellular stress factors, including hypoxia, nutrient deprivation, and ROS, are important stimuli of angiogenic signaling [220]. In addition, overexpression of Ras has been linked to vascularization of tumors [221]. Indeed, transformation by Ras stabilizes HIF-1α and upregulates the transcription of vascular endothelial growth factor-A (VEGF-A). Moreover, chemical antioxidants inhibit the mitogenic activity of Ras, indicating that ROS participate directly in malignant transformation. Finally, ROS stabilize HIF-1α protein and induce production of angiogenic factors by tumor cells [222].
The HIF system plays a significant role in angiogenesis, and the molecular mechanisms of its regulation have recently been characterized. In addition, HIF-independent mechanisms that involve a number of other molecules and transcription factors such as NF-κB and p53 have been described. p53 may interact with the HIF system but may also have direct effects on angiogenesis regulators or interfere with translation mechanisms of angiogenesis factors
One other major factor in angiogenesis is vascular endothelial growth factor (VEGF), which is produced by the cells to stimulate the growth of new blood vessels. VEGF induces angiogenesis by stimulating endothelial cell proliferation and migration primarily through the receptor tyrosine kinase VEGF receptor2, fetal liver kinase 1/ kinase insert domain receptor (Flk1/KDR). VEGF binding initiates tyrosine phosphorylation of KDR, which results in activation of downstream signaling enzymes including ERK1/2, Akt and endothelial nitric oxide synthase (eNOS), which contribute to angiogenic-related responses in endothelial cells [134]. A number of oncogenes and tumor-suppressor genes that are normally associated with cell transformation [(RAS, c-Myc, murine sarcoma 3611 oncogene (RAF), human epidermal growth factor receptor-2 (HER-2/neu), c-Jun, and steroid receptor coactivator (SRC)] regulate angiogenesis through upregulation of VEGF or downregulation of thrombospondin-1 (TSP-1), an angiogenesis suppressor [223, 224]. Furthermore, mutated p53 upregulates VEGF and in contrast, wild-type p53 decreases VEGF production and increases TSP-1 [225]. Angiogenic factors such as VEGF, fibroblast growth factor (FGF) and platelet-derived growth factor (PDGF) are released into the tumor microenvironment by tumor or inflammatory cells in response to various stimuli, such as ROS [226]. The released growth factors activate endothelial cells that give rise to new blood vessels [227, 228].
Monte et al. have demonstrated that lymphocyte-induced angiogenesis is triggered by ROS stimulation, and that this response can be blocked by the administration of a free radical scavenger to tumor bearing mice [229] [230]. In addition, the administration of H2O2 or an oxidative stress-producing drug (doxorubicin) to normal mice activated in vivo angiogenesis [229].
Due to reduced physiological tissue oxygen tension (hypoxia), which occurs during tumor initiation, tumors often become hypoxic. Under hypoxic conditions, cells activate signaling pathways, which regulate proliferation, angiogenesis, and death. Cancer cells have adapted to these pathways, effectively allowing tumors to survive and even grow under adverse hypoxic conditions [160]. This adaptation of tumor cells to hypoxia contributes to the malignant phenotype and to aggressive tumor progression [231], and low oxygen tension in tumors is associated with increased metastasis and poor survival of patients with several forms of squamous tumor [232, 233]. HIF-1α responds to these changes by specifically decreasing the oxygen (or hypoxia) level, and upregulating several genes to promote survival in low-oxygen conditions and thus promoting angiogenesis.
In conclusion, although previous sections indicate that all different sub-stages of tumor development are affected by ROS and inflammation, early stages of cancer development (e.g. cellular transformation), involving DNA damage, are however most affected by ROS generated inflammation. For example, colitis may develop into colon cancer after inflammatory infiltration, increased production of ROS, impairment of antioxidant defenses, DNA damage, and genetic and epigenetic alterations, resulting in the transformation of epithelial cells [234]. Or, bronchitis, which can lead to lung cancer, clearly links pro-oxidants, generated by cigarette smoke, to inflammation of the bronchus, and eventually transformation of lung cells into lung cancer [235]. Similarly pancreatitis and esophagitis, both induced by tobacco and alcohol, may transform normal tissue into pancreatic or esophageal cancer if the antioxidant system is not sufficiently effective [236, 237].
4f. Chemoresistance
Despite many decades of research, the mechanisms underlying chemoresistance are still poorly understood. There is growing evidence that the inflammatory tumor microenvironment modulates not only cancer development but also cancer responsiveness and resistance to conventional anticancer therapies [238]. Experimental studies have led to the identification of various cancer cell-intrinsic resistance mechanisms, e.g., activation and/or overexpression of drug transporter proteins (e.g., P-glycoprotein), altered expression of detoxifying enzymes (e.g., glutathione S-transferase) or resistance to apoptosis/senescence pathways [239-242].
For example, an inflammatory response induces changes in expression and activity of multidrug-resistance (MDR)-associated protein transporters, greatly affecting drug responses [243, 244]. It has been shown that acute inflammation suppresses the drug transporter P-glycoprotein (PGP) in the liver, whereas it activates PGP in kidneys, resulting in changes in the pharmacokinetics of the PGP substrate doxorubicin [245]. Likewise, expression of multidrug resistance-associated protein 1 (MRP1) is elevated in inflamed intestine of patients with Crohn's disease or ulcerative colitis [246]. Thus, enhanced states of inflammation influence proteins that are strongly linked with drug resistance.
In addition to the effects caused by inflammation, several chemotherapeutic agents have also been shown to activate the transcription factor NF-κB in human lung and cervical cancers and in T cells [247-249]. These agents are paclitaxel, vinblastine, vincristine, doxorubicin, daunomycin, 5-fluorouracil, cisplatin, and tamoxifen. Activation of NF-κB by these agents has been linked in turn with chemoresistance through serine phosphorylation of inhibitor of κBα (IκBα) [250, 251]. Various in vitro studies have supported a link between NF-κB activation, cytokine production and chemoresistance. One pathway via which NF-κB can be activated is the Toll-like receptor (TLR) pathway. TLRs generally signal via the adapter protein myeloid differentiation primary response gene 88 (MyD88) leading to activation of NF-κB and production of pro-inflammatory cytokines. Activation of TLR signaling in ovarian cancer cell lines by exogenously added LPS resulted in an activated NF-κB pathway, which promoted secretion of proinflammatory cytokines and subsequently conferred resistance to paclitaxel [252, 253]. Also, TNF receptor signaling promotes NF-κB activation and has been linked with chemoresistance. For example, exposure of breast cancer cells to exogenously added TNFα results in selection for breast cancer cells that overexpress NF-κB, leading to increased cancer cell survival and resistance to ionizing radiation [254]. At the same time, cytokines produced by stromal cells in the tumor microenvironment (e.g., IL-1 or TNFα) could potentially activate the NF-κB pathway in cancer cells and thus contribute to chemoresistance. These data call for functional in vivo studies to elucidate the involvement of the inflammatory tumor microenvironment in NF-κB-dependent chemoresistance.
Another mechanism that might be involved in chemoresistance is increased levels of GSH in cancer cells [92]. In particular, the overexpression of glutathione S-transferases (GST), the enzymes that catalyse the conjugation of reduced glutathione to electrophilic [255], as well as efflux pumps, may reduce the reactivity of various anticancer drugs [256]. The increase of the GST levels occurs by transcriptional activation mediated by the nuclear factor-erythroid 2 p45-related factor 2 (Nrf2) [257]. Indeed, using genetic manipulation, Lau et al. have demonstrated a strong positive correlation between Nrf2 levels and resistance of three cancer cell lines to chemotherapeutic drugs such as cisplatin, doxorubicin, and etoposide [258]. Chemical activation of Nrf2 by pretreatment with tertiary-butylhydroquinone (tBHQ) also increased survival of neuroblastoma cells in response to the three drugs tested [259]. Consistent with these findings, the role of Nrf2 in determining efficacy of cisplatin was also demonstrated in ovarian cancer cells using siRNA knockdown of Nrf2 [260]. Moreover, many kelch-like ECH-associated protein 1 (Keap1) mutations or loss of heterozygosity in the Keap1 locus have been identified in lung cancer cell lines or cancer tissues [261, 262]. Keap1 mutations or loss of heterozygosity resulted in inactivation of Keap1 or a reduced expression of Keap1, which upregulated the protein level of Nrf2 and transactivation of its downstream genes [261, 262]. Similar to Nrf2, the protective effect of heme oxygenase-1 (HMOX-1, or HO-1) in normal cells may protect from oxidative stress-related diseases. However, such an effect is undesirable in cancer because it provides a selective advantage for cancer cells to survive. Consistent with this notion, HMOX-1 has been found to be overexpressed in various tumor types. It is believed that overexpression of HMOX-1 facilitates cancer cell growth and survival in many ways, such as stimulating rapid growth of cancer cells, enhancing cancer cell resistance to stress and apoptosis, promoting angiogenesis of tumors, and aiding in metastasis of tumors [263]. In addition to HMOX-1, other Nrf2-downstream genes such as Prx1, GPx, and thioredoxin reductase (TrxR) were also upregulated in many cancer cells or tissues and may contribute to chemoresistance [264-266]. In ovarian cancer, constitutive activation of ERK activity has been associated with high tumorigenicity and chemoresistance [267, 268]. In addition, functional analyses employing knockdown of MKP3, a member of the subfamily of protein tyrosine phosphatases known as dual-specificity phosphatases (MKPs) [269, 270], and ectopic overexpression revealed the role of MKP3 in negatively regulating ERK1/2 activity and inhibiting tumorigenicity and chemoresistance in vitro and in vivo. MKP3 is capable of dephosphorylating ERK1/2 by protein-protein interactions via mitogen-activated protein kinase interaction motif within the N-terminal ERK1/2-binding domain [271].
4g. Radioresistance
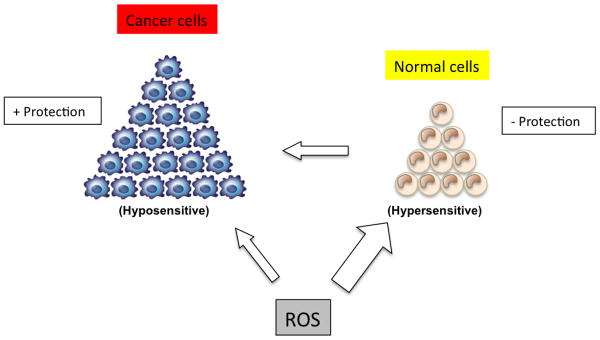
Acquired tumor radioresistance can be induced during radiotherapy owing to tumor repopulation [272]. Although tumor radioresistance stands as a fundamental barrier limiting the effectiveness of radiation therapy, the exact molecular mechanisms underlying the radioadaptive response are largely unknown (Figure 4). Olivieri et al. [273] first described an adaptive response of human lymphocytes to ionizing radiation. Since then, a substantial number of reports have made a strong case for the existence of cellular radioprotective mechanisms that can be activated in response to a small dose of ionizing radiation. It is assumed that a specific pro-survival signaling network is induced in irradiated mammalian cells.
Figure 4. Model of the sensitivity of normal cells versus cancer cells to reactive oxygen species.
Normal cells are hypersensitive to ROS if not adequately protected by anti-oxidant mechanisms, which may lead to cancer formation. Cancer cells, on the other hand, have upregulated antioxidant mechanisms (glutathione, SOD, catalase, and others) that will protect them against ROS, as can be observed in, for example, the case of radioresistance.
The elevated basal NF-κB activity in certain cancers has been linked with tumor resistance to chemotherapy and radiation [274]. NF-κB in adaptive radioresistance is evidenced in mouse epidermal cells [275] and human keratinocytes, and inhibition of NF-κB blocks the adaptive radioresistance [275]. Human breast cancer cells treated with fractional γ-irradiation show an enhanced clonogenic survival and NF-κB activation [276, 277]. Blocking NF-κB inhibited the adaptive radioresistance. These results provide the first evidence that activation of NF-κB is required for signaling the radio-adaptive resistance by exposure to radiation. Together with the assumption that NF-κB is able to regulate more than 150 effector genes, these results suggest that NF-κB plays a key role in tumor radioadaptive resistance under fractional ionizing radiation. Furthermore, in a study [278] that immunocytochemically examined the levels of activated NF-κB protein in pretreatment cancer specimens and in resected specimens of patients with chemoradiotherapy resistance, the cancers expressed higher levels of cytoplasmic NF-κB than did the adjacent nonmalignant mucosa. Furthermore, Sandur et al. suggest that transient inducible NF-κB activation provides a prosurvival response to radiation that may account for the development of radioresistance [279].
On the other hand, hypoxia is a principal signature of the tumor microenvironment and is considered to be the most important cause of clinical radioresistance and local treatment failure. The response of cells to ionizing radiation is strongly dependent upon oxygen, which is traditionally explained by the “oxygen fixation hypothesis” [280]. Oxygen is so far the best radiosensitizer. De Ridder et al. demonstrated that iNOS, activated by pro-inflammatory cytokines, can radiosensitize tumor cells through endogenous production of NO [280]. They further observed that this radiosensitizing effect is transcriptionally controlled by hypoxia and by NF-κB. Consistently, NF-κB inhibition has been used as an approach to radiosensitize tumor cells, aiming at stimulating apoptosis and inhibiting DNA repair. Moreover, the inflammatory mediators TNFα and NO have been repeatedly used as targets to radiosensitize tumor cells [281-285].
4h. Stem cell survival
Cancer stem cells (CSCs) are cancer cells that have the ability to generate tumors through the processes of self-renewal and differentiation into multiple cells. Such cells persist in tumors as a distinct population and cause relapse and metastasis by giving rise to new tumors. The existence of CSCs may have several implications in cancer treatment, including disease identification, selection of drug targets, prevention of metastasis, and development of new intervention strategies.
The first conclusive evidence for CSCs was published in 1997 [286], and to date CSCs have been isolated from both leukemias and a variety of solid tumors, including breast, brain, pancreatic, prostrate, ovary, and colon cancers [287-293]. The pathways that regulate self-renewal of CSCs include wint (Wnt), Notch, Hedgehog, and tumor-suppressor genes such as PTEN and TP53 (tumor protein 53) [294]. Although redox balance plays an important role in the maintenance of stem cell self-renewal and in differentiation, redox status in CSCs has yet to be explored. However, given the similarity between normal stem cells and CSCs and the fact that redox status plays an important role in cancer cell development, it is tempting to speculate that redox status may have a role in CSC survival. A recent study by Diehn et al. demonstrated that, similar to normal stem cells, subsets of CSCs in human and murine breast tumors have lower ROS levels than do the corresponding non-tumorigenic cells [295]. The group further showed that lower levels of ROS were associated with increased free radical scavenging systems and that pharmacologic depletion of these scavengers significantly decreased clonogenicity and resulted in radiosensitization of CSCs. Additionally, two studies showed that CD133+ CSCs conferred chemoresistance to cisplatin and doxorubicin (known ROS generators) in ovarian cancer cells [296] and hepatocellular carcinoma [297], respectively. These studies further indicate that redox status may be important in maintaining CSC survival.
4i. Stromal cell signaling
Cancer progression must involve both genetic and behavioral changes in cancer cells, and these changes are in part driven by the cancer-associated stromal cells and tumor microenvironment [298, 299]. The stromal component of the normal prostate epithelium, for example, consists of smooth muscle, fibroblasts, vascular endothelial cells, nerve cells, inflammatory cells, insoluble matrix, and soluble factors [300]. Studies by De Marzo et al. highlight the role of inflammation in prostate cancer, suggesting that atrophic lesions are an early event in prostate carcinogenesis [301]. The macrophages in the tumor microenvironment produce ROS and RNS. The resulting increases in superoxide (O2-), hydrogen peroxide (H2O2), hydroxyl radical, and free iron damage DNA, causing genetic mutations and initiating cancer progression. Tissue and cell recombination studies demonstrate the important regulatory role of fibromuscular stroma and stromal fibroblasts in prostate development and prostate carcinogenesis [300]. Cancer cells and stromal cells interact through physical contact or through soluble factors or insoluble extracellular matrix (ECM) factors. These stromal fibroblasts, which interact with cancer cells, have increased levels of brain-derived neurotropic factor, chemokines, CC chemokine ligand 5 (CCL5) and CXC chemokine lix 5 (CXCL5), versican, tenascin, connective tissue growth factor, stromal cell derived factor-1/ CXC chemokine ligand 12 (SDF-1/CXCL12), and HIF-1α [302]. Other studies have demonstrated the role of stromal soluble factors interacting with receptors on prostate cancer cells. The stromal factors include VEGF, bFGF, hepatocyte growth factor/ scatter factor (HGF/SF), transforming growth factor-β (TGF-β), insulin like growth factor-1 (IGF-1), IL-6, and keratinocyte growth factor (KGF) [303].
Several studies have found that tumors promote a constant influx of myelomonocytic cells that express inflammatory mediators supporting pro-tumoral functions. Myelomonocytic cells are key orchestrators of cancer-related inflammation associated with proliferation and survival of malignant cells, subversion of adaptive immune response, angiogenesis, stroma remodeling, and metastasis formation [304].
Tumor-derived factors, which cause sustained myelopoiesis, accumulation, and functional differentiation of myelomonocytic cells, provide an essential support for the angiogenesis and the stroma remodeling required for tumor growth [305, 306]. In addition, it has long been known that tumor growth is promoted by tumor-associated macrophages (TAM), a major leukocyte population present in tumors [65, 307-310]. Accordingly, in many but not all human tumors, a high frequency of infiltrating TAM is associated with poor prognosis. A model by which macrophages promote tumor invasion and metastasis includes expression of their proteolytic activity and subsequent breakdown of the basement membrane around the preinvasive tumors, thereby enhancing the ability of tumor cells to escape into the surrounding stroma [311]. In lung cancer, for example, TAM may favor tumor progression by contributing to stroma formation and angiogenesis through their release of platelet-derived growth factor, in conjunction with TGF-β production by cancer cells [310]. TAM produce several MMPs, such as MMP-2 and MMP-9, that degrade proteins in the extracellular matrix and also produce activators of MMPs, such as chemokines.
5. Conclusion
This review clearly implicates the role of ROS in different phases of tumorigenesis. Therefore, targeting redox-sensitive pathways and transcription factors offers great promise for cancer prevention and therapy. Numerous agents have been identified that can interfere with redox cell signaling pathways [9, 312, 313]. These include neutraceuticals derived from fruits, vegetables, spices, grains, and cereals. They have been shown to suppress tumorigenesis in preclinical models. Whether these agents can inhibit tumor growth in patients remains to be elucidated.
Acknowledgments
We thank Michael Worley for carefully editing the manuscript. Dr. Aggarwal is the Ransom Horne, Jr., Professor of Cancer Research. This work was supported by a grant from the Clayton Foundation for Research (B.B.A.), a core grant from the National Institutes of Health (CA-16672), a program project grant from National Institutes of Health (NIH CA-124787-01A2), and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center. Simone Reuter was supported by a grant from the Fonds National de la Recherche Luxembourg (PDR-08-017).
6. Abbreviations
- Akt
AKT8 virus oncogene cellular homolog
- AP-1
activator protein-1
- APC
adenomatous polyposis coli
- ATF-2
activating transcription factor-2
- Bad
Bcl-XL/Bcl-2-associated death promoter
- BH3
Bcl-2 homology3
- BRCA1
breast cancer susceptibility gene 1
- CDKN-2
cyclin-dependent kinase inhibitor-2
- COX-2
cyclooxygenase-2
- CCL5
CC chemokine ligand 5
- CSCs
cancer stem cells
- Cu-ZnSOD
copper-zinc superoxide dismutase
- CXCL5
CXC chemokine lix 5
- CXCR4
CXC chemokine receptor 4
- ECM
extracellular matrix
- EC-SOD
extracellular-superoxide dismutase
- eNOS
endothelial nitric oxide synthase
- ERK/MAPK
extracellular signal-regulated kinase/ mitogen-activated protein kinase
- FGF
fibroblast growth factor
- HIF-1α
hypoxia inducible factor-1α
- Flk1/KDR
fetal liver kinase 1/ kinase insert domain receptor
- GPx
glutathione peroxidase
- GSH
glutathione
- GSSG
glutathione disulphide
- GTPase Rac1
guanosine triphosphatase Rac1
- HER-2
human epidermal growth factor receptor-2
- HGF/SF
hepatocyte growth factor/ scatter factor
- HIF-1α
hypoxia-inducible factor-1α
- hMLH1
human mutL homolog 1
- HMOX-1
heme oxygenase-1
- 4-HNE
4-hydroxynonenal
- H2O2
hydrogen peroxide
- Hsp27
heat shock protein27
- ICAM-1
intercellular adhesion molecule-1
- IGF-1
Insulin like growth factor-1
- IκBα
inhibitor of κBα
- IL-1
interleukin-1
- IL-6
interleukin-6
- IL-8
interleukin-8
- iNOS
inducible nitric oxide synthase
- IFN
interferon
- JNK
c-Jun N-terminal kinase
- c-JUN
cellular Ju-nanna
- KGF
keratinocyte growth factor
- Keap1
Kelch-like ECH-associated protein 1
- LPS
lipopolysaccharide
- MDR
multidrug-resistance
- MDM2
murine double minute 2
- MKPs
mitogen-activated protein kinase phosphatases
- MMPs
metalloproteinases
- Mn-SOD
manganese-superoxide dismutase
- MRP1
multidrug resistance-associated protein 1
- Myc
avian myeloblastosis virus oncogene
- MyD88
myeloid differentiation primary response gene 88
- NAC
N-acetylcysteine
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor κ B
- NO
nitric oxide
- Nox
NADPH oxidase
- Nrf2
NF-E2 related factor-2
- 8-OHdG
8-hydroxydeoxyguanosine
- p27Kip1
p27 kinase inhibitor protein
- PDGF
platelet-derived growth factor
- PGP
P-glycoprotein
- PI3K
phosphoinositide 3- kinase
- PKB/Akt
protein kinase B/AKT8 virus oncogene cellular homolog
- PMA
phorbol 12-myristate 13- acetate
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PTEN
phosphatase and tensin homolog deleted from chromosome 10
- Prx
peroxiredoxins
- RAS
rat sarcoma viral oncogene
- RAF
murine sarcoma 3611 oncogene
- Rb
retinoblastoma protein
- ROS
reactive oxygen species
- RNS
reactive nitrogen species
- SDF-1/CXCL12
stromal cell derived factor-1/ CXC chemokine ligand 12
- SOD
superoxide dismutase
- SRC
steroid receptor coactivator
- STAT3
signal transducer and activator of transcription 3
- TAM
tumor-associated macrophages
- tBHQ
tertiary-butylhydroquinone
- TGF-β
transforming growth factor-β
- TLR
toll-like receptor
- TNF
tumor necrosis factor
- TSP-1
thrombospondin-1
- TrxR
thioredoxin reductase
- VEGF-A
vascular endothelial growth factor-A
- Wnt
wint
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2009 doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 2.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharmacol. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 3.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 5.Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Redox regulation of TNF signaling. Biofactors. 1999;10:145–156. doi: 10.1002/biof.5520100210. [DOI] [PubMed] [Google Scholar]
- 6.Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci U S A. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 8.Schraufstatter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988;82:1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61:290–302. doi: 10.1016/j.addr.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visconti R, Grieco D. New insights on oxidative stress in cancer. Curr Opin Drug Discov Devel. 2009;12:240–245. [PubMed] [Google Scholar]
- 12.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What's new. J Eur Acad Dermatol Venereol. 2003;17:663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 13.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 14.Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: A role for prostate cancer? Biochim Biophys Acta. 2009;1795 doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;91:31S–38S. doi: 10.1016/0002-9343(91)90281-2. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JN, Pierce JD, Clancy RL. Reactive oxygen species in acute respiratory distress syndrome. Heart Lung. 2001;30:370–375. doi: 10.1067/mhl.2001.118298. [DOI] [PubMed] [Google Scholar]
- 17.Dugan LL, Quick KL. Reactive oxygen species and aging: evolving questions. Sci Aging Knowledge Environ. 2005;2005:pe20. doi: 10.1126/sageke.2005.26.pe20. [DOI] [PubMed] [Google Scholar]
- 18.Hensley K, Butterfield DA, Hall N, Cole P, Subramaniam R, Mark R, Mattson MP, Markesbery WR, Harris ME, Aksenov M, et al. Reactive oxygen species as causal agents in the neurotoxicity of the Alzheimer's disease-associated amyloid beta peptide. Ann N Y Acad Sci. 1996;786:120–134. doi: 10.1111/j.1749-6632.1996.tb39057.x. [DOI] [PubMed] [Google Scholar]
- 19.Multhaup G, Ruppert T, Schlicksupp A, Hesse L, Beher D, Masters CL, Beyreuther K. Reactive oxygen species and Alzheimer's disease. Biochem Pharmacol. 1997;54:533–539. doi: 10.1016/s0006-2952(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- 21.Lau AT, Wang Y, Chiu JF. Reactive oxygen species: current knowledge and applications in cancer research and therapeutic. J Cell Biochem. 2008;104:657–667. doi: 10.1002/jcb.21655. [DOI] [PubMed] [Google Scholar]
- 22.Renschler MF. The emerging role of reactive oxygen species in cancer therapy. Eur J Cancer. 2004;40:1934–1940. doi: 10.1016/j.ejca.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 23.Weinberg F, Chandel NS. Reactive oxygen species-dependent signaling regulates cancer. Cell Mol Life Sci. 2009;66:3663–3673. doi: 10.1007/s00018-009-0099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res. 2004;37:1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 25.Yoshizumi M, Tsuchiya K, Tamaki T. Signal transduction of reactive oxygen species and mitogen-activated protein kinases in cardiovascular disease. J Med Invest. 2001;48:11–24. [PubMed] [Google Scholar]
- 26.Muhammad S, Bierhaus A, Schwaninger M. Reactive oxygen species in diabetes-induced vascular damage, stroke, and Alzheimer's disease. J Alzheimers Dis. 2009;16:775–785. doi: 10.3233/JAD-2009-0982. [DOI] [PubMed] [Google Scholar]
- 27.Di Virgilio F. New pathways for reactive oxygen species generation in inflammation and potential novel pharmacological targets. Curr Pharm Des. 2004;10:1647–1652. doi: 10.2174/1381612043384727. [DOI] [PubMed] [Google Scholar]
- 28.Moulton PJ. Inflammatory joint disease: the role of cytokines, cyclooxygenases and reactive oxygen species. Br J Biomed Sci. 1996;53:317–324. [PubMed] [Google Scholar]
- 29.Bolanos JP, Moro MA, Lizasoain I, Almeida A. Mitochondria and reactive oxygen and nitrogen species in neurological disorders and stroke: Therapeutic implications. Adv Drug Deliv Rev. 2009;61:1299–1315. doi: 10.1016/j.addr.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 30.Atabek ME, Vatansev H, Erkul I. Oxidative stress in childhood obesity. J Pediatr Endocrinol Metab. 2004;17:1063–1068. doi: 10.1515/jpem.2004.17.8.1063. [DOI] [PubMed] [Google Scholar]
- 31.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabner BJ, Turnbull S, El-Agnaf O, Allsop D. Production of reactive oxygen species from aggregating proteins implicated in Alzheimer's disease, Parkinson's disease and other neurodegenerative diseases. Curr Top Med Chem. 2001;1:507–517. doi: 10.2174/1568026013394822. [DOI] [PubMed] [Google Scholar]
- 33.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson's disease. IUBMB Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 34.Kamp DW, Graceffa P, Pryor WA, Weitzman SA. The role of free radicals in asbestos-induced diseases. Free Radic Biol Med. 1992;12:293–315. doi: 10.1016/0891-5849(92)90117-y. [DOI] [PubMed] [Google Scholar]
- 35.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gelderman KA, Hultqvist M, Olsson LM, Bauer K, Pizzolla A, Olofsson P, Holmdahl R. Rheumatoid arthritis: the role of reactive oxygen species in disease development and therapeutic strategies. Antioxid Redox Signal. 2007;9:1541–1567. doi: 10.1089/ars.2007.1569. [DOI] [PubMed] [Google Scholar]
- 37.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 38.Jeremy JY, Shukla N, Muzaffar S, Handley A, Angelini GD. Reactive oxygen species, vascular disease and cardiovascular surgery. Curr Vasc Pharmacol. 2004;2:229–236. doi: 10.2174/1570161043385691. [DOI] [PubMed] [Google Scholar]
- 39.Miyajima A, Nakashima J, Yoshioka K, Tachibana M, Tazaki H, Murai M. Role of reactive oxygen species in cis-dichlorodiammineplatinum-induced cytotoxicity on bladder cancer cells. Br J Cancer. 1997;76:206–210. doi: 10.1038/bjc.1997.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salganik RI, Albright CD, Rodgers J, Kim J, Zeisel SH, Sivashinskiy MS, Van Dyke TA. Dietary antioxidant depletion: enhancement of tumor apoptosis and inhibition of brain tumor growth in transgenic mice. Carcinogenesis. 2000;21:909–914. doi: 10.1093/carcin/21.5.909. [DOI] [PubMed] [Google Scholar]
- 41.Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3:323–327. doi: 10.1186/bcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma A, Rajappa M, Satyam A, Sharma M. Oxidant/anti-oxidant dynamics in patients with advanced cervical cancer: correlation with treatment response. Mol Cell Biochem. 341:65–72. doi: 10.1007/s11010-010-0437-2. [DOI] [PubMed] [Google Scholar]
- 43.Oliveira CP, Kassab P, Lopasso FP, Souza HP, Janiszewski M, Laurindo FR, Iriya K, Laudanna AA. Protective effect of ascorbic acid in experimental gastric cancer: reduction of oxidative stress. World J Gastroenterol. 2003;9:446–448. doi: 10.3748/wjg.v9.i3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calvisi DF, Ladu S, Hironaka K, Factor VM, Thorgeirsson SS. Vitamin E down-modulates iNOS and NADPH oxidase in c-Myc/TGF-alpha transgenic mouse model of liver cancer. J Hepatol. 2004;41:815–822. doi: 10.1016/j.jhep.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 45.Azad N, Rojanasakul Y, Vallyathan V. Inflammation and lung cancer: roles of reactive oxygen/nitrogen species. J Toxicol Environ Health B Crit Rev. 2008;11:1–15. doi: 10.1080/10937400701436460. [DOI] [PubMed] [Google Scholar]
- 46.Fruehauf JP, Trapp V. Reactive oxygen species: an Achilles' heel of melanoma? Expert Rev Anticancer Ther. 2008;8:1751–1757. doi: 10.1586/14737140.8.11.1751. [DOI] [PubMed] [Google Scholar]
- 47.Kuku I, Aydogdu I, Bayraktar N, Kaya E, Akyol O, Erkurt MA. Oxidant/antioxidant parameters and their relationship with medical treatment in multiple myeloma. Cell Biochem Funct. 2005;23:47–50. doi: 10.1002/cbf.1127. [DOI] [PubMed] [Google Scholar]
- 48.Sumi D, Shinkai Y, Kumagai Y. Signal transduction pathways and transcription factors triggered by arsenic trioxide in leukemia cells. Toxicol Appl Pharmacol. 244:385–392. doi: 10.1016/j.taap.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 49.van de Wetering CI, Coleman MC, Spitz DR, Smith BJ, Knudson CM. Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma. Free Radic Biol Med. 2008;44:1677–1686. doi: 10.1016/j.freeradbiomed.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahar G, Feinmesser R, Shpitzer T, Popovtzer A, Nagler RM. Salivary analysis in oral cancer patients: DNA and protein oxidation, reactive nitrogen species, and antioxidant profile. Cancer. 2007;109:54–59. doi: 10.1002/cncr.22386. [DOI] [PubMed] [Google Scholar]
- 51.Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa T, Chan KK, Ngan HY. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis. 2008;29:1742–1750. doi: 10.1093/carcin/bgn167. [DOI] [PubMed] [Google Scholar]
- 52.Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, Buchler MW, Lerch MM, Pandol SJ, Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137–1147. doi: 10.1152/ajpgi.00197.2005. [DOI] [PubMed] [Google Scholar]
- 53.Ma Q, Cavallin LE, Yan B, Zhu S, Duran EM, Wang H, Hale LP, Dong C, Cesarman E, Mesri EA, Goldschmidt-Clermont PJ. Antitumorigenesis of antioxidants in a transgenic Rac1 model of Kaposi's sarcoma. Proc Natl Acad Sci U S A. 2009;106:8683–8688. doi: 10.1073/pnas.0812688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 55.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grivennikov SI, Karin M. Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev. 20:65–71. doi: 10.1016/j.gde.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 58.Garodia P, Ichikawa H, Malani N, Sethi G, Aggarwal BB. From ancient medicine to modern medicine: ayurvedic concepts of health and their role in inflammation and cancer. J Soc Integr Oncol. 2007;5:25–37. doi: 10.2310/7200.2006.029. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol. 2009;9:351–369. doi: 10.1016/j.coph.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schetter AJ, Heegaard NH, Harris CC. Inflammation and cancer: interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990;336:357–359. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 62.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–1233. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 63.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–1592. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ekbom A, McLaughlin JK, Nyren O. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;329:1502–1503. doi: 10.1056/NEJM199311113292016. [DOI] [PubMed] [Google Scholar]
- 65.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kundu JK, Surh YJ. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 67.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 68.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hussain SP, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–2380. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 70.Federico A, Morgillo F, Tuccillo C, Ciardiello F, Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int J Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 71.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- 72.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Karihtala P, Soini Y. Reactive oxygen species and antioxidant mechanisms in human tissues and their relation to malignancies. APMIS. 2007;115:81–103. doi: 10.1111/j.1600-0463.2007.apm_514.x. [DOI] [PubMed] [Google Scholar]
- 74.Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 75.Ray G, Husain SA. Oxidants, antioxidants and carcinogenesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- 76.Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- 77.Marnett LJ. Oxyradicals and DNA damage. Carcinogenesis. 2000;21:361–370. doi: 10.1093/carcin/21.3.361. [DOI] [PubMed] [Google Scholar]
- 78.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313(Pt 1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malins DC, Haimanot R. Major alterations in the nucleotide structure of DNA in cancer of the female breast. Cancer Res. 1991;51:5430–5432. [PubMed] [Google Scholar]
- 80.Matsui A, Ikeda T, Enomoto K, Hosoda K, Nakashima H, Omae K, Watanabe M, Hibi T, Kitajima M. Increased formation of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87–95. doi: 10.1016/s0304-3835(99)00424-3. [DOI] [PubMed] [Google Scholar]
- 81.Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209–1214. doi: 10.1016/0959-8049(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 82.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 83.Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 84.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 85.Lowenstein CJ, Padalko E. iNOS (NOS2) at a glance. J Cell Sci. 2004;117:2865–2867. doi: 10.1242/jcs.01166. [DOI] [PubMed] [Google Scholar]
- 86.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 87.Zhang Y, Qi H, Taylor R, Xu W, Liu LF, Jin S. The role of autophagy in mitochondria maintenance: characterization of mitochondrial functions in autophagy-deficient S. cerevisiae strains. Autophagy. 2007;3:337–346. doi: 10.4161/auto.4127. [DOI] [PubMed] [Google Scholar]
- 88.Mathew R, Karp CM, Beaudoin B, Vuong N, Chen G, Chen HY, Bray K, Reddy A, Bhanot G, Gelinas C, Dipaola RS, Karantza-Wadsworth V, White E. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pompella A, Visvikis A, Paolicchi A, De Tata V, Casini AF. The changing faces of glutathione, a cellular protagonist. Biochem Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 90.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 91.Calvert P, Yao KS, Hamilton TC, O'Dwyer PJ. Clinical studies of reversal of drug resistance based on glutathione. Chem Biol Interact. 1998;111-112:213–224. doi: 10.1016/s0009-2797(98)00008-8. [DOI] [PubMed] [Google Scholar]
- 92.Balendiran GK, Dabur R, Fraser D. The role of glutathione in cancer. Cell Biochem Funct. 2004;22:343–352. doi: 10.1002/cbf.1149. [DOI] [PubMed] [Google Scholar]
- 93.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 94.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 95.Meira LB, Bugni JM, Green SL, Lee CW, Pang B, Borenshtein D, Rickman BH, Rogers AB, Moroski-Erkul CA, McFaline JL, Schauer DB, Dedon PC, Fox JG, Samson LD. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118:2516–2525. doi: 10.1172/JCI35073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–4642. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 97.Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, Liang J, Stine OC, Yin J, Zou TT, Abraham JM, Kong D, Wilson KT, James SP, Herman JG, Meltzer SJ. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer Res. 2000;60:4864–4868. [PubMed] [Google Scholar]
- 98.Park JH, Mangal D, Frey AJ, Harvey RG, Blair IA, Penning TM. Aryl hydrocarbon receptor facilitates DNA strand breaks and 8-oxo-2′-deoxyguanosine formation by the aldo-keto reductase product benzo[a]pyrene-7,8-dione. J Biol Chem. 2009;284:29725–29734. doi: 10.1074/jbc.M109.042143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Amstad P, Crawford D, Muehlematter D, Zbinden I, Larsson R, Cerutti P. Oxidants stress induces the proto-oncogenes, C-fos and C-myc in mouse epidermal cells. Bull Cancer. 1990;77:501–502. [PubMed] [Google Scholar]
- 100.Amstad PA, Krupitza G, Cerutti PA. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992;52:3952–3960. [PubMed] [Google Scholar]
- 101.Alexander A, Cai SL, Kim J, Nanez A, Sahin M, MacLean KH, Inoki K, Guan KL, Shen J, Person MD, Kusewitt D, Mills GB, Kastan MB, Walker CL. ATM signals to TSC2 in the cytoplasm to regulate mTORC1 in response to ROS. Proc Natl Acad Sci U S A. 107:4153–4158. doi: 10.1073/pnas.0913860107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li L, Wang J, Ye RD, Shi G, Jin H, Tang X, Yi J. PML/RARalpha fusion protein mediates the unique sensitivity to arsenic cytotoxicity in acute promyelocytic leukemia cells: Mechanisms involve the impairment of cAMP signaling and the aberrant regulation of NADPH oxidase. J Cell Physiol. 2008;217:486–493. doi: 10.1002/jcp.21523. [DOI] [PubMed] [Google Scholar]
- 103.Barlow CA, Kitiphongspattana K, Siddiqui N, Roe MW, Mossman BT, Lounsbury KM. Protein kinase A-mediated CREB phosphorylation is an oxidant-induced survival pathway in alveolar type II cells. Apoptosis. 2008;13:681–692. doi: 10.1007/s10495-008-0203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun KH, de Pablo Y, Vincent F, Shah K. Deregulated Cdk5 promotes oxidative stress and mitochondrial dysfunction. J Neurochem. 2008;107:265–278. doi: 10.1111/j.1471-4159.2008.05616.x. [DOI] [PubMed] [Google Scholar]
- 105.Burch PM, Heintz NH. Redox regulation of cell-cycle re-entry: cyclin D1 as a primary target for the mitogenic effects of reactive oxygen and nitrogen species. Antioxid Redox Signal. 2005;7:741–751. doi: 10.1089/ars.2005.7.741. [DOI] [PubMed] [Google Scholar]
- 106.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 107.Maynard S, Schurman SH, Harboe C, de Souza-Pinto NC, Bohr VA. Base excision repair of oxidative DNA damage and association with cancer and aging. Carcinogenesis. 2009;30:2–10. doi: 10.1093/carcin/bgn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Goldkorn T, Ravid T, Khan EM. Life and death decisions: ceramide generation and EGF receptor trafficking are modulated by oxidative stress. Antioxid Redox Signal. 2005;7:119–128. doi: 10.1089/ars.2005.7.119. [DOI] [PubMed] [Google Scholar]
- 109.Wartenberg M, Schallenberg M, Hescheler J, Sauer H. Reactive oxygen species-mediated regulation of eNOS and iNOS expression in multicellular prostate tumor spheroids. Int J Cancer. 2003;104:274–282. doi: 10.1002/ijc.10928. [DOI] [PubMed] [Google Scholar]
- 110.McCubrey JA, Lahair MM, Franklin RA. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid Redox Signal. 2006;8:1775–1789. doi: 10.1089/ars.2006.8.1775. [DOI] [PubMed] [Google Scholar]
- 111.Kwon D, Choi C, Lee J, Kim KO, Kim JD, Kim SJ, Choi IH. Hydrogen peroxide triggers the expression of Fas/FasL in astrocytoma cell lines and augments apoptosis. J Neuroimmunol. 2001;113:1–9. doi: 10.1016/s0165-5728(00)00321-0. [DOI] [PubMed] [Google Scholar]
- 112.Goto T, Takano M. Transcriptional role of FOXO1 in drug resistance through antioxidant defense systems. Adv Exp Med Biol. 2009;665:171–179. doi: 10.1007/978-1-4419-1599-3_13. [DOI] [PubMed] [Google Scholar]
- 113.Kietzmann T, Gorlach A. Reactive oxygen species in the control of hypoxia-inducible factor-mediated gene expression. Semin Cell Dev Biol. 2005;16:474–486. doi: 10.1016/j.semcdb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 114.Ryter SW, Choi AM. Heme oxygenase-1: redox regulation of a stress protein in lung and cell culture models. Antioxid Redox Signal. 2005;7:80–91. doi: 10.1089/ars.2005.7.80. [DOI] [PubMed] [Google Scholar]
- 115.Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y, Nakano T, Ogo T, Umei T, Niho Y. Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol. 1996;24:151–157. [PubMed] [Google Scholar]
- 116.Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49:197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- 117.Hellstrand K, Hermodsson S, Naredi P, Mellqvist UH, Brune M. Histamine and cytokine therapy. Acta Oncol. 1998;37:347–353. doi: 10.1080/028418698430566. [DOI] [PubMed] [Google Scholar]
- 118.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol. 1998;275:C1640–1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 119.Lo YY, Wong JM, Cruz TF. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem. 1996;271:15703–15707. doi: 10.1074/jbc.271.26.15703. [DOI] [PubMed] [Google Scholar]
- 120.Skinner AM, Turker MS. Oxidative mutagenesis, mismatch repair, and aging. Sci Aging Knowledge Environ. 2005;2005 doi: 10.1126/sageke.2005.9.re3. [DOI] [PubMed] [Google Scholar]
- 121.Byun YJ, Kim SK, Kim YM, Chae GT, Jeong SW, Lee SB. Hydrogen peroxide induces autophagic cell death in C6 glioma cells via BNIP3-mediated suppression of the mTOR pathway. Neurosci Lett. 2009;461:131–135. doi: 10.1016/j.neulet.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 122.Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 124.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 125.Clerkin JS, Naughton R, Quiney C, Cotter TG. Mechanisms of ROS modulated cell survival during carcinogenesis. Cancer Lett. 2008;266:30–36. doi: 10.1016/j.canlet.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 126.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 127.Han ES, Muller FL, Perez VI, Qi W, Liang H, Xi L, Fu C, Doyle E, Hickey M, Cornell J, Epstein CJ, Roberts LJ, Van Remmen H, Richardson A. The in vivo gene expression signature of oxidative stress. Physiol Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tishler RB, Calderwood SK, Coleman CN, Price BD. Increases in sequence specific DNA binding by p53 following treatment with chemotherapeutic and DNA damaging agents. Cancer Res. 1993;53:2212–2216. [PubMed] [Google Scholar]
- 129.Kanthasamy AG, Kitazawa M, Kanthasamy A, Anantharam V. Role of proteolytic activation of protein kinase Cdelta in oxidative stress-induced apoptosis. Antioxid Redox Signal. 2003;5:609–620. doi: 10.1089/152308603770310275. [DOI] [PubMed] [Google Scholar]
- 130.Rusyn I, Rose ML, Bojes HK, Thurman RG. Novel role of oxidants in the molecular mechanism of action of peroxisome proliferators. Antioxid Redox Signal. 2000;2:607–621. doi: 10.1089/15230860050192350. [DOI] [PubMed] [Google Scholar]
- 131.Cao J, Schulte J, Knight A, Leslie NR, Zagozdzon A, Bronson R, Manevich Y, Beeson C, Neumann CA. Prdx1 inhibits tumorigenesis via regulating PTEN/AKT activity. EMBO J. 2009;28:1505–1517. doi: 10.1038/emboj.2009.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chiarugi P. PTPs versus PTKs: the redox side of the coin. Free Radic Res. 2005;39:353–364. doi: 10.1080/10715760400027987. [DOI] [PubMed] [Google Scholar]
- 133.Ammendola R, Mesuraca M, Russo T, Cimino F. The DNA-binding efficiency of Sp1 is affected by redox changes. Eur J Biochem. 1994;225:483–489. doi: 10.1111/j.1432-1033.1994.t01-1-00483.x. [DOI] [PubMed] [Google Scholar]
- 134.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 135.Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- 136.Korswagen HC. Regulation of the Wnt/beta-catenin pathway by redox signaling. Dev Cell. 2006;10:687–688. doi: 10.1016/j.devcel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 137.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 138.Kerr LD, Inoue J, Verma IM. Signal transduction: the nuclear target. Curr Opin Cell Biol. 1992;4:496–501. doi: 10.1016/0955-0674(92)90017-7. [DOI] [PubMed] [Google Scholar]
- 139.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol. 2002;4:E131–136. doi: 10.1038/ncb0502-e131. [DOI] [PubMed] [Google Scholar]
- 140.Gorlach A, Kietzmann T. Superoxide and derived reactive oxygen species in the regulation of hypoxia-inducible factors. Methods Enzymol. 2007;435:421–446. doi: 10.1016/S0076-6879(07)35022-2. [DOI] [PubMed] [Google Scholar]
- 141.Liu B, Chen Y, St Clair DK. ROS and p53: a versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mantovani A. Cancer: inflammation by remote control. Nature. 2005;435:752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 143.Ames BN, Gold LS. Animal cancer tests and cancer prevention. J Natl Cancer Inst Monogr. 1992:125–132. [PubMed] [Google Scholar]
- 144.Guyton KZ, Kensler TW. Oxidative mechanisms in carcinogenesis. Br Med Bull. 1993;49:523–544. doi: 10.1093/oxfordjournals.bmb.a072628. [DOI] [PubMed] [Google Scholar]
- 145.Schulte-Hermann R, Timmermann-Trosiener I, Barthel G, Bursch W. DNA synthesis, apoptosis, and phenotypic expression as determinants of growth of altered foci in rat liver during phenobarbital promotion. Cancer Res. 1990;50:5127–5135. [PubMed] [Google Scholar]
- 146.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, Stevenson DE, Walborg EF., Jr The role of oxidative stress in chemical carcinogenesis. Environ Health Perspect. 1998;106 1:289–295. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oshima H, B H. Chronic infectious and inflammation process as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 148.Rosin MP, Saad el Din Zaki S, Ward AJ, Anwar WA. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat Res. 1994;305:283–292. doi: 10.1016/0027-5107(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 149.Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood. 1990;76:655–663. [PubMed] [Google Scholar]
- 150.Frenkel K. Carcinogen-mediated oxidant formation and oxidative DNA damage. Pharmacol Ther. 1992;53:127–166. doi: 10.1016/0163-7258(92)90047-4. [DOI] [PubMed] [Google Scholar]
- 151.Shacter E, Beecham EJ, Covey JM, Kohn KW, Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988;9:2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- 152.Wei H, Frenkel K. Suppression of tumor promoter-induced oxidative events and DNA damage in vivo by sarcophytol A: a possible mechanism of antipromotion. Cancer Res. 1992;52:2298–2303. [PubMed] [Google Scholar]
- 153.Hattori-Nakakuki Y, Nishigori C, Okamoto K, Imamura S, Hiai H, Toyokuni S. Formation of 8-hydroxy-2′-deoxyguanosine in epidermis of hairless mice exposed to near-UV. Biochem Biophys Res Commun. 1994;201:1132–1139. doi: 10.1006/bbrc.1994.1823. [DOI] [PubMed] [Google Scholar]
- 154.Weitberg AB. Effect of combinations of antioxidants on phagocyte-induced sister-chromatid exchanges. Mutat Res. 1989;224:1–4. doi: 10.1016/0165-1218(89)90002-5. [DOI] [PubMed] [Google Scholar]
- 155.Yamashina K, Miller BE, Heppner GH. Macrophage-mediated induction of drug-resistant variants in a mouse mammary tumor cell line. Cancer Res. 1986;46:2396–2401. [PubMed] [Google Scholar]
- 156.Dizdaroglu M, Olinski R, Doroshow JH, Akman SA. Modification of DNA bases in chromatin of intact target human cells by activated human polymorphonuclear leukocytes. Cancer Res. 1993;53:1269–1272. [PubMed] [Google Scholar]
- 157.Trush MA, Twerdok LE, Esterline RL. Comparison of oxidant activities and the activation of benzo(a)pyrene-7,8-dihydrodiol by polymorphonuclear leucocytes from human, rat and mouse. Xenobiotica. 1990;20:925–932. doi: 10.3109/00498259009046908. [DOI] [PubMed] [Google Scholar]
- 158.Trush MA, Kensler TW. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic Biol Med. 1991;10:201–209. doi: 10.1016/0891-5849(91)90077-g. [DOI] [PubMed] [Google Scholar]
- 159.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 160.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 161.Kroncke KD. Nitrosative stress and transcription. Biol Chem. 2003;384:1365–1377. doi: 10.1515/BC.2003.153. [DOI] [PubMed] [Google Scholar]
- 162.Cerutti PA, Trump BF. Inflammation and oxidative stress in carcinogenesis. Cancer Cells. 1991;3:1–7. [PubMed] [Google Scholar]
- 163.Cerutti PA. Prooxidant states and tumor promotion. Science. 1985;227:375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- 164.Slaga TJ, Klein-Szanto AJ, Triplett LL, Yotti LP, Trosko KE. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981;213:1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- 165.Kamata T. Roles of Nox1 and other Nox isoforms in cancer development. Cancer Sci. 2009;100:1382–1388. doi: 10.1111/j.1349-7006.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Los M, Maddika S, Erb B, Schulze-Osthoff K. Switching Akt: from survival signaling to deadly response. Bioessays. 2009;31:492–495. doi: 10.1002/bies.200900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Azad MB, Chen Y, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790. doi: 10.1089/ars.2008.2270. [DOI] [PubMed] [Google Scholar]
- 168.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435–7442. doi: 10.1038/sj.onc.1209097. [DOI] [PubMed] [Google Scholar]
- 170.Muller JM, Cahill MA, Rupec RA, Baeuerle PA, Nordheim A. Antioxidants as well as oxidants activate c-fos via Ras-dependent activation of extracellular-signal-regulated kinase 2 and Elk-1. Eur J Biochem. 1997;244:45–52. doi: 10.1111/j.1432-1033.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 171.Pennington JD, Wang TJ, Nguyen P, Sun L, Bisht K, Smart D, Gius D. Redox-sensitive signaling factors as a novel molecular targets for cancer therapy. Drug Resist Updat. 2005;8:322–330. doi: 10.1016/j.drup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 172.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 173.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 174.Gupta S, Campbell D, Derijard B, Davis RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 175.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 176.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 177.Baeuerle PA, Lenardo M, Pierce JW, Baltimore D. Phorbol-ester-induced activation of the NF-kappa B transcription factor involves dissociation of an apparently cytoplasmic NF-kappa B/inhibitor complex. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):789–798. doi: 10.1101/sqb.1988.053.01.089. [DOI] [PubMed] [Google Scholar]
- 178.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95:13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rath PC, Aggarwal BB. Antiproliferative effects of IFN-alpha correlate with the downregulation of nuclear factor-kappa B in human Burkitt lymphoma Daudi cells. J Interferon Cytokine Res. 2001;21:523–528. doi: 10.1089/10799900152434402. [DOI] [PubMed] [Google Scholar]
- 180.Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I kappa B-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47:145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 181.Delhalle S, Deregowski V, Benoit V, Merville MP, Bours V. NF-kappaB-dependent MnSOD expression protects adenocarcinoma cells from TNF-alpha-induced apoptosis. Oncogene. 2002;21:3917–3924. doi: 10.1038/sj.onc.1205489. [DOI] [PubMed] [Google Scholar]
- 182.Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, Jayawardena S, De Smaele E, Cong R, Beaumont C, Torti FM, Torti SV, Franzoso G. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 183.Schulze-Osthoff K, Ferrari D, Los M, Wesselborg S, Peter ME. Apoptosis signaling by death receptors. Eur J Biochem. 1998;254:439–459. doi: 10.1046/j.1432-1327.1998.2540439.x. [DOI] [PubMed] [Google Scholar]
- 184.Giri DK, Aggarwal BB. Constitutive activation of NF-kappaB causes resistance to apoptosis in human cutaneous T cell lymphoma HuT-78 cells. Autocrine role of tumor necrosis factor and reactive oxygen intermediates. J Biol Chem. 1998;273:14008–14014. doi: 10.1074/jbc.273.22.14008. [DOI] [PubMed] [Google Scholar]
- 185.Nomura M, Ma W, Chen N, Bode AM, Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis. 2000;21:1885–1890. doi: 10.1093/carcin/21.10.1885. [DOI] [PubMed] [Google Scholar]
- 186.Schulze-Osthoff K, Bauer MK, Vogt M, Wesselborg S. Oxidative stress and signal transduction. Int J Vitam Nutr Res. 1997;67:336–342. [PubMed] [Google Scholar]
- 187.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–599. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 188.Schmidt KN, Amstad P, Cerutti P, Baeuerle PA. The roles of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kappa B. Chem Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 189.Kass GE, Duddy SK, Orrenius S. Activation of hepatocyte protein kinase C by redox-cycling quinones. Biochem J. 1989;260:499–507. doi: 10.1042/bj2600499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Larsson R, Cerutti P. Translocation and enhancement of phosphotransferase activity of protein kinase C following exposure in mouse epidermal cells to oxidants. Cancer Res. 1989;49:5627–5632. [PubMed] [Google Scholar]
- 191.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- 192.D'Souza RJ, Phillips HM, Jones PW, Strange RC, Aber GM. Interactions of hydrogen peroxide with interleukin-6 and platelet-derived growth factor in determining mesangial cell growth: effect of repeated oxidant stress. Clin Sci (Lond) 1993;85:747–751. doi: 10.1042/cs0850747. [DOI] [PubMed] [Google Scholar]
- 193.Alliangana DM. Effects of beta-carotene, flavonoid quercitin and quinacrine on cell proliferation and lipid peroxidation breakdown products in BHK-21 cells. East Afr Med J. 1996;73:752–757. [PubMed] [Google Scholar]
- 194.Chang MC, Chan CP, Wang YJ, Lee PH, Chen LI, Tsai YL, Lin BR, Wang YL, Jeng JH. Induction of necrosis and apoptosis to KB cancer cells by sanguinarine is associated with reactive oxygen species production and mitochondrial membrane depolarization. Toxicol Appl Pharmacol. 2007;218:143–151. doi: 10.1016/j.taap.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 195.Dypbukt JM, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J Biol Chem. 1994;269:30553–30560. [PubMed] [Google Scholar]
- 196.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 197.Kundu N, Zhang S, Fulton AM. Sublethal oxidative stress inhibits tumor cell adhesion and enhances experimental metastasis of murine mammary carcinoma. Clin Exp Metastasis. 1995;13:16–22. doi: 10.1007/BF00144014. [DOI] [PubMed] [Google Scholar]
- 198.Roebuck KA. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-kappaB (Review) Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 199.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 200.Mori K, Shibanuma M, Nose K. Invasive potential induced under long-term oxidative stress in mammary epithelial cells. Cancer Res. 2004;64:7464–7472. doi: 10.1158/0008-5472.CAN-04-1725. [DOI] [PubMed] [Google Scholar]
- 201.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 202.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nelson KK, Melendez JA. Mitochondrial redox control of matrix metalloproteinases. Free Radic Biol Med. 2004;37:768–784. doi: 10.1016/j.freeradbiomed.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 204.Yoon SO, Park SJ, Yoon SY, Yun CH, Chung AS. Sustained production of H(2)O(2) activates pro-matrix metalloproteinase-2 through receptor tyrosine kinases/phosphatidylinositol 3-kinase/NF-kappa B pathway. J Biol Chem. 2002;277:30271–30282. doi: 10.1074/jbc.M202647200. [DOI] [PubMed] [Google Scholar]
- 205.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 206.Kollmar O, Rupertus K, Scheuer C, Junker B, Tilton B, Schilling MK, Menger MD. Stromal cell-derived factor-1 promotes cell migration and tumor growth of colorectal metastasis. Neoplasia. 2007;9:862–870. doi: 10.1593/neo.07559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Owen JD, Strieter R, Burdick M, Haghnegahdar H, Nanney L, Shattuck-Brandt R, Richmond A. Enhanced tumor-forming capacity for immortalized melanocytes expressing melanoma growth stimulatory activity/growth-regulated cytokine beta and gamma proteins. Int J Cancer. 1997;73:94–103. doi: 10.1002/(sici)1097-0215(19970926)73:1<94::aid-ijc15>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 208.Yuecheng Y, Xiaoyan X. Stromal-cell derived factor-1 regulates epithelial ovarian cancer cell invasion by activating matrix metalloproteinase-9 and matrix metalloproteinase-2. Eur J Cancer Prev. 2007;16:430–435. doi: 10.1097/01.cej.0000236259.88146.a4. [DOI] [PubMed] [Google Scholar]
- 209.Lu H, Ouyang W, Huang C. Inflammation, a key event in cancer development. Mol Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 210.Wen DS, Zhu XL, Guan SM, Wu YM, Yu LL, Wu JZ. Silencing of CXCR4 inhibits the proliferation, adhesion, chemotaxis and invasion of salivary gland mucoepidermoid carcinoma Mc3 cells in vitro. Oral Oncol. 2008;44:545–554. doi: 10.1016/j.oraloncology.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 211.van Wetering S, van Buul JD, Quik S, Mul FP, Anthony EC, ten Klooster JP, Collard JG, Hordijk PL. Reactive oxygen species mediate Rac-induced loss of cell-cell adhesion in primary human endothelial cells. J Cell Sci. 2002;115:1837–1846. doi: 10.1242/jcs.115.9.1837. [DOI] [PubMed] [Google Scholar]
- 212.Diers AR, Dranka BP, Ricart KC, Oh JY, Johnson MS, Zhou F, Pallero MA, Bodenstine TM, Murphy-Ullrich JE, Welch DR, Landar A. Modulation of mammary cancer cell migration by 15-deoxy-delta(12,14)-prostaglandin J(2): implications for anti-metastatic therapy. Biochem J. 430:69–78. doi: 10.1042/BJ20091193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stamatakis K, Perez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic lipid-protein interactomes. Ann N Y Acad Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 214.Stamatakis K, Sanchez-Gomez FJ, Perez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Delta12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J Am Soc Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- 215.Aldini G, Carini M, Vistoli G, Shibata T, Kusano Y, Gamberoni L, Dalle-Donne I, Milzani A, Uchida K. Identification of actin as a 15-deoxy-Delta12,14-prostaglandin J2 target in neuroblastoma cells: mass spectrometric, computational, and functional approaches to investigate the effect on cytoskeletal derangement. Biochemistry. 2007;46:2707–2718. doi: 10.1021/bi0618565. [DOI] [PubMed] [Google Scholar]
- 216.Cheng GC, Schulze PC, Lee RT, Sylvan J, Zetter BR, Huang H. Oxidative stress and thioredoxin-interacting protein promote intravasation of melanoma cells. Exp Cell Res. 2004;300:297–307. doi: 10.1016/j.yexcr.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 217.Folkman J. Tumor angiogenesis. Adv Cancer Res. 1985;43:175–203. doi: 10.1016/s0065-230x(08)60946-x. [DOI] [PubMed] [Google Scholar]
- 218.Aruoma OI, Halliwell B. Superoxide-dependent and ascorbate-dependent formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Are lactoferrin and transferrin promoters of hydroxyl-radical generation? Biochem J. 1987;241:273–278. doi: 10.1042/bj2410273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Liotta LA, Steeg PS, Stetler-Stevenson WG. Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell. 1991;64:327–336. doi: 10.1016/0092-8674(91)90642-c. [DOI] [PubMed] [Google Scholar]
- 220.North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 221.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 222.Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mazure NM, Chen EY, Yeh P, Laderoute KR, Giaccia AJ. Oncogenic transformation and hypoxia synergistically act to modulate vascular endothelial growth factor expression. Cancer Res. 1996;56:3436–3440. [PubMed] [Google Scholar]
- 224.Okada F, Rak JW, Croix BS, Lieubeau B, Kaya M, Roncari L, Shirasawa S, Sasazuki T, Kerbel RS. Impact of oncogenes in tumor angiogenesis: mutant K-ras up-regulation of vascular endothelial growth factor/vascular permeability factor is necessary, but not sufficient for tumorigenicity of human colorectal carcinoma cells. Proc Natl Acad Sci U S A. 1998;95:3609–3614. doi: 10.1073/pnas.95.7.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Van Meir EG, Polverini PJ, Chazin VR, Su Huang HJ, de Tribolet N, Cavenee WK. Release of an inhibitor of angiogenesis upon induction of wild type p53 expression in glioblastoma cells. Nat Genet. 1994;8:171–176. doi: 10.1038/ng1094-171. [DOI] [PubMed] [Google Scholar]
- 226.Longo R, Sarmiento R, Fanelli M, Capaccetti B, Gattuso D, Gasparini G. Anti-angiogenic therapy: rationale, challenges and clinical studies. Angiogenesis. 2002;5:237–256. doi: 10.1023/a:1024532022166. [DOI] [PubMed] [Google Scholar]
- 227.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 228.Eatock MM, Schatzlein A, Kaye SB. Tumour vasculature as a target for anticancer therapy. Cancer Treat Rev. 2000;26:191–204. doi: 10.1053/ctrv.1999.0158. [DOI] [PubMed] [Google Scholar]
- 229.Monte M, Davel LE, Sacerdote de Lustig E. Hydrogen peroxide is involved in lymphocyte activation mechanisms to induce angiogenesis. Eur J Cancer. 1997;33:676–682. doi: 10.1016/s0959-8049(96)00506-0. [DOI] [PubMed] [Google Scholar]
- 230.Monte M, Davel LE, de Lustig ES. Inhibition of lymphocyte-induced angiogenesis by free radical scavengers. Free Radic Biol Med. 1994;17:259–266. doi: 10.1016/0891-5849(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 231.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 232.Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer Res. 1999;59:4525–4528. [PubMed] [Google Scholar]
- 233.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 234.Roessner A, Kuester D, Malfertheiner P, Schneider-Stock R. Oxidative stress in ulcerative colitis-associated carcinogenesis. Pathol Res Pract. 2008;204:511–524. doi: 10.1016/j.prp.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 235.Martey CA, Pollock SJ, Turner CK, O'Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol Lung Cell Mol Physiol. 2004;287:L981–991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- 236.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514–529. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 237.Murphy SJ, Anderson LA, Johnston BT, Fitzpatrick DA, Watson PR, Monaghan P, Murray LJ. Have patients with esophagitis got an increased risk of adenocarcinoma? Results from a population-based study. World J Gastroenterol. 2005;11:7290–7295. doi: 10.3748/wjg.v11.i46.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.de Visser KE, Jonkers J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Curr Pharm Des. 2009;15:1844–1853. doi: 10.2174/138161209788453239. [DOI] [PubMed] [Google Scholar]
- 239.Borst P, Jonkers J, Rottenberg S. What makes tumors multidrug resistant? Cell Cycle. 2007;6:2782–2787. doi: 10.4161/cc.6.22.4936. [DOI] [PubMed] [Google Scholar]
- 240.Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM, Lowe SW. A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 2002;109:335–346. doi: 10.1016/s0092-8674(02)00734-1. [DOI] [PubMed] [Google Scholar]
- 241.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 242.Voorzanger-Rousselot N, Alberti L, Blay JY. CD40L induces multidrug resistance to apoptosis in breast carcinoma and lymphoma cells through caspase independent and dependent pathways. BMC Cancer. 2006;6:75. doi: 10.1186/1471-2407-6-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ho EA, Piquette-Miller M. Regulation of multidrug resistance by proinflammatory cytokines. Curr Cancer Drug Targets. 2006;6:295–311. doi: 10.2174/156800906777441753. [DOI] [PubMed] [Google Scholar]
- 244.Petrovic V, Teng S, Piquette-Miller M. Regulation of drug transporters during infection and inflammation. Mol Interv. 2007;7:99–111. doi: 10.1124/mi.7.2.10. [DOI] [PubMed] [Google Scholar]
- 245.Hartmann G, Vassileva V, Piquette-Miller M. Impact of endotoxin-induced changes in P-glycoprotein expression on disposition of doxorubicin in mice. Drug Metab Dispos. 2005;33:820–828. doi: 10.1124/dmd.104.002568. [DOI] [PubMed] [Google Scholar]
- 246.Blokzijl H, van Steenpaal A, Vander Borght S, Bok LI, Libbrecht L, Tamminga M, Geuken M, Roskams TA, Dijkstra G, Moshage H, Jansen PL, Faber KN. Up-regulation and cytoprotective role of epithelial multidrug resistance-associated protein 1 in inflammatory bowel disease. J Biol Chem. 2008;283:35630–35637. doi: 10.1074/jbc.M804374200. [DOI] [PubMed] [Google Scholar]
- 247.Bottero V, Busuttil V, Loubat A, Magne N, Fischel JL, Milano G, Peyron JF. Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res. 2001;61:7785–7791. [PubMed] [Google Scholar]
- 248.Das KC, White CW. Activation of NF-kappaB by antineoplastic agents. Role of protein kinase C. J Biol Chem. 1997;272:14914–14920. doi: 10.1074/jbc.272.23.14914. [DOI] [PubMed] [Google Scholar]
- 249.Piret B, Piette J. Topoisomerase poisons activate the transcription factor NF-kappaB in ACH-2 and CEM cells. Nucleic Acids Res. 1996;24:4242–4248. doi: 10.1093/nar/24.21.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ahn KS, Sethi G, Aggarwal BB. Nuclear factor-kappa B: from clone to clinic. Curr Mol Med. 2007;7:619–637. doi: 10.2174/156652407782564363. [DOI] [PubMed] [Google Scholar]
- 251.Raju U, Gumin GJ, Noel F, Tofilon PJ. IkappaBalpha degradation is not a requirement for the X-ray-induced activation of nuclear factor kappaB in normal rat astrocytes and human brain tumour cells. Int J Radiat Biol. 1998;74:617–624. doi: 10.1080/095530098141195. [DOI] [PubMed] [Google Scholar]
- 252.Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene. 2008;27:4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, Visintin I, Rutherford T, Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 254.Braunstein S, Formenti SC, Schneider RJ. Acquisition of stable inducible upregulation of nuclear factor-kappaB by tumor necrosis factor exposure confers increased radiation resistance without increased transformation in breast cancer cells. Mol Cancer Res. 2008;6:78–88. doi: 10.1158/1541-7786.MCR-07-0339. [DOI] [PubMed] [Google Scholar]
- 255.Douglas KT. Mechanism of action of glutathione-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- 256.Sau A, Pellizzari Tregno F, Valentino F, Federici G, Caccuri AM. Glutathione transferases and development of new principles to overcome drug resistance. Arch Biochem Biophys. 500:116–122. doi: 10.1016/j.abb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 257.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 258.Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD. Dual roles of Nrf2 in cancer. Pharmacol Res. 2008;58:262–270. doi: 10.1016/j.phrs.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Cho JM, Manandhar S, Lee HR, Park HM, Kwak MK. Role of the Nrf2-antioxidant system in cytotoxicity mediated by anticancer cisplatin: implication to cancer cell resistance. Cancer Lett. 2008;260:96–108. doi: 10.1016/j.canlet.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 261.Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21:689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 262.Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3:e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9:2099–2117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Brigelius-Flohe R. Selenium compounds and selenoproteins in cancer. Chem Biodivers. 2008;5:389–395. doi: 10.1002/cbdv.200890039. [DOI] [PubMed] [Google Scholar]
- 265.Kim JH, Bogner PN, Ramnath N, Park Y, Yu J, Park YM. Elevated peroxiredoxin 1, but not NF-E2-related factor 2, is an independent prognostic factor for disease recurrence and reduced survival in stage I non-small cell lung cancer. Clin Cancer Res. 2007;13:3875–3882. doi: 10.1158/1078-0432.CCR-06-2893. [DOI] [PubMed] [Google Scholar]
- 266.Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67:546–554. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- 267.Persons DL, Yazlovitskaya EM, Cui W, Pelling JC. Cisplatin-induced activation of mitogen-activated protein kinases in ovarian carcinoma cells: inhibition of extracellular signal-regulated kinase activity increases sensitivity to cisplatin. Clin Cancer Res. 1999;5:1007–1014. [PubMed] [Google Scholar]
- 268.Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL, Pescovitz OH. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase (ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol. 2004;18:2570–2582. doi: 10.1210/me.2004-0082. [DOI] [PubMed] [Google Scholar]
- 269.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 270.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 271.Nichols A, Camps M, Gillieron C, Chabert C, Brunet A, Wilsbacher J, Cobb M, Pouyssegur J, Shaw JP, Arkinstall S. Substrate recognition domains within extracellular signal-regulated kinase mediate binding and catalytic activation of mitogen-activated protein kinase phosphatase-3. J Biol Chem. 2000;275:24613–24621. doi: 10.1074/jbc.M001515200. [DOI] [PubMed] [Google Scholar]
- 272.Kim JJ, Tannock IF. Repopulation of cancer cells during therapy: an important cause of treatment failure. Nat Rev Cancer. 2005;5:516–525. doi: 10.1038/nrc1650. [DOI] [PubMed] [Google Scholar]
- 273.Olivieri G, Bodycote J, Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science. 1984;223:594–597. doi: 10.1126/science.6695170. [DOI] [PubMed] [Google Scholar]
- 274.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 275.Fan M, Ahmed KM, Coleman MC, Spitz DR, Li JJ. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res. 2007;67:3220–3228. doi: 10.1158/0008-5472.CAN-06-2728. [DOI] [PubMed] [Google Scholar]
- 276.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–2378. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Li Z, Xia L, Lee LM, Khaletskiy A, Wang J, Wong JY, Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 278.Izzo JG, Malhotra U, Wu TT, Ensor J, Luthra R, Lee JH, Swisher SG, Liao Z, Chao KS, Hittelman WN, Aggarwal BB, Ajani JA. Association of activated transcription factor nuclear factor kappab with chemoradiation resistance and poor outcome in esophageal carcinoma. J Clin Oncol. 2006;24:748–754. doi: 10.1200/JCO.2005.03.8810. [DOI] [PubMed] [Google Scholar]
- 279.Sandur SK, Deorukhkar A, Pandey MK, Pabon AM, Shentu S, Guha S, Aggarwal BB, Krishnan S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys. 2009;75:534–542. doi: 10.1016/j.ijrobp.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.De Ridder M, Verellen D, Verovski V, Storme G. Hypoxic tumor cell radiosensitization through nitric oxide. Nitric Oxide. 2008;19:164–169. doi: 10.1016/j.niox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 281.Janssens MY, Verovski VN, Van den Berge DL, Monsaert C, Storme GA. Radiosensitization of hypoxic tumour cells by S-nitroso-N-acetylpenicillamine implicates a bioreductive mechanism of nitric oxide generation. Br J Cancer. 1999;79:1085–1089. doi: 10.1038/sj.bjc.6690173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res. 1999;59:1606–1614. [PubMed] [Google Scholar]
- 283.Mitchell JB, Wink DA, DeGraff W, Gamson J, Keefer LK, Krishna MC. Hypoxic mammalian cell radiosensitization by nitric oxide. Cancer Res. 1993;53:5845–5848. [PubMed] [Google Scholar]
- 284.Sersa G, Willingham V, Milas L. Anti-tumor effects of tumor necrosis factor alone or combined with radiotherapy. Int J Cancer. 1988;42:129–134. doi: 10.1002/ijc.2910420124. [DOI] [PubMed] [Google Scholar]
- 285.Verovski VN, Van den Berge DL, Soete GA, Bols BL, Storme GA. Intrinsic radiosensitivity of human pancreatic tumour cells and the radiosensitising potency of the nitric oxide donor sodium nitroprusside. Br J Cancer. 1996;74:1734–1742. doi: 10.1038/bjc.1996.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 287.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 289.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 290.Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 291.Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, Zhan Q, Jordan S, Duncan LM, Weishaupt C, Fuhlbrigge RC, Kupper TS, Sayegh MH, Frank MH. Identification of cells initiating human melanomas. Nature. 2008;451:345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 293.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Korkaya H, Wicha MS. Selective targeting of cancer stem cells: a new concept in cancer therapeutics. BioDrugs. 2007;21:299–310. doi: 10.2165/00063030-200721050-00002. [DOI] [PubMed] [Google Scholar]
- 295.Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, Joshua B, Kaplan MJ, Wapnir I, Dirbas FM, Somlo G, Garberoglio C, Paz B, Shen J, Lau SK, Quake SR, Brown JM, Weissman IL, Clarke MF. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780–783. doi: 10.1038/nature07733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Baba T, Convery PA, Matsumura N, Whitaker RS, Kondoh E, Perry T, Huang Z, Bentley RC, Mori S, Fujii S, Marks JR, Berchuck A, Murphy SK. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 297.Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749–1758. doi: 10.1038/sj.onc.1210811. [DOI] [PubMed] [Google Scholar]
- 298.Rhee HW, Zhau HE, Pathak S, Multani AS, Pennanen S, Visakorpi T, Chung LW. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev Biol Anim. 2001;37:127–140. doi: 10.1290/1071-2690(2001)037<0127:PPAGCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 299.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 300.Josson S, Matsuoka Y, Chung LW, Zhau HE, Wang R. Tumor-stroma coevolution in prostate cancer progression and metastasis. Semin Cell Dev Biol. 21:26–32. doi: 10.1016/j.semcdb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.De Marzo AM, Nakai Y, Nelson WG. Inflammation, atrophy, and prostate carcinogenesis. Urol Oncol. 2007;25:398–400. doi: 10.1016/j.urolonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 302.Sung SY, Hsieh CL, Law A, Zhau HE, Pathak S, Multani AS, Lim S, Coleman IM, Wu LC, Figg WD, Dahut WL, Nelson P, Lee JK, Amin MB, Lyles R, Johnstone PA, Marshall FF, Chung LW. Coevolution of prostate cancer and bone stroma in three-dimensional coculture: implications for cancer growth and metastasis. Cancer Res. 2008;68:9996–10003. doi: 10.1158/0008-5472.CAN-08-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 304.Porta C, Larghi P, Rimoldi M, Totaro MG, Allavena P, Mantovani A, Sica A. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 305.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 306.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 308.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 309.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 310.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 311.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 312.Surh YJ, Kundu JK, Na HK, Lee JS. Redox-sensitive transcription factors as prime targets for chemoprevention with anti-inflammatory and antioxidative phytochemicals. J Nutr. 2005;135:2993S–3001S. doi: 10.1093/jn/135.12.2993S. [DOI] [PubMed] [Google Scholar]
- 313.Virgili F, Marino M. Regulation of cellular signals from nutritional molecules: a specific role for phytochemicals, beyond antioxidant activity. Free Radic Biol Med. 2008;45:1205–1216. doi: 10.1016/j.freeradbiomed.2008.08.001. [DOI] [PubMed] [Google Scholar]