Abstract
The most widely used approach to cancer immunotherapy is vaccines. Unfortunately, the need for multiple administrations of antigens often limits the use of one of the most effective vaccine approaches, immunogene therapy using viral vectors, because neutralizing antibodies are rapidly produced. We hypothesized that after viral immunogene therapy “primed” an initial strong antitumor immune response, subsequent “boosts” could be provided by sequential courses of chemotherapy. Three adenoviral (Ad)-based immunogene therapy regimens were administered to animals with large malignant mesothelioma and lung cancer tumors followed by three weekly administrations of a drug regimen commonly used to treat these tumors (Cisplatin/Gemcitabine). Immunogene therapy followed by chemotherapy resulted in markedly increased antitumor efficacy associated with increased numbers of antigen-specific, activated CD8+ T-cells systemically and within the tumors. Possible mechanisms included: (i) decreases in immunosuppressive cells such as myeloid-derived suppressor cells (MDSC), T-regulatory cells (T-regs), and B-cells, (ii) stimulation of memory cells by intratumoral antigen release leading to efficient cross-priming, (iii) alteration of the tumor microenvironment with production of “danger signals” and immunostimulatory cytokines, and (iv) augmented trafficking of T-cells into the tumors. This approach is currently being tested in a clinical trial and could be applied to other trials of viral immunogene therapy.
Introduction
The combination of immunotherapy with chemotherapy has traditionally not been used because of fears that post-chemotherapy leukopenia would eliminate antitumor directed lymphocytes.1 However, an increasing number of studies have recently shown that immunotherapy is not only compatible with, but may be synergistic with certain chemotherapies.2,3,4,5,6,7 Despite this growing consensus, major questions still exist about how best to combine these agents, including: (i) when in the disease process is it best to intervene, (ii) which are the best chemotherapy and immunotherapy agents to use, and (iii) what are the most effective dosing schedules? More studies to optimize the combined use of these two modalities and to understand the mechanisms of action of the augmented effect are thus needed.
Many types of combination approaches are being evaluated, for example, the delivery of immunotherapy after chemotherapy.8 However, interesting reports have appeared of clinical trials in which patients with highly resistant tumors have had remarkably good responses to chemotherapy after receiving a course of immunotherapy (reviewed in refs. 2,4). Examples include unusually high chemotherapy response rates in patients with small-cell lung cancer after receiving an Ad.p53-dendritic cell vaccine,9 advanced-stage cancer patients receiving a plasmid/microparticle vaccine directed against cytochrome P4501B1,10 and prostate cancer patients receiving a viral-based PSA/GM-CSF vaccine.11 The mechanisms responsible for this surprising efficacy of immunogene therapy and subsequent chemotherapy require further exploration.
One possible advantage to the use of chemotherapy after immunotherapy, would be the ability to use of immunotherapies that cannot be given repeatedly. One of the most widely used approaches to cancer immunotherapy is vaccine therapy that employs a traditional “prime and boost” paradigm using multiple administrations (often 5 or 6) of the antigen/adjuvant.12 The need for multiple administrations of antigens presents special problems for viral gene therapy vectors,13 because neutralizing antibodies are rapidly produced, allowing only a single or two closely spaced administrations.14 A second potential advantage could be that chemotherapy has the potential to alter the tumor microenvironment.15,16 One of the disappointments of immunotherapy, in general, has been the observation that even when lymphocytes with antitumoral activity have been generated and detected in the blood, this approach has not met with the hoped-for success clinically.12,17 A reason for this lack of clinical efficacy may be the failure of these cells to traffic into tumors and/or the inactivation or death of these lymphocytes (usually T-cells) within the local immunosuppressive microenvironment of the tumor.17 A third possible benefit could be the ability of some chemotherapy agents to counteract tumor-induced systemic immunosuppressive cell types such as myeloid-derived suppressor cells (MDSC), B-cells, or T-regulatory cells (T-regs).18,19,20
We thus hypothesized that giving immunogene therapy followed by chemotherapy would result in increased efficacy. In this approach, the viral vector is being used to “prime” an initial strong antitumor immune response, but the “boost” is provided by sequential courses of chemotherapy rather than repeated doses of vector. This “boost” is achieved by the ability of the chemotherapy agents to kill tumor cells resulting in a bolus of immunostimulatory tumor antigens along with the induction of “danger signals” within the tumor causing augmented cross-priming of tumor antigens.21 Thus, after being primed by the vaccine, the patient is boosted with his own tumor antigens, released by the chemotherapy treatment.
The goal of this study was to explore this “chemotherapy boost” hypothesis and its mechanisms, using three immunogene therapy models (two of which, adenoviral-interferon (Ad.IFN) and Ad herpes simplex virus thymidine kinase (Ad.HSVtk), are already in clinical trials). Given our group's interest in thoracic oncology, we focused on models of malignant mesothelioma and lung cancer and evaluated a drug regimen commonly used to treat these tumors (Cisplatin/Gemcitabine).
Results
Chemotherapy given after immunotherapy markedly augments antitumor efficacy in multiple tumor models
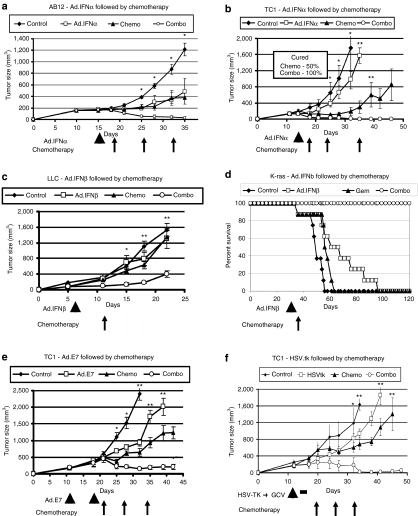
Mice-bearing large AB12 (malignant mesothelioma), TC1 (nonsmall cell lung cancer), or LKR (lung cancer K-ras, nonsmall cell lung cancer) flank tumors (~200–250 mm3), were treated with one dose of an Ad-expressing IFN-α (Ad-IFN-α) administered intratumorally, followed by weekly treatment with a chemotherapy combination of Cisplatin (3 mg/kg) and Gemcitabine (120 mg/kg). As shown in Figure 1a and b, treatment of AB12 or TC1 tumors with Ad.IFNα or chemotherapy alone slowed the growth of these tumors, but did not induce significant regressions. In contrast, Ad.IFNα followed by chemotherapy, resulted in marked shrinkage of the tumors, with tumors being significantly smaller (P < 0.05) than that of control or single treatments. In AB12 tumors (Figure 1a), combination therapy led to complete tumor regression in 13/15 animals versus only 5/15 and 2/15 in the Ad.IFNα and chemotherapy groups respectively. In the TC1 model (Figure 1b), combination therapy induced complete remission in all tumors (5/5). Chemotherapy improved the clinical effect of preceding Ad.IFNα in the LKR model as well, although to a lesser extent (Supplementary Figure S1).
Figure 1.
Chemotherapy given after viral immunotherapy markedly augments antitumor efficacy. Mice (n = 5–10 for each group) bearing large tumors, were treated in one of four ways: (i) control—no treatment (control); (ii) immunogene therapy (arrowheads); (iii) chemotherapy (arrows)—Cisplatinum (Cis) + Gemcitabine (Gem) intaperitoneal (i.p.) weekly (chemo); and (iv) immunogene therapy followed by weekly chemotherapy (combo). Immunotherapy or chemotherapy alone slowed tumor growth in all systems. However, immunotherapy followed by chemotherapy led to clear tumor regression in all regimens, with many cures. *P < 0.05 for combo versus control or immunotherapy, **P < 0.05 for combo versus all three other treatment groups. Error bars = mean ± SEM. (a) Balb/C mice injected with the AB12 mesothelioma cell line and treated with adenovirus-IFNα (Ad.IFNα) intratumorally (i.t.) as immunogene therapy. (b) C57BL/6 mice injected with the TC1 NSCLC cell line and treated with Ad.IFNα (i.t.) as immunogene therapy. (c) C57Bl/6 mice injected with the LLC NSCLC cell line and treated with Ad.IFNβ (i.t.) as immunogene therapy, followed by a single dose of gentamicine. (d) LSL KrasG12D-positive mice (that conditionally express an oncogenic KrasG12D allele) were intratracheally injected with Ad.Cre on day 0 (to activate the “floxed” mutated Kras transgene). Thirty days later, treatment groups (n = 8) were established, and mice were treated accordingly with Ad.IFN-β as immunotherapy and/or Gemcitabine. Mice were followed until they started to show signs of distress and were then sacrificed. (e) C57Bl/6 mice injected with the TC1 NSCLC cell line and vaccinated with adenovirus-HPV-E7 (Ad.E7) subcutaneously (s.c.) as immunogene therapy. (f) C57Bl/6 mice injected with the TC1 NSCLC cell line and treated with adenovirus containing the herpes simplex thymidine kinase suicide gene i.t. (Ad.HSVtk) followed by ganciclovir (GCV) i.p for activation (thick black line) as immunogene therapy. Ad, adenovirus; HSVtk, herpes simplex thymidine kinase; IFN, interferon; LLC, Lewis lung carcinoma; NSCLC, nonsmall cell lung cancer.
Similar results were obtained when we used an Ad-expressing IFN-β (Ad-IFN-β) as an immunotherapy. We evaluated this vaccine, followed by treatment with Gemcitabine in treatment of Lewis lung carcinoma flank tumors (Figure 1c), showing again that the combined treatment induced significantly better clinical response than each treatment alone. We further evaluated the effect of treating with Ad.IFNβ followed by Gemcitabine in an orthotopic transgenic activated Kras model of bronchogenic adenocarcinoma of the lung (Figure 1d). A modest improvement in survival was noted with either treatment alone. All the mice treated with the combination therapy were alive at 120 days, a time point at which none of the mice in the other three arms of treatment survived (P < 0.05 for combo compared to all three groups).
The TC1 cell line expresses the human papillomavirus (HPV)-E7 protein. We have previously shown that an Ad-E7 vaccine injected subcutaneously into the flanks of TC1 tumor-bearing mice led to significant slowing of tumor growth.22 Mice-bearing large TC1 flank tumors were treated subcutaneously with Ad.E7, followed by a booster vaccine after 1 week. Subsequent treatment with Cis + Gem caused significant and prolonged regression of the tumors compared to chemotherapy or Ad.E7 alone (Figure 1e) (P < 0.05).
Similar results were noted using “suicide gene” transfer, in which a nonreplicating Ad containing the HSVtk suicide gene (Ad.HSVtk) was administered along with ganciclovir23 causing cell death through direct and immunologic effects.24,25 Ad.HSVtk alone or Cis/Gem alone caused only a slowing in TC1 tumor growth, whereas Cis+Gem, given at the end of the Ad.HSVtk/ganciclovir therapy, induced marked and significant regression of the tumors (P < 0.05 compared to each treatment alone, Figure 1f).
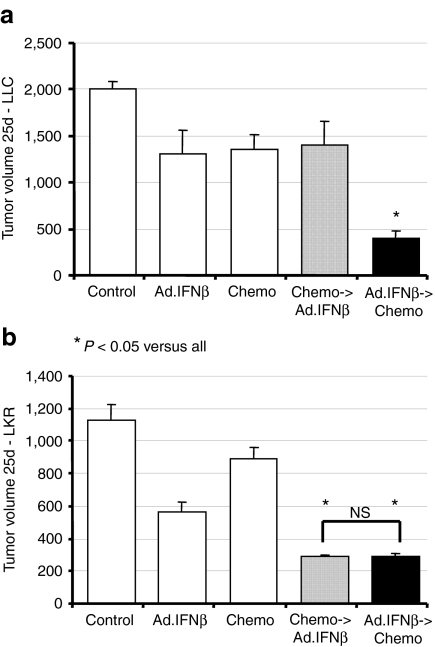
Although the main purpose of our work was to evaluate the effect of treating with immunogene therapy followed by chemotherapy, we evaluated the effect of changing the sequence of treatment, i.e., chemotherapy followed by immunotherapy (Figure 2). We found different results in different nonsmall cell lung cancer cell lines. In the nonimmunogenic Lewis lung carcinoma cell line, only treatment with immunotherapy followed by chemotherapy caused significant tumor regression (Figure 2a). In other more immunogenic cell lines (e.g., LKR), the effect of combining both treatments was similar whether the vaccine was given before chemotherapy or following it (Figure 2b).
Figure 2.
The order of treatments is important in some tumor cell lines, but not others. Mice (n = 5–10 for each group) bearing large tumors, were treated in one of five ways: (i) control—no treatment (control); (ii) Ad.IFNβ as immunotherapy on day 5; (iii) chemotherapy—120 mg/ml Gemcitibine (Gem) intraperitoneal (i.p.) on day 5; (iv) Ad.IFNβ (day 5) followed by Gem chemotherapy (day 10); and (v) Gem chemotherapy (day 5) followed by Ad.IFNβ (day 10). The volume of tumors was measured on day 25. (a) Data from C57Bl/6 mice injected with the LLC NSCLC cell line show that only immunotherapy followed by chemotherapy was significantly (*P < 0.05) better than single therapy, not when the order was reversed. (b) Data from B6129/J1 mice injected with the LKR NSCLC cell line show that both combination treatments induced significantly (*P < 0.05) more regression in tumor growth than single treatments, regardless of the order of the treatments. Error bars = mean ± SEM. Ad, adenovirus; IFN, interferon; LLC, Lewis lung carcinoma; NS, not significant; NSCLC, nonsmall cell lung cancer.
Immunogene therapy induces antigen-specific central-memory T-cells
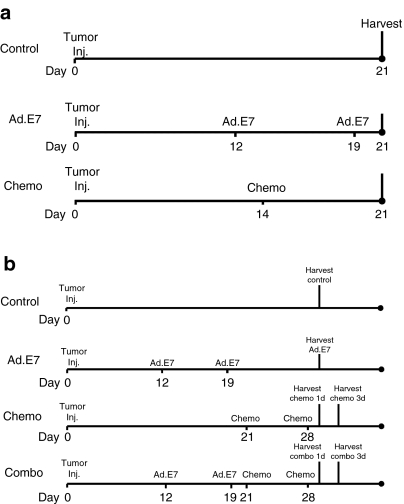
The chemotherapy-induced boost process requires immunogene therapy to induce memory T-cells that can then be activated by antigen release and cross-priming after chemotherapy. To study this, we employed the TC1/Ad.E7 system because this model enabled us to identify CD8+ T-cells with reactivity against the immunodominant HPV-E7 epitope expressed on TC1 cells using tetramers.22 Figure 3 shows the protocols used.
Figure 3.
Protocols for “reverse vaccination” evaluations. (a) Protocol for evaluation of memory cells (Figures 4a,b) mice-bearing large TC1 NSCLC tumors (~200 mm3), were treated in one of three ways: (i) control—no treatment (control); (ii) immunogene therapy—s.c. vaccine with adenovirus-HPV-E7 (Ad.E7), and a booster vaccine after a week; (iii) chemotherapy—Cisplatinum + Gemcitabine i.p. (chemo); spleens and lymph nodes were harvested for evaluations of memory T-cells at day 21 as shown. (b) Protocol for evaluation of the combined therapy (Figures 4c,d,5, 6, and Supplementary Figure S2)—mice bearing large TC1 NSCLC tumors (~200 mm3), were treated in one of four ways: (i) control—no treatment (control); (ii) immunogene therapy—s.c. vaccine with adenovirus-HPV-E7 (Ad.E7), and a booster vaccine after a week; (iii) chemotherapy—Cisplatinum + Gemcitabine i.p. weekly × 2 (chemo); and (iv) immunogene therapy (Ad.E7) followed by two weekly chemotherapy treatments (combo). Tumors, spleens, or lymph nodes were harvested for further evaluations at days 29 and 31 as shown. Ad, adenovirus; HPV, human papillomavirus; NSCLC, nonsmall cell lung cancer.
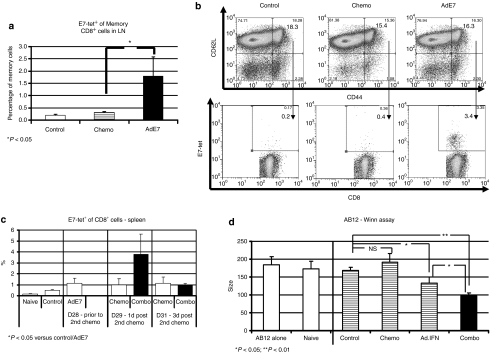
To identify antigen-specific memory cells, we evaluated draining lymph nodes (DLN) and spleens from tumor-bearing mice treated with saline, treated with one dose of chemotherapy alone or 2 days after the second vaccination with Ad.E7, an early time point, when the first dose of chemotherapy was to be delivered (see Figure 3a for details). Central-memory T-cells (identified as CD8+/CD44Hi/CD62LHi) made up ~10–15% of the CD8+ T-cells in DLNs and spleens in all groups, with no significant differences between the groups (Figure 4a, upper panels). However, antigen-specific central-memory T-cells (identified as CD8+/E7-tetramer+/CD44Hi/CD62LHi) that were <0.5% of splenocytes or lymph node cells in control or chemotherapy-treated animals, were increased four- to tenfold (P < 0.05) after Ad.E7 treatment [Figure 4a (representative tracings) and in Figure 4b (mean values)]. Thus, relatively large numbers of central-memory cells were present at the time of the first chemotherapy administration, although on average 85.9% of the CD8+/E7-tetramer+ cells in the Ad.E7-treated mice were effector cells (i.e., CD44Hi/CD62Llo), and only 14.1% of them were central-memory cells.
Figure 4.
Increased activity and antigen-specificity in CD8+ cells in spleens and draining lymph nodes with combined treatment. (a,b) Antigen-specific central-memory cells (CD8+/tetramer+/CD44hi/CD62Lhi) in draining lymph nodes (DLN) of TC1 tumor-bearing mice were evaluated by multicolor flow cytometry. Analysis was done 7 days after a dose of chemotherapy or 2 days after the second (booster) Ad.E7 vaccine. (a) A significant increase in the mean percentage of antigen-specific central-memory cells in the DLN only in the Ad.E7 group. (b) Representative FACS tracings. The population of CD44Hi/CD62LHi cells (top panel) was examined for the expression of CD8 and E7-tetramer+ (bottom panel) in each group (i.e., antigen-specific memory CD8 cells). The number in each quadrant is the percentage of the double-stained cells. (c) Multicolor flow cytometry was performed on spleens from tumor-bearing mice before and after the second treatment of Cis-Gem. The mean ± SE percentage of E7-tetramer+ out of CD8+ cells is shown at each time point, showing an increase in antigen-specific CD8+ T-cells 1 day after second chemotherapy treatment in mice previously treated with immunotherapy (Ad.E7) compared to all other groups (*P < 0.05 versus control and Ad.E7, P = 0.07 versus 1 day post-chemotherapy). Error bars = mean ± SEM. (d) A Winn assay was used to quantify the antitumor CD8+ T-cell activity. Two days after the second chemotherapy dose, CD8+ T-cells were isolated from the spleens of animals in each treatment group, combined with TC1 tumor cells at a ratio of one tumor cell to three CD8+ T-cells (n = 10/group), and the mixture was injected into the flanks of naive mice. Tumor growth was assessed 10 days later. There was no difference in tumor growth when TC1 cells were mixed with CD8+ cells from naive (nontumor-bearing) mice, untreated tumor-bearing mice (control) or from tumor-bearing mice treated with Cis + Gem. In contrast, TC1 cells mixed with CD8+ cells from animals treated with Ad.IFNα, were significantly inhibited in their growth after 10 days (*P < 0.05). TC1 cells mixed with CD8+ cells from animals treated with Ad.IFNα followed by chemotherapy were further inhibited in their growth (*P < 0.05, **P < 0.01). Ad, adenovirus; Cis, Cisplatinum; FACS, fluorescence-activated cell sorting; Gem, Gemcitabine; IFN, interferon; HPV, human papillomavirus; NSCLC, nonsmall cell lung cancer.
Chemotherapy given after immunotherapy induces increased systemic antitumor cytotoxic CD8+ T-cells
Our hypothesis also predicts that the addition of chemotherapy to mice previously treated with immunotherapy will result in increased activity and potency of antitumor cytotoxic effector CD8+ cells compared to those treated with chemotherapy or immunotherapy alone.
Using the TC1 system again, we evaluated for the presence of antigen-specific T-cells at later time points. We thus isolated splenic CD8+ T-cells 1 day after the second chemotherapy treatment (10–12 days after treatment with Ad.E7, see Figure 3b). The mean percentage of E7-tetramer+ cells out of CD8+ T-cells in the combination-therapy group, was significantly increased compared to all of the other groups (P < 0.05) (Figure 4c). These large increases in E7-antigen-specific CD8+ T-cells were also found when the total number of CD8+/E7-tetramer+ T-cells per spleen were calculated. We also evaluated cells pooled from DLN from four mice with each of the treatments. The percentage of E7-tetramer+ cells out of CD8+ cells in the combined treatment on day 1 after chemotherapy was about twofold higher than each treatment alone (Supplementary Figure S2).
To confirm these findings, we measured the activity and potency of antitumor cytotoxic CD8+ cells in the AB12/Ad.IFN model. In this model, where there are no known specific tumor antigens, we utilized the Winn assay (see Materials and Methods section) and mixed purified splenic CD8+ cells from mice in each treatment group (isolated 2 days after the second chemotherapy treatment) with AB12 tumor cells injected into the flanks of naive mice (n = 10/group). As shown in Figure 4d, addition of CD8+ spleen cells from naive, control, or Cis+Gem-treated mice did not change tumor growth. CD8+ spleen cells from Ad.IFNα-treated mice caused a mild, but significant tumor growth inhibition (P < 0.05, Ad.IFN versus control). Importantly, CD8+ spleen cells from the combination treatment mice caused significantly more tumor growth inhibition when compared to all other groups (P < 0.05) indicating increased numbers of cytotoxic T-Lymphocytes (CTL).
Chemotherapy prevents the increase in the number of systemic immune-inhibitory cells induced by immunotherapy
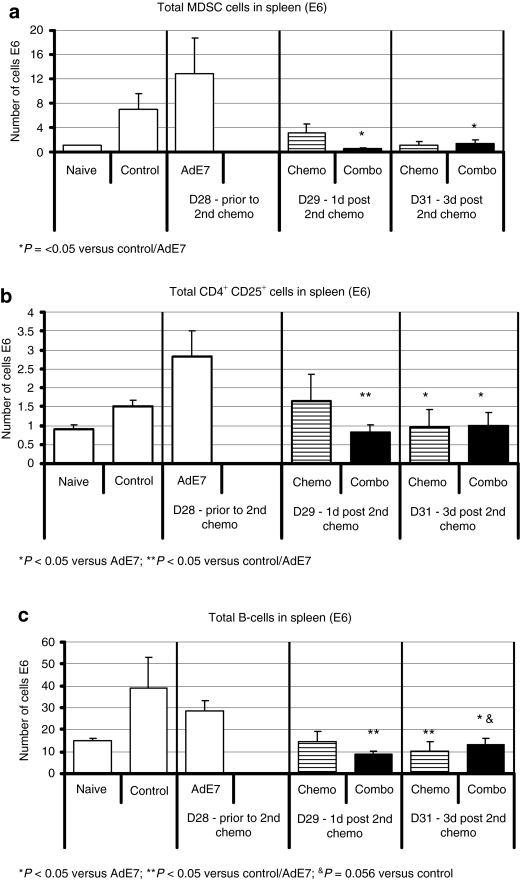
We also evaluated various populations of systemic suppressor cells.18,19,20 As expected, the number of MDSC in the spleens of control tumor-bearing animals was increased over naive nontumor-bearing animals (Figure 5a) and vaccination with Ad.E7 actually increased the number of MDSC. As we have previously shown,26 Gem markedly reduced the number of splenic MDSC cells, in all groups, including the previously vaccinated mice (P < 0.05).
Figure 5.
Chemotherapy reduces systemic immune-inhibitory cells induced by immunogene therapy. Mice (n = 5 for each group) bearing large tumors, were treated in one of four ways: (i) control—no treatment (control); (ii) Ad.E7 s.c. followed by a booster after 1 week; (iii) chemotherapy – Cis +Gem i.p weekly (chemo); and (iv) Ad.E7 treatment followed by Cis + Gem i.p weekly. At the designated time points, multicolor flow cytometry was performed on spleens, and the total number of each cell type per spleen was calculated. (a) Myeloid-derived suppressor cells (MDSC) defined as CD11b+/GR1+. Ad.E7 alone increased the number of MDSC. Chemotherapy significantly reduced the number of MDSC, with or without prior immunotherapy (*P < 0.05 versus control and Ad.E7). (b) T-regs defined as CD4+/CD25+. Ad.E7 alone increased the number of T-regs. After chemotherapy, the numbers were similar to control levels, with or without prior immunotherapy (*P < 0.05 versus Ad.E7; **P < 0.05 versus control/Ad.E7). (c) B-cells defined as B220+. Ad.E7 alone did not significantly change the number of B cells. After chemotherapy the number of B-cells were reduced, with or without prior immunotherapy (*P < 0.05 versus Ad.E7; **P < 0.05 versus control/Ad.E7; &P = 0.056 versus control). Error bars = mean ± SEM. Ad, adenovirus; T-regs, T-regulatory cells.
There was a significant difference in the total number of splenic CD4+/CD25+ T-reg cells in spleens (Figure 5b). Vaccination with Ad.E7 almost doubled the number of T-regs (P = 0.07). Chemotherapy, alone, had little effect on the T-regs. However, chemotherapy given after the vaccine, significantly reduced the number of splenic T-regs by about 50% at 1 and 3 days after the second chemotherapy treatment (P < 0.05 versus Ad.E7 alone).
The number of splenic B-cells in control, untreated tumor-bearing mice and in tumor-bearing mice vaccinated with Ad.E7 was two- to threefold higher than naive mice. However, chemotherapy reduced the number of B-cells to levels similar to those in naive mice, with or without previous immunotherapy (Figure 5c).
Adding chemotherapy in mice previously treated with immunotherapy increases the number and activity of intratumoral CD8+ T-cells
We next evaluated the number and activation state of intratumoral CD8+ T-cells at the time points shown in Figure 3b. Because the tumor data obtained either 1 day or 3 days after chemotherapy was similar, Figure 6 presents only the data obtained at day 31, 3 days after the second chemotherapy treatment.
Figure 6.
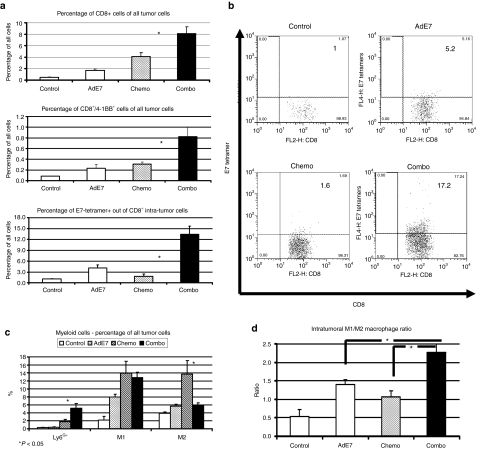
Chemotherapy following immunotherapy increases the number and activity of intratumoral CD8+ T-cells and skews the macrophage phenotype. Mice (n = 5–6 for each group) bearing large TC1 tumors, were treated as described in the legend of Figure 4, followed by multicolor flow cytometry. Data shown is from day 31 (see Figure 3b). (a,b) Changes in intratumoral CD8+ cells. A small increase in the percentage of CD8+ cells out of total tumor cells is shown after chemotherapy, but a larger increase is seen in mice treated with combination therapy (*P < 0.05, chemo versus combo, top). There is a significant increase of activated CD8+ cells (CD8+/4-1BB+ cells) out of total tumor cells in the mice treated with immunotherapy followed by chemotherapy (*P < 0.05, chemo versus combo, middle panel). There is a small increase in the percentage of intratumoral CD8+/tetramer-E7+ cells out of all CD8+ cells with immunotherapy alone, but significantly increased levels of antigen-specific cells in the combination therapy (*P < 0.05, combo versus all other groups) (lower panel). (b) Representative FACS tracings of CD8+ versus E7 tetramer+ cells in each group. The number in each quadrant is the percentage of the E7-tetramer+ cells out of CD8+ cells. (c) The percentage of intratumoral myeloid (CD11b+) cells of all tumor cells. Ad.E7 treatment increases the level of neutrophils (Ly6G+), M1 macrophages (F4/80+/CD206−) and M2 macrophages (F4/80+/CD206+). The combination therapy increases neutrophils and reduces M2 macrophages without changing M1 macrophages (*P < 0.05, combo versus chemo). (d) The ratio of M1 (antitumorigenic) to M2 (protumorigenic) macrophages. The ratio in the combination treatment group is significantly higher than either treatment alone (*P < 0.05). Error bars = mean ± SEM. Ad, adenovirus; FACS, fluorescence-activated cell sorting.
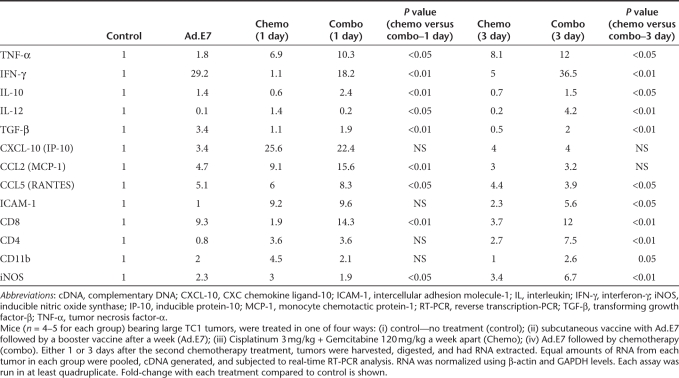
Chemotherapy alone markedly increased the percentage of intratumoral CD8+ T-cells approximately tenfold compared to control (Figure 6a, top, P < 0.05). As we have previously reported,22 the Ad.E7 vaccine induced a significant influx of CD8+ cells into the tumor as well (P < 0.05 versus control). Immunotherapy followed by chemotherapy increased intratumoral CD8+ cells 20-fold over control, twofold compared to chemotherapy and a fourfold increase compared to Ad.E7 alone (P < 0.05 versus each arm). These results were further supported by evaluating the expression levels of CD8 mRNA in the tumors using real-time reverse transcriptase (RT)-PCR (Table 1).
Table 1. Real time RT-PCR in whole tumors.
To determine the extent of intratumoral CD8+ T-cell activation, we measured the percentage of intratumoral CD8+ T-cells expressing the activation marker 4-1BB (Figure 6a, middle). The percentage of activated CD8+ cells (CD8+/4-1BB+) out of all tumor cells was threefold higher following combination therapy compared with mice treated with chemotherapy or immunotherapy alone (P < 0.05 versus each treatment alone).
We next evaluated the presence of intratumoral antigen-specific cytotoxic T-lymphocytes using tetramer staining. Immunotherapy with Ad.E7 increased the percentage of intratumoral E7-specific CD8+ cells fourfold (P < 0.05). Chemotherapy alone did not increase the percentage of E7-specific CD8+ cells. However, chemotherapy given after the Ad.E7 vaccine significantly increased the percentage of E7-tetramer+/CD8+ cells by threefold over Ad.E7 alone (P < 0.05 compared to either treatment alone). These results are summarized in Figure 6a, bottom panel, and representative tracings are shown in Figure 6b.
Immunotherapy followed by chemotherapy augments the ratio of M1/M2 tumor-associated macrophages (TAMs)
TAMs have been classified as having a differentiation state which is protumorigenic (termed M2—defined using fluorescence-activated cell sorting (FACS) as CD11b+/F4-80+/CD206+) or antitumorigenic (termed M1—defined as CD11b+/F4-80+/CD206−).27 Using these definitions, we next examined the phenotypes of TAM at 3 days post-chemotherapy. Treatment with Ad.E7, chemotherapy, or the combination increased the total number of TAM (data not shown). Changes in the percentages of M1 and M2 TAM (of all tumor cells) under each experimental condition are shown in Figure 6c. All treatments increased the percentage of both M1 and M2 TAM. More informative, however, is the determination of changes in the M1/M2 ratio (Figure 6d). After Ad.E7 and chemotherapy, the ratio rises from 1:2 (in control) to about 1:1. However, after combination therapy, the ratio is increased to above 2:1 (P < 0.05 versus all groups). These results were supported by mRNA data showing an increase in the M1 marker inducible nitric oxide synthase (iNOS) in the combined treatment compared to chemotherapy or immunotherapy alone (Table 1).
Chemotherapy also significantly increased the percentage of tumor-associated neutrophils (defined as CD11b+/Ly6G+) by more than fourfold (P < 0.05), and in mice previously treated with immunotherapy, this percentage was even higher (P < 0.05, Figure 6c, left).
Gemcitabine augments leukocyte trafficking into the tumor sites
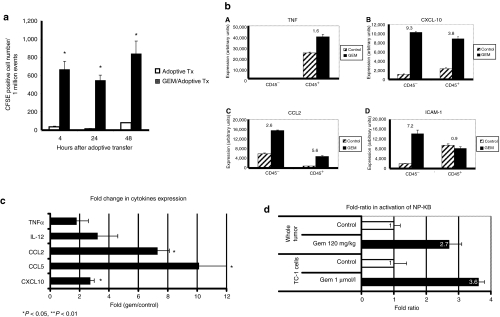
To evaluate whether chemotherapy augmented leukocyte trafficking into the tumor, we performed adoptive transfer experiments. Because activated T-cell trafficking is not antigen dependent,28 we used polyclonally activated spleen-derived T-cells (Figure 7a). As previously reported,28,29 the number of cells trafficking into flank tumors was relatively low. We found no increased trafficking in tumors from mice treated with Cis (data not shown). However, the number of carboxylfluorescein diacetate succinimidyl ester(CFSE)-positive cells in the Gem-treated TC1 tumors was markedly and significantly (P < 0.001) higher than in control tumors for at least 48 hours (Figure 7a). Immunofluorescent staining of tumors confirmed a clear increase in the number of labeled T-cells in Gem-treated tumors (data not shown).
Figure 7.
Gemcitabine increases T-cells trafficking into tumors, the secretion of inflammation cytokines by tumor and immune cells, and activation of tumor NFκB. (a) Mice-bearing TC1 tumors were treated with saline or one dose of 120 mg/kg of GEM. After 24 hours, 20 × 106 CFSE-labeled polyclonally activated T-cells were injected via tail vein. Tumors were harvested 4, 24, and 48 hours later and subjected to flow cytometry to detect CFSE positive cells. The number of CFSE-positive cells in the GEM-treated TC1 tumors (GEM/adoptive Tx) was significantly higher than in control-treated tumors (adoptive Tx). Values are mean number of CFSE+ cells out of 1 million events gated on live cells ± SE (*P <0.001). (b) Mice-bearing TC1 tumors were treated with saline or GEM. Tumors were removed 6 hours later and passed through a column separating CD45+ cells (leukocytes) and CD45− cells (tumor and stromal cells). RNA was extracted from these cell populations and analyzed by real-time RT-PCR. The relative expression levels of TNFα, CXCL-10, CCL2, and ICAM-1 mRNA's are expressed as arbitrary units to allow comparisons. (c) Mice with TC1 tumors (14 days) were given either saline or 120 mg/kg of GEM. Sixteen hours later, tumors (n = 5/group) were harvested, proteins extracted, and cytokine levels determined using a “luminex” bead assay. The fold-change in protein expression (Gem-treated over control) is plotted. *P < 0.05. (d) NFκB p65 concentration in the nucleus was used to measure NFκB activation status. “Whole tumor,” tumor-bearing mice were injected with 120 mg/kg of GEM. Three hours later, the tumors were removed and nuclear extracts prepared, showing a significant increase in O.D in extracts from Gem-treated tumors compared to tumors from untreated animals (top). “TC1 cells,” TC1 cells in culture were exposed to 1 µmol/l of GEM leading to an approximate fourfold increase in O.D. readings (bottom). (*P < 0.05; **P < 0.01). Error bars = mean ± SEM. CFSE, carboxylfluorescein diacetate succinimidyl ester; CXCL-10, CXC chemokine ligand-10; ICAM-1, intercellular adhesion molecule-1; IL, interleukin; IFN-γ, interferon-γ NFκB, nuclear factor κB; MCP-1, monocyte chemotactic protein-1; RT-PCR, reverse transcription-PCR; TGF-β, transforming growth factor-β TNF-α, tumor necrosis factor-α.
Chemotherapy augments proinflammatory cytokine changes in tumor microenvironment induced by immunotherapy
To investigate mechanisms by which chemotherapy augmented trafficking and activation of immune cells in tumor sites, we analyzed the relative mRNA expression from samples, 1 and 3 days after 2nd chemotherapy course (Table 1). When compared to control tumors (normalized to an expression level of 1.0), chemotherapy or Ad.E7 alone increased the mRNA level of several proinflammatory cytokines. In the combination group, the mRNA levels of some mediators (i.e., IFN-γ, CXC chemokine ligand-10 (CXCL-10), intercellular adhesion molecule-1 (ICAM-1) at 1 day), were similar to the highest levels induced by an individual treatment. However, for some mediators (i.e., tumor necrosis factor-α, CCL2, CCL5, ICAM-1 at 3 day), we found a significantly augmented effect of the combination compared to either treatment alone.
We next sought to isolate the effects of Gem (rather than effects due to the cells that had trafficked into the tumor), and therefore digested control or Gem-treated tumors at a very early time point (6 hours after Gem administration) and purified the CD45+ cells (leukocytes) and the CD45− cells (tumor and stromal cells). Figure 7b shows that Gem induced increases in the relative mRNA expression levels of tumor necrosis factor-α, CXCL-10, CCL2, and ICAM-1. Increases were found in both the CD45+ cells (tumor necrosis factor-α, CXCL-10 and CCL2) as well as CD45− cells (CXCL-10, CCL2, and ICAM-1).
We also validated some of these mRNA changes at the protein level 16 hours after drug administration. At this time point, we found a significant (P < 0.05) upregulation of the expression of CCL2, CCL5, and CXCL-10 induced by Gem (Figure 7c). We also saw increases of the proinflammatory adhesion molecule ICAM-1 on tumor endothelium as measured by FACS, but not in other tissues isolated from the same mice (Supplementary Figure S3).
Gemcitabine activates NFκB in tumor cells both in vivo and in vitro
Finally, we determined whether Gem was activating nuclear factor κB (NFκB) in our system by measuring NFκB p65 concentrations in the nucleus. Exposure of cultured TC1 cells to lipopolysaccharide for 3 hours induced a 16-fold increase in enzyme-linked immunosorbent assay optical density reading (data not shown). Exposure of TC1 cells to a concentration of 1 µmol/l of Gem (the estimated peak serum levels in patients30), led to 3.6-fold increase in optical density readings (Figure 7d, bottom; P < 0.05) with peak activation at 2–4 hours (data not shown).
To evaluate NFκB activation in the animal tumor model, tumor-bearing mice were injected with the standard dose of 120 mg/kg of Gem. Three hours later, extracts from Gem-treated tumors showed a significant 2.7-fold increase in optical density compared to tumors from untreated animals (Figure 7d, top; P < 0.01). Increased NFκB activation was seen in both CD45− and CD45+ cells in the Gem-treated animals (data not shown).
Discussion
The present study demonstrates the potential feasiblity of using chemotherapy as a way to “boost” the immune effects of the administration of viral immunogene therapy and suggests multiple mechanisms for the augmented effect. In most models described, a single dose (or two closely spaced doses) of vector were administered to animals-bearing large tumors followed by three weekly administrations of Cis/Gem chemotherapy. Because mouse tumors grow much more rapidly than human cancers, this treatment schedule was compressed in time compared to human cancer treatment, however, we believe these models represent a reasonable murine approximation of what might be approached clinically. Whereas each treatment alone slowed tumor growth, combination therapy led to tumor regresssions with cures in the majority of animals in all of the models (Figure 1). Our data showed that in some of the lines, namely those that are less immunogenic (Lewis lung carcinoma), the sequence of immunotherapy followed by chemotherapy was crucial to obtain the impressive clinical effect. We think that the vaccine in these tumors is necessary in order to initially induce effective cytotoxic CD8+ cells, that can then be reactivated (boosted) when chemotherapy is subsequently given.
We observed similar increases in efficacy using three different Ad-based immunogene therapy regimens that stimulate immune responses via different mechanisms. This included a vaccine directed against a single known viral tumor antigen (Ad.E7 directed against the HPV-E7 antigen),22 and vectors that induce more broad antitumor immune responses via production of type 1 IFNs (Ad.IFN),14 or by killing cells in an “immunogenic fashion” (Ad.HSVtk).23,24,25 All three of these approaches are in use clinically today, but primarily as monotherapy. Our data suggests that trials combining any of these approaches are reasonable.
We hypothesize that the augmented antitumor effect of the combination treatment is due to vaccine boosting process in which viral immunogene therapy induces effector and central-memory antitumor T-cells (the “prime”). As the effector cells disappear, chemotherapy agents then serve to “boost” the immune response by: (i) providing antigens and tumor danger signals to antigen-presenting cells that activate the virally induced memory antitumor T-cells, and (ii) altering the tumor microenvironment. To evaluate this hypothesis, we examined the animals well after the viral therapy, at a time point shortly after the second dose of chemotherapy was delivered. At these time points, the tumors in all groups (especially the three treatment groups) were fairly similar in size, so that the changes observed could not be attributed to differences in tumor burden.
In the Ad.E7 model, where we could measure tumor antigen-specific tetramers, we saw a clear increase in E7-tetramer+ CD8 cells in splenic and DLN cells, but only in the combination group (Figure 4c and Supplementary Figure S2). These data fit well with the observations that chemotherapy (and specifically Gemcitabine) can release antigen and induce cross priming.5,21 When cured mice were rechallenged, we noted that in all mice treated with chemotherapy alone, the tumors regrew, whereas only about a third of tumors regrew tumor in the vaccine alone, and in the combination-therapy groups (data not shown). These data suggest that chemotherapy does not abrogate the memory effect induced by immunotherapy. However, the number of rechallenged mice was too small in the “chemo alone” and “vaccine alone” groups to properly evaluate the long-term memory by rechallenge.
Although we did not attempt to directly evaluate this in our models, our data are consistent with the increasing number of reports showing that chemotherapy can kill tumor cells in an “immunogenic” fashion through multiple mechanisms, including upregulation of surface molecules like calreticulin and/or release of danger signals like high mobility box 1 group protein, uric acid, and heat-shock proteins.31,32,33 Thus, our results support a key component of vaccination boosting, that is, the generation of increased numbers of antitumor T-cells in the combination group. It has been recently described in several combination studies that chemotherapy can upregulate tumor-antigen expression.34,35 We did not directly evaluate this possibility, however it is possible that this mechanism contributes to the clinical effect of combining immunotherapy with chemotherapy.
An additional prediction in our vaccination boost model is that the tumor microenvironment would be changed to augment T-cell trafficking and or persistence. We saw an increased ratio of M1 to M2-type TAMs in the combination group (Figure 6c,d), and were able to show that Gem alone induced upregulation of the mRNA and protein levels of immunostimulatory cytokines and chemokines, such as tumor necrosis factor-α, IFNγ, CXCL-10, CCL2, and CCL5, that was accompanied by an increase in the cell adhesion molecule ICAM-1 (Table 1 and Figure 7c). These changes were even more prominent in the combination groups. Both chemotherapy and combination therapy were associated with increased numbers of CD8+ T-cells within the tumors (Figure 6 and Table 1). In the combination group, this resulted in marked increases in activated and tumor-antigen specific CD8+ T-cells (Figure 6a,b). Although some of this increase may be due to enhanced persistence, our adoptive transfer data (Figure 7a) suggests that there was also an enhancement of T-cell trafficking to the tumors.
It appears that many of these tumor microenvironment effects were due to the ability of Gem to activate NFκB within the tumor cells. It has been well established that many chemotherapeutic agents, including Gem, have the ability to activate NFκB in tumor cells in cell culture.36,37 Although activation of NFκB has generally been considered an undesirable effect of chemotherapeutic drugs because it makes the cells more resistant to apoptosis,38 our data suggest that in the context of vaccination boosting where antitumor cytotoxic T lymphocyte are present, this activation plays a positive role by enhancing migration of these cells into the tumor.
Although not part of our general vaccination boost hypothesis, we also observed important systemic changes in suppressor cells induced by Cis/Gem chemotherapy that likely contributed to the increased CD8+ T-cell activity that we saw in the combination treatment. Vaccination therapy led to increases in the number of both MDSC and T-regs in the spleens. As we have previously shown,15 Gem chemotherapy was quite effective in reducing both MDSC (Figure 5a) and T-regs (Figure 5b) when used alone or in the combination group. Gem also reduced the number of splenic B-cells. We have previously shown that B-cells are immunosuppressive in this model and that depletion with a B-cell specific antibody was associated with enhanced efficacy of the Ad.E7 vaccine.20 We were not able to determine the specific contribution of each of the changes in suppressor immune cells (i.e., MDSC, T-regs, and B-cells) in these studies, but based on other studies showing their individual importance (e.g., see ref. 21), we believe that the combination of these mechanisms had an important role in inducing the clinical effect seen in animals treated with chemotherapy following immunotherapy.
This study raises a number of questions that will require further study. We studied four different immunogene therapy regimens in three different mouse strains, but focused on only one chemotherapy regimen using Cisplatin and Gemcitabine. Gemcitabine is a nucleoside analog that is used in a wide variety of tumors including pancreas, breast. lung, and mesothelioma. Work from Nowak et al. suggested that Gem seems to preferentially deplete B-cells while sparing T-lymphocytes, properties that would be especially useful to combine with immunotherapy.30 In fact, follow-up studies showed that Gem could enhance immunotherapy using an activating CD40 antibody.3 As previously mentioned, our group, and others, showed that Gem could also selectively deplete myeloid suppressor cells and that much of the antitumor effects seen in mouse syngeneic tumors were actually immunologically mediated.15,27 It is not clear which of the many immunostimulatory functions of Gem might be the most important (i.e., activation of NFκB) and how well-these findings would apply to other chemotherapy agents. However, data exists in the literature to suggest that other drugs such as taxanes and anthracyclines6,39,40,41,42 will also function as effective vaccination boosting regimens. Administration of multiple courses of chemotherapy at certain doses can markedly suppress T-cell function,40 so dosing will likely play an important role. Finally, in this study, we did not directly measure cross-priming or explore mechanisms related to the ability of chemotherapy agents to enhance the sensitivity of tumor cells to cytotoxic T-lymphocytes, as has been reported for Cisplatin43 and, more recently for taxol, doxorubicin and Cisplatin via a dramatic perforin-independent increase in permeability to granzyme B via upregulation of the mannose-6-phosphate receptor on the surface of tumor cells.35
In summary, these studies have demonstrated that a major potential limitation of viral immunogene therapy (the inability to give multiple doses) can be overcome by using Cisplatin/Gemcitabine chemotherapy to “boost” the initial vaccination. This approach is currently being tested in a clinical trial using Ad.IFN in combination with Cis/Gem and Cis/pemetrexed in patients with mesothelioma and could be applied to other trials of viral immunogene therapy.
Materials and Methods
Animals. Female Balb/C and C57BL/6 mice (6–8 weeks old, 19–24 g) were purchased from Taconic Labs (Germantown, NY). Female C57BL/6J X 129P3/J hybrids (B6-129/J1) were purchased from Jackson Labs (Bar Harbor, ME). Breeding pairs of Lox-Stop-Lox (LSL) KrasG12D mice (on mixed 129Sv.J and C57BL/6 background) used in the orthotopic lung model were initially provided by Dr David Tuveson of the University of Pennsylvania (Philadelphia, PA).44 The Animal Use Committee of the University of Pennsylvania approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
Cell lines. TC1 cells were derived from mouse lung epithelial cells from a C57B6 mouse, immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene.45
The murine malignant mesothelioma cell line, AB12 was derived from an asbestos-induced tumor in a Balb/C mouse.46 The murine lung cancer line LKR was derived from an explant of a pulmonary tumor from an activated Kras G12D mutant mouse that had been induced in an F1 hybrid of 129Sv.J and C57BL/6.47 Cell lines were regularly tested and maintained negative for mycoplasma spp.
Animal immunotherapy models. We used three different Ad immunogene therapy models:
1. Ad expressing a hybrid interferon-α2α (Ad.IFNα) with activity in mice was received from Schering-Plough (Kenilworth, NJ).48 Ad-expressing murine interferon-β (Ad.IFNβ) was constructed as previously described.44 We used one dose of 1 × 109 plaque-forming units of virus injected intratumorally or intranasally in the orthotopic lung model.
2. An E1/E3-deleted type 5 Ad expressing the HPV-E7 protein under control of a cytomegalovirus promoter (Ad.E7) as previously described.22 Animals-bearing TC1 tumors were vaccinated subcutaneously. Contralateral to the tumor with 1 × 109 plaque-forming units of Ad.E7 vector. Seven days following the initial vaccination, mice received a booster vaccine of 1 × 109 plaque-forming units of Ad.E7.
3. Ad encoding the HSVtk gene (Ad.HSVtk)49 was injected intratumorally at a dose of 1 × 109 plaque-forming units. Forty-eight hours following inoculation, ganciclovir [100 mg/kg intraperitoneal (i.p.) for 5 days] was given to the animals.
Tumor models and schedules (see Figure 3). Mice were injected on the right flank with 1 × 106 TC1, AB12, or LKR tumor cells in the appropriate syngeneic host. The flank tumors were allowed to reach an average size of 200–250 mm3 (~12–15 days). Mice were treated in one of four groups: (i) control-untreated, (ii) chemotherapy—3 mg/kg of Cisplatinum (Cis) and 120 or 60 mg/kg of Gemcitabine (Gem) given i.p. once per week, (iii) immunogene therapy—either Ad.IFNα, Ad.IFNβ, Ad.E7, or Ad.HSVtk as described above, or (iv) the combination of immunotherapy followed by weekly i.p. chemotherapy. Tumors were measured twice weekly. All experiments had at least five mice per group and were repeated at least two times. When needed, as described in Figure 3 (i.e., for FACS, RNA, cell subsets isolation, etc.), flank tumors were harvested from the mice, minced, and digested with 2 mg/ml DNase I (Sigma, St Louis, MO) and 4 mg/ml collagenase type IV (Sigma) at 37 °C for 1 hour.
Evaluation of splenic CD8+ antitumor activity (Winn assay). To determine the amount of suppressive activity found in CD8+ splenocytes from animals-bearing large tumors, we used the Winn assay, in which CD8+ cells are mixed with tumor cells and injected into flanks of naive animals, as previously described.15
Flow cytometric analysis of tumors and spleen after SM16 treatment. Splenocytes, lymph nodes, and tumor cells were studied by FACS analysis as previously described.22 All fluorescently labeled antibodies were purchased from BD Biosciences (San Jose, CA), except CD206-PE [obtained from Serotec (Oxford, UK)], 4-1BB (CD137)-PE [obtained from Abcam (Cambridge, UK)], and GR-1-FITC [obtained from eBioscience (San Diego, CA)]. The allophycocyanin-labeled H-2Db tetramer (1:200 dilution) loaded with E7 peptide (RAHYNIVTF) was obtained from the National Institute of Allergy and Infectious Diseases Tetramer Core.
RNA isolation and real-time, reverse transcription-PCR (RT-PCR). To evaluate changes in tumor microenvironment induced by the different treatments, mice with tumors (~200 mm3) were treated with either one of the four treatments detailed above (n = 5 in each group). Tumors were removed 1 or 3 days after the second chemotherapy dose, flash frozen, and the RNA from each tumor isolated. For each treatment condition, a pool of RNA was created by adding the same amount of RNA from each of the five tumors within the group. Complementary DNA was made from each pool, RNA levels were normalized to GAPDH levels, and quantification of tumor mRNA levels was performed as previously described.15 Relative levels of expression of each of the selected genes (fold-change in each treatment versus control) in whole tumors were determined. Each sample was run in quadruplicate and the experiment was repeated at least once. Primer sequences are given in Supplementary Table S1.
Separation of leukocytes from tumor and stromal cells. TC1 flank tumors were harvested from C57/B6 mice 6 hours after treatment of the experimental mice with 120 mg/kg Gem and digested as described above. Six to eight tumors from control or Gem-treated mice were pooled, and cells were sorted for leukocytes using CD45 magnetic beads (Miltenyi Biotec, Auburn, CA). The positive fraction was confirmed by flow cytometry to be ~90% CD45+. We compared by real-time RT-PCR changes induced by Gem in the positive (CD45+) and negative (CD45−) fractions of the isolation.
Analysis of trafficking of activated T-cells to tumor sites. Spleen cells from naive C57BL/6 mice were isolated and activated with 2 µg/ml of anti-CD3 Ab (BD Biosciences) for 48 hours followed by further incubation with 20 ng/ml of mouse recombinant interleukin-2 (R&D Systems, Minneapolis, MN) for 7 days. Media-containing fresh interleukin-2 was changed every other day. These polyclonally activated T-cells were labeled with carboxylfluorescein diacetate succinimidyl ester (Molecular Probes, Eugene, OR) per manufacturer's instructions. To evaluate effects of Gem on trafficking of activated T-cells to tumor sites, TC1 tumors were grown to a minimal volume of 200 mm3, and injected with either saline or 120 mg/kg of Gem i.p. on day 7 (n = 3). The next day, animals were injected with 20 × 106 carboxylfluorescein diacetate succinimidyl ester-labeled polyclonally activated T-cells via tail vein. Four, twenty-four, and forty-eight hours after adoptive transfer, tumors from three mice of each group were harvested and digested by a 60-minute incubation with 400 units/ml collagenase type V (Sigma) and 0.4 mg/ml DNase I (Roche, Basel, Switzerland) at 37 °C to prepare a single-cell suspensions. The CSFE labeled cells were identified by FACS.
Protein expression of various cytokines and chemokines in tumors. Production of various cytokines and chemokines that could be potentially involved in immune cell trafficking into tumors were measured using a multiplex Luminex bead assay system.22 Briefly, 13 days after TC1 cell (1 × 106) injection, mice were given either saline or 120 mg/kg of Gem. After 16 hours, the tumors were removed and processed for cytokine measurements as described.50
Effects of Gem on NFκB activation. To determine the ability of Gem to activate NFκB, TC1 cells were exposed to 1 µmol/l Gem, the estimated peak concentration of Gem in patients. Nuclear fractions were prepared using a nuclear extract kit (Active Motif, Carlsbad, CA), as per the manufacturer. For in vivo experiments, animals-bearing TC1 tumors were left untreated or given one i.p. dose of 120 mg/kg Gem. Three hours later, the tumors were harvested and the tissues were diced, homogenized, and cell pellets further processed for nuclear fractions following the same process as for the cells above.
NFκB activation was measured semiquantitatively using the “TransAM NFκB p65” kit (Active Motif) using manufacturer's instructions. Ten microgram of nuclear extract from each sample were added to each well, and the primary antibody that only recognizes NFκB p65 when activated and bound to its target DNA was added. An horseradish peroxidase-conjugated secondary antibody was used for detection and quantified by measuring optical density using spectrophotometry. Each sample was run in duplicate or triplicate.
Statistical analyses. For the RT-PCR, FACS studies, and flank tumor studies comparing differences between two groups, we used unpaired Student t-tests. For FACS, RT-PCR, and flank tumor studies comparing more than two groups, we used one-sided analysis of variance with appropriate post hoc testing. Differences were considered significant when P < 0.05. Data are presented as mean ± SEM.
SUPPLEMENTARY MATERIAL Figure S1. Chemotherapy given after immunotherapy augments antitumor efficacy in the NSCLC line LKR. B6129/J1 mice (n = 5 for each group) bearing large tumors (approximately 200 mm3), were treated in one of four ways: 1) control – no treatment (control); 2) Adenovirus-IFNα i.t. as immuno-gene therapy (Ad.IFNα); 3) Chemotherapy – Cisplatinum (Cis) +Gemcitabine (Gem) intaperitoneal (i.p) weekly (chemo); and 4) Ad.IFNα followed by weekly chemotherapy (Combo). Treatment with Ad.IFNα or chemotherapy alone mildly slowed tumor growth. The combined treatment augmented the anti-tumor effect (**=p<0.05 for Combo vs. all three other treatment groups). Figure S2. Chemotherapy following immunotherapy increases the percentage of antigen-specific CD8+ T-cells in draining lymph nodes (DLN). Mice (n=3 for each group) bearing large TC1 tumors, were treated in one of four ways: 1) control – no treatment (control); 2) Ad.E7 s.c. followed by a booster after 1 week. 3) Chemotherapy – Cis +Gem i.p weekly (chemo); and 4) Ad.E7 treatment followed by Cis +Gem i.p weekly. At the proper time (see figure 2), DLN were harvested, pooled per group, and multi-color flow cytometry was performed. The percentage of CD8+/tetramer-E7+ directed against the immunodominant epitope of the HPV-E7 protein, out of all CD8+ cells is shown. There is a mild increase with immunotherapy alone, and significant increased levels of antigen-specific cells in the combination therapy 1 day after second chemotherapy treatment. The experiment was repeated with similar results. Figure S3. Gemcitabine increases the level of ICAM-1 in tumor endothelial cells (EC), but not in normal lung EC. Mice bearing TC1 tumors (approximately 200 mm3 in size) were treated with saline or one dose of 120 mg/kg of GEM. After 24 hours, tumors were digested and subjected to flow cytometry. The mean fluorescence intensity of ICAM-1 on CD45−/CD31+ cells (endothelium) from tumors was increased 1.8-fold in Gem-treated mice compared to endothelium from control TC-1 tumors (Panel a). Values are MFI ± SE (*=P <0.05). ICAM-1 expression on lung endothelial cells from the same mice showed no up-regulation of ICAM-1 on endothelial cells (Panel b). Table S1. Primer sequences.
Acknowledgments
This work was funded by NCI PO1 CA 66726. We thank Dr Robert Streiter for help in measuring cytokine and chemokines from tissue samples. We thank Dr Beth Hutchens for the gift of Ad.IFNα.
Supplementary Material
Chemotherapy given after immunotherapy augments antitumor efficacy in the NSCLC line LKR. B6129/J1 mice (n = 5 for each group) bearing large tumors (approximately 200 mm3), were treated in one of four ways: 1) control – no treatment (control); 2) Adenovirus-IFNα i.t. as immuno-gene therapy (Ad.IFNα); 3) Chemotherapy – Cisplatinum (Cis) +Gemcitabine (Gem) intaperitoneal (i.p) weekly (chemo); and 4) Ad.IFNα followed by weekly chemotherapy (Combo). Treatment with Ad.IFNα or chemotherapy alone mildly slowed tumor growth. The combined treatment augmented the anti-tumor effect (**=p<0.05 for Combo vs. all three other treatment groups).
Chemotherapy following immunotherapy increases the percentage of antigen-specific CD8+ T-cells in draining lymph nodes (DLN). Mice (n=3 for each group) bearing large TC1 tumors, were treated in one of four ways: 1) control – no treatment (control); 2) Ad.E7 s.c. followed by a booster after 1 week. 3) Chemotherapy – Cis +Gem i.p weekly (chemo); and 4) Ad.E7 treatment followed by Cis +Gem i.p weekly. At the proper time (see figure 2), DLN were harvested, pooled per group, and multi-color flow cytometry was performed. The percentage of CD8+/tetramer-E7+ directed against the immunodominant epitope of the HPV-E7 protein, out of all CD8+ cells is shown. There is a mild increase with immunotherapy alone, and significant increased levels of antigen-specific cells in the combination therapy 1 day after second chemotherapy treatment. The experiment was repeated with similar results.
Gemcitabine increases the level of ICAM-1 in tumor endothelial cells (EC), but not in normal lung EC. Mice bearing TC1 tumors (approximately 200 mm3 in size) were treated with saline or one dose of 120 mg/kg of GEM. After 24 hours, tumors were digested and subjected to flow cytometry. The mean fluorescence intensity of ICAM-1 on CD45−/CD31+ cells (endothelium) from tumors was increased 1.8-fold in Gem-treated mice compared to endothelium from control TC-1 tumors (Panel a). Values are MFI ± SE (*=P <0.05). ICAM-1 expression on lung endothelial cells from the same mice showed no up-regulation of ICAM-1 on endothelial cells (Panel b).
Primer sequences.
References
- Harris J, Sengar D, Stewart T., and, Hyslop D. The effect of immunosuppressive chemotherapy on immune function in patients with malignant disease. Cancer. 1976;37 2 suppl:1058–1069. doi: 10.1002/1097-0142(197602)37:2+<1058::aid-cncr2820370813>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Gulley JL, Madan RA., and, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25 suppl 2:B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak AK, Robinson BW., and, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–4496. [PubMed] [Google Scholar]
- Ramakrishnan R, Antonia S., and, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Most RG, Currie A, Robinson BW., and, Lake RA. Cranking the immunologic engine with chemotherapy: using context to drive tumor antigen cross-presentation towards useful antitumor immunity. Cancer Res. 2006;66:601–604. doi: 10.1158/0008-5472.CAN-05-2967. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F., and, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, André F, Tesniere A., and, Kroemer G. The anticancer immune response: indispensable for therapeutic success. J Clin Invest. 2008;118:1991–2001. doi: 10.1172/JCI35180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake RA., and, Robinson BW. Immunotherapy and chemotherapy–a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12 3 Pt 1:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- Gribben JG, Ryan DP, Boyajian R, Urban RG, Hedley ML, Beach K, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. The promise of cancer vaccines. Nat Rev Cancer. 2004;4:401–411. doi: 10.1038/nrc1359. [DOI] [PubMed] [Google Scholar]
- Harrop R., and, Carroll MW. Viral vectors for cancer immunotherapy. Front Biosci. 2006;11:804–817. doi: 10.2741/1838. [DOI] [PubMed] [Google Scholar]
- Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, et al. A phase I clinical trial of single-dose intrapleural IFN-β gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin Cancer Res. 2007;13 15 Pt 1:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR., and, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, et al. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res. 2007;13 18 Pt 1:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. Does the immune system see tumors as foreign or self. Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. 2008;20:241–246. doi: 10.1016/j.coi.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Fridlender ZG, Dunn R, Kehry MR, Kapoor V, Blouin A, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31:446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- Haas AR, Sun J, Vachani A, Wallace AF, Silverberg M, Kapoor V, et al. Cycloxygenase-2 inhibition augments the efficacy of a cancer vaccine. Clin Cancer Res. 2006;12:214–222. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- Lambright ES, Amin K, Wiewrodt R, Force SD, Lanuti M, Propert KJ, et al. Inclusion of the herpes simplex thymidine kinase gene in a replicating adenovirus does not augment antitumor efficacy. Gene Ther. 2001;8:946–953. doi: 10.1038/sj.gt.3301489. [DOI] [PubMed] [Google Scholar]
- Vile RG, Nelson JA, Castleden S, Chong H., and, Hart IR. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994;54:6228–6234. [PubMed] [Google Scholar]
- Shibata MA, Morimoto J, Akamatsu K., and, Otsuki Y. Antimetastatic effect of suicide gene therapy for mouse mammary cancers requires T-cell-mediated immune responses. Med Mol Morphol. 2008;41:34–43. doi: 10.1007/s00795-007-0388-1. [DOI] [PubMed] [Google Scholar]
- Suzuki E, Sun J, Kapoor V, Jassar AS., and, Albelda SM. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol Ther. 2007;6:880–885. doi: 10.4161/cbt.6.6.4090. [DOI] [PubMed] [Google Scholar]
- Sica A, Schioppa T, Mantovani A., and, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kjaergaard J., and, Shu S. Tumor infiltration by adoptively transferred T cells is independent of immunologic specificity but requires down-regulation of L-selectin expression. J Immunol. 1999;163:751–759. [PubMed] [Google Scholar]
- Cohen PA, Peng L, Kjaergaard J, Plautz GE, Finke JH, Koski GK, et al. T-cell adoptive therapy of tumors: mechanisms of improved therapeutic performance. Crit Rev Immunol. 2001;21:215–248. [PubMed] [Google Scholar]
- Nowak AK, Robinson BW., and, Lake RA. Gemcitabine exerts a selective effect on the humoral immune response: implications for combination chemo-immunotherapy. Cancer Res. 2002;62:2353–2358. [PubMed] [Google Scholar]
- Spisek R., and, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6:1962–1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- Apetoh L, Ghiringhelli F, Tesniere A, Criollo A, Ortiz C, Lidereau R, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- Vo DD, Prins RM, Begley JL, Donahue TR, Morris LF, Bruhn KW, et al. Enhanced antitumor activity induced by adoptive T-cell transfer and adjunctive use of the histone deacetylase inhibitor LAQ824. Cancer Res. 2009;69:8693–8699. doi: 10.1158/0008-5472.CAN-09-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R, Assudani D, Nagaraj S, Hunter T, Cho HI, Antonia S, et al. Chemotherapy enhances tumor cell susceptibility to CTL-mediated killing during cancer immunotherapy in mice. J Clin Invest. 2010;120:1111–1124. doi: 10.1172/JCI40269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt A, Gehrz A, Müerköster S, Vorndamm J, Kruse ML, Fölsch UR, et al. Role of NF-kappaB and Akt/PI3K in the resistance of pancreatic carcinoma cell lines against gemcitabine-induced cell death. Oncogene. 2003;22:3243–3251. doi: 10.1038/sj.onc.1206390. [DOI] [PubMed] [Google Scholar]
- Jones DR, Broad RM, Madrid LV, Baldwin AS., Jr, and, Mayo MW. Inhibition of NF-kappaB sensitizes non-small cell lung cancer cells to chemotherapy-induced apoptosis. Ann Thorac Surg. 2000;70:930–6; discussion 936. doi: 10.1016/s0003-4975(00)01635-0. [DOI] [PubMed] [Google Scholar]
- Haefner B. NF-kappa B: arresting a major culprit in cancer. Drug Discov Today. 2002;7:653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, et al. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61:3689–3697. [PubMed] [Google Scholar]
- Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, et al. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- Prell RA, Gearin L, Simmons A, Vanroey M., and, Jooss K. The anti-tumor efficacy of a GM-CSF-secreting tumor cell vaccine is not inhibited by docetaxel administration. Cancer Immunol Immunother. 2006;55:1285–1293. doi: 10.1007/s00262-005-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett CT, Schlom J., and, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008;14:3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt RE, Mahtabifard A, Yamada RE, Crystal RG., and, Korst RJ. Cisplatin augments cytotoxic T-lymphocyte-mediated antitumor immunity in poorly immunogenic murine lung cancer. J Thorac Cardiovasc Surg. 2003;126:1609–1617. doi: 10.1016/s0022-5223(03)00707-4. [DOI] [PubMed] [Google Scholar]
- Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A, et al. Intrapulmonary IFN-β gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res. 2005;65:8379–8387. doi: 10.1158/0008-5472.CAN-05-0920. [DOI] [PubMed] [Google Scholar]
- Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–26. [PubMed] [Google Scholar]
- Fitzpatrick DR, Bielefeldt-Ohmann H, Himbeck RP, Jarnicki AG, Marzo AL., and, Robinson BW. Transforming growth factor-β: antisense RNA-mediated inhibition affects anchorage-independent growth, tumorigenicity and tumor-infiltrating T-cells in malignant mesothelioma. Growth Factors. 1994;11:29–44. doi: 10.3109/08977199409015049. [DOI] [PubMed] [Google Scholar]
- Wilderman MJ, Kim S, Gillespie CT, Sun J, Kapoor V, Vachani A, et al. Blockade of TNF-α decreases both inflammation and efficacy of intrapulmonary Ad.IFNβ immunotherapy in an orthotopic model of bronchogenic lung cancer. Mol Ther. 2006;13:910–917. doi: 10.1016/j.ymthe.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Brin E, Atencio I, Helmich BK, Maneval D., and, Laface D. Adenovirus delivery provides extended interferon-α exposure and augments treatment of metastatic carcinoma. Cancer Gene Ther. 2006;13:664–675. doi: 10.1038/sj.cgt.7700942. [DOI] [PubMed] [Google Scholar]
- Smythe WR, Hwang HC, Amin KM, Eck SL, Davidson BL, Wilson JM, et al. Use of recombinant adenovirus to transfer the herpes simplex virus thymidine kinase (HSVtk) gene to thoracic neoplasms: an effective in vitro drug sensitization system. Cancer Res. 1994;54:2055–2059. [PubMed] [Google Scholar]
- Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res. 2005;65:11752–11761. doi: 10.1158/0008-5472.CAN-05-1658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Chemotherapy given after immunotherapy augments antitumor efficacy in the NSCLC line LKR. B6129/J1 mice (n = 5 for each group) bearing large tumors (approximately 200 mm3), were treated in one of four ways: 1) control – no treatment (control); 2) Adenovirus-IFNα i.t. as immuno-gene therapy (Ad.IFNα); 3) Chemotherapy – Cisplatinum (Cis) +Gemcitabine (Gem) intaperitoneal (i.p) weekly (chemo); and 4) Ad.IFNα followed by weekly chemotherapy (Combo). Treatment with Ad.IFNα or chemotherapy alone mildly slowed tumor growth. The combined treatment augmented the anti-tumor effect (**=p<0.05 for Combo vs. all three other treatment groups).
Chemotherapy following immunotherapy increases the percentage of antigen-specific CD8+ T-cells in draining lymph nodes (DLN). Mice (n=3 for each group) bearing large TC1 tumors, were treated in one of four ways: 1) control – no treatment (control); 2) Ad.E7 s.c. followed by a booster after 1 week. 3) Chemotherapy – Cis +Gem i.p weekly (chemo); and 4) Ad.E7 treatment followed by Cis +Gem i.p weekly. At the proper time (see figure 2), DLN were harvested, pooled per group, and multi-color flow cytometry was performed. The percentage of CD8+/tetramer-E7+ directed against the immunodominant epitope of the HPV-E7 protein, out of all CD8+ cells is shown. There is a mild increase with immunotherapy alone, and significant increased levels of antigen-specific cells in the combination therapy 1 day after second chemotherapy treatment. The experiment was repeated with similar results.
Gemcitabine increases the level of ICAM-1 in tumor endothelial cells (EC), but not in normal lung EC. Mice bearing TC1 tumors (approximately 200 mm3 in size) were treated with saline or one dose of 120 mg/kg of GEM. After 24 hours, tumors were digested and subjected to flow cytometry. The mean fluorescence intensity of ICAM-1 on CD45−/CD31+ cells (endothelium) from tumors was increased 1.8-fold in Gem-treated mice compared to endothelium from control TC-1 tumors (Panel a). Values are MFI ± SE (*=P <0.05). ICAM-1 expression on lung endothelial cells from the same mice showed no up-regulation of ICAM-1 on endothelial cells (Panel b).
Primer sequences.