Abstract
Liver-directed gene therapy with adeno-associated virus (AAV) vectors effectively treats mouse models of lysosomal storage diseases (LSDs). We asked whether these results were likely to translate to patients. To understand to what extent preexisting anti-AAV8 antibodies could impede AAV8-mediated liver transduction in primates, commonly preexposed to AAV, we quantified the effects of preexisting antibodies on liver transduction and subsequent transgene expression in mouse and nonhuman primate (NHP) models. Using the highest viral dose previously reported in a clinical trial, passive transfer of NHP sera containing relatively low anti-AAV8 titers into mice blocked liver transduction, which could be partially overcome by increasing vector dose tenfold. Based on this and a survey of anti-AAV8 titers in 112 humans, we predict that high-dose systemic gene therapy would successfully transduce liver in >50% of human patients. However, although high-dose AAV8 administration to mice and monkeys with equivalent anti-AAV8 titers led to comparable liver vector copy numbers, the resulting transgene expression in primates was ~1.5-logs lower than mice. This suggests vector fate differs in these species and that strategies focused solely on overcoming preexisting vector-specific antibodies may be insufficient to achieve clinically meaningful expression levels of LSD genes using a liver-directed gene therapy approach in patients.
Introduction
Systemic administration of adeno-associated virus (AAV) vectors has been used to transduce the liver for the subsequent production of a therapeutic protein. This approach has shown robust efficacy in mouse models for several lysosomal storage diseases (LSDs).1,2,3,4 For example, an AAV8 vector bearing α-galactosidase A (αgal) was used to transduce the liver of a mouse model for Fabry disease, resulting in the correction of both biochemical and functional deficits.1 This same strategy has been used successfully to generate factor IX (FIX) in mice,5,6,7,8,9 dogs,10,11,12 nonhuman primates (NHPs),8,13,14,15 and hemophilia B patients.16
Although host immune responses have been the major concern in patients, there have also been anecdotal reports that the expression levels produced from AAV transduction of mouse liver exceed those that can be obtained from primates.7,15,17 Thus, for a well-secreted protein like FIX, expression levels attained in patients are generally less than those seen in mouse models.9,16 Compared to FIX, the secretion efficiency of LSD proteins is significantly lower, and the target blood levels for therapy are significantly higher. For example, FIX levels of 200 ng/ml are considered sufficient, while for αgal, serum levels approaching 1,000 ng/ml are likely to be required1 because αgal must be taken up from the circulation into the lysosomes of the target endothelial cells. Thus, generating necessary serum levels of an LSD protein such as αgal in primates using a liver-directed approach may represent a higher hurdle than an analogous approach for a well-secreted protein like FIX.
Primates, both monkeys18 and humans,19,20 are known to have prior exposure to AAV, although the fraction of the population with identified exposure may vary by viral serotype and assay used to characterize that exposure. By any measure, a significant fraction of NHPs have been exposed to AAV, and in those with high neutralizing anti-AAV titers, attempts to transduce the liver are largely blocked. Indeed, recent studies have pointed out that very low levels of neutralizing antibodies are sufficient to prevent liver transduction by AAV.7,15,17 However, neither the relationships between viral dose, preexisting anti-AAV antibody level and liver transduction, nor between total and neutralizing anti-AAV antibodies are well characterized.
Prior exposure of the primate liver to AAV also has the potential to alter viral trafficking and transgene expression. For example, latent AAV in mammalian hepatocytes is likely maintained by low levels of viral rep expression.21 How this might impact a subsequent transduction of the same hepatocyte by a gene therapy vector is largely unknown. By quantifying the role played by preexisting anti-AAV antibodies in expression from the primate liver, we reasoned that any remaining differences between mouse and primate expression from the same vector would be attributable to either fundamental differences between vector fate in mouse and primate hepatocytes, or would be related to the prior exposure of the primate liver to AAV.
To address possible translational issues related to the prior exposure of primates to AAV, we have used identical dosing [in DNase-resistant particles (drp)/kg] of a single preparation of an AAV2/8-DC190-hα-gal (AAV8-αgal) vector in mice and NHPs. Here, the use of one preparation is valuable as differences between preparations may impact vector expression levels. In our study, at equivalent vector dose the resulting expression levels in NHPs averaged 1.5-logs lower than those seen in mice. Through experiments in mouse and primate primary hepatocytes, we show that these in vivo differences in expression are unlikely to reflect species-specific differences in relative vector or promoter efficiency or in the efficiency of transgene translation or secretion. The potential role of preexisting antivector antibodies was characterized using the passive transfer of NHP serum into mice followed by vector administration. We thereby determined the relationships between preexisting antiviral titers, vector dose, vector genome copies in the liver and expression. These passive transfer results in mice were compared to results obtained in NHPs, and the resulting wide discrepancy in transgene expression suggests that under equivalent antibody/vector conditions there are significant differences between vector fate in mouse and primate liver. To determine the implications of preexisiting humoral immunity to the AAV8 vector in potential patients, we surveyed total and neutralizing anti-AAV8 antibody titers in 112 human subjects. These included a subset in which plasmapheresis was used to reduce antibody levels. Based on our passive transfer and NHP studies, we would predict that systemic gene therapy might successfully transduce the liver in roughly 50% of potential patients if treated with vector at high dose. With the addition of pretreatment by plasmapheresis, this percentage is predicted to increase to roughly 90%. Our results suggest that although preexisting antiviral antibodies in humans present a barrier that can be overcome, additional barriers to clinically significant expression of LSD proteins may exist in the primate liver.
Results
Expression cassette is equally active in mouse and primate primary hepatocytes
In these studies, species-dependent differences in the efficiency of the vector-utilized promoter or in αgal translation or secretion could lead to marked differences in the resulting serum αgal levels that would not reflect the relative efficacy of systemic AAV-mediated gene therapy in mice and primates. Considering this, we sought to determine whether the hepatocyte-specific DC190 promoter had equivalent activity in mouse and primate primary hepatocytes. To assess relative promoter efficiencies in these two species, we packaged the DC190-αgal transgene in AAV8 and AAV2 vectors. AAV2, which shows substantially lower vector potency than AAV8 in vivo,1,22 is nevertheless more potent than AAV8 in vitro—both in primary hepatocytes (Figure 1) and in human liver cell lines (data not shown). Though robust transgene expression following AAV-vector treatment in vivo has been widely observed,1,4,22 AAV-based vectors exhibit relatively lower potency in vitro than adenovirus (Figure 1). Therefore, to ensure efficient transduction of primary cells, we also constructed an adenovirus-2 vector (Ad2-αgal) containing the DC190 promoter and human αgal complementary DNA (cDNA) to test in parallel with the analogous AAV vectors. Mouse and cynomolgus macaque primary hepatocytes were transduced with AAV8-, AAV2-, and Ad2-αgal at a multiplicity of infection (MOI) of 1,000 for the AAV vectors and 75 for Ad2. Figure 1a shows the vector genomes per cell at 48 hours post-treatment as measured by quantitative PCR. Significantly more vector copies per cell were observed in Ad2- and AAV2/2-DC190-hα-gal (AAV2-αgal)-treated mouse primary hepatocytes than in primate (3- and 1.8-fold more, respectively); however, per-cell vector copy numbers did not differ significantly by species after AAV8-αgal treatment. At the same MOI, vector genomes per cell were >2-logs higher in AAV2-treated samples compared to AAV8-treated samples.
Figure 1.
Primary mouse and primate hepatocytes show equivalent αgal expression and secretion in response to vector treatment. Mouse and cynomolgus macaque primary hepatocytes (light gray and black bars, respectively) were transduced with Ad2, AAV8, or AAV2 vectors carrying the DC190-αgal cassette at a multiplicity of infection of 75 for Ad2, and 1,000 for AAV2 and AAV8. Untreated primary hepatocytes of both species were included as controls. For each treatment N = 3 and error bars indicate the SEM. P values were determined by unpaired two-tailed Student's t-test. (a) Vector copy numbers per cell in mouse and primate primary hepatocytes are shown 48 hours after treatment with vector. All vector-treated samples differed significantly in vector copies per cell relative to untreated controls with a P value ≤1 × 10−6. # indicates a statistically significant difference in vector copies per cell between mouse and cynomolgus macaque primary hepatocytes with a P value ≤0.05 by unpaired two-tailed t-test; ‡ indicates a P value of ≤0.001. (b) 18S-normalized mRNA levels of the αgal transgene at 48 hours post-treatment are shown (arbitrary units). All vector-treated samples differed significantly in transgene-specific mRNA relative to untreated controls with a P value ≤0.05. ‡ indicates a statistically significant difference in transgene mRNA between mouse and cynomolgus macaque primary hepatocytes with a P value of 0.008. (c) Levels of secreted αgal in cell culture media 48 hours post-treatment. All Ad2-αgal treated samples differed significantly from untreated controls in levels of secreted αgal with a P value ≤0.001. Apart from detectable secreted αgal in the media of mouse primary hepatocyte cultures treated with AAV8-αgal, neither AAV8 nor AAV2-αgal treatment resulted in secreted αgal levels significantly above that of untreated controls. # indicates a statistically significant difference in secreted αgal between mouse and cynomolgus macaque primary hepatocytes treated with Ad2-αgal with a P value 0.03. (d) Vector-normalized αgal transgene expression indicates DC190 promoter efficiency in mouse and primate primary hepatocytes. Transgene-specific mRNA levels at 48 hours post-treatment are shown after normalization to vector copies per cell. # indicates a statistically significant difference in vector-normalized transgene mRNA between mouse and cynomolgus macaque primary hepatocytes treated with AAV2-αgal with a P value 0.02. (e) For Ad2-αgal treatment (the only group with secreted αgal levels at 48 hours above background for both mouse and primate samples) αgal levels are shown after normalization to transgene-specific mRNA levels. No statistically significant difference was observed between mouse and primate groups suggesting equivalent αgal translation and secretion efficiency in mouse and primate primary hepatocytes. (f) Secreted αgal levels after normalization to vector copies per cell are shown 48 hours after treatment with Ad2-αgal. No statistically significant difference was observed between mouse and primate groups. AAV, adeno-associated virus; Ad2, adenovirus-2; αgal, α-galactosidase A.
Transgene-specific mRNA was also measured at 48 hours (Figure 1b). Though significantly higher transgene expression was observed after Ad2-αgal treatment in mouse than in primate hepatocytes, no species-specific difference in αgal mRNA expression was observed after treatment with either AAV vector. Consistent with the robust αgal mRNA levels seen after treatment with Ad2-αgal, secreted αgal levels were readily detectable in the media of mouse or primate cell cultures (Figure 1c), and mirrored the differences in vector copies per cell and αgal transcript levels. Levels of secreted αgal were not significantly above background after treatment with either AAV vector (Figure 1c).
To determine relative DC190 promoter efficiency in mouse and primate primary hepatocytes, αgal transcript levels were normalized to vector copies per cell (Figure 1d). No difference in DC190-driven transcription was observed for Ad2-αgal between mouse and primate cells. For AAV-based vectors, DC190 in primates showed a trend toward improved efficiency in primates over that observed in mouse cells, with a statistically significant improvement seen in treatment with AAV2-αgal. To measure the relative efficiency of αgal translation and secretion, secreted αgal levels at 48 hours were normalized by mRNA expression levels for the groups treated with Ad2-αgal, which led to detectable secretion (Figure 1c). No difference was observed in secreted αgal levels per unit of transgene expression between mouse and primate hepatocytes (Figure 1e). Secretion levels were similarly normalized to vector copies per cell and no significant species difference was observed (Figure 1f). Taken together, these results suggest that no hepatocyte-intrinsic barrier exists that would prevent a response to systemic gene therapy in macaques comparable to that seen in mice.
Serum αgal levels after systemically administered AAV8-αgal are significantly lower in primates than mice
In male mice dosed with 2 × 1013 drp/kg of AAV8-αgal, serum αgal levels after 4 weeks reached ~600 ng/ml (data not shown; J.B. Nietupski, G.D. Hurlbut, R.J. Ziegler, Q. Chu, B.L. Hodges, K.M. Ashe et al., unpublished results). However, in nine male rhesus macaques with very low anti-AAV8 total antibody titers (100–200, Table 1) dosed with 2 × 1013 drp/kg of the same vector preparation, serum αgal levels at 4 weeks averaged 18 ng/ml, i.e., 1.5-logs lower than observed in mice. To examine whether these species-dependent expression differences led to comparable differences in long-term sustained expression, we followed serum αgal levels in three rhesus macaques for 17 weeks post-treatment and compared these with plateau serum levels in mice treated with the same vector at one-quarter the dose (5 × 1012 drp/kg). Figure 2 shows that in both species, relatively stable transgene expression levels were achieved by week 2. At a fourfold increased vector dose, plateau serum αgal levels in primates were, nevertheless, ~1/10th that observed in mice (~20 versus ~260 ng/ml, respectively). After a spike in expression to average levels of ~80 ng/ml in macaques at day 4 post-treatment, serum αgal levels fall to ~20 ng/ml by week 2. The kinetics of this decrease are far too rapid to be explained by a primary immune response to αgal. As αgal levels remain essentially unchanged from week 2 to 17, it is also unlikely that a humoral response affects levels over this time frame. Because it was not clear what effects the low total anti-AAV8 antibody titers detected in primates might have on liver transduction and subsequent expression in NHPs, we next used a passive transfer paradigm to characterize the relationships between total antibody titer, viral dose, liver transduction, and αgal expression.
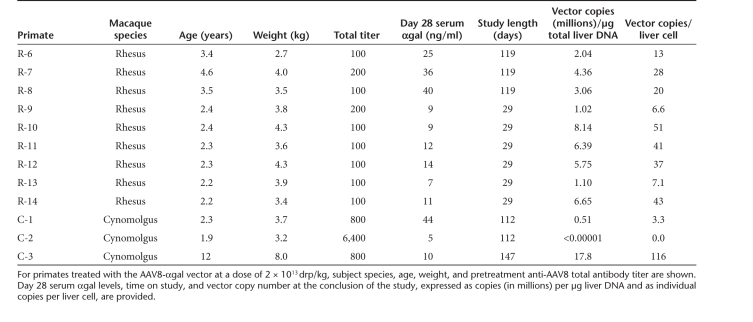
Table 1. Summary of vector-treated primates with their serum αgal levels and liver vector copy numbers.
Figure 2.
Sustained serum αgal levels are substantially lower in primates than in mice. Serum human αgal levels are shown in mice for 16 weeks after treatment with AAV8-αgal at 5 × 1012 drp/kg (light gray diamonds, N = 10 to week 11 and N = 5 thereafter) and rhesus macaques (R-6 to R-8, Table 1) for 17 weeks after treatment with the same vector at four times the dose (2 × 1013 drp/kg, black squares, N = 3). Error bars indicate the SEM. After expression stabilization (after 14 days), average serum αgal levels were ~260 ng/ml in mice and ~20 ng/ml in macaques. AAV, adeno-associated virus; αgal, α-galactosidase A.
Passive transfer of low anti-AAV8 antibody titer NHP serum reduces mouse liver transduction by AAV8-αgal
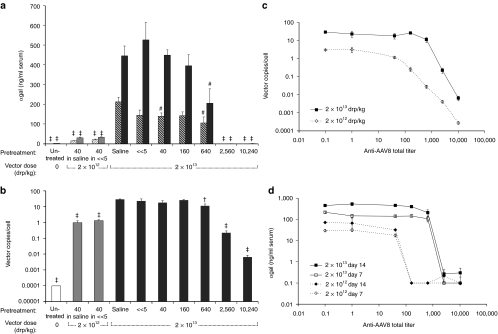
To assess the potential impact of preexisting antibodies to AAV8 on liver transduction after systemic administration of vector, serum αgal levels were measured in transduced mice pretreated with sera from NHPs with varying levels of humoral immunity to the vector. Serum (200 µl) from 5 NHPs with low anti-AAV8 total antibody titers was administered to mice intravenously by tail-vein injection, reaching (based on dilution) titers in vivo of ~10% of neat NHP serum levels. This provided passive antibody-mediated immunity to the vector over a range of low total titers, and was followed ~1 hour later by low-dose vector administration (2 × 1012 drp/kg) by tail-vein injection. Although 2 × 1012 drp/kg is a low dose relative to our NHP studies, it was the highest dose used in a clinical trial for liver-directed gene therapy.16 Groups of mice receiving no treatment or dosed with vector after pretreatment with pooled naive mouse serum or NHP serum with high anti-AAV8 total antibody titer (102,400) were included as controls. Levels of human αgal were measured in serum collected from treated animals 1 week after vector administration. Compared to pretreatment with naive mouse serum, anti-AAV8 total titers over an in vivo titer range of 5–10 did not reduce serum αgal levels (Figure 3a). However, passive transfer of high titer NHP serum to a final in vivo titer of 10,240 suppressed serum human αgal to near undetectable levels (Figure 3a).
Figure 3.
Passive transfer of low anti-AAV8 antibody titer nonhuman primate (NHP) serum reduces mouse liver transduction by AAV8-αgal. (a) Passive transfer of naive mouse serum or diluted low-titer NHP serum has no effect on expression levels; high-titer NHP serum abrogates expression. Mice were pretreated with naive mouse serum, serum from five different NHPs (R-23 to R-27, Supplementary Table S1) to an anti-AAV8 total antibody titer in the mouse of ≤10, or serum from an NHP with a high anti-AAV8 total titer (R-28, Supplementary Table S1) to an in vivo titer of 10,240, followed 1 hour later by intravenous administration of the AAV8-αgal vector at 2 × 1012 drp/kg or saline vehicle without vector (Untreated). Mean serum αgal levels 1 week post-treatment are shown. For each group N = 4 and error bars indicate SEM. Compared to mice pretreated with naive mouse serum, no statistically significant reduction in serum αgal levels was observed for mice pretreated with low-titer NHP serum to in vivo anti-AAV8 total titers of ≤10. However, for mice pretreated with high-titer NHP serum to an in vivo titer of 10,240, ‡ indicates a statistically significant reduction in serum αgal relative to controls pretreated with naive mouse serum (P value ≤0.001 by unpaired two-tailed t-test). In b and c, high-titer inhibitory NHP serum (R-28, Supplementary Table S1) was serially diluted into NHP serum with no detectable anti-AAV8 titer (R-23, Supplementary Table S1) and passively transferred into mice to obtain a range of in vivo anti-AAV8 total antibody titers. Pretreatment with sera or a saline control was followed 1 hour later by intravenous administration of AAV8-αgal at 2 × 1012 drp/kg or saline vehicle as indicated. Error bars indicate SEM and 4–5 animals were included in each group. (b) Pretreatment to in vivo anti-AAV8 titers of ≥160 blocks gene transfer after low-dose AAV8-αgal. Mean serum αgal levels are shown 1 (checked light gray bars) and 2 weeks (solid light gray bars) after vector administration. # indicates a statistically significant difference in serum αgal levels at that time point relative to mice administered vector after saline pretreatment with a P value ≤0.05 by unpaired two-tailed t-test; ‡ indicates a P value of ≤0.001. (c) Decreased serum αgal correlates with reduced vector copies per liver cell. Mean vector copies per liver cell are shown 2 weeks after treatment with vehicle (white bar) or the AAV8-αgal vector at 2 × 1012 drp/kg (light gray bars). No vector was detected in control animals treated with vehicle alone (arbitrarily assigned a vector copy per cell value of 0.0001 for log-scale display). # indicates a statistically significant difference in vector copies/cell relative to mice administered vector after saline pretreatment with a P value ≤0.05 by unpaired two-tailed t-test; ‡ indicates a P value of ≤0.001. αgal, α-galactosidase A.
To define a preexisiting total antibody titer threshold permissive to vector-mediated transduction, high anti-AAV8 titer inhibitory serum was diluted serially fourfold with permissive NHP serum, i.e., serum with no detectable anti-AAV8 antibodies, to create a range of NHP sera of defined anti-AAV8 total titers from 400 to 102,400. Mice were injected intravenously with 200 µl of these sera followed ~1 hour later by the AAV8-αgal vector at low dose (2 × 1012 drp/kg). Groups receiving no vector or vector after pretreatment with saline or permissive NHP serum were included as controls. Levels of human αgal were measured in mouse serum 1 and 2 weeks after vector treatment. At either time point, no difference in αgal levels was observed between groups pretreated with saline or permissive NHP serum (Figure 3b). However, 1 week after vector administration, mice pretreated with NHP serum to a diluted in vivo anti-AAV8 total antibody titer of 40 showed a statistically significant reduction in αgal levels compared to animals treated with saline or permissive NHP serum (Figure 3b). At both 1 and 2 weeks after vector administration, serum αgal levels were undetectable in animals pretreated with NHP serum to in vivo anti-AAV8 total titers of ≥160, suggesting complete antibody-mediated inhibition of vector-mediated liver transduction (Figure 3b). No significant difference in serum αgal levels was seen after pretreatment with naive mouse or NHP sera compared to saline at either time point (Figure 3a,b).
To determine whether decreased transgene expression correlated with reduced vector transduction of the liver, animals were killed 2 weeks after vector administration and vector copies per liver cell determined. Figure 3c shows that for animals treated with saline, permissive NHP serum, or NHP serum diluted with permissive serum to a final titer of ≤40, low-dose vector (2 × 1012 drp/kg) delivery resulted in ~3 vector copies/cell. Animals pretreated with NHP serum to a final titer of 40 had a statistically significant (P = 0.035), 1.8-fold reduction in vector copy number per liver cell, consistent with the measured decrease in αgal levels at week 1 (Figure 3b). Animals pretreated with NHP serum to a final anti-AAV8 total titer of 160 had an 11-fold reduction in vector copies per cell compared to animals pretreated with saline. For each further fourfold increase in titer, an additional log decrease in vector copy number was observed (Figure 3c).
Increasing vector dose tenfold partially overcomes preexisting antibodies to AAV8 and correlates with increased serum αgal in the passive transfer model
To assess the potential of increased vector dose to overwhelm preexisting anti-AAV8 antibodies, AAV8-αgal was administered at 2 × 1013 drp/kg (high dose) to mice pretreated with serially diluted inhibitory NHP sera. Serum αgal levels were measured 1 and 2 weeks after vector administration. Groups receiving no vector, or vector after pretreatment with saline or permissive NHP serum were included (Figure 4a). To control for both vector potency and the inhibitory activity of sera, additional groups were pretreated with NHP serum diluted either in saline or permissive NHP serum to a final in vivo anti-AAV8 titer of 40, and were dosed with vector at 2 × 1012 drp/kg as in the previous study (Figure 3b). No significant difference was observed in serum αgal levels between these low-dose control groups and the similarly treated groups shown in Figure 3b. Consistent with a tenfold increase in vector dose, after pretreatment to an in vivo titer of 40, day 14 serum αgal levels were ~1.4-logs higher in animals dosed with vector at 2 × 1013 drp/kg than in those dosed at 2 × 1012 drp/kg (Figure 4a). Comparing groups pretreated with saline or permissive NHP serum, a similar ~1-log increase in αgal levels corresponded with the tenfold increase in vector dose (Figures 3b and 4a). Unlike low-dose vector treatment, at a dose of 2 × 1013 drp/kg, animals pretreated with NHP serum to a titer of 160 showed no significant reduction in serum αgal levels relative to saline pretreatment at either time point (Figure 4a). However, at high vector dose, mice pretreated to an in vivo anti-AAV8 total antibody titer of 640 showed a statistically significant reduction in αgal levels compared to those pretreated with saline (P = 0.035) or NHP serum without detectable titer (P = 0.031; Figure 4a). At in vivo titers of ≥2,560 expression levels of αgal were undetectable. Relative to the low-dose result (Figure 3b), with vector dose increased tenfold, the maximum anti-AAV8 titer permitting αgal expression at levels equivalent to saline pretreatment was increased fourfold (Figure 4a).
Figure 4.
Increasing vector dose tenfold partially overcomes preexisiting antibodies to AAV8. To assess the potential of higher vector dose to overwhelm existing anti-AAV8 antibodies, 2 × 1013 drp/kg (high dose) AAV8-αgal was administered to mice pretreated with saline or serially diluted inhibitory nonhuman primate (NHP) sera to obtain a range of in vivo anti-AAV8 total antibody titers by passive transfer as shown. Mice injected with vector-free vehicle without pretreatment (Untreated) were included for comparison. To ensure inhibitory activity was comparable to Figure 3, additional control groups were treated with vector at 2 × 1012 drp/kg after pretreatment with inhibitory NHP serum, diluted in either saline or naive NHP serum, to the minimum in vivo inhibitory titer of 40 as determined for that dose in Figure 3b. Error bars indicate SEM and 4–5 animals were included in each group. P values were determined by unpaired two-tailed Student's t-test. (a) Mean serum αgal levels are shown at a vector dose of 2 × 1012 (gray bars) and 2 × 1013 (black bars) at 1 (striped bars) and 2 weeks (solid bars) after administration. For low-dose controls pretreated to a titer of 40, serum αgal levels are similar to those of Figure 3b. # indicates a statistically significant difference in serum αgal levels at that time point relative to mice administered 2 × 1013 drp/kg vector after saline pretreatment with a P value ≤0.05 by unpaired two-tailed t-test; ‡ indicates a P value of ≤0.001. (b) Mean vector copies per cell are shown 2 weeks after treatment with saline only (white bar) or the AAV8-αgal vector at a dose of 2 × 1012 drp/kg (gray bars) or 2 × 1013 drp/kg (black bars) after passive transfer of inhibitory serum to the in vivo titer indicated. No vector was detected in vehicle-treated control animals, which were arbitrarily assigned a vector copy per cell value of 0.0001 for log-scale display. Roughly 25 vector copies/cell were detected in animals dosed with vector at 2 × 1013 drp/kg after pretreatment with saline or permissive NHP serum without detectable anti-AAV8 antibodies (R-23, Supplementary Table S1). † indicates a statistically significant difference in vector copies/cell relative to mice administered 2 × 1013 drp/kg vector after saline pretreatment with a P value ≤0.01; ‡ indicates a P value of ≤0.001. No statistically significant difference in vector copies/cell was seen for controls treated with low-dose vector (2 × 1012 drp/kg) after pretreatment with NHP serum to an in vivo anti-AAV8 titer of 40 (gray bars) compared to the similarly treated groups in Figure 3c. (c) Mean vector copies per cell 2 weeks after administration the AAV8-αgal vector at a drp/kg dose of 2 × 1012 (open diamonds with dashed connecting line) or 2 × 1013 (filled squares with solid connecting line) are shown as a function of the anti-AAV8 total titer in the mouse passive transfer model (Figures 3 and 4). In c and d, saline pretreatment was arbitrarily assigned an anti-AAV8 titer of 0.1, and permissive NHP serum, without detectable anti-AAV8 antibodies, a titer of 1 for the purpose of log-scale display. (d) Mean serum αgal levels in Figures 3 and 4 are shown as a function of in vivo anti-AAV8 total antibody titers in the mouse passive transfer model. For the purposes of log-scale display, αgal levels below the limit of detection were arbitrarily assigned a value of 0.1 ng/ml. Squares with solid connecting lines show serum αgal levels at 1 (open squares) and 2 weeks (filled squares) after treatment with a vector dose of 2 × 1013 drp/kg. Diamonds with dashed connecting lines show serum αgal levels at 1 (open diamonds) and 2 weeks (filled diamonds) after treatment with a vector dose of 2 × 1012 drp/kg at the indicated titer. AAV, adeno-associated virus; αgal, α-galactosidase A.
As in the low-dose study, vector copies per liver cell were also determined after high-dose administration. Figure 4b shows that after pretreatment with saline or naive NHP serum, ~25 vector copies/cell were detected in animals dosed with 2 × 1013 drp/kg AAV8-αgal. Thus, a tenfold increase in vector dose resulted in a roughly tenfold increase in vector copies per cell. In controls pretreated to a final in vivo anti-AAV8 titer of 40 and given vector at low dose, no significant difference in vector copies per cell was observed relative to similarly treated groups in the previous study (Figures 3c and 4b). Compared to saline pretreatment, at high vector dose pretreatment with NHP serum to an in vivo anti-AAV8 titer of 640 resulted in a statistically significant (P = 0.004), 2.6-fold reduction in vector copy number per cell (Figure 4b). At a final in vivo titer of 2,560, a 2-log reduction in vector copies per cell from control levels was observed. As with groups treated with vector at lower dose (Figure 3c), decreased vector copy number correlated with decreased transgene expression (Figure 4a,b). Although at the lower dose, copy number reduction was seen after pretreatment to an anti-AAV8 titer of 40, in mice treated at high dose, vector copies per cell were equivalent to control levels up to a titer of 160.
Figure 4c,d illustrates the dependence of liver copy number and resulting αgal expression levels on anti-AAV8 titers and vector dose. Figure 4c demonstrates the expected tenfold increase in liver copy number achieved by increasing vector dose tenfold, over the entire range of anti-AAV8 titers in the passive transfer model. At low vector dose, liver copy number falls off rapidly for titers above ~40, whereas for the higher dose, this fall off occurs for titers above ~640. Similarly, Figure 4d shows that serum αgal expression levels in the passive transfer model paralleled copy number in terms of their dependence on vector dose and anti-AAV8 titers, with decreases shifted to higher titers as a result of treatment with higher vector dose. Of note is the more precipitous decrease in expression relative to copy number as a function of anti-AAV8 titer.
Serum αgal levels correlate with AAV8 vector copy number per liver cell in mice but not in NHPs
Next, we compared results in the passive transfer model to those obtained in NHPs. Nine rhesus and three cynomolgus macaques (Table 1) were dosed intravenously with 2 × 1013 drp/kg of the same AAV8-αgal vector preparation used in the mouse passive transfer experiments. In the rhesus macaques, anti-AAV8 total antibody titers were ≤200 (Table 1). Based on the passive transfer model, it would be predicted that this vector dose would overwhelm the preexisting anti-AAV8 antibody load and transduce the liver to a degree similar to that achievable in animals totally naive to AAV8. The three cynomolgus macaques treated had titers ≥800 (Table 1). Given these higher titers, lower levels of vector transduction were predicted, especially for the cynomolgus macaque with a titer of 6,400 (C-2, Table 1), which is well outside the permissive range defined by passive transfer. Indeed, as the data in Table 1 demonstrate, at the end of the study vector copy numbers in the livers of NHPs with low antibody titers were in the same range as those seen in the mouse passive transfer experiments when in vivo antibody levels were at permissive levels (Table 1, Figure 4c). In the high-titer cynomolgus macaque, no vector copies were detectable in the liver at the end of the study. This is consistent with passive transfer results where pretreatment to achieve high in vivo anti-AAV8 titers blocked vector transduction even at the higher vector dose (Figure 4a–d). Although the time after vector administration at which vector copy number was measured varied between NHPs, in rhesus macaques no statistically significant difference was seen in groups where copy number was determined at day 29 versus day 119. However, in all NHPs with detectable vector, serum αgal levels were dramatically lower than those attained in mice at the same copy number.
Figure 5 compares the relationships between serum αgal level and vector copy number in the liver for both mouse and monkey. In the mouse, vector copies/cell >0.8 led to detectable αgal expression. At day 14, αgal expression levels correlate linearly (R2 = 0.93) with, and can be predicted from, the number of vector copies per liver cell. A similar relationship was seen for day 7 αgal levels (data not shown). However, no such correlation was observed in NHPs (Table 1), where expression did not increase significantly with copy number (Figure 5). As an example of this discrepancy, the mouse data predict that a vector copy number of 116/liver cell would result in a serum αgal level >2,000 ng/ml. However, in a monkey with that copy number, a serum level of only 10 ng/ml was detected—a ~200-fold reduction.
Figure 5.
Serum αgal levels correlate with AAV8 vector copy number per cell in mice but not in nonhuman primates (NHPs). Mean serum αgal levels as a function of vector copies per liver cell are compared between the mouse passive transfer and NHP models. In mice, αgal expression and vector copy number in Figures 3 and 4 were positively correlated (R2 = 0.93). Vector copies above 0.8/cell led to detectable αgal expression, which increased linearly according to the equation y = 18.0x + 11.2. In NHPs (Table 1), however, no correlation was observed. Linear trend lines are shown for mouse (solid line) and NHP (dashed line) data. Error bars indicate SEM. AAV, adeno-associated virus; αgal, α-galactosidase A.
Passive transfer results suggest that high vector dose should lead to transduction of human liver
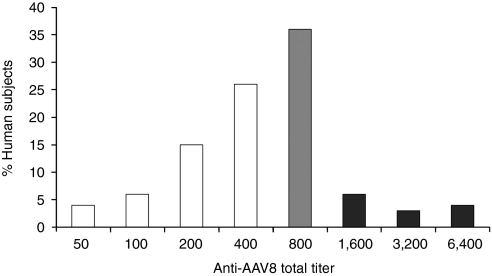
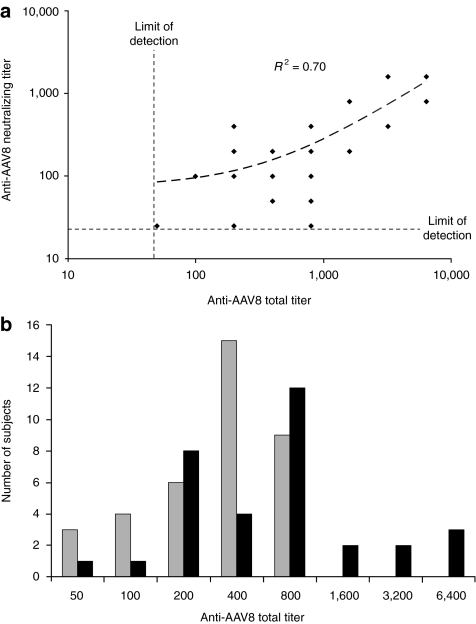
To determine the implications of these results for systemic AAV8-mediated gene transfer to human liver, anti-AAV8 total antibody titers were measured in 100 normal human subjects by the same assay used to measure NHP titers. Figure 6 shows that anti-AAV8 antibodies were detected in all subjects tested, the majority of which had total titers ≤400. In humans, anti-AAV8 total titers correlated linearly (R2 = 0.7) with neutralizing titers (Figure 7a). Significant total titers (400–800) were also measured in the majority of individuals with neutralizing titers below the limit of detection (Figure 7b). As such, total titer appears a more sensitive measure of prior AAV exposure than neutralizing titer. This is an important point, as the passive transfer model shows relatively low preexisting total titers could reduce vector copies per liver cell and the resulting serum αgal levels. At a vector dose of 2 × 1012 drp/kg, given that titers of ≥40 significantly reduced vector copy number in the passive transfer model, preexisting anti-AAV8 antibodies in all normal human subjects would be predicted to reduce vector copy number from levels theoretically possible in humans naive to AAV8 (Figure 6). In the passive transfer model, a total titer of 160 was sufficient to reduce αgal expression to levels undetectable in serum after treatment with vector at a dose of 2 × 1012 drp/kg. Only ~10% of tested human subjects had titers below this threshold. However, based on the passive transfer and NHP models (Figure 4, Table 1), a higher vector dose of 2 × 1013 drp/kg would be predicted to overwhelm preexisting antibodies to AAV8 in the ~52% of individuals with total titers ≤400, allowing systemic vector-mediated transgene delivery to the liver at a vector copy per cell ratio of >5. Because titers ≥640 suppressed αgal expression and vector copy numbers even at high vector dose, a liver-directed gene therapy approach utilizing systemic administration is predicted to be of limited efficacy in individuals with high total titer. This conclusion is supported by the absence of detectable human αgal in serum or vector copies in the liver of the high-titer (6,400) cynomolgus macaque C-2. Although a permissive threshold for AAV8 transduction of the liver was not directly determined in primates, it appears to be close to a total titer of 800 at high vector dose. In the two macaques with a preexisting titer of 800, C-1 showed a reduction in liver vector copy number to 10% of the average of NHPs with any detectable copies; however, C-3 had a high vector copy number (Table 1).
Figure 6.
Anti-AAV8 titers in human subjects. Anti-AAV8 total antibody titers were determined for 112 human volunteers. Percent of individuals with each titer are shown. Extrapolating from the passive transfer model, a vector dose of 2 × 1013 drp/kg would be predicted to overcome preexisting antibodies to AAV8, allowing systemic vector-mediated transgene delivery to the liver at >5 vector copies/cell in humans with titers ≤640 (white bars), or ~52% of individuals. An anti-AAV8 titer of 800 (gray bar) would be predicted to be borderline permissive to liver transduction at high vector dose (2 × 1013). Individuals with titers >800 (black bars) would be predicted to be nonpermissive to liver transduction (even at high dose) based both on the passive transfer and NHP data (Table 1). AAV, adeno-associated virus.
Figure 7.
Anti-AAV8 total titers are a more sensitive indicator of prior virus exposure in humans than neutralizing titers. (a) Total versus neutralizing anti-AAV8 antibody titers in human subjects. Neutralizing antibody and total anti-AAV8 antibody titers were measured in 70 human subjects (Supplementary Table S3). Although all 70 had measurable anti-AAV8 total antibody titers, only 33 had a detectable neutralizing titer (titer ≥25). In individuals where both total and neutralizing titers could be determined, total titer and neutralizing titer correlated linearly (dashed line) with an R2 value of 0.70, as shown. Limits of detection for total and neutralizing antibody assays are indicated by dashed lines. (b) Distribution of anti-AAV8 total antibody titers in 70 human subjects for which neutralizing titers were also determined. The distribution of anti-AAV8 total antibody titers is shown for subjects with neutralizing antibody titers above (≥25, black bars) and below (<25, gray bars) the limit of detection for the neutralizing titer assay. AAV, adeno-associated virus.
Anti-AAV8 total titers were also measured in 12 additional human subjects pre- and postplasmapheresis. Supplementary Table S2 shows that a single course of plasmapheresis reduced total titers in these subjects on average by ~2.9-fold. Thus, the segment of the population with anti-AAV8 titers of 800 in Figure 6 could be reduced to ≤400 with plasmapheresis, potentially sufficient to allow for vector-mediated liver transduction after systemic high-dose vector administration. The passive transfer results thus predict that with plasmapheresis the proportion of subjects in which significant vector copy number could reach the liver should increase to ~87%. Taken together, these results suggest that with high vector dose, it should be possible to transduce the liver of most patients. At the low dose, however, ~10% of patients would be predicted to be treatable, increasing to ~25% with added plasmapheresis pretreatment. However, given the discrepancy between mouse and NHP expression levels shown in Figure 5, it is likely that successful transduction of the liver in patients would nonetheless result in relatively low expression levels for an LSD protein like αgal.
Discussion
Liver-directed gene therapy using AAV vectors is seen as a promising approach for generating systemic levels of a transgene product at clinically relevant concentrations. For example, extensive preclinical studies in mice, dogs, and NHPs with AAV-derived FIX led to a clinical trial in hemophilia B patients.16 In the human population, preexisting antibodies to AAV vectors, including AAV8, are well documented, and recognized as a barrier to gene transfer to hepatocytes.16,23 Indeed, recent studies using the passive transfer approach clearly demonstrate that the ability of low neutralizing titers to neutralize vector in vivo is underestimated by in vitro neutralizing assays.23,24 We undertook these studies to determine what effects prior exposure to AAV would have on our ability to transduce the primate liver with an AAV8 vector and generate clinically significant expression levels of a model LSD enzyme, namely, αgal.
Using NHP serum with known anti-AAV8 total antibody titers coupled with the passive transfer of this serum into mice, we characterized the relationships between anti-AAV8 total titers and the ability to deliver an AAV8 vector to the liver as well as the subsequent ability of that vector to express the encoded transgene. These results demonstrate that relatively low total antibody titers (≥40) can reduce vector copy number in the liver and subsequent expression from a low dose (2 × 1012 drp/kg) of vector. We show that this reduction can be partially overcome by increasing vector dose. Of note, the AAV8 dose used to achieve this (2 × 1013 drp/kg) is ~10× the highest dose used in the AAV2 FIX clinical trial.16
Based on the prevalence of anti-AAV8 titers in the human population (Figures 6 and 7a,b) and the ability of high vector dose to overcome this barrier, especially when it is reduced by plasmapheresis (Supplementary Table S2), we do not believe that the preexisting antibody problem should present an insurmountable obstacle to AAV8 liver-directed gene transfer in the majority of humans. However, in the absence of an immune suppressive regime, treatment with vector would result in significantly higher antivector titers post-treatment. Given that anti-AAV8 titers as low as 640 significantly reduced αgal expression after vector administration at high dose, repeat treatments utilizing the same vector could present a substantial practical challenge.
As the data in Figure 5 indicate, simply getting the vector to NHP liver was not sufficient to result in expression of the transgene product at levels observed in the mouse. These data therefore suggest that there are significant differences between the fate of AAV8 in murine and NHP livers. Such a difference can be noted anecdotally in the literature.7,15,17 The studies presented here, which use the identical vector preparation for both mouse and NHP transduction, serve to solidify this observation.
Several possible hypotheses can be advanced to account for the dramatic differences observed here between the expression levels achieved in mice and NHPs with similar liver copy numbers and antibody titers (Figure 5). One possibility is that the AAV vector is processed differently by mouse and monkey hepatocytes, resulting in viral DNA structures that lead to sustained expression in mice but only transient expression in primates. Though the significance of this remains undetermined, differences in AAV vector structure have been observed in mice and primates after liver-directed gene transfer.25 It is noteworthy that no intrinsic primate-specific block to vector expression appeared to exist in primary hepatocytes (Figure 1). However, this in vitro model is limited to short-term studies (<1 week) and later vector fate could vary significantly in these species. Indeed, after treatment with the AAV8 vector, mice and rhesus monkeys had similar initial serum αgal levels (Figure 2). However, later serum levels differed greatly, suggesting that in primates effective stabilization of transcription-competent vector forms may have failed to occur. In primates, the decreased αgal expression shown here at the protein level was mirrored by a similar decrease in steady-state vector-encoded transcript levels (Nietupski et al., unpublished results). Though potential differences in vector stabilization could account for decreased transcription, it is also possible that, perhaps after an early permissive phase, mechanisms that lead to vector silencing or decreased transcript stability are triggered in primates. We are currently exploring these possibilities.
Using pharmacologic approaches, we have evaluated several additional hypotheses that might affect vector expression in primates, such as androgen effects6 and vector methylation and acetylation, and these are the subject of a companion paper (Nietupski et al., unpublished results). Differing mechanisms of antibody-bound vector clearance may also account for the observed differences in vector expression in these two species. It has been shown recently that preexisting antibodies can shunt AAV to the spleen in the mouse passive antibody transfer model.24 In our study, equivalent vector copy numbers per liver cell in NHPs and mice suggest that differences in splenic clearance alone are unlikely to explain the observed differences in expression. Another possibility is that in the presence of low circulating antibody levels, vector sequestration within resident liver Kupffer cells differs between NHPs and mice. Indeed, subneutralizing levels of anti-AAV antibodies have been shown to increase the uptake and subsequent transduction of macrophage-like cell lines. However, upon transduction these cells do express transgene.26 Although Kupffer cells are numerous, comprising ~15% of all liver cells (in rat27), a dramatic species-dependent difference in the efficiency of Kupffer-cell-mediated AAV8 clearance would likely be required for animals with similar anti-AAV8 titers to have such different transgene expression levels at equivalent vector copies/cell. Here, in situ hybridization experiments could provide an answer.
Two additional, yet speculative, possibilities for the relatively low expression levels observed in NHPs stem from the likelihood that virtually all the NHPs used in these studies, and likely all studies of this kind, have been previously infected by AAV. Indeed, one may speculate that most monkey livers have been infected by multiple AAV serotypes. In this context, given that recombination between AAV genomes appears to be a surprisingly common event in primate tissue18 and the number of vector genomes delivered to the primate liver in these experiments was relatively low, it is possible that recombination occurred between preexisting integrated or persistent nonintegrated AAV genomes in the liver and the incoming AAV8-αgal genome, resulting in recombinants with reduced αgal expression. Finally, given that low level AAV rep expression is required to maintain vector persistence in infected cells,21 and Rep protein is known to suppress expression,28,29,30 it is possible that hepatic expression of AAV8-αgal in NHPs was suppressed by the expression of rep from previous AAV infection(s). This is plausible as the inclusion of Rep binding sites is a requirement for vector packaging.
In summary, we have shown that preexisting anti-AAV8 antibodies in NHPs (and by inference, most humans) can likely be overwhelmed by increasing the viral dose. However, even when this is accomplished and significant vector copies reach the primate liver, compared to mice there remains a dramatically (1.5 logs) reduced ability of the vector to express the encoded transgene in primates. In the case of LSD enzymes, e.g., αgal, the resulting expression levels that we have observed in primates are substantially below theoretical levels sufficient for clinically meaningful substrate clearance.1 Therefore, to the extent that these results in macaques are relevant to humans, methods that improved expression from liver-targeted AAV vectors in primates would represent a significant advance toward the clinical application of this approach.
Materials and Methods
Vectors. The human αgal cDNA and the hepatocyte-specific vector construct AAV8-αgal used here have been described previously.1 This vector cassette contains a human serum albumin promoter together with two copies of the human prothrombin enhancer (DC190) and the bovine growth hormone polyadenylation sequence (BGH polyA). Virus was purified from cell pellets (University of Pennsylvania Vector Core Facility) by an Iodixanol/Q column method.31 Each virus preparation was analyzed for impurities by gel electrophoresis (purity estimated at >95% VP1,2,3 on a silver stained gel), assayed for endotoxin (<0.3 EU/ml) and bioburden (no growth) levels, and then evaluated for expression in male C57/Bl6 mice. A total of 75 individual preparations meeting these criteria were pooled and titered for drp using real-time Taqman PCR (ABI 7500; Applied Biosystems, Foster City, CA) with primers specific to the BGH polyA. The final viral pool of ~2 × 1015 drp was adjusted to 1 × 1013 drp/ml in 165 mmol/l NaCl, 2 mmol/l MgCl2,10% glycerol, 10 mmol/l NaH2PO4 pH 7.5, and frozen in aliquots at −80 °C. Virus from this one pool was used for all in vivo mouse and primate experiments.
The AAV8-αgal vector was characterized further by determining the empty/full ratio using negatively stained (2% uranyl acetate) transmission electron micrographs. Using empty and full AAV particles isolated from a CsCl density–gradient as transmission electron micrograph standards, micrographs were taken (Microview Labs, Hayward, CA) of three randomly chosen regions of 3–4 transmission electron micrograph grids each for the AAV8 standards and the pooled AAV8-αgal vector, which were then manually counted. To control for possible solvent artifacts in the negative stain process, micrographs of the CsCl standards were also obtained after dilution into the AAV8-αgal vector buffer (earlier text). By this analysis the pooled vector used in these experiments contained 57 ± 1% full virions.
For in vitro studies, human αgal was also expressed using AAV2- and Ad2-based vectors containing the DC190 promoter and BGH polyA. AAV2-αgal was produced as previously described.22 For the Ad2-αgal, a plasmid was constructed containing the DC190 promoter, human α-gal cDNA, and BGH polyA, flanked by adenoviral sequences. Virus was produced by the method used in Armentano et al.32 and was purified from 293 cell lysates by a step-gradient and two equilibrium–gradient cesium chloride purification steps. Purified vector was dialyzed to remove cesium chloride, aliquoted, and stored at –80 °C.
Primary hepatocyte experiments. Freshly prepared primary hepatocytes derived from a 3-year-old male cynomolgus macaque and four 10-week-old male CD1 mice (CellzDirect, Durham, NC) were seeded on collagen I coated 6-well tissue culture plates (CellzDirect) according to the supplier protocol and were maintained in Clonetics HCM Hepatocyte Culture Medium (Lonza, Walkersville, MD) under standard incubation conditions. At 12 hours postseeding, cells were transduced in triplicate with the AAV8-, AAV2-, or Ad2-αgal vectors at an MOI of 1,000 for AAV2 and AAV8 or 75 for Ad2. Vector-treated cells and untreated controls were incubated for an additional 48 hours under standard conditions. Medium was then removed, centrifuged at 10,000g for 10 minutes at 4 °C to remove cells and cell debris, shock frozen on dry ice, stored at −80 °C, and later assayed for αgal levels by enzyme-linked immunosorbent assay as described later. Adherent cells were washed twice with 2 ml of 1× phosphate buffered saline, pH 7.2 (Life Technologies, Carlsbad, CA), incubated in a 0.25% solution of trypsin with EDTA (Life Technologies) at 37 °C to dissociate cells completely and release any remaining surface-associated vector. Cells were centrifuged at 75g for 5 minutes at 4 °C, the supernatant removed, and the cells washed three times by resuspension in 2 ml of phosphate buffered saline at 4 °C followed by cell recovery via centrifugation (as described earlier) and phosphate buffered saline removal. Cell pellets were lysed, and DNA and RNA purified according to the Qiagen AllPrep DNA/RNA Mini Kit protocol (Qiagen, Valencia, CA).
Animal experiments. Use of all animals in these experiments was governed by the rules set forth in the NRC Guide for the Care and Use of Laboratory Animals. All procedures were performed in accordance with the guidelines of each institution's Institutional Animal Care and Use Committee.
Liver-directed gene transfer in primates and mice. Male rhesus macaques were housed at two facilities, Charles River Laboratories (Sparks, NV; of Chinese origin, designated in Table 1 as R-6 through R-8), and the New England Primate Research Center (Southborough, MA; of Indian origin, R-9 through R-14). Male cynomolgus macaques (designated in Table 1 as C-1 through C-3) were housed at the Mannheimer Foundation (Homestead, FL). Macaques from each facility were prescreened for anti-AAV8 total antibody titers (see later text for the method). Table 1 lists the study monkeys with their corresponding anti-AAV8 total antibody titers at the time of vector administration (day 0). For systemic delivery, the AAV8-αgal vector was administered at 2 × 1013 drp/kg (1 × 1013 drp/ml) as a slow bolus injection (50–100 seconds) into the saphenous or cephalic vein of sedated monkeys. Blood was collected pretreatment and for up to 17 weeks post-treatment by venipuncture under anesthesia with 15 mg/kg Ketamine HCl administered by intramuscular injection.
For the long-term expression study in mice, 15 male C57BL/6 mice were treated systemically by tail-vein injection with the AAV8-αgal vector at a dose of 5 × 1012 drp/kg in 100 µl of saline. Blood was collected pretreatment and post-treatment weekly for 17 weeks from the retro-orbital sinus under anesthesia with 3–5% isoflurane in oxygen.
Mouse passive antibody transfer. Ten-week-old male BALB/cJ mice were obtained from Taconic (Hudson, NY). For passive antibody transfer studies, 200 µl of NHP serum (neat or diluted) from animals of varying anti-AAV8 total antibody titer (Supplementary Table S1), naive mouse serum, or saline was administered by tail-vein injection to 4–5 mice/treatment. After ~1 hour, saline or the AAV8-αgal vector was administered by the same route in 100 µl of saline at a dose of 2 × 1012 (low dose) or 2 × 1013 drp/kg (high dose). Due to the dilution of the transfused serum in the total blood volume of a mouse (~2 ml in a 25 g mouse),33 we approximate that the in vivo anti-AAV8 antibody titers resulting from passive transfer at the time of vector administration were reduced to ~10% of the levels measured in neat serum. Blood was collected from the retro-orbital sinus under anesthesia with 3–5% isoflurane in oxygen 1 and 2 weeks after vector administration. At the end of study, animals were killed and liver tissue harvested.
NHP serum. Serum samples for passive antibody transfer studies were obtained from additional male rhesus macaques of Indian origin also housed at the New England Primate Research Center, Southborough, MA (Supplementary Table S1).
Human serum. Human serum samples were obtained from 100 normal volunteers. Additional human sera from 12 patients, collected pre- and postplasmapheresis, were obtained from Biological Specialty Corporation (Colmar, PA). For plasma exchange, 50 ml of patient plasma/kg of body weight (up to a 4.5 l maximum) was replaced with an equal volume of 5% albumin in 0.9% sodium chloride over a 75–90 minute period. Serum was collected immediately before and after the procedure.
Anti-AAV8 titers. To determine anti-AAV8 total titers, enzyme-linked immunosorbent assay plates (Corning, Oneonta, NY) were coated overnight at 4 °C with 2 × 109 drp/well of AAV8-αgal in 0.1 mol/l NaHCO3 at pH 9.2. Plates were washed and blocked with 5% (wt/vol) milk in Tris-buffered saline with 0.1% vol/vol Tween-20 for a minimum of 1 hour at 37 °C. After removal of the blocking solution and plate wash, serum samples were diluted twofold serially in duplicate across the plate with a starting dilution of 1:100 and incubated for 1 hour at 37 °C. A 1:10,000 dilution of the horseradish peroxidase–conjugated goat anti-monkey or anti-human IgG secondary antibody (Immunology Consultants Laboratory, Newburg, OR) was applied to the plates and incubated for 1 hour at 37 °C. The enzyme-linked immunosorbent assay was developed using TMB One Component Microwell Substrate (BioFX Labs, Owings Mills, MD) in the dark for 30 minutes. The reaction was stopped with 450 nm Stop Reagent (BioFX Labs) and the plates read in a plate reader (Molecular Devices Spectra Max Plus, Molecular Devices, Sunnyvale, CA) at 450 nm. Titers are expressed as the reciprocal of the minimum serum dilution giving an OD450 ≤0.1.
To determine neutralizing titers, HeLa cells (American Type Culture Collection, Manassas, VA) were plated at 20,000 cells per well in flat-bottom 96-well tissue culture plates (Corning) in 50 µl culture media [Dulbecco's modified Eagle's medium, 10% heat-inactivated fetal bovine serum, 1% penicillin/streptomycin, and 1% -glutamine (Life Technologies)] and incubated for 2 hours at 37 °C in a 5% CO2 atmosphere until cells became adherent. Culture media was removed and replaced with 25 µl of culture media to which wild-type Ad2 helper virus was added at an MOI of 5 and the plates then incubated for 4 hours at 37 °C in a 5% CO2 atmosphere.
Serum samples to be assayed for neutralizing titer were heat inactivated for 30 minutes at 56 °C and then serially diluted twofold with culture media from 1:12.5 to 1:12,800. An AAV2/8 vector encoding cytomegalovirus-driven LacZ, namely AAV2/8-CMVLacZ1 (University of Pennsylvania Vector Core Facility), at 4 × 105 drp/ml in culture media was incubated 1:1 with either the diluted serum or culture media for 1 hour at 37 °C in 5% CO2 to allow antibodies potentially present in the serum to bind to the AAV vector. Medium containing the Ad2 helper virus was removed from HeLa cells, replaced with 150 µl culture medium and 50 µl of the serum-incubated AAV2/8 vector added to each well at an MOI of 1,000. Cells were incubated at 37 °C in a 5% CO2 atmosphere for 3 days. Medium was removed and β-galactosidase activity was assayed using the Galacto-Star kit (Life Technologies) according to the protocol provided by the manufacturer. Luminescence was measured in opaque 96-well assay plates (Corning) on a Tropix TR717 microplate luminometer (Perkin Elmer, Waltham, MA). Neutralizing titer is reported as the reciprocal of the serum dilution that decreases β-galactosidase expression by 50% or more from levels in controls where the AAV vector was incubated in culture medium alone.
Vector copy number determination. To determine vector copy number in liver, samples of mouse or primate liver were collected at necropsy, flash frozen on dry ice, and stored at −80 °C. For mouse studies (4–5 mice/treatment group) one sample of liver was analyzed per mouse and the group average reported. For primates, 2–3 pieces of liver were analyzed for each animal and averaged. DNA and RNA were purified from liver samples using the Qiagen AllPrep DNA/RNA Mini Kit (Qiagen) according to the manufacturer's instructions. Approximately 20 mg fragments of frozen liver were placed into buffer RLT (supplied in the AllPrep kit) containing guanidine hydrochloride and then immediately homogenized with stainless steel beads using a TissueLyser (Qiagen). The remainder of the purification procedure was carried out as recommended. For PCR assays, liver- and primary cell–derived DNA (prepared as described earlier) was adjusted to a concentration of ~100 ng/µl and 5 µl used per reaction. To prevent possible crossreaction with primate genomic DNA, vector-specific primers were designed that spanned the region between the human αgal cDNA and the bovine growth hormone polyadenylation signal sequence. Vector copies per 500 ng DNA were determined in duplicate reactions using a real-time TaqMan PCR assay (ABI PRISM 7700; Applied Biosystems) and a standard curve of serially diluted plasmid containing the vector sequence at known concentration. Vector copies per diploid cell were calculated from the copy numbers per µg of input DNA using estimates of the haploid genome weight (C-value) for each species: 3.136 pg for the mouse and 3.256 pg for the macaque. These C-values were obtained by averaging the genome size in base pairs34,35 multiplied by the per nucleotide gram weight of 1.096 × 1021 with published Feulgen densitometry-based C-value estimates.36,37,38
αGal protein and mRNA levels. The αgal protein levels were measured by enzyme-linked immunosorbent assay from serum and cell culture media as previously described.1 Serum was collected from mice by retro-orbital bleed and from monkeys by standard phlebotomy. Liver- and primary cell–derived RNA (described earlier) was assessed for vector-specific mRNA copy number. For tissue samples, a reverse transcriptase (RT) primer (Integrated DNA Technologies, Coraville, IA) was designed to overlap with the vector region amplified by the PCR reaction (described earlier). Reactions were carried out using Promega M-MLV RT (Promega, Madison, WI), as described by the manufacturer, to generate vector-specific cDNA. Briefly, samples were adjusted to ~200 ng/ml RNA, and 10 µl used per reaction (~2 ng). Samples were mixed with 5× buffer, dNTPs, RNasin (as per Promega protocol), and primer to a final volume of 48 µl. Sample mixtures were spilt into two aliquots, and 1 µl M-MLV RT added to one of the aliquots. Both aliquots were then incubated sequentially at 42 °C (60 minutes) followed by 95 °C (2 minutes) for cDNA synthesis. For primary cell samples cDNA was generated from RNA pretreated with DNAse (DNA-free DNase Treatment; Applied Biosystems) and reverse transcribed with random primers using the High-Capacity cDNA RT Kit (Applied Biosystems) according to protocol. Vector expression levels were determined by real-time TaqMan PCR (ABI PRISM 7700; Applied Biosystems) of input cDNA using the vector-specific PCR reaction and standard curve described earlier. Aliquots untreated by RT were included as controls for residual vector DNA contamination. For primary hepatocyte samples, expression levels of the αgal transgene were normalized to 18S rRNA levels as determined by real-time TaqMan PCR using an 18S Ribosomal RNA Control Assay (Applied Biosystems).
Statistics and error analysis. Values shown represent means, and error bars depict SEM. The Student's t-test was used to test differences between group means for statistical significance, for which a threshold of P < 0.05 was used.
SUPPLEMENTARY MATERIAL Table S1. NHP sera used for passive transfer studies. Table S2. Anti-AAV8 total antibody titers in patients pre- and post-plasmapheresis. Table S3. Anti-AAV8 total and neutralizing antibody titers in 112 human subjects.
Acknowledgments
We thank Keith Mansfield and Angela Carville of the New England Primate Research Center, and Julie Bell and Kelly Hopper of the Mannheimer Foundation for help with these studies. We also thank Jeffrey Widdoss of Biological Specialty Corporation for assisting us with human serum samples from patients pre- and postplasmapheresis.
Supplementary Material
NHP sera used for passive transfer studies.
Anti-AAV8 total antibody titers in patients pre- and post-plasmapheresis.
Anti-AAV8 total and neutralizing antibody titers in 112 human subjects.
REFERENCES
- Ziegler RJ, Cherry M, Barbon CM, Li C, Bercury SD, Armentano D, et al. Correction of the biochemical and functional deficits in fabry mice following AAV8-mediated hepatic expression of alpha-galactosidase A. Mol Ther. 2007;15:492–500. doi: 10.1038/sj.mt.6300066. [DOI] [PubMed] [Google Scholar]
- Ziegler RJ, Bercury SD, Fidler J, Zhao MA, Foley J, Taksir TV, et al. Ability of adeno-associated virus serotype 8-mediated hepatic expression of acid alpha-glucosidase to correct the biochemical and motor function deficits of presymptomatic and symptomatic Pompe mice. Hum Gene Ther. 2008;19:609–621. doi: 10.1089/hum.2008.010. [DOI] [PubMed] [Google Scholar]
- Passini MA, Bu J, Fidler JA, Ziegler RJ, Foley JW, Dodge JC, et al. Combination brain and systemic injections of AAV provide maximal functional and survival benefits in the Niemann-Pick mouse. Proc Natl Acad Sci USA. 2007;104:9505–9510. doi: 10.1073/pnas.0703509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern KA, Nietupski JB, Chuang WL, Armentano D, Johnson J, Hutto E, et al. AAV8-mediated expression of glucocerebrosidase ameliorates the storage pathology in the visceral organs of a mouse model of Gaucher disease. J Gene Med. 2006;8:719–729. doi: 10.1002/jgm.901. [DOI] [PubMed] [Google Scholar]
- Wang L, Takabe K, Bidlingmaier SM, Ill CR., and, Verma IM. Sustained correction of bleeding disorder in hemophilia B mice by gene therapy. Proc Natl Acad Sci USA. 1999;96:3906–3910. doi: 10.1073/pnas.96.7.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff AM, Ng CY, Zhou J, Spence Y., and, Nathwani AC. Sex significantly influences transduction of murine liver by recombinant adeno-associated viral vectors through an androgen-dependent pathway. Blood. 2003;102:480–488. doi: 10.1182/blood-2002-09-2889. [DOI] [PubMed] [Google Scholar]
- Davidoff AM, Gray JT, Ng CY, Zhang Y, Zhou J, Spence Y, et al. Comparison of the ability of adeno-associated viral vectors pseudotyped with serotype 2, 5, and 8 capsid proteins to mediate efficient transduction of the liver in murine and nonhuman primate models. Mol Ther. 2005;11:875–888. doi: 10.1016/j.ymthe.2004.12.022. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder RO, Miao CH, Patijn GA, Spratt SK, Danos O, Nagy D, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, et al. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Mount JD, Herzog RW, Tillson DM, Goodman SA, Robinson N, McCleland ML, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Wang L, Nichols TC, Read MS, Bellinger DA., and, Verma IM. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Davidoff AM, Hanawa H, Hu Y, Hoffer FA, Nikanorov A, et al. Sustained high-level expression of human factor IX (hFIX) after liver-targeted delivery of recombinant adeno-associated virus encoding the hFIX gene in rhesus macaques. Blood. 2002;100:1662–1669. doi: 10.1182/blood-2002-02-0589. [DOI] [PubMed] [Google Scholar]
- Jiang H, Couto LB, Patarroyo-White S, Liu T, Nagy D, Vargas JA, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108:3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Gao GP, Lu Y, Sun X, Johnston J, Calcedo R, Grant R, et al. High-level transgene expression in nonhuman primate liver with novel adeno-associated virus serotypes containing self-complementary genomes. J Virol. 2006;80:6192–6194. doi: 10.1128/JVI.00526-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, Lin J., and, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns KI. Parvovirus replication. Microbiol Rev. 1990;54:316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler RJ, Lonning SM, Armentano D, Li C, Souza DW, Cherry M, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9:231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, Patarroyo-White S, Sommer JM, Zhou S, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Wang H, Bell P, Grant R, Vandenberghe LH, et al. The pleiotropic effects of natural AAV infections on liver-directed gene transfer in macaques. Mol Ther. 2010;18:126–134. doi: 10.1038/mt.2009.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Lu Y, Bish LT, Calcedo R, Wilson JM, Gao G. Molecular analysis of vector genome structures after liver transduction by conventional and self-complementary adeno-associated viral serotype vectors in murine and nonhuman primate models. Hum Gene Ther. 2010;21:750–761. doi: 10.1089/hum.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Takeuchi T., and, Kanda T. Antibody-dependent enhancement of adeno-associated virus infection of human monocytic cell lines. Virology. 2008;375:141–147. doi: 10.1016/j.virol.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Blouin A, Bolender RP., and, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat PL. Down-regulation of the human c-fos and c-myc proto-oncogene promoters by adeno-associated virus Rep78. Cancer Lett. 1994;81:129–136. doi: 10.1016/0304-3835(94)90193-7. [DOI] [PubMed] [Google Scholar]
- Hörer M, Weger S, Butz K, Hoppe-Seyler F, Geisen C., and, Kleinschmidt JA. Mutational analysis of adeno-associated virus Rep protein-mediated inhibition of heterologous and homologous promoters. J Virol. 1995;69:5485–5496. doi: 10.1128/jvi.69.9.5485-5496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Kozuka T, Nakagawa K, Aoki Y, Ohtomo K, Yoshiike K, et al. Adeno-associated virus type 2 nonstructural protein Rep78 suppresses translation in vitro. Virology. 2000;266:196–202. doi: 10.1006/viro.1999.0061. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Armentano D, Sookdeo CC, Hehir KM, Gregory RJ, St George JA, Prince GA, et al. Characterization of an adenovirus gene transfer vector containing an E4 deletion. Hum Gene Ther. 1995;6:1343–1353. doi: 10.1089/hum.1995.6.10-1343. [DOI] [PubMed] [Google Scholar]
- Riches AC, Sharp JG, Thomas DB., and, Smith SV. Blood volume determination in the mouse. J Physiol (Lond) 1973;228:279–284. doi: 10.1113/jphysiol.1973.sp010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church DM, Goodstadt L, Hillier LW, Zody MC, Goldstein S, She X, Mouse Genome Sequencing Consortium et al. Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biol. 2009;7:e1000112. doi: 10.1371/journal.pbio.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Rhesus Macaque Genome Sequencing and Analysis Consortium et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- Manfredi Romanini MG. The nuclear content of deoxyribonucleic acid and some problems of Mammalian phylogenesis. Mammalia. 1985;49:369–385. [Google Scholar]
- Manfredi Romanini MG. Nuclear DNA content and area of primate lymphocytes as a cytotaxonomical tool. J Hum Evol. 1972;1:23–40. [Google Scholar]
- Gregory TR. Nucleotypic effects without nuclei: genome size and erythrocyte size in mammals. Genome. 2000;43:895–901. doi: 10.1139/g00-069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NHP sera used for passive transfer studies.
Anti-AAV8 total antibody titers in patients pre- and post-plasmapheresis.
Anti-AAV8 total and neutralizing antibody titers in 112 human subjects.