Abstract
Peptide for ocular delivery (POD) is a novel cationic cell-penetrating peptide (CPP) which, when conjugated with polyethylene glycol (PEG-POD), can deliver plasmid DNA to the retinal pigment epithelium (RPE) of adult murine retina. PEG-POD nanoparticles containing an expression cassette for glial cell line–derived neurotrophic factor (PEG–POD~GDNF) were investigated for their ability to inhibit light-induced photoreceptor apoptosis. PEG-POD~GDNF, control nanoparticles, or buffer were injected into the subretinal space of adult murine retina and retinal degeneration induced by blue light. Animals injected with PEG-POD~GDNF showed a significant reduction (3.9–7.7 fold) in apoptosis relative to control-injected animals. The thickness of the outer nuclear layer (ONL) of the superior retina of PEG-POD~GDNF-injected eyes was significantly greater (23.6–39.3%) than control-injected retina 14 days post-light treatment. PEG-POD~GDNF-injected eyes showed a 27–39% greater functional response relative to controls, as measured by electroretinogram (ERG) 7 days post-light treatment. This is one of only two studies demonstrating histological and functional rescue of a mouse model of retinal degeneration following nonviral administration of a transgene into adult retina. Although rescue is short lived for clinical application, this study represents an important step in the development of nonviral gene therapy for retinal diseases.
Introduction
Retinitis pigmentosa (RP) and age-related macular degeneration (AMD) comprise two of the most common causes of blindness in the developed world.1 Because of the chronic nature of many ocular diseases, including RP and AMD, gene therapy may offer an ideal form of treatment. While nonviral vectors have the potential to offer a safe and scalable approach to ocular gene therapy, such strategies have been impeded by very limited gene transfer efficiency relative to viral vectors in vivo. Despite substantial progress in preclinical and clinical ocular gene therapy, there are few reports of nonviral rescue of retinal degeneration. To date, only one study has demonstrated histological and functional rescue of retinal degeneration after delivery of a nonviral vector to the postmitotic adult eye.2
Some proteins, known as cell-penetrating peptides (CPPs), possess the ability to move across the plasma membrane and into the cytoplasm.3 CPPs have been shown to be effective in delivering various cargoes, such as proteins, oligonucleotides, plasmid DNA, and liposomes into the cell cytoplasm. Based on the clinical implications of these peptides for delivery of macromolecules, we have been interested in using CPPs as vectors to deliver proteins and DNA to retinal tissue in vivo.4,5 The glycosaminoglycans chondroitin sulphate and heparan sulfate are present at high levels in the adult retina. To specifically target retinal cells, we have synthesized a number of peptides based on a consensus recognition sequence for glycosaminoglycan. The novel synthetic peptide CGGG(ARKKAAKA)4, molecular weight = 3.5 kd termed peptide for ocular delivery (POD) was previously described by us as a potential CPP capable of crossing the plasma membrane of cells in vitro as well as most ocular neuronal cell types in vivo, including retinal pigment epithelium (RPE), photoreceptors, and ganglion cells.6 POD retains this cell-penetrating ability even when fused with much larger molecules, such as green fluorescent protein or quantum dots.6,7 More recently, we demonstrated that PEGylation of POD (PEG-POD) permits compaction of plasmid DNA into 120–150 nm nanoparticles.8 Upon ocular delivery, PEG-POD nanoparticles transfect the postmitotic RPE ~200-fold more efficiently than plasmid DNA.8
Over 40 different genes have been documented to cause RP, with over 100 novel mutations in the rhodopsin gene alone (RetNet at http://www.sph.uth.tmc.edu/retnet/). AMD is a complex disease involving a variety of genetic and environmental influences. The broad etiological spectrum of retinal degeneration makes it practical to consider the use of neurotrophic factors, which can allow for a mutation independent approach to treatment. Such factors have been shown to rescue a number of animal models of retinal degeneration.9 A recent phase II clinical trial in which RP patients received intravitreal implants of encapsulated cells secreting ciliary neurotrophic factor found that a subset of patients exhibited an improvement in visual acuity.10 Glial cell line–derived neurotrophic factor (GDNF) is a member of a family of neurotrophic factors whose activity has been shown to prevent photoreceptor cell loss in both inherited models of retinal degeneration,11,12,13 as well as environmentally induced models of retinal damage.14
Apoptosis represents a final step in retinal degeneration for both RP15 and AMD.16 Apoptosis-mediated cell death has been induced in rodent retina by exposure to light, causing synchronized and rapid cell death within the retina. Studies in rats exposed to high levels of light have found morphological changes to both the RPE and photoreceptors, indicating that both of these cell types are affected by light exposure.17 The pathogenic effects of lipofuscin deposits, such as A2E, observed in AMD patients has been partially attributed to blue and visible light exposure.18 Light itself has been found to be an accelerator of RP in patients19 and animal models of RP.9,20,21,22 Thus, light-induced retinal degeneration provides an in vivo model applicable across a wide spectrum of degenerative disorders.
To examine the efficiency of PEG-POD-mediated gene therapy, PEG-POD nanoparticles were generated expressing GDNF (PEG-POD~GDNF) and investigated for their ability to rescue photoreceptor degeneration in adult mice exposed to bright blue light. We found that retinas treated with PEG-POD~GDNF nanoparticles exhibit significantly reduced photoreceptor loss, resulting in a significant increase in their functional response as measured by electroretinography (ERG). These data provide evidence that PEG-POD is able to successfully deliver genes to murine retina at levels sufficient to permit partial structural and functional rescue of a model of acute retinal damage.
Results
PEG-POD can form GDNF-expressing nanoparticles
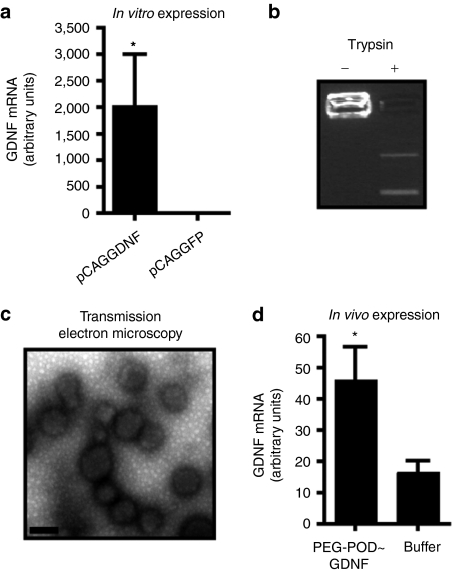
Previously, we have demonstrated that PEG-POD is able to compact plasmid DNA containing various reporter genes into discrete and homogeneous nanoparticles that can transduce a variety of tissues in vivo, including the RPE.8 In order to examine the potency of these nanoparticles in rescuing a model of retinal degeneration, the rat GDNF complementary DNA was cloned downstream of a chicken β-actin promoter (pCAGGDNF). Expression of rat GDNF mRNA was confirmed in human embryonic retinoblasts by transfection in vitro (Figure 1a). When pCAGGDNF was compacted using PEG-POD (PEG-POD~GDNF), electrophoretic migration of the plasmid DNA was retarded, and could be relieved by trypsin-mediated digestion of the POD peptide (Figure 1b). PEG-POD~GDNF was examined by transmission electron microscopy and found to form discrete spherical nanoparticles (Figure 1c), similar to those previously described with a luciferase-expressing plasmid.8 Analysis of the transmission electron microscopic images revealed a mean particle diameter of 175.9 ± 28.6 nm. When PEG-POD~GDNF nanoparticles were injected into the subretinal space of adult mice, there was a detectable level of rat-specific GDNF transcript above control buffer-injected retinas 48 hours postinjection (P < 0.05) (Figure 1d).
Figure 1.
PEG-POD forms GDNF-expressing nanoparticles. (a) A plasmid (pCAGGDNF) containing an expression cassette for rat GDNF was shown to express GDNF mRNA in vitro. Both conditions, n = 3. Mean ± SEM. (b) PEG-POD compaction of pCAGGDNF prevents electrophoretic migration of the plasmid, which can be relieved by trypsin-mediated digestion of the protein. (c) PEG-POD compacted pCAGGDNF (PEG-POD~GDNF) was examined by transmission electron microscopy (TEM) and found to form discrete spherical particles, similar to those previously described. Analysis of the TEM images showed a mean particle diameter of 175.9 ± 28.6 nm. Bar = 200 nm. (d) Injection of PEG-POD compacted pCAGGDNF into the subretinal space results in a detectable level of rat GDNF mRNA expression (P < 0.05). Both conditions, n = 9. Mean ± SEM. GDNF, glial cell line–derived neurotrophic factor; PEG, polyethylene glycol; POD, peptide for ocular delivery.
Bright blue light activates caspase-3/7 and induces retinal degeneration that can be modulated by damage due to a subretinal injection
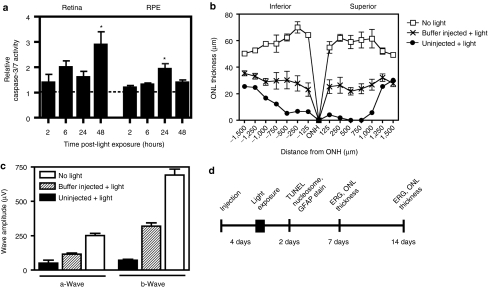
Because the mechanism of light damage has been shown to be highly dependent on the intensity and type of light used,9,17,23 we were interested in characterizing the specific mechanism of photoreceptor degeneration induced by the regimen in our studies. Caspase-3-mediated apoptosis has been shown to be important in a number of retinal degeneration models.24,25,26 Following dark adaptation, mice were exposed to bright blue light for 4 hours. To confirm caspase-3/7 activation in mouse retina, light exposed eyes were harvested at 2, 6, 24, and 48 hours post-light treatment, the retina separated from the RPE/choroid/sclera, and caspase-3/7 activity quantified relative to nonlight-treated eyes (Figure 2a). We observed a 2.9-fold increase in caspase-3/7 activity above nonlight-treated eyes in the retina 48 hours post-light exposure (P < 0.05) and a 1.9-fold increase in caspase-3/7 activity in the RPE/choroid/sclera 24 hours post-light exposure (P < 0.05).
Figure 2.
Bright blue light induces caspase-3/7 activation and retinal degeneration that is modulated by subretinal injection. (a) Caspase-3/7 activity was measured at various time points following light treatment. There was a 2.9-fold increase in activity in the retina 48 hours post-light exposure (P < 0.05), as well as a 1.9-fold increase in activity in the RPE 24 hours post-light exposure (P < 0.05), relative to the respective tissues of nonlight-treated mice. Two and forty-eight hours, n = 4; 6 and 24 hours, n = 6. Mean ± SEM. (b) Photoreceptor degeneration was evaluated by measuring the thickness of the ONL. The ONL of uninjected mice showed marked loss of photoreceptor nuclei post-light treatment, which was partially rescued by a subretinal injection of buffer. (c) Electroretinograms (ERGs) were recorded in mice 7 days after light exposure. In the absence of any injection, there was a significant decrease in the amplitudes of both the a-wave (P < 0.001) and the b-wave (P < 0.001). The impairment in light-response was partially prevented by subretinal injection of a buffer solution, although both the a- and b-waves were significantly lower in amplitude than those of nonlight-treated mice (P < 0.001). Buffer, n = 11; uninjected, n = 3; nonlight-treated, n = 4. Mean ± SEM. (d) To examine the effects of PEG-POD~GDNF, retinal degeneration was induced 4 days following subretinal injection by exposure to 4 hours of bright blue light. Mice were injected into the superior hemisphere with either PEG-POD~GDNF nanoparticles, PEG-POD~Lux (sham nanoparticles), or buffer alone. Following light exposure, effects of the treatment were assessed by measuring Müller cell activation (GFAP), apoptosis (TUNEL and nucleosome release), and ONL thickness and functional response (ERG) at 2, 7, and 14 days post-light-treatment as indicated. GFAP, glial fibrillary acidic protein; ONH, optic nerve head; ONL, outer nuclear layer; TUNEL, TdT-dUTP terminal nick-end labeling.
It has previously been shown that subretinal injection alone is sufficient to provide partial rescue of light-induced damage.27 Hence, before injection of PEG-POD~GDNF nanoparticles, we wished to test the level of rescue provided by a sham subretinal injection in our hands. Mice were injected in the subretinal space with 5% dextrose solution and 4 days later exposed to bright blue light for 4 hours. Eyes were harvested, sectioned, and nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI) such that the extent of photoreceptor degeneration could be evaluated by measuring the thickness of the outer nuclear layer (ONL). Because degeneration in the light damage model has been previously reported to occur predominantly in the central retina and at higher levels in the superior hemisphere of the eye,28 eyecups were sectioned for histological analysis along the vertical meridian in a section bisecting the optic nerve head (ONH). Although the ONL of the superior hemisphere showed a marked decrease in average thickness post-light treatment (10.6 ± 5.6 µm) compared to nonlight-treated eyes (P < 0.001, 57.4 ± 2.0 µm), a significant reduction in photoreceptor loss (as measured by ONL thickness) was observed in those eyes receiving a subretinal injection of buffer (5% dextrose, P < 0.001, 25.4 ± 1.7 µm) before light exposure (Figure 2b). Five percent dextrose buffer was used in all subsequent experiments and is the solution used to suspend the nanoparticle formulations.
To measure any potential change in retinal function, ERGs were recorded in buffer-injected mice 7 days after light exposure (Figure 2c) and compared with those of uninjected light-treated mice. Consistent with the histological data, subretinal injection provided partial ERG rescue, with a 2.3- and 4.5-fold increase observed in the amplitudes of the a- and b-waves, respectively, relative to those of the uninjected light-treated mice. However, both the a- and b-wave amplitudes were substantially impaired relative to those of nonlight-treated eyes (P < 0.001). We now wished to determine whether PEG-POD~GDNF nanoparticles could provide significantly greater levels of rescue relative to that provided by sham subretinal injection. We employed the same regimen of light exposure (Figure 2d), and harvested eyes at different time points for measurement of rescue.
PEG-POD~GDNF reduces light-induced apoptosis
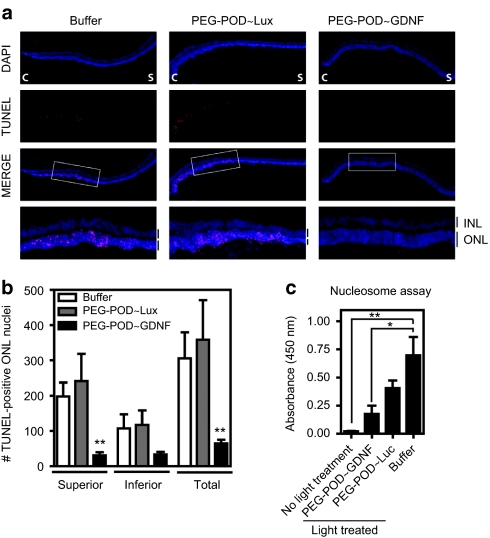
We next examined the ability of PEG-POD~GDNF to inhibit the apoptotic cascade and ensuing photoreceptor cell death. Eyes of 6–8-week-old BALB/cJ mice were injected with either PEG-POD~GDNF, PEG-POD~Lux, or buffer alone into the subretinal space of the superior hemisphere. Eyes were harvested and sectioned 48 hours post-light treatment. Sections bisecting the ONH were stained using a TdT-dUTP terminal nick-end labeling (TUNEL) method (Figure 3a) and the TUNEL-positive nuclei counted in both the superior and inferior hemispheres (Figure 3b). Eyes injected with PEG-POD~GDNF showed a significant decrease in the number of TUNEL-positive nuclei (in the ONL) in the superior pole (31.00 ± 8.5 nuclei/section) relative to PEG-POD~Lux (241.2 ± 77.6 nuclei/section, P < 0.01) and buffer (197.9 ± 39.7, P < 0.01) -injected eyes.
Figure 3.
PEG-POD~GDNF nanoparticles result in decreased apoptosis of photoreceptors. (a) TUNEL stain of retinal sections 48 hours after light exposure showed a significant reduction in apoptotic photoreceptors in PEG-POD~GDNF-injected eyes. C, central; S, superior. (b) TUNEL-positive nuclei in the ONL of both the superior and inferior hemispheres. Eyes injected with PEG-POD~GDNF showed a significant decrease in the number of TUNEL-positive nuclei in the ONL of the superior pole (31.00 ± 8.5 nuclei/section) compared to PEG-POD~Lux (241.2 ± 77.6 nuclei/section, P < 0.01) and buffer- (197.9 ± 39.7, P < 0.01) injected eyes. PEG-POD~GDNF, n = 10; PEG-POD~Lux, n = 5; buffer, n = 7. Mean ± SEM. (c) Retinal tissue was harvested 48 hours post-light treatment and apoptosis evaluated by nucleosome release ELISA. Lower absorbance is observed with less nucleosome release and is thus an indicator of decreased apoptosis. Eyes injected with PEG-POD~GDNF showed 2.3-fold lower absorbance than PEG-POD~Lux-injected eyes (P > 0.05) and 3.9-fold lower than buffer-injected eyes (P < 0.05). Nonlight treated and PEG-POD~GDNF, n = 5; PEG-POD~Lux, buffer, and no light treatment, n = 5. Mean ± SEM. GDNF, glial cell line–derived neurotrophic factor; INL/ONL, inner/outer nuclear layer; PEG, polyethylene glycol; POD, peptide for ocular delivery; TUNEL, TdT-dUTP terminal nick-end labeling.
Consistent with the subretinal injections being performed in the superior hemisphere, the decrease in apoptosis in the inferior hemisphere was not found to be significant when analyzed alone. However, the total decrease in TUNEL-positive nuclei across the entire retina was significant comparing PEG-POD~GDNF to PEG-POD~Lux and buffer (P < 0.01).
Although the use of TUNEL staining revealed a significant decrease in the level of apoptosis, it was only evaluated in the areas most significantly affected by light exposure (the vertical meridian at the ONH). The nucleosome release assay would permit a more global evaluation of tissue apoptosis by evaluating cell death throughout the retina. Retinal tissue was harvested 48 hours post-light exposure and the level of apoptosis evaluated by nucleosome release enzyme-linked immunosorbent assay (Figure 3c). This assay detects histone-associated DNA fragments that occur as a result of internucleosomal genomic DNA degradation during apoptosis. An increase in absorbance due to antibody detection of histone bound DNA is therefore correlated with increased cell apoptosis. Eyes injected with PEG-POD~GDNF had a 3.9-fold lower absorbance than buffer-injected eyes (P < 0.05) and a 7.4-fold lower absorbance than uninjected light-treated eyes (P < 0.001, data not shown). Although PEG-POD~GDNF was observed to have a 2.3-fold lower absorbance than PEG-POD~Lux-injected eyes, this difference was not significant and suggests a possible therapeutic effect from a PEG-POD nanoparticle. Collectively, these data suggest that PEG-POD~GDNF decreases the level of apoptosis in eyes that have undergone injury via light exposure.
GDNF nanoparticles prevent photoreceptor cell loss
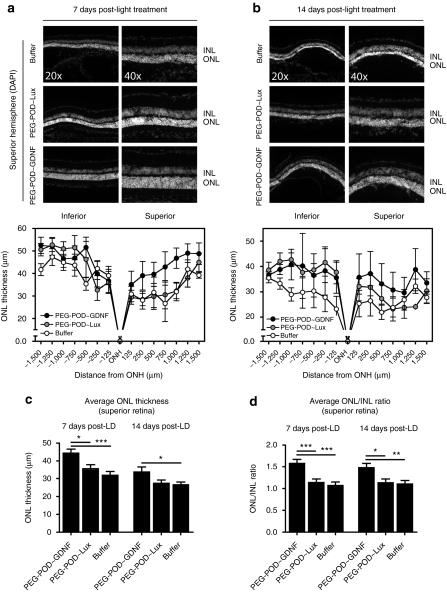
Although injection of PEG-POD~GDNF nanoparticles significantly reduced apoptosis, we had yet to determine whether this would lead to a preservation of the photoreceptor cells. The number of photoreceptor nuclei can be approximated by measuring the thickness of the ONL (Figure 4). To examine the differences in ONL thickness, eyes injected with either PEG-POD~GDNF, PEG-POD~Lux, or buffer were analyzed as in Figure 2b by sectioning along the vertical meridian and staining with DAPI at a section bisecting the ONH. ONL and inner nuclear layer (INL), the nuclear layer of bipolar cells, thickness was measured at 250 µm intervals along the length of the retina. We examined the effect of PEG-POD~GDNF at 7 and 14 days post-light treatment.
Figure 4.
Injection of PEG-POD~GDNF results in decreased photoreceptor cell loss. To examine the effect on photoreceptor cell loss, ONL thickness was measured in retinas injected with either PEG-POD~GDNF, PEG-POD~Lux, or buffer harvested (a) 7 days or (b) 14 days post-light exposure. ONL thickness was measured at 250 µm intervals extending from the optic nerve. Representative images of the superior hemisphere adjacent to the ONH are shown. Average thickness of the ONL of the superior retina was calculated from measurements taken between 250 and 1,500 µm from the optic nerve. Similar measurements were taken for the INL and the average ONL/INL ratio calculated. (c) PEG-POD~GDNF-injected eyes showed a significant increase in the ONL thickness of the superior hemisphere by 24.5% when compared to PEG-POD~Lux (P < 0.05) or by 39.3% buffer (P < 0.001)-injected eyes 7 days post-light treatment and 27.7% relative to buffer (P < 0.05) 14 days post-light treatment. (d) Similar results were obtained when analyzing the ONL/INL ratio of the superior hemisphere. At 7 days the ratio was higher in eyes injected with PEG-POD~GDNF by 38.5% compared to those injected with PEG-POD~Lux (P < 0.001) or 47.3% compared to buffer (P < 0.001). The ONL/INL ratio remained higher at 14 days post-light exposure in PEG-POD~GDNF-treated eyes by 30.4% compared to PEG-POD~Lux (P < 0.05) and by 33.9% compared to buffer (P < 0.01). 7 days: PEG-POD~GDNF and PEG-POD~Lux, n = 6; buffer, n = 4. 14 days: PEG-POD~GDNF, n = 4; PEG-POD~Lux, n = 7; buffer, n = 6. Mean ± SEM. DAPI, 4′,6-diamidino-2-phenylindole; GDNF, glial cell line–derived neurotrophic factor; INL, inner nuclear layer; LD, light degeneration; ONH, optic nerve head; ONL, outer nuclear layer; PEG, polyethylene glycol; POD, peptide for ocular delivery.
Eyes injected with PEG-POD~GDNF were compared to PEG-POD~Lux and buffer 7 days (Figure 4a) and 14 days (Figure 4b) post-light exposure. Because TUNEL staining was observed almost exclusively in the photoreceptor nuclei, the thickness of the INL was also measured and used as an internal control by determining the ONL/INL ratio (Supplementary Figure S1a,b). To quantify rescue, the average thickness of the ONL of the superior retina was then calculated from measurements taken between 250 and 1,500 µm from the ONH, proximal to the site of injection (Figure 4c). We expected to see the greatest rescue in the superior portion of the eye as this was the site of injection. The average ONL thickness of PEG-POD~GDNF injected retinas (44.37 ± 2.154 µm) was greater at 7 days post-light treatment by 24.5% relative to PEG-POD~Lux injected retinas (35.63 ± 2.198 µm, P < 0.05) and by 39.3% compared to those injected with buffer alone (31.86 ± 2.184 µm, P < 0.001) (Figure 4c). At 14 days post-light treatment, PEG-POD~GDNF-injected retinas had an average ONL thickness (33.83 ± 2.696 µm) which was significantly greater by 27.7% compared to the ONL thickness of retinas injected with buffer alone (26.50 ± 1.549 µm, P < 0.05). Though not significant by analysis of variance, the mean ONL thickness of PEG-POD~GDNF-injected eyes increased by 23.6% compared to that of PEG-POD~Lux (27.36 ± 1.812 µm). There was no significant difference between the average ONL thickness of PEG-POD~Lux and buffer-injected eyes at either 7 or 14 days post-light treatment (P > 0.05).
Similar results were obtained when analyzing the average ONL/INL ratio of the superior hemisphere (Figure 4d). At 7 days, the ratio was higher in eyes injected with PEG-POD~GDNF compared to those injected with PEG-POD~Lux by 38.5% (P < 0.001) or buffer by 47.3% (P < 0.001). The ONL/INL ratio remained higher at 14 days post-light treatment in PEG-POD~GDNF-injected eyes compared to PEG-POD~Lux by 30.4% (P < 0.05) and buffer-injected eyes by 33.9% (P < 0.01). Together these data strongly indicate that subretinal injection of PEG-POD~GDNF promotes photoreceptor survival.
We also analyzed the thickness of the ONL and INL of the inferior hemisphere of the treated eye cups (Supplementary Figure S2a,b). Because the injections were localized in the superior hemisphere before light treatment we hypothesized that there may be little rescue if any in the inferior hemisphere. This theory was supported by the increased rescue in the superior hemisphere observed via TUNEL stain (Figure 3b). Although at 7 days post-light exposure there was no significant difference in the average ONL thickness or ONL/INL ratio between conditions (P > 0.05), there were differences observed after 14 days. At 14 days post-light exposure there was a significant 40.2% increase in the ONL thickness of PEG-POD~Lux-treated eyes compared to those treated with buffer alone (P < 0.01), but no significant difference between PEG-POD~GDNF and PEG-POD~Lux-injected eyes. The ONL/INL ratio was significantly higher 14 days post-light exposure by 37.0% in PEG-POD~GDNF-treated eyes (P < 0.01) and by 30.6% in PEG-POD~Lux-treated eyes compared to buffer (P < 0.001). There was no significant difference in the ratios for PEG-POD~GDNF and PEG-POD~Lux (P > 0.05).
Interestingly, regardless of treatment group, there appeared to be a significant decrease in the ONL thickness between 7 and 14 days post-light treatment in PEG-POD~GDNF (P < 0.005), PEG-POD~Lux (P < 0.005), and buffer-injected eyes (P < 0.05), indicating that, while degeneration is slowed following PEG-POD~GDNF injection, it is not completely inhibited.
Injection of GDNF nanoparticles leads to functional rescue
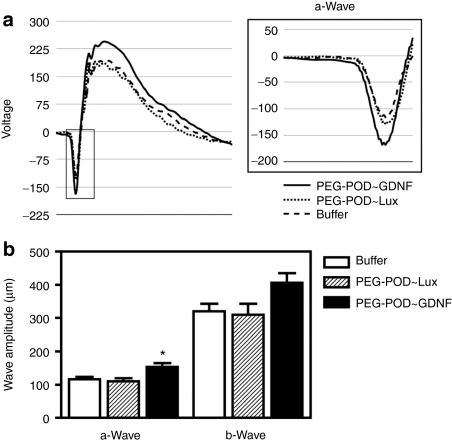
In order to evaluate the functional rescue of the retina, ERGs were performed 7 days post-light treatment. The amplitude of both the a- and b-waves were measured and compared between injection conditions (Figure 5a,b). PEG-POD~GDNF-injected eyes showed a 39% increase in amplitude in the a-wave relative to PEG-POD~Lux (P < 0.05) and 32% relative to buffer-injected eyes (P < 0.01).
Figure 5.
Injection of PEG-POD~GDNF results in partial functional rescue of light-damaged retina. In order to evaluate the functional rescue of PEG-POD~GDNF-treated retinas, electroretinograms (ERGs) were performed 7 days post-light exposure. (a) Averages of scotopic ERG recordings for all eyes in each treatment condition. The a-wave is boxed in gray and shown in more detail. (b) The amplitude of both the a- and b-waves were measured and compared between injection conditions. PEG-POD~GDNF-injected eyes showed a 39% increase in amplitude in the a-wave compared to PEG-POD~Lux (P < 0.05) and 32% compared to buffer injected eyes (P < 0.01). Though not significant, the mean b-wave amplitude was increased in PEG-POD~GDNF-injected eyes by 31% compared to PEG-POD~Lux and by 27% compared to buffer-injected eyes. PEG-POD~GDNF, n = 12; PEG-POD~Lux, n = 9; buffer, n = 11. Mean ± SEM. GDNF, glial cell line-derived neurotrophic factor; PEG, polyethylene glycol; POD, peptide for ocular delivery.
The amplitude of the b-wave was increased in PEG-POD~GDNF-injected eyes by 31% compared to PEG-POD~Lux injected and by 27% compared to buffer-injected eyes. Although the increase in the PEG-POD~GDNF b-wave amplitude was not significant by analysis of variance post hoc analysis, the analysis of variance itself was significant between these three conditions (P < 0.05). There was no significant difference between PEG-POD~Lux and buffer in the amplitude of either the a- or b-wave (P > 0.05). These data correlate with the previously noted relative ONL thicknesses between conditions (Figure 4).
We also measured ERGs at later time points, comparing PEG-POD~GDNF with buffer injections alone (Supplementary Figure S3). There was an increased ERG amplitude compared to buffer-injected eyes 10 days after light exposure of 49% in the a-wave (P < 0.005) and 29% in the b-wave (P < 0.05). However, there was no significant difference between the two conditions 14 days after light treatment (P > 0.05). This finding agrees with the ONL thickness data in which a decrease in the effects of PEG-POD~GDNF rescue were observed over time (Figure 4c).
Activation of Müller cells by PEG-POD~GDNF nanoparticles
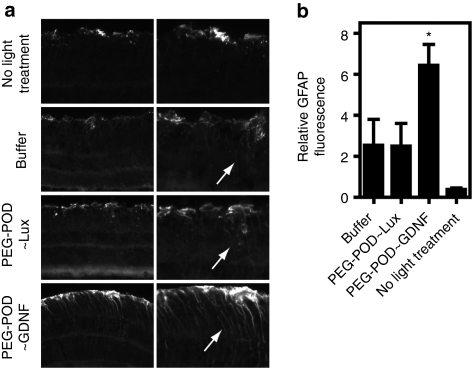
Injection of GDNF protein into the subretinal space of rd/rd mice has been shown to increase glial fibrillary acidic protein (GFAP) immunoreactivity in Müller glia.11 It has been speculated that GDNF may act via an indirect neuroprotective pathway through activation of Müller glia, a hypothesis supported by findings indicating that GDNF stimulates neurotrophic factor release from Müller cells.29 To investigate the effect of PEG-POD~GDNF on Müller cell activation in our light damage model, light-treated eyes injected with PEG-POD~GDNF, PEG-POD~Lux, or buffer alone were harvested 48 hours postinjection, sectioned, and stained for GFAP. Increased expression of GFAP in the Müller cell body of the inner retina was found to occur following light treatment irrespective of the injection condition, relative to nonlight-treated eyes (Figure 6a, arrows). However, a quantification of GFAP signal in the inner retina revealed a significant 2.6-fold increase in GFAP in PEG-POD~GDNF-injected eyes relative to control-injected eyes (P < 0.05) (Figure 6b). This observation suggests that the level of GDNF expression in cells transduced by the PEG-POD~GDNF nanoparticles is sufficient to activate Müller glia in the retina, and provides supporting evidence for a potential indirect mechanism for GDNF-mediated rescue in our light-induced model of retinal degeneration.
Figure 6.
Injection of PEG-POD~GDNF nanoparticles causes Müller cell activation. (a) Sections of retinas injected with PEG-POD~GDNF, PEG-POD~Lux, or buffer were stained for GFAP and compared to GFAP staining of retinas that had not been exposed to light. Eyes treated with light showed a redistribution of GFAP (arrows) regardless of injection condition, whereas nonlight-treated eyes showed a restriction of GFAP localization to astrocytes and the end feet of Müller cells at the inner limiting membrane. Eyes injected with PEG-POD~GDNF showed an increased level of GFAP staining throughout the Müller cell extending toward the outer limiting membrane (arrows) compared to other injections. (b) Quantification of GFAP fluorescence in the inner retina revealed a significant increase in GFAP in the inner retina in PEG-POD~GDNF injected eyes compared to both injection controls (P < 0.05). PEG-POD~GDNF, n = 7; PEG-POD~Lux and buffer, n = 6; no light treatment, n = 3. Mean ± SEM. GDNF, glial cell line-derived neurotrophic factor; GFAP, glial fibrillary acidic protein; PEG, polyethylene glycol; POD, peptide for ocular delivery.
Discussion
This study demonstrates the ability of a PEGylated CPP, PEG-POD to deliver a therapeutic GDNF transgene in vivo and partially rescue a model of retinal degeneration both histologically and functionally. In this study, PEG-POD~GDNF was injected before the light-induced apoptosis. Although the majority of RP cases are due to mutations in rod photoreceptors, loss of fine vision and complete blindness develops as a result of loss of cone photoreceptors. It has been suggested that the rods produce factors that promote the survival of cones. Hence, therapeutic interventions such as exogenous delivery of growth factor may promote the survival of the cones as a consequence of rod preservation.
Activation of caspase-3 has been shown to be an important mediator of apoptosis in inherited24 and acute26 or blue light-induced retinal degeneration.25 We confirmed induction of caspase-3/7 activity in photoreceptors following acute exposure to bright blue light 48 hours post-treatment (Figure 2a). In rat, RPE cells have been shown to undergo apoptosis in response to bright light at a time point delayed from that of photoreceptors30 and with transient morphological changes.17 RPE cells exposed to the lipofuscin fluorophore A2E were specifically shown to initiate apoptosis in vitro after exposure to blue light in a caspase-3-dependent manner.31 Consistent with these observations, we found increased caspase-3/7 activity in the RPE/choroid/sclera after 24 hours (Figure 2a), with no TUNEL stain observed in these tissues at the 48-hour time-point (data not shown). Interestingly, the Chesapeake Bay Waterman study reported a correlation between patients with the most advanced AMD and a high incidence of exposure to blue or white light,32 pointing to blue light as a possible contributing factor in the pathogenesis of AMD.33 Because of the link between caspase-3 and inherited models of retinal degeneration, we conclude that exposure to bright blue light is a viable model in which to test the efficacy of PEG-POD~GDNF nanoparticles to rescue photoreceptor degeneration.
GDNF has been proposed to mediate neuroprotection by both a direct mechanism through photoreceptor signaling and an indirect mechanism of signaling through Müller cells.29,34 Injection of GDNF protein into mouse retina has been shown to cause activation of Müller glia.11 Consistent with this observation, we have found that retinas injected with PEG-POD~GDNF nanoparticles had significantly increased and redistributed GFAP expression relative to those injected with either control nanoparticles or buffer alone, indicative of Müller cell activation (Figure 6b).
GDNF has been demonstrated to rescue the inherited rd/rd (rd1) mouse model of RP,11 the rhodopsin mutant S334ter rat,12 the RCS rat,13 damage induced by retinal detachment14 as well as ischemia-reperfusion injury.35 Similar to the model of light injury used here, the S334ter rat model has been shown to upregulate caspase-3 activity,24 indicating that GDNF antiapoptotic activity may be effective in preventing the caspase-3–initiated cell death cascade. While a previous report has shown that GDNF delivered via adeno-associated virus did not rescue retinal degeneration in mice as measured by ERG or histology,36 that study employed prolonged low-intensity white light as opposed to acute blue light, a light regimen that has been shown to cause cell death via a different apoptotic pathway.33
PEG-POD~GDNF appears to prevent apoptosis most efficiently in the superior hemisphere in proximity to the site of injection (Figure 3b). This is especially significant when considering blue light exposure that has been shown to preferentially damage the superior hemisphere of the eye.25 Significant rescue was observed following PEG-POD~GDNF injection compared to controls in the superior hemisphere histologically after 7 days (Figure 4c,d). Interestingly, at 14 days there was a significant photoreceptor rescue also of the inferior hemisphere following injection of PEG-POD~Lux compared to buffer by both ONL thickness (P < 0.001) (Supplementary Figure S2a) and ONL/INL ratio (P < 0.01) (Supplementary Figure S2b). These findings suggest that the POD peptide component of the nanoparticle, or the nanoparticle itself, may potentially have additional therapeutic effects. This is consistent with the observation that, when looking at whole retina apoptosis by the nucleosome release assay, there was no significant difference between PEG-POD~Lux and PEG-POD~GDNF (P > 0.05) (Figure 3c). The effect of PEG-POD complexed to DNA alone could be due to increased or prolonged detachment, growth factor release following the initial injection27, or by downregulation of proteins of the phototransduction cascade that play a role in mediating light damage.37 However, delivery of a nanoparticle containing the therapeutic (GDNF) transgene significantly altered the course of degeneration beyond effects of the vector or injection alone.
ERGs measured 7 days post-light treatment showed a 32–39% increase in the a-wave and a 27–31% increase in the b-wave amplitudes (Figure 6a,b), with only the a-wave being significant (P < 0.05). Because in our model apoptosis occurs almost exclusively within photoreceptors, the a-wave, which primarily measures photoreceptor function, may be a more sensitive measurement of rescue. Analysis of function 10 days post-light exposure showed a significant rescue in PEG-POD~GDNF-injected eyes compared to buffer by 49% in the a-wave (P < 0.005) and by 29% in the b-wave (P < 0.05). Interestingly, there was no significant difference between the two conditions 14 days after light treatment (Supplementary Figure S3). This is consistent with the histological findings. There was less rescue as measured by the average ONL thickness 14 days compared to 7 days after light treatment (Figure 4c).
One potential cause for the attenuation of rescue could be a lack of long-term expression. Evaluation of a luciferase transgene compacted into PEG-POD nanoparticles showed a significant decrease in expression after 1 week.8 A number of different strategies have been employed to increase the longevity of gene expression including using nonviral tissue-specific promoters, linearized DNA, and plasmid sequence modifications, such as scaffold/matrix attachment regions (MAR or S/MARs). Nonviral vectors-containing scaffold/matrix attachment region (S/MAR) have been shown to allow long-term expression,38 with preliminary data also indicating increased efficacy during ocular transduction.39
Although there have been a number of recent advances in the field of nonviral ocular gene therapy, translation to the clinic has been hampered by low-transfection efficiency, with few vectors demonstrating in vivo rescue in animal models. Those few reports of rescue of models of retinal degeneration have employed either the use of positively charged peptides to create electrostatically charged peptide/DNA complexes40,41 or mechanical disruption by iontophoresis or electroporation.40,42,43 Subretinal injection of DNA compacted with the two peptides K8 and JTS-1 (K8/JTS-1) led to transfection of RPE and choroidal cells in the RCS rat and a significant increase relative to control injection in the retinal area displaying delayed retinal degeneration.40 Because the ONL thickness and function of these retinas was not determined, it is hard to make direct comparisons with our study. The PEGylated poly-lysine peptide PEG-CK30 has been recently used to deliver the peripherin/rds transgene into the subretinal space of heterozygous rds mutant (rds+/−) mice in the developing (postnatal day 5, P5) and postmitotic (P22) retina.2,41 At both injection time points, PEG-CK30 transfection led to a 2-4-fold increase in peripherin/rds detectable up to 120 days postinjection, resulting in improved scotopic and photopic ERGs, as well as ultrastructural rescue by electron microscopy.2,41
A study employing transscleral iontophoresis of β-PDE cDNA in the rd1 mouse model found a significant increase in the thickness of the peripheral retina and detectable ERG recordings at P23.42 However, iontophoresis was performed at P8, before completion of retinal development.44 Although promising, it will be interesting to observe whether iontophoresis gene delivery to retinal tissue can be used to rescue disease after retinal development. In the adult RCS rat model, electroporation was found to deliver DNA to the RPE and retinal ganglion cells.43 Delivery of a brain-derived neurotrophic factor (BDNF) transgene by this method resulted in decreased TUNEL-positive photoreceptor nuclei and increased photoreceptor survival43 but functional rescue was not determined. Although electroporation using similar voltage levels in mice has shown efficient gene delivery, it was also documented to cause a corresponding high level of toxicity and substantial decrease in ERG amplitudes.45
This report on the use of PEG-POD as a therapeutically relevant nonviral DNA delivery vehicle is only the second nonviral gene therapy study to report functional rescue in an adult model of retinal degeneration. Because the apoptotic pathways observed in the light-induced degeneration model used in this study is similar to those observed in inherited models of retinal degeneration, the rescue effects observed with PEG-POD~GDNF may have therapeutic implications across a wide spectrum of retinal dystrophies. Although there are still significant improvements necessary before clinical use, PEG-POD nanoparticles appear to be an important step in the development of nonviral gene therapy for retinal degeneration.
Materials and Methods
Materials and reagents. pCAGLuc was cloned by first placing the Luciferase complementary DNA from pGL3-control (Promega, Madison, WI) into pBluescript II KS (Stratagene, La Jolla, CA) using HindIII and XbaI. An XhoI/NotI fragment of this plasmid was then inserted into pCAGEN (kindly provided by C. Cepko). The rat GDNF trangene was cloned from pGDNF3a (kindly provided by M. Bohn) in to pCAGEN using a NotI/XhoI to generate pCAGGDNF. POD, CGGG(ARKKAAKA)4, was generated at Tufts University Peptide Synthesis Core Facility (Tufts University School of Medicine, Boston, MA) and purified by high-performance liquid chromatography. Human embryonic retinoblasts 911 cells46 were grown in Dulbecco's modified Eagle's media supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Animals. All experiments involving animals were in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research, set out by the Association for Research in Vision and Ophthalmology. BALB/cJ mice were purchased from Jackson Laboratories, Bar Harbor, ME and maintained in 12-hour dark light cycles in accordance with federal, state, and local regulations. Mice were anesthetized by intraperitoneal injection of xylazine (Xyla-Ject; Phoenix Pharmaceutical, St Joseph, MO, 10 mg/ml)/ketamine HCl (Ketaset; Fort Dodge Animal Health, Fort Dodge, IA, 1 mg/ml). Subretinal injections were performed with a 32G needle (Becton Dickinson, Franklin Lakes, NJ) and a 5-µl glass syringe (Hamilton, Reno, NV) by a transscleral transchoroidal approach. Animals were sacrificed by CO2 inhalation followed by cervical dislocation.
Preparation and characterization of PEG-POD/DNA compactions. PEG-POD was prepared and characterized as previously described.8 Briefly, peptides were resuspended in 0.1 mol/l sodium phosphate (pH 7.2), 5 mmol/l EDTA to form a 20 mg/ml solution. An equimolar amount of methoxy-PEG-maleimide-10 kd (Nektar Transforming Therapeutics, San Carlos, CA) was resuspended in the same volume of dimethyl sulfoxide. PEG was added drop wise to the peptide over ~10 minutes, vortexing between drops. The solution was shaken overnight at room temperature, and dialyzed using a Bio-gel P6 column (Bio-Rad, Hercules, CA) into 0.1% trifluoroacetic acid. The PEGylated POD (PEG-POD) was quantified by Coomassie stain of a Tris-HCl gel (Bio-Rad) using a POD standard curve. DNA was compacted by diluting the plasmid in water to a final concentration of 0.2 µg/µl and added drop wise to PEG-POD with a final ratio 1.8 nmol/l peptide: 2 µg DNA (0.466 pmol pCAGLuc or 0.396 pmol pCAGGDNF), vortexing to mix. The nanoparticles were dialyzed three times in 5% dextrose using Biomax 10K centrifugal filter (Ultrafree; Millipore, Billerica, MA) and stored at 4 °C. Plasmid compaction was verified by reduced mobility through a 1% agarose gel (Invitrogen), which was relieved after 15-minute incubation with 0.25% Trypsin (Invitrogen) at 37 °C. PEG-POD particles were analyzed by incubation on glow-discharged Formvar copper grids (Electron Microscopy Sciences, Hatfield, PA), stained with 0.4% uranyl acetate, and visualized using CM10 transmission electron microscope (FEI, Hillsboro, OR) using Digital Micrograph software (Gatan, Pleasanton, CA).
In vitro and in vivo expression of GDNF. The plasmid was transfected in triplicate into 911 cells using Lipofectamine 2000 (Invitrogen) in accordance with the manufacturers protocol. Cells were harvested after 48 hours by removing media, adding RNA Stat-60 (Tel-Test, Friendswood, TX), and storing at –80 °C. 1.2 µg PEG-POD~GDNF was injected into the subretinal space of 6–8-week-old BALB/cJ and tissue was harvested after 48 hours. The posterior eyecup was isolated and homogenized in Stat-60 and stored at –80 °C. RNA was isolated using chloroform extraction and precipitated in isopropanol. DNase treatment was performed using Turbo DNase. Samples were run using a iScript One-Step RT-PCR Kit for Probes (Bio-Rad) on an iQ-5 Thermal Cycler (Bio-Rad) and probed using a rat GDNF primer/probe (Assay #Rn00569510_m1, Taq Man Gene Expression Assay; Applied Biosystems, Carlsbad, CA) and normalized to human GAPDH primer/probe (Assay #4332649, Taq Man Gene Expression Assay; Applied Biosystems) in 911 cells and mouse β-actin primer/probe (Assay #4352663; Taq Man Gene Expression Assay, Applied Biosystems) in mouse tissue.
Light-induced retinal degeneration. Mice were injected into the subretinal space and dark-adapted for 4 days. The eyes of the mice were dilated in the dark and left for 2 minutes just before light exposure. Mice were unrestrained and exposed to 8,500–11,000 lux (Light Meter, Lux/FC 840020; Sper Scientific, Scottsdale, AZ) of 450-nm saturated blue light (Bili Blue; Interlectric, Warren, PA) for 4 hours. Following light exposure, mice were kept in the dark until sacrificed.
Caspase-3/7 activity. Caspase 3/7 activity was measured using a Caspase-Glo assay kit (Promega) and modified from a previously published protocol.47 Briefly, cytosolic extracts from retina and RPE tissue harvested at various time points and prepared by homogenization in 50 mmol/l Tris–HCl, pH 8.0, 150 mmol/l with protease inhibitors and centrifuged for 15 minutes, at 2,000 r.p.m., at 4 °C. The protein concentration was measured using Quick-Start Bradford Dye Reagent (Bio-Rad) and concentration was adjusted to 0.15 mg/ml and stored at –80 °C. An equal volume of Caspase reagent and protein extract were mixed and incubated at room temperature for 1 hour. The luminescence was measured using a Glomax-20/20 (Promega).
GFAP staining. The eyes were harvested 48 hours after light exposure and fixed for 24 hours in 4% paraformaldehyde, dehydrated in 15 and 30% sucrose solution overnight, embedded in Tissue-Tek optimal cutting temperature compound (Sakura Finetek, Torrance, CA), and 14-µm frozen sections were collected along the vertical meridian. Sections were blocked in 6% (wt:vol) normal goat serum (Jackson ImmunoResearch, West Grove, PA), 0.25% (vol:vol) Triton X-100 (Fischer Bioreagents, Fair Lawn, NJ) in phosphate-buffered saline or 1 hour. Rabbit anti-GFAP antibody (Novus Biologicals, Littleton, CO) was diluted 1:500 in the blocking solution and incubated on the section for 2.5 hours. The section was washed three times in phosphate-buffered saline followed by incubation with CY3-goat anti-rabbit secondary antibody (Jackson ImmunoResearch) diluted 1:500 in the blocking buffer. For all sections, light and fluorescent microscopy was performed using an Olympus IX51 with differential interference contrast and appropriate fluorescent filters. Images were captured using a Retiga 2000R FAST camera and QCapture Pro 5.0 (QImaging, Surrey, British Columbia, Canada). Images were analyzed by removing autofluorescence by consistent manipulation of the levels in Photoshop CS and by measuring the signal in the lower retina, excluding the inner limiting membrane, in Image J.
Assessment of apoptosis. To detect and localize apoptosis TUNEL staining was performed 48 hours after light exposure. The eyes were fixed as above for 24 hours in 4% paraformaldehyde, dehydrated, embedded in OCT, and 14-µm sections were collected along the vertical meridian. TUNEL stain was performed using an In situ Cell Death Detection Kit, TMR Red (Roche Applied Science, Indianapolis, IN) in accordance with the manufacturers protocol, counterstained with 0.1 µg/ml 4′,6-diamidino-2-phenylindole and coverslipped. TUNEL-positive photoreceptor nuclei were quantified in a vertical section through the optic nerve in both the superior and inferior hemisphere of each eye. The presence of nucleosomes released into the cytoplasm was assessed using a Cell Death Detection enzyme-linked immunosorbent assay (Roche Applied Science). Briefly, the retina was removed and homogenized in 400 µl isolation buffer using a VWR PowerMax AHS 200 homogenizer and incubated for 30 minutes at room temperature. After spinning at 20,000g for 10 minutes, the supernatant was removed and diluted 1:200 in isolation buffer and used according to the manufacturer's protocol.
Histological analysis. Eyes were harvested 7 and 10 days after light exposure and fixed as above for 24 hours in 4% paraformaldehyde, dehydrated, embedded in OCT, and 14 µm sections were collected along the vertical meridian. Sections that approximately bisected the optic nerve were 4′,6-diamidino-2-phenylindole stained, coverslipped, and photographed. Images were analyzed using QCapture Pro 5.0 (QImaging). Images were spatially calibrated and manual measurements of the thickness of the ONL and INL nuclear layers were performed. Measurements of ONL and INL thickness were taken at a distance of 125, 250, 500, 750, 1,000, 1,250, and 1,500 µm from the optic nerve. The thickness was averaged between sections in a treatment group and the ONL/INL ratio determined by the quotient of the thicknesses.
ERGs. Mice were dark-adapted following light exposure. The mice were anaesthetized as described above, pupils dilated with 1% Tropicamide (Akorn, Lake Forest, IL), and scotopic ERGs recorded at a flash intensity of 1 dB using contact lens electrodes and the UTAS system with BigShot ganzfeld (LKC Technologies, Gaithersburg, MD). Five flashes were averaged and smoothed using the UTAS software.
Statistical analysis. All data analyses were performed using Prism Software 4 (GraphPad Software, La Jolla, CA). In experiments comparing three or more sample conditions, data were analyzed using a one-way analysis of variance with a post hoc Student–Newman–Keuls multiple comparison test for significance (Figures 2a–c, 3b,c, 4c,d, 5b, 6b, and Supplementary Figure S2a,b). All other statistical tests were performed using an unpaired Students t-test. Outliers were determined using the Quartile or Fourth-Spread method.48 All data is presented as the mean ± SE.
SUPPLEMENTARY MATERIAL Figure S1. Subretinal injection of PEG-POD~GDNF results in an increased ONL/INL ratio. (a) PEG-POD∼GDNF-injected eyes showed an increase in the ONL/INL ratio compared to PEG-POD∼Lux or buffer-injected eyes 7 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. Mean ±SEM. (b) The ONL/INL ratio of PEG-POD∼GDNF-injected eyes was greater than PEG-POD∼Lux or buffer-injected eyes 14 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM. Figure S2. Little significant change in the average ONL thickness and ONL/INL ratio of the inferior hemisphere. Average thickness of the ONL of the inferior retina calculated from measurements taken between 250-1500μm from the optic nerve. Similar measurements were taken for the INL and the average ONL/INL ratio calculated. (a) There was no significant difference in the average ONL thickness of retinas 7 days post-light exposure in eyes injected with PEG-POD∼GDNF, PEG-POD∼Lux or buffer (p>0.05). At 14 days post-light exposure there was a significant 40.2% PEG-POD∼Lux increase in thickness compared to buffer alone (p<0.01). There was no significant difference between PEG-POD∼GDNF and PEG-POD∼Lux or buffer injected eyes 14 days post-light exposure (p>0.05). 7 days: PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. 14 days: PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM. Figure S3. The functional rescue observed 7 days after subretinal injection of PEG-POD~GDNF is lost by 14 days post-light treatment. Electroretinograms (ERGs) were performed 10 and 14 days post-light treatment as in Fig. 8. (a) 10 days post-light treatment, the ERG recordings of PEG-POD∼GDNF-injected eyes were significantly higher than those of buffer injected eyes in both the a-wave (p<0.005) and b-wave (p<0.05). PEG-POD∼GDNF, n=7; Buffer, n=10. Mean ±SEM. (b) 14 days post-light treatment, the functional response of PEG-POD∼GDNF-injected eyes was not significantly higher than that of buffer injected eyes (p>0.05). PEG-POD∼GDNF, n=4; Buffer, n=6. Mean ±SEM.
Acknowledgments
This study was supported by grants to R.K.-S. from The Ellison Foundation, the National Institutes of Health/National Eye Institute (EY014991 and EY013887), the Virginia B Smith Trust and grants to the Department of Ophthalmology at Tufts University from the Lions Eye Foundation and Research to Prevent Blindness.
Supplementary Material
Subretinal injection of PEG-POD~GDNF results in an increased ONL/INL ratio. (a) PEG-POD∼GDNF-injected eyes showed an increase in the ONL/INL ratio compared to PEG-POD∼Lux or buffer-injected eyes 7 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. Mean ±SEM. (b) The ONL/INL ratio of PEG-POD∼GDNF-injected eyes was greater than PEG-POD∼Lux or buffer-injected eyes 14 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM.
Little significant change in the average ONL thickness and ONL/INL ratio of the inferior hemisphere. Average thickness of the ONL of the inferior retina calculated from measurements taken between 250-1500μm from the optic nerve. Similar measurements were taken for the INL and the average ONL/INL ratio calculated. (a) There was no significant difference in the average ONL thickness of retinas 7 days post-light exposure in eyes injected with PEG-POD∼GDNF, PEG-POD∼Lux or buffer (p>0.05). At 14 days post-light exposure there was a significant 40.2% PEG-POD∼Lux increase in thickness compared to buffer alone (p<0.01). There was no significant difference between PEG-POD∼GDNF and PEG-POD∼Lux or buffer injected eyes 14 days post-light exposure (p>0.05). 7 days: PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. 14 days: PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM.
The functional rescue observed 7 days after subretinal injection of PEG-POD~GDNF is lost by 14 days post-light treatment. Electroretinograms (ERGs) were performed 10 and 14 days post-light treatment as in Fig. 8. (a) 10 days post-light treatment, the ERG recordings of PEG-POD∼GDNF-injected eyes were significantly higher than those of buffer injected eyes in both the a-wave (p<0.005) and b-wave (p<0.05). PEG-POD∼GDNF, n=7; Buffer, n=10. Mean ±SEM. (b) 14 days post-light treatment, the functional response of PEG-POD∼GDNF-injected eyes was not significantly higher than that of buffer injected eyes (p>0.05). PEG-POD∼GDNF, n=4; Buffer, n=6. Mean ±SEM.
REFERENCES
- Klein R, Klein BE., and, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99:933–943. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ., and, Naash MI. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2010;24:1178–1191. doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M, Hällbrink M, Prochiantz A., and, Langel U. Cell-penetrating peptides. Trends Pharmacol Sci. 2000;21:99–103. doi: 10.1016/s0165-6147(00)01447-4. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Morris DJ., and, Kumar-Singh R. Evidence of protein transduction but not intercellular transport by proteins fused to HIV tat in retinal cell culture and in vivo. Mol Ther. 2003;8:130–142. doi: 10.1016/s1525-0016(03)00131-x. [DOI] [PubMed] [Google Scholar]
- Cashman SM, Sadowski SL, Morris DJ, Frederick J., and, Kumar-Singh R. Intercellular trafficking of adenovirus-delivered HSV VP22 from the retinal pigment epithelium to the photoreceptors–implications for gene therapy. Mol Ther. 2002;6:813–823. doi: 10.1006/mthe.2002.0806. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Read SP., and, Kumar-Singh R. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2010;50:686–697. doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM., and, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read SP, Cashman SM., and, Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med. 2010;12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A, Grimm C, Samardzija M., and, Remé CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005;24:275–306. doi: 10.1016/j.preteyeres.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasson M, Picaud S, Léveillard T, Simonutti M, Mohand-Said S, Dreyfus H, et al. Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest Ophthalmol Vis Sci. 1999;40:2724–2734. [PubMed] [Google Scholar]
- McGee Sanftner LH, Abel H, Hauswirth WW., and, Flannery JG. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622–629. doi: 10.1006/mthe.2001.0498. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Keegan DJ, Muir EM, Coffey PJ, Rogers JH, Wilby MJ, et al. Transplantation of Schwann cell line clones secreting GDNF or BDNF into the retinas of dystrophic Royal College of Surgeons rats. Invest Ophthalmol Vis Sci. 2004;45:267–274. doi: 10.1167/iovs.03-0093. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen SL, Xiao X, Chen TL, Tsai RJ, et al. Gene therapy for detached retina by adeno-associated virus vector expressing glial cell line-derived neurotrophic factor. Invest Ophthalmol Vis Sci. 2002;43:3480–3488. [PubMed] [Google Scholar]
- Chang GQ, Hao Y., and, Wong F. Apoptosis: final common pathway of photoreceptor death in rd, rds, and rhodopsin mutant mice. Neuron. 1993;11:595–605. doi: 10.1016/0896-6273(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Dunaief JL, Dentchev T, Ying GS., and, Milam AH. The role of apoptosis in age-related macular degeneration. Arch Ophthalmol. 2002;120:1435–1442. doi: 10.1001/archopht.120.11.1435. [DOI] [PubMed] [Google Scholar]
- Gorgels TG., and, van Norren D. Ultraviolet and green light cause different types of damage in rat retina. Invest Ophthalmol Vis Sci. 1995;36:851–863. [PubMed] [Google Scholar]
- Weale RA. Do years or quanta age the retina. Photochem Photobiol. 1989;50:429–438. doi: 10.1111/j.1751-1097.1989.tb04181.x. [DOI] [PubMed] [Google Scholar]
- Paskowitz DM, LaVail MM., and, Duncan JL. Light and inherited retinal degeneration. Br J Ophthalmol. 2006;90:1060–1066. doi: 10.1136/bjo.2006.097436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naash ML, Peachey NS, Li ZY, Gryczan CC, Goto Y, Blanks J, et al. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest Ophthalmol Vis Sci. 1996;37:775–782. [PubMed] [Google Scholar]
- Organisciak DT, Darrow RM, Barsalou L, Kutty RK., and, Wiggert B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest Ophthalmol Vis Sci. 2003;44:486–492. doi: 10.1167/iovs.02-0708. [DOI] [PubMed] [Google Scholar]
- Vaughan DK, Coulibaly SF, Darrow RM., and, Organisciak DT. A morphometric study of light-induced damage in transgenic rat models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:848–855. doi: 10.1167/iovs.02-0709. [DOI] [PubMed] [Google Scholar]
- Hao W, Wenzel A, Obin MS, Chen CK, Brill E, Krasnoperova NV, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Peng M, Laties AM., and, Wen R. Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J Neurosci. 1999;19:4778–4785. doi: 10.1523/JNEUROSCI.19-12-04778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Gorman A, Zhou X, Sandra C., and, Chen E. Involvement of caspase-3 in photoreceptor cell apoptosis induced by in vivo blue light exposure. Invest Ophthalmol Vis Sci. 2002;43:3349–3354. [PubMed] [Google Scholar]
- Perche O, Doly M., and, Ranchon-Cole I. Caspase-dependent apoptosis in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:2753–2759. doi: 10.1167/iovs.06-1258. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT., and, LaVail MM. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Chen E., and, Söderberg PG. Failure of ascorbate to protect against broadband blue light-induced retinal damage in rat. Graefes Arch Clin Exp Ophthalmol. 1999;237:855–860. doi: 10.1007/s004170050323. [DOI] [PubMed] [Google Scholar]
- Hauck SM, Kinkl N, Deeg CA, Swiatek-de Lange M, Schöffmann S., and, Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol Cell Biol. 2006;26:2746–2757. doi: 10.1128/MCB.26.7.2746-2757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi F, Marti A, Munz K., and, Remé CE. Light-induced apoptosis: differential timing in the retina and pigment epithelium. Exp Eye Res. 1997;64:963–970. doi: 10.1006/exer.1997.0288. [DOI] [PubMed] [Google Scholar]
- Sparrow JR., and, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci. 2001;42:1356–1362. [PubMed] [Google Scholar]
- Taylor HR, West S, Muñoz B, Rosenthal FS, Bressler SB., and, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- Wu J, Seregard S., and, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006;51:461–481. doi: 10.1016/j.survophthal.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Harada C, Harada T, Quah HM, Maekawa F, Yoshida K, Ohno S, et al. Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light-induced retinal degeneration. Neuroscience. 2003;122:229–235. doi: 10.1016/s0306-4522(03)00599-2. [DOI] [PubMed] [Google Scholar]
- Wu WC, Lai CC, Chen SL, Sun MH, Xiao X, Chen TL, et al. GDNF gene therapy attenuates retinal ischemic injuries in rats. Mol Vis. 2004;10:93–102. [PubMed] [Google Scholar]
- Allocca M, Di Vicino U, Petrillo M, Carlomagno F, Domenici L., and, Auricchio A. Constitutive and AP20187-induced Ret activation in photoreceptors does not protect from light-induced damage. Invest Ophthalmol Vis Sci. 2007;48:5199–5206. doi: 10.1167/iovs.07-0140. [DOI] [PubMed] [Google Scholar]
- Rattner A, Toulabi L, Williams J, Yu H., and, Nathans J. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. J Neurosci. 2008;28:9880–9889. doi: 10.1523/JNEUROSCI.2401-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraghy N, Gaussin A., and, Mermod N. Sustained transgene expression using MAR elements. Curr Gene Ther. 2008;8:353–366. doi: 10.2174/156652308786071032. [DOI] [PubMed] [Google Scholar]
- Koirala A, Cooper MJ., and, Naash MI.2009The role of S/MAR on the efficiency of ocular non-viral gene delivery to the RPE American Society Gene TherMay 27–30, 2009 Abstract 129.
- Neuner-Jehle M, Berghe LV, Bonnel S, Uteza Y, Benmeziane F, Rouillot JS, et al. Ocular cell transfection with the human basic fibroblast growth factor gene delays photoreceptor cell degeneration in RCS rats. Hum Gene Ther. 2000;11:1875–1890. doi: 10.1089/10430340050129495. [DOI] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ., and, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souied EH, Reid SN, Piri NI, Lerner LE, Nusinowitz S., and, Farber DB. Non-invasive gene transfer by iontophoresis for therapy of an inherited retinal degeneration. Exp Eye Res. 2008;87:168–175. doi: 10.1016/j.exer.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mo X, Fang Y, Guo W, Wu J, Zhang S, et al. Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr Eye Res. 2009;34:791–799. doi: 10.1080/02713680903086018. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M., and, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachi S, Oshima Y, Esumi N, Kachi M, Rogers B, Zack DJ, et al. Nonviral ocular gene transfer. Gene Ther. 2005;12:843–851. doi: 10.1038/sj.gt.3302475. [DOI] [PubMed] [Google Scholar]
- Fallaux FJ, Kranenburg O, Cramer SJ, Houweling A, Van Ormondt H, Hoeben RC, et al. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- Liu D, Li C, Chen Y, Burnett C, Liu XY, Downs S, et al. Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem. 2004;279:48434–48442. doi: 10.1074/jbc.M407190200. [DOI] [PubMed] [Google Scholar]
- Devore JL. Probability and Statistics for Engineering and the Sciences. Pacific Grove, CA; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subretinal injection of PEG-POD~GDNF results in an increased ONL/INL ratio. (a) PEG-POD∼GDNF-injected eyes showed an increase in the ONL/INL ratio compared to PEG-POD∼Lux or buffer-injected eyes 7 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. Mean ±SEM. (b) The ONL/INL ratio of PEG-POD∼GDNF-injected eyes was greater than PEG-POD∼Lux or buffer-injected eyes 14 days post-light treatment. ONH, Optic Nerve Head; INL, Inner Nuclear Layer; ONL, Outer Nuclear Layer. PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM.
Little significant change in the average ONL thickness and ONL/INL ratio of the inferior hemisphere. Average thickness of the ONL of the inferior retina calculated from measurements taken between 250-1500μm from the optic nerve. Similar measurements were taken for the INL and the average ONL/INL ratio calculated. (a) There was no significant difference in the average ONL thickness of retinas 7 days post-light exposure in eyes injected with PEG-POD∼GDNF, PEG-POD∼Lux or buffer (p>0.05). At 14 days post-light exposure there was a significant 40.2% PEG-POD∼Lux increase in thickness compared to buffer alone (p<0.01). There was no significant difference between PEG-POD∼GDNF and PEG-POD∼Lux or buffer injected eyes 14 days post-light exposure (p>0.05). 7 days: PEG-POD∼GDNF and PEG-POD∼Lux, n=6; Buffer, n=4. 14 days: PEG-POD∼GDNF, n=4; PEG-POD∼Lux, n=7; Buffer, n=6. Mean ±SEM.
The functional rescue observed 7 days after subretinal injection of PEG-POD~GDNF is lost by 14 days post-light treatment. Electroretinograms (ERGs) were performed 10 and 14 days post-light treatment as in Fig. 8. (a) 10 days post-light treatment, the ERG recordings of PEG-POD∼GDNF-injected eyes were significantly higher than those of buffer injected eyes in both the a-wave (p<0.005) and b-wave (p<0.05). PEG-POD∼GDNF, n=7; Buffer, n=10. Mean ±SEM. (b) 14 days post-light treatment, the functional response of PEG-POD∼GDNF-injected eyes was not significantly higher than that of buffer injected eyes (p>0.05). PEG-POD∼GDNF, n=4; Buffer, n=6. Mean ±SEM.