Abstract
Ginger, the rhizome of Zingiber officinale, is a traditional medicine with carminative effect, anti-nausea, anti-inflammatory, and anti-carcinogenic properties. This study examined the growth inhibitory effects of [8]-shogaol, one of pungent phenolic compounds in ginger, on human leukemia HL-60 cells. It demonstrated that [8]-shogaol was able to induce apoptosis in a time- and concentration-dependent manner. Treatment with [8]-shogaol caused a rapid loss of mitochondrial transmembrane potential, stimulation of reactive oxygen species (ROS) production, release of mitochondrial cytochrome c into cytosol, and subsequent induction of procaspase-9 and procaspase-3 processing. Taken together, these results suggest for the first time that ROS production and depletion of the glutathione that committed to [8]-shogaol-induced apoptosis in HL-60 cells.
Keywords: [8]-shogaol, human leukemia HL-60 cells, apoptosis, reactive oxygen species
Introduction
A large number of chemopreventive and therapeutic agents that induce apoptosis in malignant cells have shown promise in the fight against cancer (1). The consumption of fruits and vegetables is associated with a protective action for many human cancers. Phytochemicals such as curcumin, zerumbone, and tea polyphenols are known to induce apoptosis through downregulation of apoptotic suppressor, Bcl-2 and Bcl-XL, in several tumor cell lines (2–4). Ginger (Zingiber officinale) is widely used as a spice, and as a medicinal herb in traditional herbal medicine. In traditional medicine, ginger contains pungent phenolic substances such as gingerols, shogaols, paradols and gingerdiols with pronounced antioxidative, anti-inflammatory, and anti-carcinogenic activities (5, 6). In the fresh ginger rhizome, the gingerols were identified as the major active components, and 6-gingerol is the most abundant constituent (7). Recent reports have shown that shogaols have much stronger anti-inflammatory activity and growth inhibitory effects on cancer cells than gingerols (8). Among shogaols, [6]-, [8], [10]-shogaols exhibited similar extent of growth inhibitory effects against human cancer cell lines (9). Previously, we have reported the molecular mechanism of 6-shogal-inducing apoptosis in human colon carcinoma COLO 205 cells (8). In our recent study, we found that the amount of [8]-shogaol may reach as high as 8–12 mg/100 g sample in ground dried ginger powder (Sang et al. unpublished data).
Apoptosis is defined as a type of cell death, involving the concerted action of a number of intracellular signaling pathways, including members of the caspases family of cysteine proteases, stored in most cells as zymogens or procaspases (10). The two main apoptotic pathways, but ultimately converging, apoptosis signaling pathways: the death receptor (extrinsic), which is activated by death receptors; and mitochondrial (intrinsic) pathways. The intrinsic pathway is characterized by the permeabilization of outer mitochondrial membrane and release of several pro- apoptotic factors into the cytoplasm. (11). Moreover, the mitochondrial pathway is regulated by the Bcl-2 family of proteins, including anti-apoptotic proteins such as Bcl-2 and Bcl-XL and pro-apoptotic proteins such as Bad, Bid, Bim, Bax, and Bak (12).
In this study, we investigated the mechanism of apoptosis by [8]-shogaol, one of the major shogaol in dried ginger, in human leukemia HL-60 cells and the possible involvement of mitochondria. The role of Bcl-2 family in the modulation of membrane potential was also investigated.
Materials and methods
Cell culture and chemicals
Human promyelocytic leukemia (HL-60) cells obtained from American Type Culture Collection (Rockville, MD) were grown in 90% RPMI 1640 and 10% fetal bovine serum (GIBCO BRL, Grand Island, NY), supplemented with 2 mM glutamine (GIBCO BRL), 1% penicillin/streptomycin (10000 units of penicillin/mL and 10 mg streptomycin/mL). Medium was normally changed to phenol red-free RPMI 1640 before [8]-shogaol treatment. Propidium iodide was obtained from Sigma Chemical Co. (St. Louis, MO).
Isolation of [8]-shogaol from ginger extract
Crude ginger extract was a gift from Sabinsa Corporation (Piscataway, NJ). It was subjected to solvent extraction prior to column chromatography. The methanolic extract of ginger (50 g) was dissolved in ethyl acetate and partitioned with water. The ethyl acetate portion was collected, evaporated and the residue dissolved in methanol. This was further partitioned with hexane and the methanolic portion evaporated and used for the isolation of gingerols and shogaols.
The methanolic extract obtained by the above solvent extraction step was loaded onto a Sephadex column and eluted with 95% ethanol to yield gingerols, shogaols and related compounds. These extracts were analyzed by thin layer chromatography (using a 2:1 hexane: ethyl acetate solvent system) and similar fractions were combined. The extracts were then subjected to Diaon HP-20 column chromatography and eluted with 30%– 70% ethanol. Similar fractions were combined to give rise to gingerol mixture and shogaol mixture. [8]-shogaol were purified and identified from ginger extract in our laboratory (9) and the purify was assessed as 99.8% by HPLC analysis. An HPLC-ECD/UV system (ESA, Chelmsford, MA, USA) consisting of an ESA model 584 HPLC pump, an ESA model 542 autosampler, an ESA organizer, an ESA coularray detector coupled with two ESA model 6210 four sensor cells, and an ESA 526 UV detector was used in our study. Chromatographic analysis was performed on a 150 mm × 4.6 mm, 5 µm, SupelcosilTM LC-18 column. The mobile phases were consisted of solvent A (30 mM sodium phosphate buffer, pH 3.35) and Solvent B (15 mM sodium phosphate buffer containing 58.5% acetonitrile and 12.5% tetrahydrofuran, pH 3.45). The gradient elution had the following profile: 50% – 55% B from 0 min to 10 min; 55% – 60% B from 10 min to 14 min; 60% – 65% B from 14 min to 15 min; 65% – 100% B from 15 min to 40 min; then 50% B from 40.1 min to 53 min with a flow rate of 1.0 mL/min.
Cell Viability Assay
Cell viability was assayed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). Briefly, human leukemia HL-60 cells were plated at a density of 1×105 cells/mL into 24 well plates. After overnight growth, cells were pretreated with a series of concentration of [8]-shogaol for 24 h. The final concentration of dimethyl sulfoxide (DMSO) in the culture medium was < 0.05%. At the end of treatment, 30 µL of MTT was added, and the cells were incubated for a further 4 h. Cell viability was determined by scanning with an enzyme-linked immunosorbent assay reader with a 570 nm filter (13).
Flow cytometry
Cells (2 × 105) were seeded into 60-mm Petri dishes and treated with [8]-shogaol for different concentrations and time periods at 37 °C for 24 h. The cells were then harvested, washed with PBS, resuspended in 200 µL of PBS, and fixed in 800 µL of 100% ethanol at −20 °C. After being left to stand overnight, the cell pellets were collected by centrifugation, resuspended in 1 mL of hypotonic buffer (0.5% Triton X-100 in PBS and 0.5 µg/mL RNase), and incubated at 37 °C for 30 min. Next, 1 mL of propidium iodide solution (50 µg/mL) was added, and the mixture was allowed to stand in crushed water ice for 30 min. Fluorescence emitted from the propidium iodide-DNA complex was quantitated after excitation of the fluorescent dye by FACScan cytometry (Becton Dickinson, San Jose, CA). Quantitation of the fraction of each cell cycle stage was performed with ModFit LT for Mac 3.0 software (Becton Dickinson, San Jose, CA).
Determination of ROS production and intracellular GSH depletion
ROS production was monitored by flow cytometry using DCFH-DA. This dye is a stable, nonpolar compound that readily diffuses into cells and is hydrolyzed by intracellular esterase to yield the DCFH, trapped within the cells. Hydrogen peroxide, or low molecular weight peroxides, produced by the cells oxidize DCFH to the highly fluorescent compound 2′,7′-dichlorofluorescein (DCF). Thus, the fluorescence intensity is proportional to the amount of hydrogen peroxide produced by the cells. Dihydroethidium (DHE) was used as a probe, recognizing mainly the oxygen species superoxide anion. The thio-reactive fluorescent dye 5-chloromethylfluorescein diacetate (CMFDA) was assayed for GSH determination. CMFDA, a nonfluorescence compound, can enter the intracellular compartment and react with esterase to allow CMF trapped inside the cell. The interaction of CMF with thio group of GSH will result in the generation of fluorescence (14). Cells were treated with [8]-shogaol (30 µM) for different time periods, and CMFDA (30 µM), D CFH-DA (20 µM), and DHE (20 µM), respectively, was added to the medium for a further 30 min at 37 °C. Histograms were analyzed using Cell Quest software and were compared with histograms of untreated control cells.
Analysis of mitochondrial trans-membrane potential
The change of the mitochondrial trans-membrane potential was monitored by flow cytometry. Briefly, HL-60 cells were treated with [8]-shogaol (30 µM) for different time periods, and the mitochondrial trans-membrane potential was measured directly using 40 nM 3,3′-dihexyloxacarbocyanine [DiOC6(3)] (Molecular Probes, Eugene, OR). Fluorescence was measured after staining the cells for 30 min at 37 °C. Histograms were analyzed using Cell Quest software and were compared with histograms of untreated, control cells.
Preparation of subcellular fraction
Subcellular fraction was performed as described previously (15). Briefly, cells were trypsinized and washed once with ice-cold phosphate-buffered saline following lysed in lysis buffer and homogenized (20 strokes with a B-pestle Dounce homogenizer). The lysate was centrifuged (750 ×g for 5 min) to remove nuclei and unlysed cells and centrifuged again (10,000 ×g for 10 min) to obtain the mitochondria fraction (pellet). The supernatant was centrifuged at 14,000 ×g for 1 h to obtain the cytosolic fraction.
Western blotting
For the determination of the expression of Bcl-2 family, Bid, Fas, and FasL in HL-60 cells, the nuclear and cytosolic proteins were isolated f rom HL-60 cells in Petri dishes with a cell scraper after treatment with 30 µM [8]-shogaol for 0, 1, 2, 3, 6, 9, and 12 h. Nuclear and cytoplasmic extracts were prepared according to a modification of the method as described previously. Briefly, at the end of culture, the cells were suspended in hypotonic buffer A (10 mM HEPES, pH7.6, 10 mM KCl, 0.1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride) for 10 min on ice, and vortexed for 10 sec. Nuclei were pelleted by centrifugation at 12000 ×g for 20 sec, and the supernatants, containing cytosolic proteins, were collected. The pellet, were then suspended in buffer C (20 mM HEPES pH 7.6, 25% glycerol, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride) for 30 min on ice. The supernatants containing nuclear proteins, were collected by centrifugation at 12000× g for 20 min and stored at −70 °C. Western blotting analysis was performed by the method described previously (8) . Briefly, cell lysates were prepared, electrotransferred, immunoblotted with antibodies, and then visualized by measuring the chemiluminescence of the blotting agent (ECL, Amersham Corp., Arlington Heights, IL). The mitochondria and cytosolic fractions isolated from the cells were used for immunoblot analysis of cytochrome c as previously described (16). The cytochrome c protein was detected using an anti-cytochrome c antibody (Research Diagnostic Inc., Flanders, NJ).
Activity of caspases
After [8]-shogaol treatment, cells were collected and washed with PBS and suspended in 25 mM HEPES (pH 7.5), 5 mM MgCl2, 5 mM EDTA, 5 mM dithiothione, 2 mM phenylmethanesulfonyl fluoride, 10 µg/mL pepstatin A, and 10 µg/mL leupeptin after treatment. Cell lysates were clarified by centrifugation at 12000g for 20 min at 4 °C. Caspase activity in the supernatant was determined as described previously (17).
Statistical analysis
Data are presented as means ± SE for the indicated number of independently performed experiments. Significant differences from the respective controls for each experimental test condition were assayed using Student's t-test for each paired experiment. A P-value of < 0.05 was considered statistically significant.
Results
[8]-shogaol caused dose-dependent reduction in leukemic cell viability
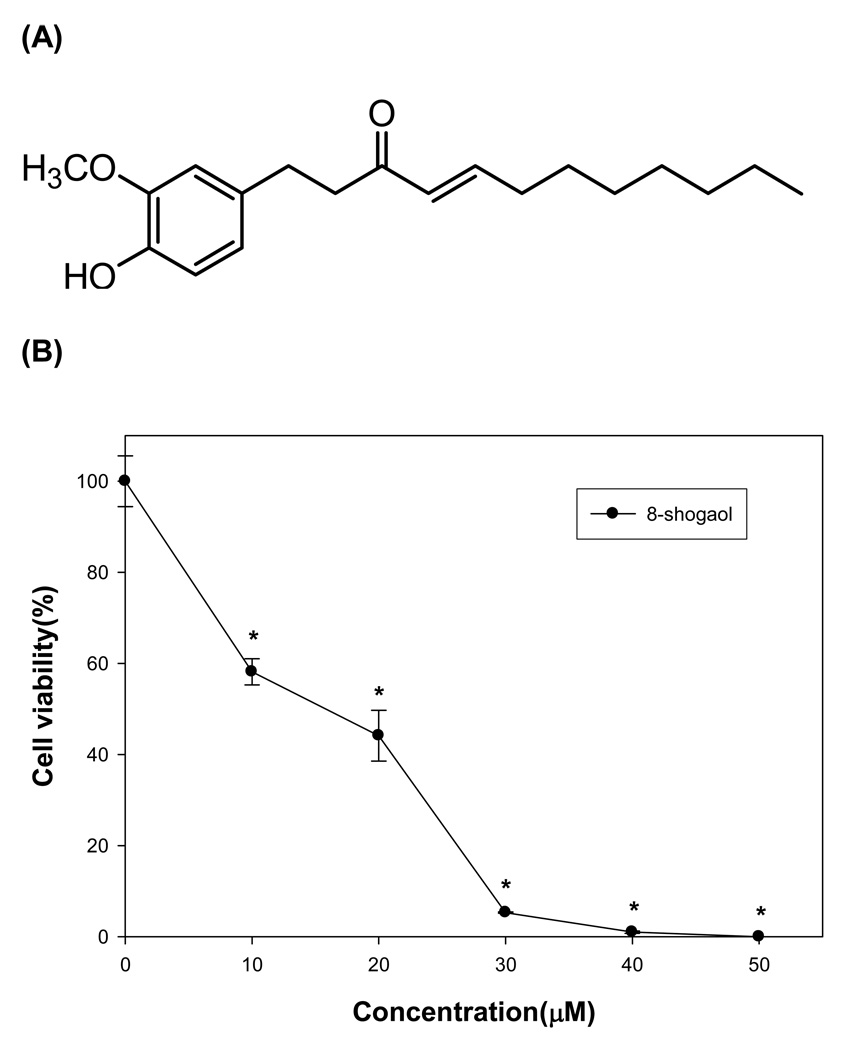
We evaluated the growth-inhibitory potential of [8]-shogaol (Figure 1A) on human leukemia HL-60 cell line. Various doses of [8]-shogaol was added to exponential HL-60 cells for 24 h of incubation and the curve of cell growth was determined by means of MTT method. As shown in Figure 1B, [8]-shogaol exhibits the cytotoxic effects on HL-60 cell growth in a concentration-dependent manner within the range of low concentration. The data implicates that [8]-shogaol might suppress cancer cell proliferation or induce cancer cell undergoing apoptosis that lead to cell growth-inhibition.
Figure 1. Effect of [8]-shogaol on cell cytotoxicity.
(A) Chemical structure of [8]-shogaol. (B) HL-60 cells were treated with different concentration of [8]-shogaol (0, 10, 20, 30, 40, and 50 µM) for 24 h. Cell viability then was determined by the MTT assay. The values are expressed as means ± SD of triplicates tests. *P < 0.001 indicate statistically significant difference from control.
[8]-shogaol promoted HL-60 cells undergoing apoptosis
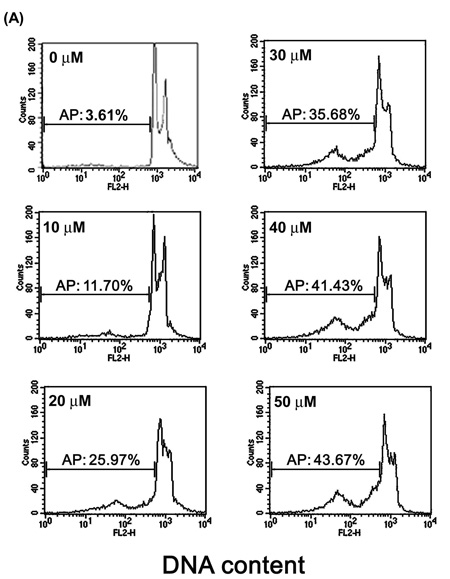
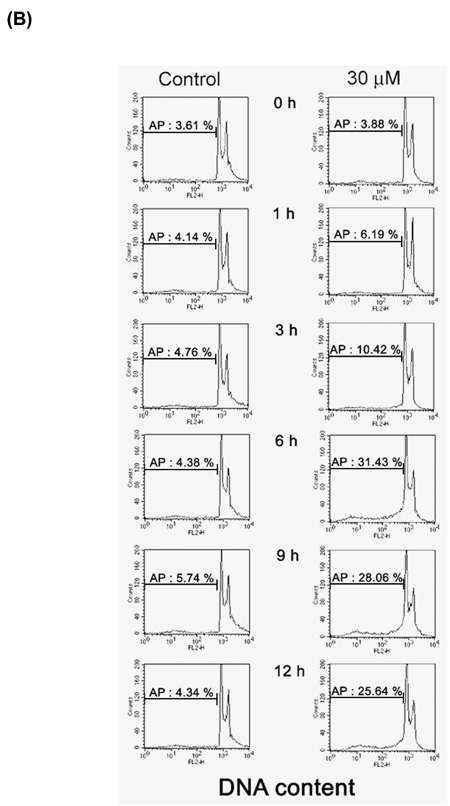
We studied the mechanisms by which [8]-shogaol suppresses cell viability. To investigate the effect of [8]-shogaol on cell cycle progression, the DNA content of HL-60 cells treated with [8]-shogaol at various concentrations and for various time periods was analyzed by flow cytometry. A sub-G1 DNA peak, which has been suggested to be the apoptotic DNA (18), was detected in [8]-shogaol-treated cells, and the percentages of apoptotic HL-60 cells (sub-G1 population) were gradually increased by 11.70%, 25.97%, 35.68%, 41.43%, and 43.67% for 10, 20, 30, 40, and 50 µM of treatment, respectively (Figure 2A). Time-corresponding increases in sub-G1 population of HL-60 cells were also found for the indicated times with 30 µM of [8]-shogaol treatment (Figure 2B).
Figure 2. Determination of sub-G1 cells in [8]-shogaol-treated HL-60 cells by flow cytometry.
HL-60 cells were treated with (A) different concentration of [8]-shogaol (0, 10, 20, 30, 40, and 50 µM) for 24 h or (B) treated with 30 µM of [8]-shogaol for indicated time. The method of flow cytometry used is descried under Materials and Methods. AP (apoptotic peak) represents apoptotic cells with a lower DNA content. The data presented are representative of three independent experiments.
Involvement of ROS production, GSH depletion, mitochondrial dysfunction, and release of cytochrome c from mitochondria to cytosol in [8]-shogaol-induced apoptosis
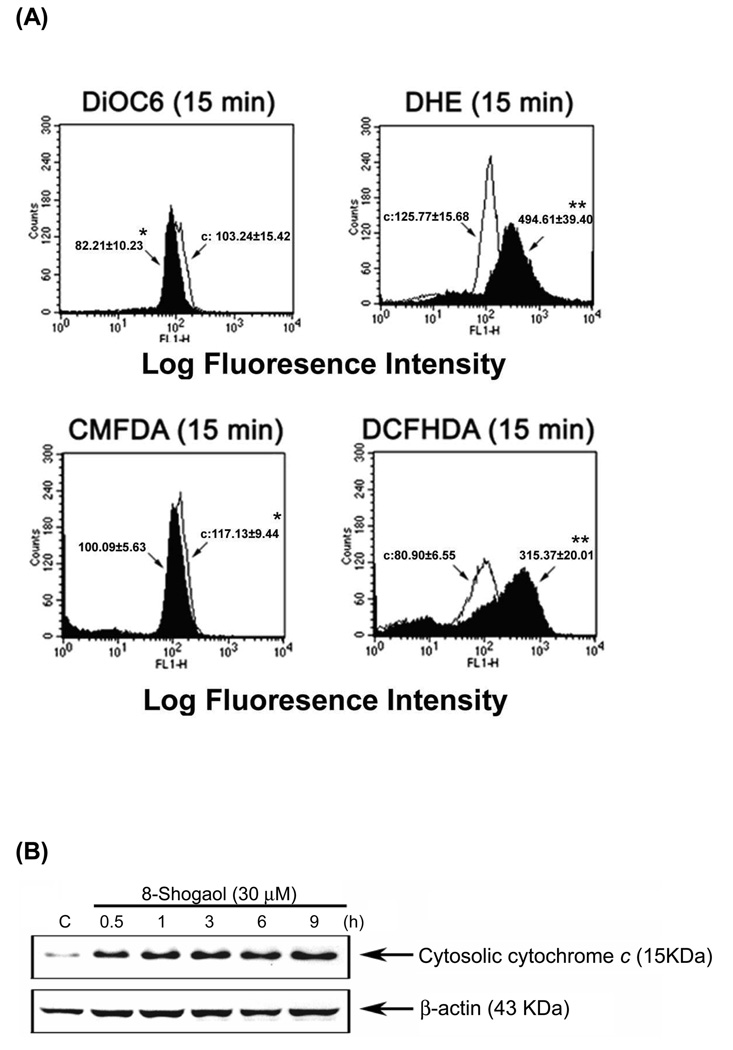
We further investigated if ROS production is involved in [8]-shogaol-induced apoptosis. ROS such as superoxide and hydrogen peroxide have been indicated to play an important role in the induction of apoptosis (19). To assess whether the reduction of GSH by [8]-shogaol would result in ROS generation, the levels of superoxide and hydrogen peroxide were determined by flow cytometry using fluorescent probes DHE (specific for superoxide) and DCFH-DA (specific for hydrogen peroxide). After 15 min of incubation with [8]-shogaol, the increased levels of intracellular peroxide were clearly detected, and the means of fluorescence intensity emitted by DHE and DCFH-DA increase from 125.77 to 494.61 and from 80.90 to 315.37, respectively (Figure 3A). We then studied the possibility that [8]-shogaol could deplete intracellular GSH content using the fluorescent probe CMFDA, the decrease in fluorescence reflects the depletion of the availability of GSH. Treatment with [8]-shogaol resulted in decreases in the means of CMF fluorescence intensity from 117.13 to 100.09. Combined with these findings, we suggest that there is a little depletion of GSH levels due to the high levels of ROS as a consequence of mitochondrial dysfunction caused [8]-shogaol.
Figure 3. Induction of mitochondrial dysfunction, reactive oxygen species (ROS) generation, GSH depletion, and cytochrome c release in [8]-shogaol-induced apoptosis.
(A) HL-60 cells were treated with 30 µM [8]-shogaol for indicated times and were then incubated with 3, 3'-dihexyloxacarbocyanine (40 nM), DCFH-DA (20 µM), DHE (20 µM), CMFDA (20 µM) respectively and analyzed by flow cytometry. Data are presented as log fluorescence intensity. C: control. (B) Cells were treated with 30 µM [8]-shogaol for 15 min. Subcellular fractions were prepared and cytosolic cytochrome c was analyzed by Western blotting as described in the Material and Methods section. These experiments were performed at least three times, and a representative experiment is presented. *P < 0.05 and **P < 0.01 indicate statistically significant difference from control.
A decreasing mitochondrial membrane potential (ΔΨm) has been shown to be a pivotal factor to control the induction of apoptosis (20). Therefore, we investigated the change of ΔΨm in [8]-shogaol-induced apoptosis. HL-60 cells were treated with 30 µM of [8]-shogaol for 15 min and then exposed to a fluorescent probe DiOC6(3) that is taken up by mitochondria and undergoes a red shift in emission spectrum during changes in ΔΨm. As shown in Figure 3A, which compares HL-60 cells exposed to [8]-shogaol and control cells, the DiOC6(3) fluorescence intensity shifted to the left from 103.24 to 82.21 in [8]-shogaol-induced apoptotic HL-60 cells at 15 min. These findings show that [8]-shogaol has an effect on mitochondrial function and accumulation of ROS. To determine whether the reduction of ΔΨm by [8]-shogaol could lead to cytochrome c release from mitochondria into cytosol, cell lysates were subfractionated and cytosolic cytochrome c was determined by Western blotting. Consistent with the timing of loss of ΔΨm, cytochrome c release into cytosol began at 0.5 h exposure of [8]-shogaol and increased progressively up to 9 h (Figure 3B). Therefore, these results suggest that mitochondrial dysfunction caused cytochrome c to be the cascade between caspase-9 and caspase-3.
Caspase activation and PARP, DFF-45 degradation in [8]-shogaol-treated HL-60 Cells
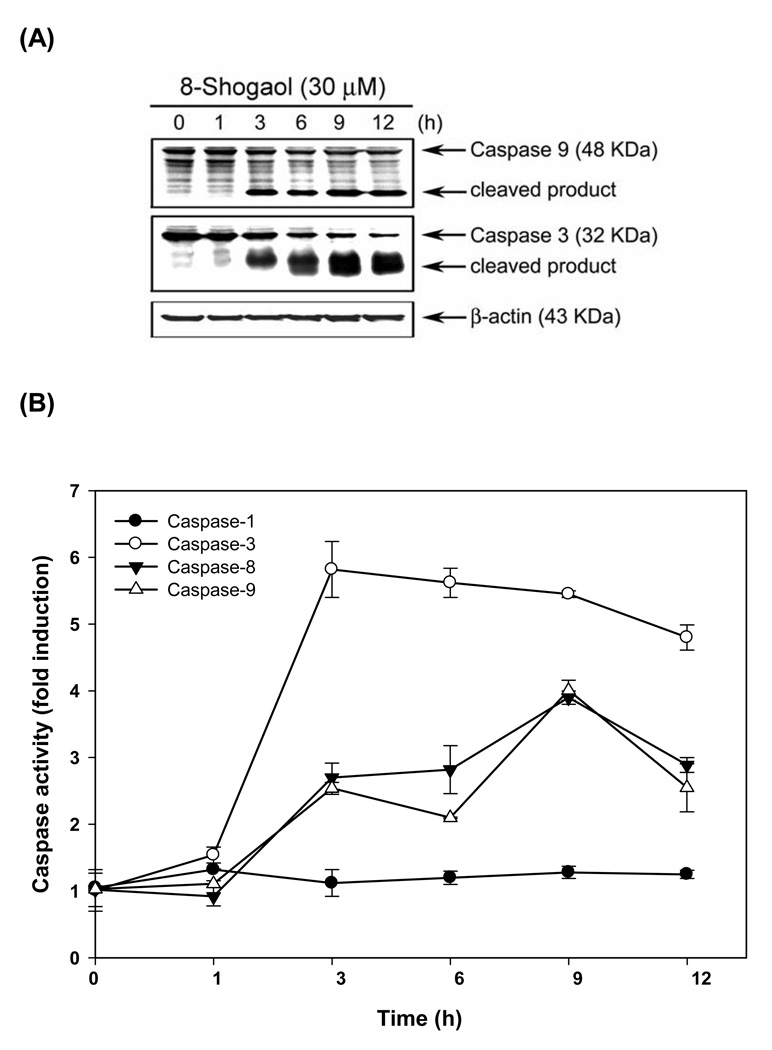
To further demonstrate whether cytochrome c release by [8]-shogaol treatment would subsequently result in the processing of caspase-9, Western blotting was performed to detect the cleavage of pro-caspase-9 by [8]-shogaol treatment. Along with the timing of cytochrome c release, the time-dependent cleavage of pro-caspase-9 was sequentially observed at 3 h and increased up to 12 h after 30 µM of [8]-shogaol (Figure 4A). Since activation of caspase-9 is accompanied with the sequential processing and activation of caspase cascades, e.g. caspase-3, whose activity has been considered to essentially involved in many types of stimuli-induced apoptosis (20), we next evaluated whether caspase-3 activity would be involved in [8]-shogaol-induced apoptosis. Figure 4A displays that [8]-shogaol led to a time-dependent increase in caspase-3 cleavage, suggesting the activation of caspase cascades in [8]-shogaol-induced apoptosis. These observations suggest that [8]-shogaol triggers an apoptosis-inducing mechanism via mitochondria in HL-60 cells. To monitor the enzymatic activity of caspase-1, -3, -8, and -9, the caspase activity was measured following treatment of HL-60 cells with 30 µM [8]-shogaol for various times. We measured the enzymatic activity of caspases using four fluoroegenic peptide substrates as follows: Ac-YVAD-AMC, Ac-DEVE-AMC, Ac-IETD-AMC and Ac-LEHD-AMC, which are specific substrates for caspase-1, -3, -8 and -9, respectively. As illustrated in Figure 4B, showing the kinetic activity of various caspases, [8]-shogaol induced a rapid rise in caspase-3, -8 and -9 activities. Notably, the increases in caspase-9 and -3 activities by [8]-shogaol are closely correlated with the processing of pro-caspase-9 and -3, respectively, as demonstrated in Figure 4A. In contrast to the significant increase in caspase-3, -8, and -9, a negligible increase in caspase-1 was observed.
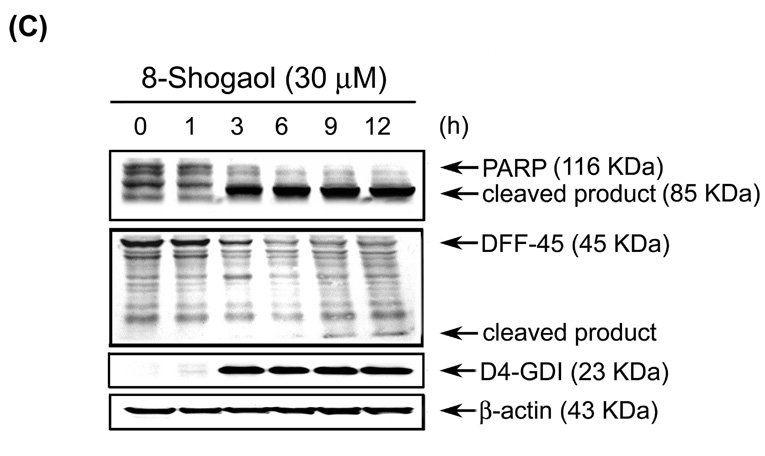
Figure 4. Induction of caspase activities, PARP cleavage, DFF-45 degradation in [8]-shogaol-induced apoptosis in HL-60 cells.
(A) [8]-Shogaol induced caspase-9 processing and further caused caspase-3 activation. Total cell lysates were prepared from HL-60 cells treated with 30 µM [8]-shogaol in a time-dependent manner and analyzed by Western blotting. Degradation of pro-caspase protein represents its activation. (B) Kinetics of caspase activation in HL-60 cells. Cells were treated with 30 µM [8]-shogaol for different times. Caspase activities were analyzed as described in the Materials and Methods section. Data represent means ± SD for three determinations. (C) Cleavage of PARP, DFF-45, and D4-GDI induced by [8]-shogaol was time-dependent. HL-60 cells were treated as indicated and analyzed by Western blotting as described in the Materials and Methods. These experiments were performed at least three times, and a representative experiment is presented.
Caspase-3 activity contributes to the proteolytic cleavage of a number of proteins, such as D4-GDI and poly(ADP-ribose) polymerase (PARP), which is cleaved by activated caspase-3 to yield the characteristic 85 kDa fragment during apoptosis (20). Another caspase-3 substrate is DNA fragmentation factor-45 (DFF-45), which complexes with caspase-activated DNase (CAD), retains CAD in cytosol, and inhibits its function in living cells (20). During apoptosis, caspase-3 cleaves DFF-45, resulting in the release and activation of CAD, which translocates to nucleus and degrades chromosomal DNA to produce interchromosomal DNA fragmentation. To demonstrate that activation of caspase-3 was followed by the cleavage of PARP and DFF-45 during [8]-shogaol-induced apoptosis, the cleavage of PARP and DFF-45 was analyzed by Western blotting as demonstrated in Figure 5C. The disappearance of 110 kDa PARP was accompanied with the accumulation of the 85-kDa cleaved species after time-treatment of [8]-shogaol for 1-12 h, and an increase in the cleaved form of the caspase-3 substrate, D4-GDI protein, were detected in [8]-shogaol treated HL-60 cells. Taken together, the current data suggest that [8]-shogaol would lead to loss of ΔΨm, cytochrome c release, subsequently activating the caspase cascades, all of which cause the induction of apoptosis in HL-60.
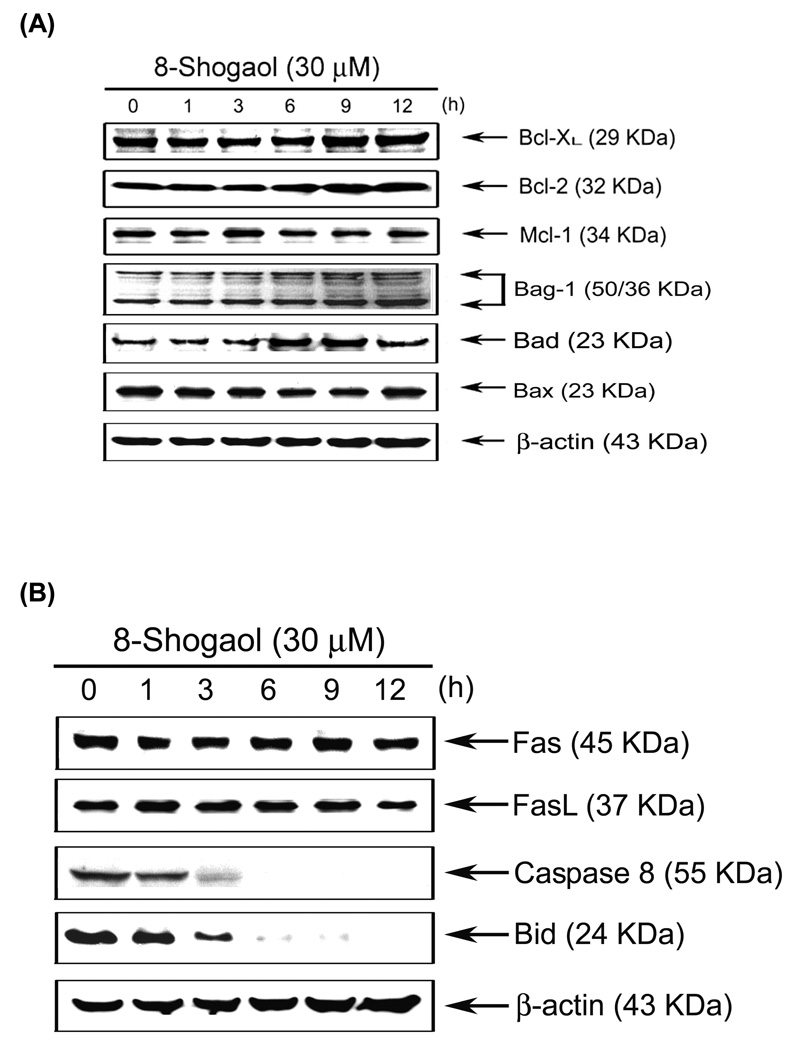
Figure 5. Effect of [8]-shogaol on Bcl-2 family protein, Fas, Fas L, and Bid protein expression in [8]-shogaol-treated HL-60 cells.
HL-60 cells were treated with 30 µM [8]-shogaol for indicated time. The expression of Bcl-XL, Bcl-2, MCl-1, Bag-1, Bad, and Bax (A), Fas, FasL, caspase-8 and Bid (B) were analyzed by Western blotting as described in the Material and Methods. This experiment was repeated three times with similar results.
Effects of [8]-shogaol on the expression of Bcl-2 family proteins, Fas and FasL in HL-60 Cells
Members of Bcl-2 family of proteins are the most important apoptotic regulators to maintain the integrity of mitochondrial membrane and are located upstream of caspase activation(20). Members of Bcl-2 family can be classified as anti- and pro-apoptotic proteins whose imbalance by certain stimulus is one of the major mechanisms underlying the ultimate fate of cells during apoptosis. To check the change of members of Bcl-2 family during [8]-shogaol treatment, the time course effects of [8]-shogaol on protein expression of Bcl-2 family were evaluated by Western blotting. As shown in Figure 5A, [8]-shogaol only promoted Bad protein expression. To assess whether [8]-shogaol promoted apoptosis via a receptor-mediated pathway, the levels of Fas, Fas ligand (FasL), caspase-8, and Bid proteins were determined by Western blotting. Results of Figure 5B show that a decrease in the levels of the preform of caspase-8 and Bid were detected in [8]-shogoal-treated HL-60 cells. These data suggested that the cleavage of Bid by active caspase-8 may be one of the mechanisms that contributed to the activation of mitochondrial pathway during [8]-shogaol-induced apoptosis.
Discussion
The results in the present study ascertain the capacity of [8]-shogaol to inhibit growth and induce apoptosis in human leukemia cell line HL-60. As shown in Figure. 2 and 4, [8]-shogaol was a potent and rapid inducer of apoptosis, concurrent with sub-G1 peak appearance. Indeed, treatment with [8]-shogaol caused the activation of caspase-3, -8 and -9, but not caspase-1, associated with the degradation of PARP, DFF-45, and, D4-GDI which preceded the onset of apoptosis. Exploring the possible molecular mechanisms underlying HL-60 cell apoptosis by [8]-shogaol, we found that the pro-apoptotic activity of [8]-shogaol was accompanied by accumulation of ROS including superoxide anion and hydrogen peroxide (Figure 3A), suggesting that leukemia cell death by [8]-shogaol is a ROS-dependent process. ROS are a family of active molecules containing free radicals and involved in the modulation of biological cell functions. However, excessive ROS bring about oxidative stresses that cause injury to various cellular constituents such as lipid, protein, and DNA, finally resulting in growth arrest, senescence, or apoptosis (20). Intracellular ROS can be generated from aberrant mitochondria, which are well known as sites of ROS generation and targets for ROS action (20). Here, we demonstrated that [8]-shogaol induced the loss of mitochondrial membrane potential accompanied with increased ROS generation in HL-60 cells. It provides a possible fundamental explanation for why [8]-shogaol brings about excessive ROS generation in HL-60 cells. We suggest that [8]-shogaol might lead to ROS accumulation by disrupting the integrity of mitochondrial membrane, subsequently interrupting the electron transport assembly and generating ROS by one-electron transfer. Besides mitochondrial decay, the other possibility for excessive ROS production by [8]-shogaol in leukemia cells is GSH depletion, as demonstrated in Figure 3A. GSH is an important defense mechanism against potentially toxic hydrogen peroxide by glutathione peroxidase, which reduces hydrogen peroxide to water, along with that the oxidation of GSH. Decreased GSH concentrations might be related to susceptibility to injury by [8]-shogaol in leukemia cells. The decrease in GSH levels might be attributable to several different mechanisms. One possibility is that excessive ROS generation modulated by [8]-shogaol facilitates the consumption of GSH. Another possible mechanism is that [8]-shogaol might directly or indirectly diminish GSH synthesis or regeneration. However, the details of how [8]-shogaol caused the decrease in GSH levels are not yet clear and require further study.
Previous studies suggest thatα,β-unsaturated carbonyls are very susceptible to nucleophilic addition reactions with thiols such as glutathione; the most abundant nonprotein thiol in vivo (21). It is clear that α,β-unsaturated ketone moiety is essential for exerting cytotoxic activity via depletion of intracellular GSH (22). Recent studies indicated that intracellular GSH depletion could result in mitochondria dysfunction (23, 24). Herein, we demonstrated that [8]-shogaol could disrupt the functions of mitochondria at the early stages of apoptosis and subsequently coordinate caspase-9 activation, but not caspase-1, through the release of cytochrome c. Therefore, we speculated that intracellular generation of ROS could be an important factor in [8]-shogaol-induced apoptosis. Although [8]-shogaol can change the integrity of the mitochondrial membrane, however, early stage not by regulating the expression of Bcl-2 family, Fas, and FasL proteins (Figure 5), again, we do not rule out a possible mechanism in which [8]-shogaol penetrate cells and directly targets mitochondria or ER, thereby increasing membrane permeability with an attendant decrease of ΔΨm, accompanied by ROS production. Bad is one the ‘death-promoting’ members of Bcl-2 family , and its pro-apoptotic activity is upregulated by [8]-shogaol (Figure 5A). Based on the results, we postulate that Bad proteins may differentially induce apoptosis by binding and antagonizing the anti-apoptotic Bcl-2 family members, thereby participates in [8]-shogaol-induced apoptosis.
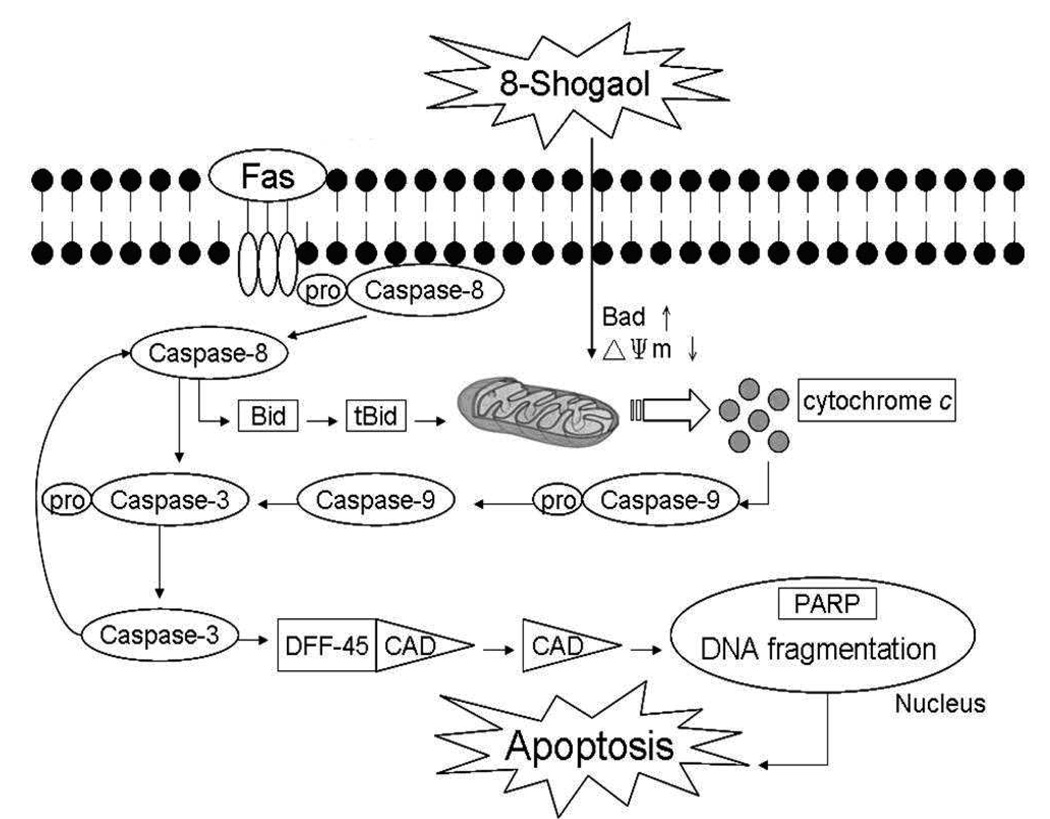
Taken collectively, the current findings can be interpreted to propose a temporal sequence of events related to the effects of [8]-shogaol on HL-60 cells. The initial event induced by [8]-shogaol could induce ROS generation and disrupt the functions of mitochondria at the early stages of apoptosis then cause the release of cytochrome c to cytosol subsequently coordinating caspase-9 activation in HL-60 cells. Thus, we propose that caspase-8 is involved in mitochondria-mediated apoptosis and participates in a feedback amplification loop involving caspase-3 in [8]-shogaol-treated HL-60 cells. However, other genes may also be involved in the cellular responses to [8]-shogaol exposure, an involvement that may eventually lead to the cells undergoing apoptosis (Figure 6). Previous studies, we found that [6]-shogaol, [8]-shogaol analog, induced apoptosis in COLO 205 cells via ROS production, caspase activation, DNA damage, and GADD 153 gene expression (17). Further studies are needed to determine whether DNA damage directly initiate apoptosis in HL-60 cells exposed to [8]-shogaol. Because induction of apoptosis is considered an important mechanism of prevention/treatment of cancer by chemopreventive agent(s), further elucidation of the mechanism during [8]-shogaol-mediated apoptosis would provide useful information concerning the basis for its use as a potential therapeutic and chemopreventive agent.
Figure 6. Schematic representation of mechanisms of action by which [8]-shogaol-induced apoptosis in HL-60 cells.
Acknowledgment
This study was supported by the National Science Council NSC 98-2313-B-022-002-MY3, NSC 98-2622-B-127-001-CC3, and NIH grant CA138277 to Dr. S. Sang
Abbreviations Used
- DFF
DNA fragmentation factor
- PARP
poly(ADP-ribose) polymerase
- GSH
glutathione
- DCFH-DA
dichlorodihydrofluorescein diacetate
- D4-GDI
a Rho guanosine diphosphate (GDP) dissociation inhibitor
- CMFDA
5-chloromethylfluorescein diacetate
- DHE
dihydroethidium
- MTT
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- ROS
reactive oxygen species
- GADD153
growth arrest and DNA damage-inducible gene 153
Literature Cited
- 1.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–530. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol. Pharmacol. 2006;69:195–206. doi: 10.1124/mol.105.017400. [DOI] [PubMed] [Google Scholar]
- 3.Takada Y, Murakami A, Aggarwal BB. Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion. Oncogene. 2005;24:6957–6969. doi: 10.1038/sj.onc.1208845. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa T, Nakajima T, Moriguchi M, Jo M, Sekoguchi S, Ishii M, Takashima H, Katagishi T, Kimura H, Minami M, Itoh Y, Kagawa K, Okanoue T. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006;44:1074–1082. doi: 10.1016/j.jhep.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 5.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998;129:139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 6.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin. Chim. Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 7.Baranowski JD. Low-Temperature Production of Urocanic Acid by Spoilage Bacteria Isolated from Mahimahi (Coryphaena hippurus) Appl. Environ. Microbiol. 1985;50:546–547. doi: 10.1128/aem.50.2.546-547.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan MH, Hsieh MC, Hsu PC, Ho SY, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol suppressed lipopolysaccharide-induced up-expression of iNOS and COX-2 in murine macrophages. Mol. Nutr. Food Res. 2008;52:1467–1477. doi: 10.1002/mnfr.200700515. [DOI] [PubMed] [Google Scholar]
- 9.Sang S, Hong J, Wu H, Liu J, Yang CS, Pan MH, Badmaev V, Ho CT. Increased growth inhibitory effects on human cancer cells and anti-inflammatory potency of shogaols from Zingiber officinale relative to gingerols. J. Agric. Food Chem. 2009;57:10645–10650. doi: 10.1021/jf9027443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- 11.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Li P, Nijhawan D, Wang X. Mitochondrial activation of apoptosis. Cell. 2004;116:S57–S59. 2. doi: 10.1016/s0092-8674(04)00031-5. [DOI] [PubMed] [Google Scholar]
- 13.Pan MH, Huang MC, Wang YJ, Lin JK, Lin CH. Induction of apoptosis by hydroxydibenzoylmethane through coordinative modulation of cyclin D3, Bcl-X(L), and Bax, release of cytochrome c, and sequential activation of caspases in human colorectal carcinoma cells. J. Agric. Food Chem. 2003;51:3977–3984. doi: 10.1021/jf034094i. [DOI] [PubMed] [Google Scholar]
- 14.Pan MH, Huang YT, Ho CT, Chang CI, Hsu PC, Sun PB. Induction of apoptosis by Meretrix lusoria through reactive oxygen species production, glutathione depletion, and caspase activation in human leukemia cells. Life Sci. 2006;79:1140–1152. doi: 10.1016/j.lfs.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 16.Pan MH, Lin JH, Lin-Shiau SY, Lin JK. Induction of apoptosis by penta-O-galloyl-beta-D-glucose through activation of caspase-3 in human leukemia HL-60 cells. Eur. J. Pharmacol. 1999;381:171–183. doi: 10.1016/s0014-2999(99)00549-x. [DOI] [PubMed] [Google Scholar]
- 17.Pan MH, Hsieh MC, Kuo JM, Lai CS, Wu H, Sang S, Ho CT. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res. 2008;52:527–537. doi: 10.1002/mnfr.200700157. [DOI] [PubMed] [Google Scholar]
- 18.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 19.Shin DY, Kim GY, Li W, Choi BT, Kim ND, Kang HS, Choi YH. Implication of intracellular ROS formation, caspase-3 activation and Egr-1 induction in platycodon D-induced apoptosis of U937 human leukemia cells. Biomed. Pharmacother. 2009;63:86–94. doi: 10.1016/j.biopha.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Pan MH, Ghai G, Ho CT. Food bioactives, apoptosis, and cancer. Mol. Nutr. Food Res. 2008;52:43–52. doi: 10.1002/mnfr.200700380. [DOI] [PubMed] [Google Scholar]
- 21.Boyland E, Chasseaud LF. Enzymes catalysing conjugations of glutathione with alpha-beta-unsaturated carbonyl compounds. Biochem. J. 1968;109:651–661. doi: 10.1042/bj1090651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atsmon J, Freeman ML, Meredith MJ, Sweetman BJ, Roberts LJ. Conjugation of 9-deoxy-delta 9,delta 12(E)-prostaglandin D2 with intracellular glutathione and enhancement of its antiproliferative activity by glutathione depletion. Cancer Res. 1990;50:1879–1885. [PubMed] [Google Scholar]
- 23.Friesen C, Kiess Y, Debatin KMA. A critical role of glutathione in determining apoptosis sensitivity and resistance in leukemia cells. Cell Death. Differ. 2004;11 Suppl 1:S73–S85. doi: 10.1038/sj.cdd.4401431. [DOI] [PubMed] [Google Scholar]
- 24.Vesce S, Jekabsons MB, Johnson-Cadwell LI, Nicholls DG. Acute glutathione depletion restricts mitochondrial ATP export in cerebellar granule neurons. J. Biol. Chem. 2005;280:38720–38728. doi: 10.1074/jbc.M506575200. [DOI] [PubMed] [Google Scholar]