Abstract
The goal of this paper was to examine the literature related to stromal cell-derived factor 1-alpha (SDF-1alpha) and its receptor CXCR4 in endometrial cancer, as expression of these biomarkers has been implicated in an aggressive phenotype in other common epithelial cancers. We conducted a qualitative review of all published studies examining the role of SDF-1alpha/CXCR4 in endometrial cancer progression and prognosis. Pubmed and Ovid MEDLINE databases were searched in order to identify relevant studies for this qualitative review. Four studies have examined the role of the SDF-1alpha/CXCR4 pathway on endometrial cancer progression. The findings were contradictory; two studies reported an inverse association between overexpression and mortality while two studies reported overexpression to be associated with hallmarks of aggressive endometrial cancer. Expression of stromal-derived proteins can potentially serve as biomarkers of aggressive disease as well as biomarkers for remission monitoring, however the endometrial cancer literature has lagged behind in this area. Furthermore, the current research suffers from lack of comparability among different studies due to the utilization of different tools and lack of common outcome definitions. Future studies in this area should use clinically meaningful protein expression categories, widely accepted outcome definitions, and larger samples of patients. Finally, although standard immunohistochemistry is a mainstay in tumor marker studies, automated detection methods may be more suitable as they do not rely on subjective interpretation.
Keywords: Chemokines, CXCR4, Endometrial cancer, Stromal cell-derived factor 1-alpha, Tumor markers, Tumor microenvironment
Introduction
Although endometrial cancer is the most common gynecologic malignancy, the mortality associated with this disease is relatively low. Typically, tumors present as well-differentiated, low stage masses that are confined to the uterus and are effectively managed by hysterectomy [1]. The major burden of disease associated with endometrial cancer occurs from aggressive tumors characterized by nonendometrioid histology, high stage, poor differentiation, and metastasis to regional nodes. Patients with these types of tumors are more likely to suffer from recurrence as well as have a poor overall survival, and therefore are prime candidates for adjuvant therapy [2]. Despite this knowledge, surgical factors alone are not sufficient to make recommendations regarding specific chemotherapies that would be most beneficial for the individual patient. Moreover, the toxicities associated with these therapies require selection criteria that will predict which patients would most likely benefit from such treatments. The addition of tumor markers to the current endometrial cancer prognostic panel would significantly aid in the stratification of patients into appropriate subgroups for treatment, similar to the work that has been done in the breast cancer field.
The tumor microenvironment is composed of cancer cells as well as stromal cells and the extracellular matrix [3]. Common epithelial mutations which give rise to endometrial tumors include PTEN inactivation, beta-catenin, k-ras, p53, and HER2/neu mutations, as well as microsatellite instability [4]. Importantly, cancer cells continue to communicate with host stromal cells after acquiring these initial mutations. Moreover, cancer cells are proficient in exploiting a wide array of signaling pathways regulated by stromal-derived proteins with the purpose of maintaining cancer proliferation and promoting metastasis; one such class of signaling molecules is the chemokine family. Chemokines are chemotactic cytokines that direct the movement of cells; cells which express the appropriate chemokine receptors migrate towards high concentrations of chemokines along a chemokine gradient [5]. Therefore, their role in tumor invasion and metastasis has been explored frequently in the cancer literature. Furthermore, chemokines and their receptors are known to play an important role in immune responses, and recent evidence suggests that a particular CXC receptor, CXCR4 (CXC motif receptor 4), is the predominately expressed chemokine receptor in many human cancers [6]. CXCR4 is involved in chemotaxis, hematopoiesis, and tumor metastasis in breast, ovarian, and thyroid cancers [5, 7–9].
The CXCR4 receptor and its ligand, stromal cell-derived factor 1-alpha (SDF-1alpha, CXCL12) may potentially enhance endometrial tumor progression and metastasis. In vitro studies report that SDF-1alpha is a potent stimulator of endometrial cancer cell proliferation [10, 11], yet the association between expression of these markers and prognostic factors is inconsistent in the literature [12–15]. Understanding the association between these proteins and clinical factors may inform therapeutic protocols to better impact survival. The goal of this paper was to review the literature related to endometrial cancer and the SDF-1alpha/CXCR4 pathway in order to characterize the current state of knowledge regarding this relationship.
Methods
Literature Search Strategy
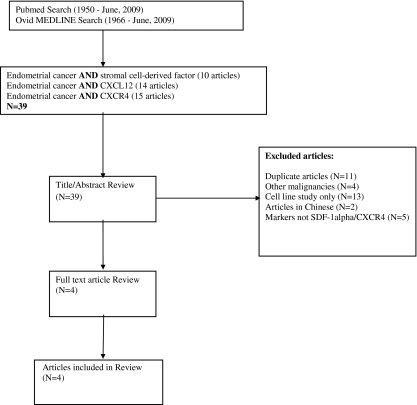
To identify published studies on SDF-1alpha/CXCR4 in endometrial cancer, two electronic databases, Pubmed (1950-June 8, 2009) and Ovid MEDLINE (1966-June 8, 2009), were accessed through the Health Sciences Library System at the University of Pittsburgh. Three separate searches were performed in each database with the following keywords: 1.) endometrial cancer AND stromal cell-derived factor 2.) endometrial cancer AND CXCL12 3.) endometrial cancer AND CXCR4. No restrictions on language or human subjects were used. Unpublished reports, including dissertations and conference abstracts, were not considered.
Inclusion and Exclusion Criteria
All titles and abstracts of the retrieved articles from Pubmed and Ovid MEDLINE were reviewed. Studies were included if the following criteria were met 1.) human tissue (ex vivo) was studied, 2.) mRNA or protein expression of SDF-1alpha or CXCR4 was characterized, and 3.) the association between pathologic variables and/or outcomes in relation to expression were explored. Some of the ex vivo studies that met the inclusion criteria for this study also included in vitro analyses of the migratory and proliferative effects of the markers. When this was the case, these results were also summarized in this review.
The major reasons for exclusion were 1.) endometrial cancer was not the primary cancer, 2.) studies examined the invasive or mitogenic capacity of the proteins in endometrial cancer cell lines only, and 3.) the markers studied were not SDF-1alpha or CXCR4. Figure 1 outlines the complete search strategy used for this review.
Fig. 1.
Flow chart of search strategy for article identification
Data Extraction
Data from each study included in the review were extracted by one reviewer. Extracted data elements included: sample population, study design and laboratory assays, important covariates, primary results related to expression and prognostic factors, and secondary findings. Table 1 summarizes the data elements abstracted from each article included in this review.
Table 1.
Studies examining the role of SDF-1alpha/CXCR4 in endometrial cancer (N = 4)
| Author | Sample population | Study design and lab methods | Important covariates | Primary results with estimates | Secondary findings |
|---|---|---|---|---|---|
| Mizokami (2004) |
Human samples Human endometrial cancers (N = 41) obtained following hysterectomy—source of sample not described Cell lines Five endometrial cancer cell lines: AMEC, Ishikawa, HEC1A, HEC50, RL95—purchased from commercial source |
Cross-sectional: association between protein expression (SDF-1alpha and CXCR4) and histologic grade Lab methods 1.) RT-PCRa 2.) IHCb 3.) Western blot |
Exposure Mean protein expression of SDF-1alpha and CXCR4 in endometrial cancer tissues Outcome Histologic grade (grade 1 and 2 tumors vs. grade 3 tumors) |
Mean CXCR4 expression was greater in Grade 1/2 tumors vs. Grade 3 tumors (p < 0.05) -nonparametric Mann-Whitney U-test Mean SDF-1alpha expression was greater in Grade 1/2 tumors vs. Grade 3 tumors (p < 0.05) -nonparametric Mann-Whitney U-test |
CXCR4 mRNA and protein was detected in cell lines, the normal endometrium of the secretory phase, and endometrial cancer tissue (RT-PCR & Western blot) Stromal expressions of SDF-1alpha and CXCR4 were not significantly different among tumors of different grade (IHC) |
| Kodama (2007) |
Human samples Human endometrial cancers (N = 166) obtained following hysterectomy and oophorectomy at the Okayama Hospital (Japan) between Jan, 1997 and Nov, 2004 -patients with distant metastases excluded |
Cross-sectional: association between CXCR4 protein expression and clinicopathological features (listed in important covariates) Longitudinal: association between CXCR4 expression and disease free survival Lab methods 1.) IHC 2.) RT-PCR |
Exposure Dichotomous CXCR4 protein expression (positive vs. negative) in endometrial cancer tissues Outcomes FIGO stage (late/early), myometrial invasion (pos/neg), cervical involvement (pos/neg), lymphovascular invasion (pos/neg), lymph node metastasis (pos/neg), ovarian metastasis (pos/neg) Disease free survival and overall survival |
Positive CXCR4 was significantly associated with positive myometrial invasion, late FIGO stage, positive lymphovascular invasion, and positive ovarian metastasis (p < 0.05) -chi-square test Disease free survival rates were not significantly different in patients with positive CXCR4 expression compared to negative expression (p = 0.089) -log rank test statistic Overall survival rates were significantly different in patients with positive CXCR4 expression compared to negative expression (p = 0.035) -log rank test statistic |
CXCR4 expression was not an independent prognostic factor for endometrial cancer patient survival -Cox proportional hazards model CXCR4 mRNA levels (RT-PCR) were observed to be significantly higher in tumors with positive CXCR4 protein (IHC) expression (p < 0.0001) |
| Tsukamoto (2007) |
Human samples Human endometrial adenocarcinomas (N = 34) obtained from patients at Nagoya University Hospital between 1994 and 2002 Cell lines Five endometrial cancer cell lines: AMEC, Ishikawa, HEC1A, HEC50, RL95—purchased from commercial source |
Cross-sectional: association between CXCR4 protein expression and myometrial invasion Lab methods 1.) IHC 2.) Western blot 3.) ELISAd 4.) Migration assay |
Exposure Mean CXCR4 protein expression in endometrial cancer tissues Outcomes Myometrial invasion (more than 1/2 invaded vs. less than 1/2 invaded) |
Mean CXCR4 expression was significantly greater in tumors that invaded more than half of the myometrium compared to tumors that invaded less than one half of the myometrium (p < 0.05) -nonparametric Mann-Whitney U-test |
CXCR4 protein was expressed in endometrial cancer cell lines (Western blot) Endometrial cancer cells co-cultured with uterine smooth muscle cells increased migration of cells compared to cells cultured alone (MAc) Normal human uterine smooth muscle cells produce SDF-1alpha (ELISA) SDF-1alpha activated the PI-3 K/Akt pathway (Western blot) Migration was inhibited by CXCR4 antibodies and antagonists (MA) |
| Gelmini (2009) |
Human samples Endometrial cancer patients (N = 41) consecutively admitted for surgery at the Department of Gynecology (University of Florence) from November, 2006 to January, 2008 Experimental component in vivo mouse model |
Cross-sectional: association between mRNA and protein expression (CXCR4 and SDF-1alpha) and histologic grade Lab methods 1.) RT-PCR 2.) IHC 3.) in vivo model |
Exposures Median CXCR4 mRNA expression Median SDF-1alpha mRNA expression Outcomes Histologic grade (early grade vs. late grade) |
Median CXCR4 mRNA expression was significantly lower in grade 1 tumors compared to grade 2/3 tumors (p = 0.035) -statistical method not described—assumption: Mann-Whitney U-test or Student’s t-test Median SDF-1alpha mRNA expression was not significantly different between grade 1 tumors and grade 2/3 tumors -statistical method not described |
SDF-1alpha mRNA expression was significantly higher in normal tissues compared to cancers (RT-PCR) CXCR4 mRNA was significantly higher in cancers than in normal counterparts (RT-PCR) All endometrial cancers had a high, uniform expression of CXCR4 protein regardless of grade (IHC) After injection of cancer cells, metastases were evident. Treatment with a CXCR4 neutralizing monocolonal antibody reduced the number and size of all metastases (in vivo model) |
aRT-PCR: Reverse transcription polymerase chain reaction bIHC: Immunohistochemistry cMA: Migration assay dELISA: enzyme-linked immunosorbent assay
Results
Four studies have analyzed the SDF-1alpha/CXCR4 axis in endometrial cancer, however, the conclusions regarding the prognostic role of these proteins are conflicting. Mizokami et al. [12] studied the relationship between histological grade, an important prognostic factor in endometrial cancer, and protein expression of SDF-1alpha and CXCR4 in 41 endometrial cancer cases. Tissues obtained from hysterectomy specimens were analyzed immunohistochemically (IHC) and staining was classified on a 3-level ordinal scale: negative or weakly positive, moderately positive, and strongly positive. Both SDF-1alpha and CXCR4 expression were inversely associated with histological grade in the carcinoma compartment of the tissue. In grade 1 and 2 tumors, the mean level of SDF-1alpha staining was 0.54 while grade 3 tumors had a mean level of 0.10, which was statistically significant at p = 0.05. The mean level of CXCR4 staining in grade 1 and 2 tumors was 0.73 while grade 3 tumors had a mean expression of 0.17 (p < 0.05) [12]. Stromal expression of SDF-1alpha and CXCR4 was also assessed with IHC, however no significant difference in stromal SDF-1alpha or CXCR4 expression among different grade cancers was detected.
Similarly, Kodama et al. [16] reported CXCR4 expression to be significantly lower in patients (N = 55) with characteristics of advanced endometrial cancer. Protein expression was analyzed by IHC and dichotomized as positive or negative. Tissue specimens were labeled as ‘positive’ when treatment with the appropriate antibody stained more than 50% of cells. In the unadjusted models, CXCR4 expression was negative in patients with late stage tumors (p = 0.004), deep muscular invasion (p = 0.05), lymph node metastasis (p = 0.03), ovarian metastasis (p = 0.003), and positive peritoneal cytology (p = 0.001); all of which indicate an aggressive cancer phenotype. Additionally, survival following surgery was examined; CXCR4 expression was not an independent predictor of either overall or disease-free survival adjusted for other known endometrial cancer prognostic factors [13]. Results from these two studies conflict with the notion that the SDF-alpha/CXCR4 pathway is involved in an aggressive endometrial cancer phenotype, as tumors with poor prognostic traits were less likely to have strong positive staining.
The third study to examine this pathway was performed by Tsukamoto et al. [14] which investigated the interaction between SDF-1alpha and CXCR4 on the ability of endometrial tumors to invade the muscular layer of the endometrium. Muscular infiltration is an important prognostic factor in endometrial cancer. Regional node metastases and distant organ metastases are significantly more likely to occur as the depth of muscular invasion increases [15]. Five human endometrial cancer cell lines and endometrial cancer tissues resected from endometrial cancer patients (N = 34) were examined. CXCR4 protein expression was analyzed by IHC and scored on a 3-titered scale similar to Mizokami et al. [12]. The outcome, muscular invasion, was classified on the basis of depth: more than half of the muscle layer invaded vs. less than half of the muscle layer invaded. Mean expression of CXCR4 was significantly higher in those tumors that invaded more than half of the myometrium compared to those tumors with superficial invasion (p < 0.05). The in vitro assays revealed two key findings: 1.) SDF-1alpha activates the phosphoinositide 3-kinase/Akt pathway as shown by Western blot analysis using a p-Akt-specific antibody and 2.) Akt activation is required for uterine smooth muscle cell-induced endometrial cancer cell migration, as treatment with a PI3K inhibitor significantly impeded cell migration [14]. This study suggests that the SDF-1alpha/CXCR4 axis plays a significant role in endometrial cancer invasion, as overexpression of the receptor was associated with greater depth of muscle invasion.
Most recently, Gelmini et al. [15] examined mRNA and protein expression of CXCR4, CXCR7, and SDF-1alpha in 41 patients who underwent hysterectomy for the treatment of endometrial cancer. CXCR7 was identified by Burns et al. [17] as a novel receptor for SDF-1alpha; in vitro, the interaction of SDF-1alpha and CXCR7 enhanced growth and adhesion of cells from several different cell lines. Tumor samples and adjacent non-neoplastic tissues were analyzed by reverse transcriptase-polymerase chain reaction (RT-PCR) and IHC for the detection of mRNA and protein, respectively. The main outcome in the ex vivo portion of the study was histological grade, which was dichotomized as grade 1 tumors vs. grades 2 and 3 tumors. As in the previous studies, IHC analyses reported semi-quantitative scoring of protein expression (3-titered scale) whereas RT-PCR analysis of mRNA expression generates a continuous measure. CXCR4 mRNA expression was significantly higher in endometrial cancers compared to the paired normal tissue (medians: 10.3 vs. 2.9, p = 0.035). Conversely, SDF-1alpha mRNA expression was significantly higher in normal tissues compared to corresponding cancer tissues (medians: 15.6 vs. 5.1, p = 0.002). This relationship mirrors the paradigm of expression noted in other cancers; CXCR4-expressing cancer cells are able to migrate to non-cancerous tissues where SDF-1alpha is high in concentration, leading to invasion and metastasis in distant sites. Additionally, CXCR4 mRNA expression was significantly lower in grade 1 tumors compared to grades 2 and 3 tumors indicating that CXCR4 expression tends to increase in poorly differentiated cancers [15]. With regard to CXCR7, no significant difference in mRNA expression between the cancer and paired normal tissues was noted, suggesting that this receptor is not an important factor in endometrial cancer prognosis. Importantly, no difference in CXCR4 protein expression by IHC was detected; all endometrial carcinomas, regardless of histologic grade, showed uniform and high expression of the CXCR4 protein.
In addition to exploring this pathway in human endometrial cancer tissues, an in vivo mouse model was used to explore the invasive capacity of CXCR4 [15]. Following intraperitoneal injection of the HEC1A human endometrium adenocarcinoma cell line, experimental mice developed distant metastases in the lung, liver, and peritoneum. Nude mice treated with an anti-CXCR4 antibody had complete regression of liver and lung metastases compared to the control mice. In anti-CXCR4 treated mice the metastatic index of the peritoneum was 2.5% compared to a metastatic index of 70% in the control mice. The implications from this study are significant; treatment with chemokine antagonists may reduce distant metastases in patients where CXCR4 is highly expressed.
Discussion
The literature reviewed in this article demonstrates that the role of the SDF-1alpha/CXCR4 pathway in endometrial cancer progression is still conflicting. Of the four studies that examined this pathway, two reported that increasing levels of protein expression of either or both markers to be inversely associated with higher histologic grade, advanced stage, and other poor prognostic factors in endometrial cancer [12]. The remaining two studies reported a direct association between greater expression and poor prognostic factors for endometrial cancer [14, 15]. These contradictory findings are intriguing but may be explained by differences in study design. First, different prognostic factors were examined between the four studies, including histologic grade [12, 15], FIGO stage [13], depth of muscle invasion [13, 14], cervical involvement, ovarian metastasis, peritoneal cytology, and lymph node metastasis [13]. Although these are indicators of an aggressive endometrial cancer phenotype, the proteins under study may be differently associated with each factor. Furthermore, when similar prognostic factors were explored, different groupings were used. For example, Mizokami et al. [12] grouped grade 1 and 2 tumors together whereas the study by Gelmini et al. [15] combined grade 2 and 3 tumors as a distinct group. The choice for groupings is arbitrary, however, this may influence the direction of the findings.
Second, the protein expression classifications among the four studies varied. Two standard methods for scoring protein expression in IHC exist: the first is the 3-titered scale (1 = weakly positive, 2 = moderately positive, and 3 = strongly positive) where staining intensity is scored relative to negative controls. The second method is the H score which is a product of staining intensity and the percent of cells stained [18]. In this review, two of the studies used a 3-titer scale system [12, 14], one used an H score method [15], and one used a broad classification for protein expression, positive vs. negative, based on whether 50% or more of cells in the specimen stained positive[13]. Comparing results from different pathologists across studies is not ideal, especially when similar scoring methods are not used. The lack of standardization among IHC protocols is not a limitation of each study, per say, however the inability to directly compare studies is a limitation of the current state of knowledge.
Even though immunohistochemistry is the gold standard for the detection and localization of antigens in tissues, this molecular tool may lack the sensitivity to place patients into meaningful risk strata. Countless studies review the limitations presented by IHC studies, however no cost-effective, easily implemented alternative currently exists [19, 20]. Furthermore, subtle differences in the optimization of the assay can significantly change the association between the biomarker and the outcome. McCabe et al. [18] showed that diluting the concentration of the antibody used in IHC reversed the association between p53 expression and survival in breast cancer patients. When a high antibody concentration was used, low expression of p53 was significantly associated with poorer survival, yet when a low antibody concentration was used, high expression of p53 was associated with poorer survival. Future studies that explore the role of these biomarkers should consider automated techniques that are less subject to inconsistencies. The automated quantitative analysis (AQUA) system is a tool that eliminates subjectivity of traditional scoring methods and generates continuous and reproducible scoring of protein expression [18]. Combining automated techniques with tissue microarrays, which removes differences in antigen retrieval and staining protocols, may produce more valid and reliable associations between protein expression and outcomes.
The main limitation of this review is the small number of studies that met the inclusion criteria. The small number of existing studies in the area of endometrial cancer can be partially attributed to the high laboratory costs associated with the investigation of tumor microenvironment. The inconsistency between studies can be resolved with more research in this area. Furthermore, the mechanism by which CXCR4 and SDF-1alpha enhance cancer progression has been frequently studied in other epithelial cancers. While not directly relevant for endometrial cancer, research from other cancers can serve as clues for the direction of future studies in endometrial cancer research.
For example, Zhang et al. [21] reported SDF-1alpha and CXCR4 to be overexpressed in prostate cancers compared to hyperplastic prostate tissues. Furthermore, overexpression of SDF-1alpha was associated with an increase in perineural invasion of the prostate cancer cells, which can lead to invasion and metastasis. Similarly, Kajiyama et al. [22] reported overall survival was significantly worse among epithelial ovarian cancer patients who had positive expression of CXCR4 compared to those with negative expression. An in vitro experiment confirmed the functional ability of SDF-1alpha to increase peritoneal metastasis via an enhanced attachment of the ovarian tumor cells to the cells lining the peritoneal cavity [22]. The breast cancer literature has seen a similar difficulty in ascribing a protective or negative role of the SDF-1alpha/CXCR4 pathway and poor outcomes. SDF-1alpha expression was reported to be an independent marker of disease free and overall survival in breast cancer. Patients with high levels of SDF-1alpha were 21% less likely to have a local recurrence compared to those with low expression of SDF-1alpha [23]. Conversely, Liu [24] recently reported that increased expressions of the SDF-1alpha/CXCR4 proteins may be an important molecular mechanism facilitating lymph node metastasis in invasive micropapillary carcinoma of the breast, a breast cancer subtype that often carries an unfavorable prognosis. Invasive micropapillary carcinomas showed increased expression of SDF-1 and CXCR4 compared with control cases of invasive ductal carcinoma; moreover, SDF-1alpha and CXCR4 correlated positively with the rate and extent of lymph node metastasis.
Despite the controversy surrounding the role of this pathway in cancer progression, the efficacy of CXCR4 antagonists is currently being tested in clinical trials for the management of epithelial and hematopoietic tumors. Therefore, the importance of this pathway is even more salient as the proper selection of patients for treatment with such inhibitors can directly impact survival outcomes. In order to add to existing knowledge in this area, forthcoming studies need to carefully describe the study population that is being examined with respect to demographic factors and risk factors, when possible. A general limitation of tissue studies is that the surgical pathology report is the only available source of information for these specimens. As such, details such as race and other important exposures are generally missing. Studies using cancer registry data and banked specimens can avoid this pitfall, as important data elements are retrievable. Moreover, laboratory analyses utilizing IHC should carefully optimize reagents and antibodies used, as this can significantly impact the results. Where possible and practical, tissue microarray analyses may be more suitable for the study of biomarkers in large samples of patients compared to whole-tissue section analyses. Finally, use of small molecule inhibitors of the SDF-1alpha and CXCR4 molecules may be attractive treatment alternatives where fertility conservation is desired; a careful evaluation of the outcomes related to less invasive procedures in comparison to hysterectomy will need to be fully evaluated prior to clinical recommendations.
Acknowledgements
This research was supported by a National Institutes of Health grant R25-CA57703.
References
- 1.Sohaib SA, et al. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62(1):28–34. doi: 10.1016/j.crad.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Amant F, et al. Endometrial cancer. Lancet. 2005;366(9484):491–505. doi: 10.1016/S0140-6736(05)67063-8. [DOI] [PubMed] [Google Scholar]
- 3.Itano N, Zhuo L, Kimata K. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 2008;99(9):1720–1725. doi: 10.1111/j.1349-7006.2008.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiozawa T, Konishi I. Early endometrial carcinoma: clinicopathology, hormonal aspects, molecular genetics, diagnosis, and treatment. Int J Clin Oncol. 2006;11(1):13–21. doi: 10.1007/s10147-005-0546-1. [DOI] [PubMed] [Google Scholar]
- 5.Balkwill F. Cancer and the chemokine network. Nat Rev, Cancer. 2004;4(7):540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 6.Zlotnik A. Chemokines in neoplastic progression. Semin Cancer Biol. 2004;14(3):181–185. doi: 10.1016/j.semcancer.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Hall JM, Korach KS. Stromal cell-derived factor 1, a novel target of estrogen receptor action, mediates the mitogenic effects of estradiol in ovarian and breast cancer cells. Mol Endocrinol. 2003;17(5):792–803. doi: 10.1210/me.2002-0438. [DOI] [PubMed] [Google Scholar]
- 8.Scotton CJ, et al. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002;62(20):5930–5938. [PubMed] [Google Scholar]
- 9.Hwang JH, et al. CXC chemokine receptor 4 expression and function in human anaplastic thyroid cancer cells. J Clin Endocrinol Metab. 2003;88(1):408–416. doi: 10.1210/jc.2002-021381. [DOI] [PubMed] [Google Scholar]
- 10.Zhao D, et al. Stromal cell-derived factor 1alpha stimulates human endometrial carcinoma cell growth through the activation of both extracellular signal-regulated kinase 1/2 and Akt. Gynecol Oncol. 2006;103(3):932–937. doi: 10.1016/j.ygyno.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 11.Li XP, et al. Growth and activation of PI-3K/PKB and Akt by stromal cell-derived factor 1alpha in endometrial carcinoma cells with expression of suppressor endoprotein PTEN. Chin Med J (Engl) 2006;119(5):378–383. [PubMed] [Google Scholar]
- 12.Mizokami Y, et al. Stromal cell-derived factor-1alpha-induced cell proliferation and its possible regulation by CD26/dipeptidyl peptidase IV in endometrial adenocarcinoma. Int J Cancer. 2004;110(5):652–659. doi: 10.1002/ijc.20183. [DOI] [PubMed] [Google Scholar]
- 13.Kodama J, et al. Expression of the CXCR4 and CCR7 chemokine receptors in human endometrial cancer. Eur J Gynaecol Oncol. 2007;28(5):370–375. [PubMed] [Google Scholar]
- 14.Tsukamoto H, et al. Uterine smooth muscle cells increase invasive ability of endometrial carcinoma cells through tumor-stromal interaction. Clin Exp Metastasis. 2007;24(6):423–429. doi: 10.1007/s10585-007-9079-5. [DOI] [PubMed] [Google Scholar]
- 15.Gelmini S, et al. The CXCR4/CXCL12 axis in endometrial cancer. Clin Exp Metastasis. 2009;26(3):261–268. doi: 10.1007/s10585-009-9240-4. [DOI] [PubMed] [Google Scholar]
- 16.Kodama J, et al. Prognostic significance of stromal versican expression in human endometrial cancer. Ann Oncol. 2007;18(2):269–274. doi: 10.1093/annonc/mdl370. [DOI] [PubMed] [Google Scholar]
- 17.Burns JM, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCabe A, et al. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97(24):1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 19.Henson DE. Back to the drawing board on immunohistochemistry and predictive factors. J Natl Cancer Inst. 2005;97(24):1796–1797. doi: 10.1093/jnci/dji449. [DOI] [PubMed] [Google Scholar]
- 20.Cregger M, Berger AJ, Rimm DL. Immunohistochemistry and quantitative analysis of protein expression. Arch Pathol Lab Med. 2006;130(7):1026–1030. doi: 10.5858/2006-130-1026-IAQAOP. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, et al. Chemokine CXCL12 and its receptor CXCR4 expression are associated with perineural invasion of prostate cancer. J Exp Clin Cancer Res. 2008;27:62. doi: 10.1186/1756-9966-27-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajiyama H, et al. Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. Int J Cancer. 2008;122(1):91–99. doi: 10.1002/ijc.23083. [DOI] [PubMed] [Google Scholar]
- 23.Mirisola V, et al. CXCL12/SDF1 expression by breast cancers is an independent prognostic marker of disease-free and overall survival. Eur J Cancer. 2009;45(14):2579–2587. doi: 10.1016/j.ejca.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, et al. Increased expression of SDF-1/CXCR4 is associated with lymph node metastasis of invasive micropapillary carcinoma of the breast. Histopathology. 2009;54(6):741–750. doi: 10.1111/j.1365-2559.2009.03289.x. [DOI] [PubMed] [Google Scholar]