Abstract
More than fifty years has passed since the first allogeneic hematopoietic stem cell transplant in patients, however, the promise of other stem cell populations for tissue replacement and repair remains unachieved. When considering cell-based interventions for personalized medicine, the factors influencing therapeutic success and safety are more complicated than for traditional small-molecule pharmacological agents and protein biologics. Failure to progress personalized stem cell therapies to the clinic has resulted from complications that include an incomplete understanding of stem cell development programs and the diversity of host-donor interactions between patients and in different microenvironments within the same patient. In order to more rapidly extend the use of non-hematopoietic stem cells to the clinic, a better understanding of the different stem cell sources and the implications of their host interactions is required. In this review, we introduce currently available stem cell sources and highlight recent literature that instructs the potential and limitations of their use, with a focus on mesenchymal stem cells.
Lessons from the success of hematopoietic stem cell transplant
Hematopoietic stem cell transplant (HSCT) was first successfully applied in 1959 when bone marrow (BM) cells were transplanted from the identical twin of a patient suffering from acute leukemia who was treated with supralethal whole body irradiation2. Although the radiation ultimately failed to cure the leukemia, there was sufficient evidence of BM replacement by the isologous cells to offer proof of principle of BM transplantation2. Fifty years after this clinical breakthrough, HSCT remains the only stem cell therapy widely used in clinical practice, despite extensive research to advance other stem cell populations into the clinic. There are many lessons, however, that have been learned from more than fifty years of HSCT that may apply to the transplant of other stem cells into patients.
The most important breakthrough in extending the clinical application of HSCT came from a better understanding of host-donor interactions. The discovery of major histocompatibility complex (MHC) molecules and the understanding of the importance human leukocyte antigen (HLA) matching allowed the first successful use of non-identical HSCs for transplant in 19743. This allowed patients without HLA-identical sibling donors to receive autologous HSCT. This seminal work paved the way for the development of the National Marrow Donor Program (NMDP) in 1986 that now maintains HLA information on millions of potential volunteer bone marrow donors, enormously increasing the chances of a needy patient finding an appropriate allograft4.
Even with optimal HLA matching and pharmacological prophylaxis, however, there is still much room for improvement. As many as 60% of patients receiving HLA-identical allogeneic sibling transplants suffer from acute graft versus host disease (GvHD), which occurs when immune cells derived from the grafted cells attack the donor tissue5. This immune attack by the grafted cells against the host is not always undesirable, however, as the transplanted cells can also target cancer cells in a process termed the graft versus tumor (GvT) effect, reducing rates of malignant relapse6.
Another scientific advance that has yielded great clinical benefit is the discovery of more efficient methods to harvest HSCs from a donor than standard bone marrow aspirations. The first successful HSCT donor in 1959 was subjected to twenty or more BM aspirations on four separate occasions in order to yield sufficient cell numbers for transplant2. The discovery that granulocyte colony-stimulating factor (GCSF) can efficiently mobilize HSCs from the BM to the peripheral blood has made HSC donation a much less painful process and facilitated the expansion of bone marrow registries7. Storing collected HSCs, however, remains inefficient and transplants are most often performed from freshly-isolated cells because cryopreservation results in reduced cell viability8. Attempts to expand HSCs in culture prior to transplant are being explored in the laboratory, but these technologies have not yet reached the clinic9.
It is also important to note that there are phenotypic differences between HSCs of different sources. HSCT performed from peripheral blood HSCs repopulates the hematopoietic system more rapidly than transplanted bone marrow10. Peripheral blood stem cells, however, confer increased risk of chronic GvHD when compared to bone marrow-derived cells, although this feature may be beneficial if a GvT effect is desired11.
Differences in clinical outcomes depend not only on the transplanted cells, but also on the recipient. As a general rule, younger transplant patients fare much better than older patients9. Adding further complexity, it appears that different stem cell sources perform differently in different patient populations. While the vast majority of adult HSCTs are performed using peripheral blood stem cells mobilized with GCSF because of their ease of harvest, pediatric patients have better outcomes when they receive BM transplants rather than HSCs from peripheral blood9.
HSCT has been a great success in the field of regenerative medicine and clinical outcomes continue to improve as technologies and the science becomes more advanced. By comparison, nearly all other types of stem cell transplant technologies remains in their infancy. Many of the lessons learned from HSCT may help instruct these new technologies as they continue to advance. In the remainder of this article we review other sources of stem cells and introduce some of the potential therapeutic uses of these cell populations and then discuss the challenges of translating these stem cell technologies to the clinic.
Non-hematopoietic stem cells
There are many types of stem cells from different sources with differing capacities for differentiation, ranging from pluripotent embryonic stem (ES) cells to organ-specific adult tissue stem cells such as HSCs. Since the discovery of blood-lineage repopulating stem cells in the BM, stem cells for many other tissues have been discovered, including stem cell populations in the brain, skin, intestine and heart1. The only two defining characteristics of a stem cell are their ability to self-renew and their capacity to differentiate into more specialized cell types, whereas the differences between different stem cell populations can be large and significantly impact their potential for use in regenerative medicine. Below we will discuss some of these different stem cell populations and how they might be used as personalized therapeutics.
Embryonic stem cells
Embryonic stem (ES) cells have significant implication in studying developmental biology and provide a vast potential for clinical application. Human ES cells are derived by explanting the inner cell mass of a four- or five-day-old blastocyst from a donated embryo produced by in vitro fertilization12. Whereas adult stem cells are limited to differentiating along restricted lineages, ES cells are pluripotent and retain the ability to form all three embryonic germ layers, as well as the peripheral blastocyst, or trophoblast layer both in vivo and in vitro12, 13. Scientists are able to manipulate ES cells into various cell types in vitro because of their extensive developmental potential. Human ES cells have been induced to form cell types such as pancreatic beta cells, cardiomyocytes, and osteoblasts14–16.
Although embryonic stem cell research is very promising, scientists face many technical hurdles that impede translation into clinical settings. Most notably, there are challenges in controlling cell growth. In vitro, ES cells replicate indefinitely, even in the absence of the stem cell-associated factor human leukemia inhibitory factor (LIF)12. In vivo, ES cells form teratoma tumors when injected into immunodeficient SCID mice12. The propensity for tumor formation has led scientists to believe that there may be a relationship between human ES cells and embryonic carcinoma (EC) cells. Both cell types express transcription factor Oct-4, which is downregulated at during differentiation17. The parallels between human ES and EC cells suggests that certain factors must be closely regulated before ES cells can be put into clinical use.
In January of 2009, the FDA approved the first human clinical trial of GRNOPC1, an ES cell based therapy for spinal cord injury. This study was led by Dr. Hans Keirstead and funded by the biopharmaceutical company, Geron18. Based on small animal models, researchers hypothesized that human ES cell-derived oligodendrocyte progenitor cells injected into the damaged spinal cords of patients before the formation of scar tissue will lead to remyelination and the eventual restoration of motor function18. The FDA temporarily halted the trial in August 2009 due to microscopic cysts found in rat models, however, the trial is expected to resume by the end of 2010.
To further complicate this area of research, there is still much ethical debate over the derivation of human embryonic stem cell lines and the potential for human cloning. In the United States, the federal government regulates funding and usage of embryonic stem cell lines, although much regulation remains in the hands of state legislators. Individual states may further regulate or provide funding for ES cell research. Privately funded institutions are not subject to the same federal regulation. As of July 2010, the National Institutes of Health reported sixty-four eligible cell lines, with twelve additional lines pending approval (http://grants.nih.gov/stem_cells/registry/current.htm).
Outside of the US, each country differs in terms of regulation. Although the European Union does not have a common policy, there are four main positions that represent the diversity of moral stances on embryonic stem cell research across Europe: permissive, permissive with restrictions, restrictive, or no position due to nonspecific or indirect legislation19. Belgium, Spain, Sweden, and the UK are amongst the countries that hold a permissive position and specific legislation for the use and derivation of embryonic stem cells19. Policy in Asia also varies across the continent, with China having the most unrestrictive embryonic stem cell research policy in the world20. In contrast to the US, countries such as South Africa, Israel, Belgium, Sweden, UK, Russia, Japan, China, Singapore and Turkey all allow for the creation of embryos specifically for embryonic stem cell research (http://www.isscr.org/public/regions/index.cfm).
The discovery of induced pluripotent stem (iPS) cells was a breakthrough in stem cell research that has offered hope to assuage ethical concerns, as well as address technical issues such as immune rejection. iPS cells are adult somatic cells reprogrammed to exhibit embryonic stem cell identity21. In 2007, Dr. James Thomson of the University of Wisconsin led a team of scientists who were able to derive human induced pluripotent stem cells through somatic cell nuclear cell transfer and induction with four transcription factors, OCT4, SOX2, NANOG, and LIN2822. These cells were demonstrated to be pluripotent, self-renewing and posses normal karyotype and telomerase activity22. Because the reprogramming mechanism is still not fully understood, directed differentiation into specific cell types is poorly controlled. In addition, it is still unknown if IPS cells are truly pluripotent23. Although research is thus far limited, it serves a potential alternative to the use of embryonic stem cells.
Fetal stem cells
Fetal stem cells are self-renewing cells located in various types of fetal tissue, including umbilical cord blood, umbilical cord matrix, fetal blood and the amniotic membrane24–28. Mesenchymal stem cells (MSCs) and HSCs are two of the more accessible stem cell populations amongst the fetal stem cell populations. Fetal MSCs can be found in the peripheral blood, bone marrow and liver of the first trimester fetus28.
There are many advantages to using fetal stem cells that have led scientists to explore these cell types for regenerative therapy. First, the fetal stem cells have shorter doubling times than adult stem cells28. They demonstrate greater telomere lengths and their plasticity is superior to that of adult stem cells. Thus their self-renewal potential may be greater than adult cells and they may possess greater expansion and growth potential without becoming senescent28. Another benefit to using of fetal stem cells is that umbilical cord blood, which is a major source of fetal stem cells, can be readily obtained in emergent situations as ‘off-the-shelf’ stem cell therapy29. In addition to these advantages, fetal stem cells appear to be more immunologically naïve than adult stem cells, allowing for enhanced transplantation efficiency25, 30, 31. They express low levels of MHC class I and nearly undetectable levels of MHC class II, and mismatch between donor fetal stem cells and host tissue is better tolerated than for adult stem cells31, 32. Despite the potent immunosuppressive properties of certain fetal stem cell types, fetal stem cells may possess some immunogenic potential which could result in graft rejection, and therefore further research into the immunobiology of fetal stem cells is required prior to implementation of allogeneic fetal stem cell transplants in the clinic29, 33.
Despite these advantages that fetal stem cell therapy have over adult stem cell therapy, the use of fetal stem cells for transplant is met with numerous drawbacks. While fetal tissue contains abundant HSC and MSC populations, other fetal stem cell populations are difficult to obtain in sufficient numbers with current technologies. Furthermore, as with ESCs, the use of fetal stem cells for research and therapeutic purposes carry many ethical questions. For example, fetal stem cells arise from the fusion of sperm and oocyte, and they are frequently obtained from terminated pregnancies or after in vitro fertilization, therefore consent cannot be reliably obtained for these fetuses.
Other major drawbacks to fetal stem cell therapy are the technical challenges of their implementation. Allogeneic stem cell transplants using HSCs are most successful when the cells are harvested immediately prior to transplant since they do not maintain viability well during cryopreservation8. However, fetal stem cells from umbilical cord blood cells are cryopreserved in banks for storage and later use. Thus, it has been suggested that there is limited utility in having a mother’s cord blood stored in a bank for future use. Research into the optimal methods of cryopreservation is ongoing and thus far indicates that program freezing confers the best cryoprotection34. The limited number of cells that can be isolated from the umbilical cord blood poses another technical challenge26. The umbilical cord matrix has been proposed to harbor MSCs with the better expansion potential than umbilical cord blood26. The stem cell field should seek to optimize procedures for the isolation and expansion of these potentially valuable cells.
Despite these challenges, fetal stem cells are the most clinically-advanced stem cell population after BM HSCs. Since umbilical cord blood was first used as a stem cell source for allogeneic transplant in 1988, more than 5000 patients have received cord blood transplants35. This number has the potential to grow, as more than 90,000 units of cord blood are currently being stored in international registries36.
Adult stem cells
Like BM that contains multipotent HSCs capable of replenishing mature blood cells, most adult tissues contain multipotent stem cells that can give rise to organ or tissue specific lineages, including the brain, skin, muscle and intestine1. In addition to HSCs, he BM is also a residence for mesenchymal stem cells. MSCs were first described in 1974 as mutipotential stromal precursor cells isolated from the BM37. MSCs can be readily isolated from adult tissue, in addition to fetal tissue and umbilical cord blood, making MSCs one of the most available stem cell populations for research and transplantation. In the adult, MSCs have also been identified outside of the BM in adipose tissue and in dental pulp38, 39. Like MSCs derived from fetal tissue, adult MSCs are multipotent cells that readily differentiate into cells of mesodermal origin such as osteoblasts, chondrocytes and adipocytes37, 40. MSCs also have transdifferentiation potential, and researchers have even been able to induce differentiation into cells from other embryonic lineages, including neurons41, astrocytes42 and hepatocyte-like cells43 (Figure 1). Due to lack of a single marker to define a MSC and variation in MSCs derived from different sources, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy has adopted a set of criteria regarding marker expression, differentiation potential and in vitro culture capacity to more clearly define this population of cells44. Robust protocols exist for the isolation of MSC populations from bone marrow aspirates and adipose tissue, however, the low MSC yields from BM requires in vitro expansion for therapeutic use45. Caution must be exercised in expanding MSCs in culture, however, as significant differences in gene expression have been observed between early and late passage MSC cultures46.
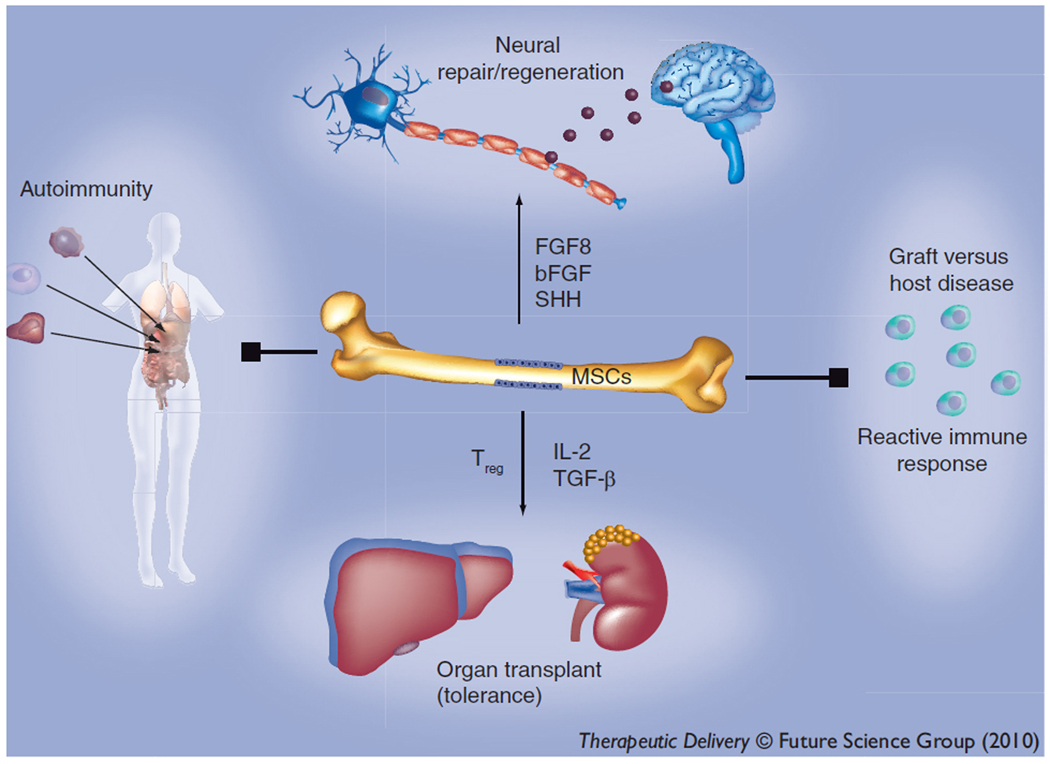
Figure 1.
Diverse applications of mesenchymal stem cells (MSCs). MSCs can be transdifferentiated into functional neurons, with therapeutic prospects for degenerative states of the nervous system. They have been shown to suppress autoimmunity and promote graft tolerance through the induction of Tregs. Their modulatory effects on graft-versus-host disease are currently being explores in clinical trials.
As has been described for HSCs, differences also exist in gene expression between MSCs of different sources. The pluripotency gene Oct-4 has been found to be expressed at lower levels in MSCs derived from adult tissue than from umbilical cord blood and amniotic tissue, suggesting differences in differentiation potential of cells derived from different sources47. However, the cytokine profile of MSCs derived from different origins is largely the same, varying instead by cellular morphology, suggesting that cell culture conditions may influence the immunomodulatory effects of MSCs more that the MSC source48. Genetic and epigenetic abnormalities have been observed in cultured MSCs, including polyploidy and upregulation of microRNAs, suggesting a possible transformation risk of in vitro expansion of MSCs prior to transplantation49, 50.
Also like HSCs, donor age may introduce an additional source of variability in harvested MSCs. Stolzing et al. argue a reduction in stem cell numbers as well as lower “fitness” with age due to increased p53, p21 and reactive oxygen species (ROS) levels in MSCs obtained from the BM of adults compared to children51. Elderly individuals or individuals with systemic disease who are most likely to benefit from stem cell transplants may have reduced or defective MSCs in the BM, making autologous MSC transplant undesirable or challenging52, 53. This problem demonstrates the need for developing allogeneic MSC transplant technologies and highlights the need for ‘off-the-shelf’ cell therapy.
One of the best arguments for the use of MSCs for transplant is their weak immunogenicity. This feature makes MSC populations ideal cells for use as ‘off-the-shelf’ stem cell therapeutics, especially for acute administration in which allogeneic cells may be the only option. Both animal and human studies have demonstrated the broad tolerance for non-HLA matched MSC transfusions54, 55. This feature of MSCs can be explained in part by their cytokine production. The repertoire of cytokines secreted by MSCs includes macrophage colony stimulating factor (M-CSF), IL-6, IL-8, IL-12, hepatocyte growth factor, tumor necrosis factor-alpha (TNF-α) and the immunosuppressive cytokines IL-10 and TGF-β56, 57. Furthermore, MHC-II is decreased when the MSCs differentiate into specialized cells, such as neurons58. Due to these characteristics, MSCs have been extensively explored for applications in regenerative medicine.
Application of mesenchymal stem cells transplant
MSCs are an ideal stem cell source for ‘off-the-shelf’ stem cell therapy because of their low immunogenicity, ease of availability and their unique biological properties that include a broad differentiation potential and immunomodulatory effects. Because of their role in contributing to the supportive stroma of the BM stem cell niche for HSCs, early attempts at MSC therapy were intended to enhance engraftment of HSCT59. MSC transplants have subsequently been used for the replacement of damaged or congenitally defective tissue, and also as adjuvant therapy owing to their immunomodulatory and specific homing properties.
Tissue Replacement
As HSCT has been applied to replace the hematopoietic system of patients with inborn blood disorders and immunodeficiencies, MSCs have been used to replace inborn genetic defects in cells of mesenchymal origin. Culture-expanded MSCs infused into irradiated mice were found to account for 1.5–12% of cells in bone and cartilage months after transplant60. More than ten years ago, three children with the inheritable bone disease osteogenesis inperfecta (OI) were transplanted intravenously with HLA-matched unmanipulated bone marrow. Three months after the BM transplant, 1.5–2% of osteoblasts cultured from bone marrow biopsy were identified as donor-derived, demonstrating MSC engraftment. This engraftment of cells without the type I collagen defect encoded by the OI gene conferred measurable benefit to the three transplant patients61. Engraftment of purified BM-derived MSCs conferred similar benefit, demonstrating the MSC population as the engrafting cells, which were identified as residing in bone, skin, and marrow stroma62. Autologous transplant can also accomplish a similar goal through the use of ex vivo gene therapy. For example, a patient’s own MSCs can be harvested and genetically engineered, then re-implanted to restore inherited defects28. Such methods have been successfully employed to introduce the wild-type collagen gene into MSCs harvested from an OI patient63.
Another major field of investigation regarding the use of stem cells for regenerative purposes after injury with some preclinical success is cardiac medicine. In 2001, labeled lin- c-kit+ bone marrow injected into the infarcted heart muscle of mice following coronary ligation were found to compose the majority of the regenerating myocardium64. Fetal MSCs have also been shown to have therapeutic potential in the treatment of infarcted myocardium. Umbilical cord blood-derived MSCs have been shown to acquire phenotypic and functional properties of cardiac cells when co-cultured with cardiac myocytes in vitro65. These cells expressed cardiac troponin-I and connexin 43 and demonstrate electrical and contractile properties resembling true myocardial cells65. Amniotic fluid MSCs can also demonstrate cardiac differentiation66 and umbilical cord blood harbors CD133(+) progenitors which have been used to generation of heart valves67.
Immunomodulatory effects
In addition for use in tissue replacement, MSCs may have utility as adjuvant therapies due to their immunomodulatory effects. In recent years the anti-inflammatory effects of MSCs on both the innate and adaptive immune systems have been demonstrated to be broad. MSCs are potent inhibitors of T-cell activation and proliferation54, 68, dendritic cell maturation69, 70, and proliferation and cytotoxic activity of NK cells71. MSCs have also been recently demonstrated to stimulate proliferation of Regulatory T Cells72. MSC effects on B-cells, however, are less clear and in vitro experiments have demonstrated both activating and inhibitory effects73, 74.
Due to these potent and diverse immunomodulatory effects, the most advanced clinical use of MSCs is to minimize the effects of GvHD upon HSCT. In 2004 haploidentical MSCs were transplanted into one patient with severe acute GvHD and provided striking remission of symptoms55. However, the recently failed clinical trial by Orsiris Therapeutics demonstrated that the basic biology of MSCs and their interactions with their target microenvironment must be better understood before these cells can be widely used in the clinic.
MSC transplant for immune modulation have been attempted experimentally to influence other injury responses as well. In an experiment in which rats with ischemia-reperfusion-induced acute renal failure were infused with MSCs, the stem cells protected renal cells against apoptosis and improved renal function without significant engraftment or differentiation into glomerular cells, suggesting instead a beneficial immunomodulatory effect75. Similarly, MSCs were found to improve engraftment of islet cells in diabetes rat model76. The paracrine immunomodulatory effects of transplanted MSCs has also been demonstrated to have anti-cancer effects. In a mouse hepatoma xenograft tumor model, MSCs have been demonstrated to reduce cancer cell proliferation and invasion77.
MSCs as tumor homing cells
Aside from regenerative and immunomodulatory applications, MSCs have also been shown to serve as an effective vehicle for the delivery of beneficial gene products, especially to tumor. MSCs have been shown to preferentially engraft at sites of injury and tumor78. MSCs when cultured in hypoxic conditions proliferate 30-fold faster than in normoxic conditions, providing a possible advantage in treating tumors which are often hypoxic79. Furthermore, MSC proliferation has been demonstrated to be increased by coculture with glioma cells in the absence of hypoxia80. BM-derived MSCs have been shown to efficiently home to multiple tumor types in xenograft models, including glioma, melanoma, breast cancer and colon cancer81–84. MSC migration is enhanced by many cytokines and secreted growth factors, including IL-1, IL-8, TNFα, TGFβ, EGF, PDGF and SDF-1, factors commonly secreted in injury and by tumors81. These properties of MSCs could potentially be harnessed to employ these cells as anti-cancer delivery vehicles. For example, multiple research groups have successfully loaded MSCs with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by viral transduction methods and found that transduced MSCs home to tumor and reduce tumor burden in multiple zenograft mouse tumor models85–87. Adenoviral introduction of IFN-β into MSCs has also been demonstrated to have similar benefit in a melanoma xenograft mouse model83. Currently, the use of viral transduction for MSC-mediated gene delivery is not universally supported due to risk of oncogenic transformation of the transplanted MSCs, but these favorable in vivo findings merit further investigations of this modality of therapy.
Hurdles to advancing stem cell regenerative therapies
Since the first hematopoietic stem cell transplant more than fifty years ago, the potential of stem cells for regenerative medicine has been investigated with only limited translation to the clinic. The reasons for the lack of successes are multifactorial and future progress is plagued by many uncertainties in this still nascent field. The use of stem cells for tissue regeneration and disease modulation carries concerns about appropriate tissue homing and rejection/engraftment. Once cells have been targeted to the correct location, there is uncertainty that they will differentiate appropriately into functional tissue or maintain their immunomodulatory characteristics. Perhaps the most concerning uncertainty is the risk of initiating a tumor from the transplanted stem cells.
One of the major reasons for success of HSCT is that once infused, hematopoietic stem cells migrate to the BM where they occupy the native stem cell niche and function like native HSCs in replenishing blood cells. The question of how well other stem cell populations will home to and engraft at their native stem cell niches, and continue to self-renew and differentiate appropriately remains unanswered. While there is preclinical and clinical evidence for successful engraftment of transplanted MSCs60, 61, the efficiency and duration of engraftment remains unknown. Furthermore, as MSCs are discussed for use as ‘off-the-shelf’ therapeutics, the effects of in vitro culture and storage must also be rigorously assessed. Cell culture conditions and culture time have been demonstrated to influence MSC tropism once transplanted in experimental settings. For example, human MSCs expanded in adherent culture conditions home much less efficiently to the BM and sites of injury due to loss of CXCR4 expression, whereas culture of MSCs as spheroids retains CXCR4 expression and homing ability48.
Use of non-autologous cells for transplant also requires that one consider the possibility of graft rejection. While MSCs are a desirable stem cell source for ‘off-the-shelf’ therapy because of their weak immunogenicity, in vitro studies demonstrate re-expression of MHC-II on the MSC-derived neurons in the presence of low levels of IFNγ58. This finding is highly significant, as future therapies with MSCs need to address the possibility that there could be eventual rejection of the implanted cells by the host immune system. This re-expression of MHC-II could occur at times long after implantation. If MHC-II is re-expressed, future therapies will need to consider methods that induce tolerance to the implanted cells.
There is also uncertainty in the outcome of removing multipotent stem cells with self-renewal capacity from their native niche and transplanting the cells into a patient where they will be exposed to varied cellular and tissue microenvironments. Will they differentiate appropriately into the desired specialized tissue? Will MSCs maintain the immunomodulatory characteristics that make them attractive cells for treating GvHD or cancer? Most concerning, is there potential for these transplanted stem cells to become transformed themselves and initiate a cancer?
MSC infusion for purposes of tissue replacement has achieved mixed results. While donor MSCs transplanted into osteogenesis imperfecta patients were found in the bone marrow and as appropriately differentiated cells in bone and skin62, not all transplants for purposes of tissue replacement have achieved similar success. Autologous MSCs administered to sheep subjected to bilateral renal ischemia were found to engraft in the kidney, however, they did not differentiate into glomerular cells nor did they provide any therapeutic benefit to renal function88. MSCs mobilized from the BM with GMSCF following myocardial damage in mice homed to the heart but did not differentiate into cardiac myocytes and did not repair the damaged heart89.
Owing to their self-renewal capacity and the similarities between stem cells and cancer cells, there is reasonable concern about initiating a tumor in a patient receiving a stem cell transplant. The tumor risk of transplanting ES cells is well known12. The risk of malignant transformation of transplanted fetal stem cells was tragically demonstrated in a recent intrathecal administration of fetal neural stem cells to a boy with the ataxia-telangiectasia, a degenerative condition of the central nervous system90. The patient developed a glial tumor derived from multiple origins, including the donor fetal neural stem cells90. From these studies it is clear that some stem cell populations pose a cancer risk, however, HSCT has been employed successfully for 50 years without conferring malignancy, demonstrating that not all stem cells pose cancer risk.
The risk of initiating a tumor by MSC transplant has been much discussed in the field. It has been suggested that human MSCs could not spontaneously transform in culture whereas mouse MSCs could, suggesting a limitation on what could be inferred about human cells from studies with mouse cells46. Similar studies, however, have demonstrated that postsenescent human MSC frequently do become transformed91. The tissue source of MSCs for transplantation may also influence transformation potential. One recent study demonstrated that adipose tissue-derived MSCs transform more readily than BM-derived cells92, however other groups have found that even BM-derived MSCs possess a significant transformation risk93.
The risk of tumor formation from transplanting MSCs into patients remains undetermined, and further studies need to be performed to clarify this risk and determine how the potential for transformation is influenced by the stem cell tissue source and in vitro culture conditions. This is particularly important if MSC populations are going to be expanded significantly for ‘off-the-shelf’ uses, or maintained in culture or transduced for gene delivery. Furthermore, rigorous methods to identify transformed cells prior to transplantation should be adopted. Several studies of the transformation process in MSCs have identified morphological changes, transcriptional changes, as well as changes in cell surface expression of MSC markers such as CD34, CD90, CD105 and VEGF receptor upon transformation91, 94–96.
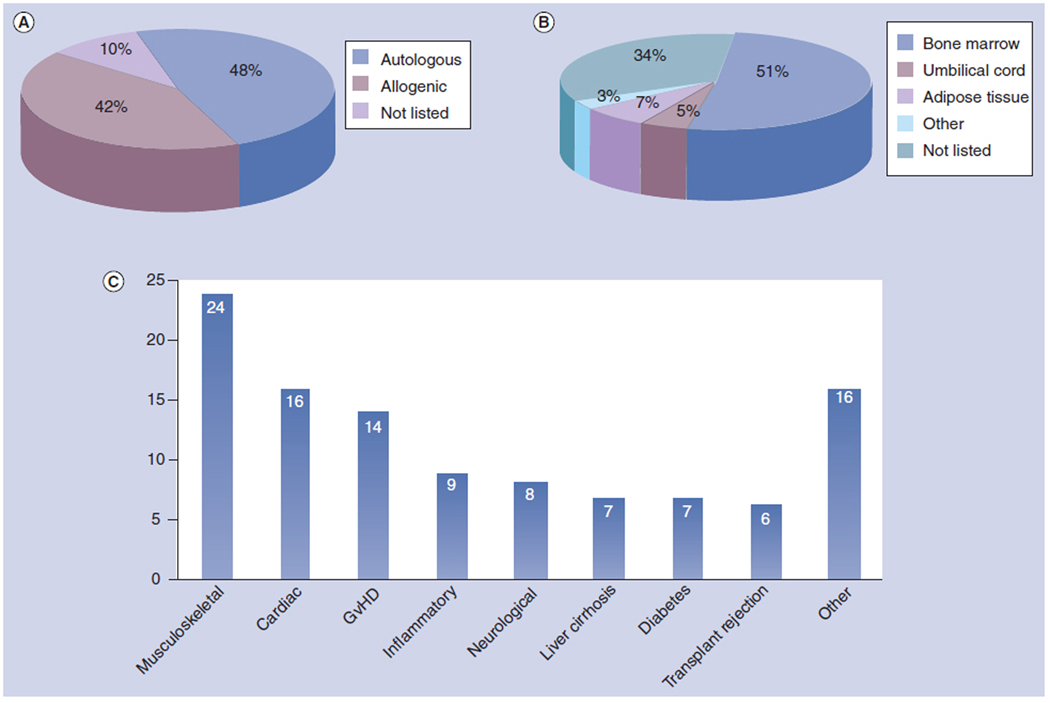
As of this writing, 105 clinical trials involving MSC transplantation have been registered with the US Food and Drug Administration (Table 1; www.clinicaltrials.gov). While most of the studies are currently ongoing or are small Phase I and Phase II safety trials, current findings suggest that MSC transplants are safe and offer no suggestion of malignancy risk. Human trials of MSC transplantation are roughly evenly divided between the use of autologous and allogeneic cells (Figure 1a) and these trials employ both freshly-isolated and ex-vivo culture expanded cell populations. While most MSCs used for transplant are derived from BM, cells isolated from adipose, umbilical cord, and other MSC sources such as peripheral blood liver are being employed (Figure 2b). These clinical trials employ MSCs for a multitude of different purposes in different disease states, including tissue replacement in musculoskeletal, cardiac and liver diseases, and as immunomodulatory cells to mitigate GvHD, organ transplant rejection and autoimmune disorders (Figure 2c).
Figure 2.
Summary of human clinical trials with mesenchymal stem cells (MSCs). MSCs have been applied to humans in 105 clinical trails registered with the FDA. (a) 48% of trails involving MSC transplants have been performed with autologous cells and 42% of transplants have employed allogeneic cells. (b) The MSCs used for transplant have primarily been isolated from BM (51%) and also from adipose tissue (7%), umbilical cord blood (5%) as well as other sources such as liver and peripheral blood (3%). (c) MSC transplants have been tested to treat a wide variety of diseases, including musculoskeletal disorders (24 trials), cardiac disease (16), GvHD (14), inflammatory diseases such as Crohn’s disease (9), neurological disease (8), cirrhosis and liver disease (7), type I and type II diabetes (7), organ transplant rejection (6), as well as other conditions (16).
Overcoming the hurdles
While the stem cell transplantation field awaits the results of the many ongoing clinical trials, scientists are working to devise strategies to better control the outcome of introducing multipotent stem cells into varied cellular environments. Different methods have been employed to influence these factors, including predifferentiating stem cells in vitro, introducing cells directly at the site of action or with synthetic scaffolds, and manipulating the cells genetically prior to transplant. Predifferentiation strategies have been employed with preclinical success to MSCs. MSCs that are cultured into chondrocytes prior to transplantation enhances engraftment for cartilage repair97. Injecting MSCs along with a physical scaffold may also enhance engraftment rate and direct differentiation programs more successfully. Seeding of BM-derived MSCs in 3D collagen gel matrix induced BMP-2 expression and osteogenic differentiation98. Flexible bone implants of poly(lactide-co-glycolide) (PLGA)/amorphous tricalcium phosphate (ATCP) nanocomposite also permitted osteogenic differentiation of MSCs99.
The route of delivery of MSCs is another factor that may significantly influence engraftment and therapeutic benefit. While MSCs often migrate to sites of injuries or tumors after peripheral infusion, direct injection at the desired site of action may provide enhanced targeting and engraftment of these cells. Paraspinally-injected MSCs transduced with BMP-9 were found to engraft with vertebrae at the site of injection without nerve compression or demonstrating other toxicity52. In adult dogs in heart block due to experimental radioablation of the atrioventricular node, MSCs transduced with the cardiac pacemaker gene HCN2 and transplanted into the left ventricular wall engrafted successfully at the site of injection and conferred biological pacemaker activity100.
While preclinical studies manipulating the differentiation state and delivery method of transplanted cells may lead to more efficacious stem cell therapies, the long-term outcomes of these manipulations must be thoroughly evaluated. Answers to these are questions are required for efficient and safe stem cell treatments. Since there is a limit to the type of studies that can be done in human, robust analyses are required by in vitro and in vivo systems. Since both systems have significant drawbacks, the limitations will need to be considered when establishing clinical trials. A major limitation of the in vitro system is its failure to recapitulate an in vivo microenvironment. The in vivo models use different species of animal, all of which show some difference from human.
Future Perspective
Scientists and clinicians throughout the world are investigating ways to mitigate the challenges and risks of stem cell therapy. As discussed above, MSCs are already in human trials and to date, these stem cells appear to be safe with regards to tumor formation55, 101. Once the important safety concerns regarding malignant transformation of stem cells have been addressed, the major challenges will then be centered around homing the transplanted cells to the desired location and directing the cells to function appropriately in that location. Future work will be required better understand the host-stem cell interactions and how placing stem cells at any site of tissue injury might not provide the expected outcome. It is paramount to consider the tissue microenvironment and pro-inflammatory and anti-inflammatory cytokines and other mediators that could establish a crosstalk with the implanted stem cells or their differentiated cells (Figure 1). This interaction could be different between different stem cell populations and undifferentiated stem cells versus specialized cells. Furthermore, if the stem cells are dispersed within the site of tissue injury, there will be lack of synchrony with regards to the developmental stage of the stem cells, and also the types of receptors on each cells. Therefore crosstalk between the cells and mediators within the microenvironment of tissue damage would vary within a particular region of tissue damage. Despite these challenges, the great success of HSCT offers hope and instruction in progressing other stem cell populations into the clinic for regenerative medicine.
Acknowledgements
K.Y.H. is a Howard Hughes Medical Institute Medical Research Training Fellow. The authors acknowledge the support of the FM Kirby Foundation.
Key Terms
- Autologous Transplant
Transplant of cells or tissue into the same individual from which the transplant was harvested. Isologous transplant involves the transplant from a separate, but genetically identical individual, such as an identical twin
- Allogeneic Transplant
Transplant of cells or tissue into a different individual of the same species from which the transplant was harvested
- Graft versus Host Disease (GvHD)
A common side effect of transplanting allogeneic immune cells (from blood transfusion or bone marrow transplant) in which the donor immune cells recognize the host tissue as foreign and mount an immune attack
- Embryonic Stem Cell (ESC)
ESCs are pluripotent cells derived from the inner cell mass of an early blastocyst. They retain the ability to form all three embryonic germ layers, as well as the trophoblast layer
- Adult Tissue Stem Cell
Adult tissue stem cells multipotent stem cells that can give rise to organ or tissue specific lineages. They are found in most if not all adult tissues, including the brain, skin, muscle and intestine
- Mesenchymal Stem Cell (MSC)
MSCs are adult tissue stem cells found in multiple locations in the adult, including bone marrow, adipose tissue and dental pulp. MSCs are multipotent cells that readily differentiate into cells of mesodermal origin, such as osteoblasts, chondrocytes and adipocytes, but also can transdifferentiate into cells from other embryonic lineages
Footnotes
This work was done at UMDNJ-New Jersey Medical School, Department of Medicine, Newark, NJ 07103.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
REFERENCES
- 1.Alison MR, Islam S. Attributes of adult stem cells. J Pathol. 2009;217:144–160. doi: 10.1002/path.2498. [DOI] [PubMed] [Google Scholar]
- 2. Thomas ED, Lochte HL, Jr, Cannon JH, Sahler OD, Ferrebee JW. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. doi: 10.1172/JCI103949.This seminal paper describes the first successful bone marrow transplant between genetically identical individuals.
- 3. O'Reilly RJ, et al. Reconstitution in severe combined immunodeficiency by transplantation of marrow from an unrelated donor. N Engl J Med. 1977;297:1311–1318. doi: 10.1056/NEJM197712152972403.This report describes the first repair of congenital immune defficiency in a patient with severe combined immunodeficiency by allogeneic bone marrow transplant.
- 4.McCullough J, Perkins HA, Hansen J. The National Marrow Donor Program with emphasis on the early years. Transfusion. 2006;46:1248–1255. doi: 10.1111/j.1537-2995.2006.00842.x. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Sun K, Welniak LA, Murphy WJ. Immunomodulation and pharmacological strategies in the treatment of graft-versus-host disease. Expert Opin Pharmacother. 2008;9:2305–2316. doi: 10.1517/14656566.9.13.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 7.Molineux G, Pojda Z, Hampson IN, Lord BI, Dexter TM. Transplantation potential of peripheral blood stem cells induced by granulocyte colony-stimulating factor. Blood. 1990;76:2153–2158. [PubMed] [Google Scholar]
- 8.Beshlawy AE, Metwally HG, Khalek KA, Hammoud RF, Mousa SM. The effect of freezing on the recovery and expansion of umbilical cord blood hematopoietic stem cells. Exp Clin Transplant. 2009;7:50–55. [PubMed] [Google Scholar]
- 9. Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Nat Rev Cancer. 10:213–221. doi: 10.1038/nrc2804.This is an excellent Timeline article reviewing the history of HSCT as well as perspectives on future advances of the technology to treat cancer.
- 10.Bensinger WI, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–181. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 11.Cutler C, et al. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685–3691. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 12. Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145.This important work describes the generation of embryonic stem cell lines from blastocycts.
- 13.Gerrard L, Zhao D, Clark AJ, Cui W. Stably transfected human embryonic stem cell clones express OCT4-specific green fluorescent protein and maintain self-renewal and pluripotency. Stem Cells. 2005;23:124–133. doi: 10.1634/stemcells.2004-0102. [DOI] [PubMed] [Google Scholar]
- 14.Bonner-Weir S. Stem cells in diabetes: what has been achieved. Horm Res. 2003;60 Suppl 3:10. doi: 10.1159/000074493. [DOI] [PubMed] [Google Scholar]
- 15.Schwanke K, et al. Generation and characterization of functional cardiomyocytes from rhesus monkey embryonic stem cells. Stem Cells. 2006;24:1423–1432. doi: 10.1634/stemcells.2005-0380. [DOI] [PubMed] [Google Scholar]
- 16.zur Nieden NI, Kempka G, Ahr HJ. In vitro differentiation of embryonic stem cells into mineralized osteoblasts. Differentiation. 2003;71:18–27. doi: 10.1046/j.1432-0436.2003.700602.x. [DOI] [PubMed] [Google Scholar]
- 17.Andrews PW, et al. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochem Soc Trans. 2005;33:1526–1530. doi: 10.1042/BST0331526. [DOI] [PubMed] [Google Scholar]
- 18.Keirstead HS, et al. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci. 2005;25:4694–4705. doi: 10.1523/JNEUROSCI.0311-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Druml C. Stem cell research: toward greater unity in Europe? Cell. 2009;139:649–651. doi: 10.1016/j.cell.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 20.Dhar D, Hsi-En Ho J. Stem cell research policies around the world. Yale J Biol Med. 2009;82:113–115. [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 22.Yu J, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 23.Kang L, Kou Z, Zhang Y, Gao S. Induced pluripotent stem cells (iPSCs)-a new era of reprogramming. J Genet Genomics. 37:415–421. doi: 10.1016/S1673-8527(09)60060-6. [DOI] [PubMed] [Google Scholar]
- 24.Reinisch A, Strunk D. Isolation and animal serum free expansion of human umbilical cord derived mesenchymal stromal cells (MSCs) and endothelial colony forming progenitor cells (ECFCs) J Vis Exp. 2009 doi: 10.3791/1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jager M, Zilkens C, Bittersohl B, Krauspe R. Cord blood--an alternative source for bone regeneration. Stem Cell Rev. 2009;5:266–277. doi: 10.1007/s12015-009-9083-z. [DOI] [PubMed] [Google Scholar]
- 26.Zeddou M, et al. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol Int. 34:693–701. doi: 10.1042/CBI20090414. [DOI] [PubMed] [Google Scholar]
- 27.Magatti M, et al. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells. 2008;26:182–192. doi: 10.1634/stemcells.2007-0491. [DOI] [PubMed] [Google Scholar]
- 28.Chong MS, Chan J. Lentiviral vector transduction of fetal mesenchymal stem cells. Methods Mol Biol. 614:135–147. doi: 10.1007/978-1-60761-533-0_9. [DOI] [PubMed] [Google Scholar]
- 29.Rameshwar P. Casting doubt on the safety of "off-the-shelf" mesenchymal stem cells for cell therapy. Mol Ther. 2009;17:216–218. doi: 10.1038/mt.2008.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eapen M, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 31.Reimann V, Creutzig U, Kogler G. Stem cells derived from cord blood in transplantation and regenerative medicine. Dtsch Arztebl Int. 2009;106:831–836. doi: 10.3238/arztebl.2009.0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotherstrom C, et al. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239–245. doi: 10.1016/j.ajog.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 33.Neri M, et al. Robust generation of oligodendrocyte progenitors from human neural stem cells and engraftment in experimental demyelination models in mice. PLoS One. 5:e10145. doi: 10.1371/journal.pone.0010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todorov P, Hristova E, Konakchieva R, Michova A, Dimitrov J. Comparative studies of different cryopreservation methods for mesenchymal stem cells derived from human fetal liver. Cell Biol Int. 34:455–462. doi: 10.1042/CBI20090127. [DOI] [PubMed] [Google Scholar]
- 35.Barker JN, Rocha V, Scaradavou A. Optimizing unrelated donor cord blood transplantation. Biol Blood Marrow Transplant. 2009;15:154–161. doi: 10.1016/j.bbmt.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Karanes C, et al. Twenty years of unrelated donor hematopoietic cell transplantation for adult recipients facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14:8–15. doi: 10.1016/j.bbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 39.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pittenger MF, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143.In this important report, mesenchymal stem cells were identified as multipotent cells residing in adult bone marrow that differentiate into cells of mesodermal lineage.
- 41.Cho KJ, et al. Neurons derived from human mesenchymal stem cells show synaptic transmission and can be induced to produce the neurotransmitter substance P by interleukin-1 alpha. Stem Cells. 2005;23:383–391. doi: 10.1634/stemcells.2004-0251. [DOI] [PubMed] [Google Scholar]
- 42.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen BE, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 44.Dominici M, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 45.Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 46.Lepperdinger G, Brunauer R, Jamnig A, Laschober G, Kassem M. Controversial issue: is it safe to employ mesenchymal stem cells in cell-based therapies? Exp Gerontol. 2008;43:1018–1023. doi: 10.1016/j.exger.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Alviano F, et al. Term Amniotic membrane is a high throughput source for multipotent Mesenchymal Stem Cells with the ability to differentiate into endothelial cells in vitro. BMC Dev Biol. 2007;7:11. doi: 10.1186/1471-213X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potapova IA, Brink PR, Cohen IS, Doronin SV. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izadpanah R, et al. Long-term in vitro expansion alters the biology of adult mesenchymal stem cells. Cancer Res. 2008;68:4229–4238. doi: 10.1158/0008-5472.CAN-07-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner W, et al. Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS One. 2008;3:e2213. doi: 10.1371/journal.pone.0002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stolzing A, Jones E, McGonagle D, Scutt A. Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Dumont RJ, et al. Ex vivo bone morphogenetic protein-9 gene therapy using human mesenchymal stem cells induces spinal fusion in rodents. Neurosurgery. 2002;51:1239–1244. doi: 10.1097/00006123-200211000-00020. discussion 1244–5. [DOI] [PubMed] [Google Scholar]
- 53.El-Badri NS, Hakki A, Ferrari A, Shamekh R, Good RA. Autoimmune disease: is it a disorder of the microenvironment? Immunol Res. 2008;41:79–86. doi: 10.1007/s12026-007-0053-8. [DOI] [PubMed] [Google Scholar]
- 54.Bartholomew A, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 55. Le Blanc K, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7.Description of the striking benefit of mesenchymal stem cell therapy to mitigate the effects of GvHD, first demonstrating the immunomodulatory effects of MSCs in vivo.
- 56.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 57.Rossignol J, et al. Mesenchymal stem cells induce a weak immune response in the rat striatum after allo or xenotransplantation. J Cell Mol Med. 2009;13:2547–2558. doi: 10.1111/j.1582-4934.2008.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castillo MD, Trzaska KA, Greco SJ, Ponzio NM, Rameshwar P. Immunostimulatory effects of mesenchymal stem cell-derived neurons: implications for stem cell therapy in allogeneic transplantations. Clin Transl Sci. 2008;1:27–34. doi: 10.1111/j.1752-8062.2008.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Almeida-Porada G, Porada CD, Tran N, Zanjani ED. Cotransplantation of human stromal cell progenitors into preimmune fetal sheep results in early appearance of human donor cells in circulation and boosts cell levels in bone marrow at later time points after transplantation. Blood. 2000;95:3620–3627. [PubMed] [Google Scholar]
- 60.Pereira RF, et al. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529.This study describes the first successful transplant of mesenchymal stem cells for tissue regeneration in humans to treat the genetic bone disorder osteogenesis imperfecta.
- 62.Horwitz EM, et al. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci U S A. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pochampally RR, Horwitz EM, DiGirolamo CM, Stokes DS, Prockop DJ. Correction of a mineralization defect by overexpression of a wild-type cDNA for COL1A1 in marrow stromal cells (MSCs) from a patient with osteogenesis imperfecta: a strategy for rescuing mutations that produce dominant-negative protein defects. Gene Ther. 2005;12:1119–1125. doi: 10.1038/sj.gt.3302514. [DOI] [PubMed] [Google Scholar]
- 64.Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 65.Nishiyama N, et al. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–2024. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 66.Walther G, Gekas J, Bertrand OF. Amniotic stem cells for cellular cardiomyoplasty: promises and premises. Catheter Cardiovasc Interv. 2009;73:917–924. doi: 10.1002/ccd.22016. [DOI] [PubMed] [Google Scholar]
- 67.Sodian R, et al. Use of human umbilical cord blood-derived progenitor cells for tissue-engineered heart valves. Ann Thorac Surg. 89:819–828. doi: 10.1016/j.athoracsur.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 68.Di Nicola M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 69.Jiang XX, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–4126. doi: 10.1182/blood-2004-02-0586. [DOI] [PubMed] [Google Scholar]
- 70.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080–2087. doi: 10.4049/jimmunol.177.4.2080. [DOI] [PubMed] [Google Scholar]
- 71.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006;107:1484–1490. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]
- 72.Selmani Z, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 73.Corcione A, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 74.Rasmusson I, Le Blanc K, Sundberg B, Ringden O. Mesenchymal stem cells stimulate antibody secretion in human B cells. Scand J Immunol. 2007;65:336–343. doi: 10.1111/j.1365-3083.2007.01905.x. [DOI] [PubMed] [Google Scholar]
- 75.Togel F, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 76.Figliuzzi M, et al. Bone marrow-derived mesenchymal stem cells improve islet graft function in diabetic rats. Transplant Proc. 2009;41:1797–1800. doi: 10.1016/j.transproceed.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 77.Qiao L, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–507. doi: 10.1038/cr.2008.40. [DOI] [PubMed] [Google Scholar]
- 78.Kidd S, et al. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27:2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochem Biophys Res Commun. 2007;358:948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 80.Birnbaum T, et al. Malignant gliomas actively recruit bone marrow stromal cells by secreting angiogenic cytokines. J Neurooncol. 2007;83:241–247. doi: 10.1007/s11060-007-9332-4. [DOI] [PubMed] [Google Scholar]
- 81.Nakamizo A, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 82.Studeny M, et al. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 83.Studeny M, et al. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 84.Xin H, et al. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 85.Kim SM, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614–9623. doi: 10.1158/0008-5472.CAN-08-0451. [DOI] [PubMed] [Google Scholar]
- 86.Loebinger MR, Eddaoudi A, Davies D, Janes SM. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69:4134–4142. doi: 10.1158/0008-5472.CAN-08-4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grisendi G, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 88.Behr L, et al. Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif. 2009;42:284–297. doi: 10.1111/j.1365-2184.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tatsumi K, et al. Granulocyte-colony stimulating factor increases donor mesenchymal stem cells in bone marrow and their mobilization into peripheral circulation but does not repair dystrophic heart after bone marrow transplantation. Circ J. 2008;72:1351–1358. doi: 10.1253/circj.72.1351. [DOI] [PubMed] [Google Scholar]
- 90.Amariglio N, et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009;6:e1000029. doi: 10.1371/journal.pmed.1000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rubio D, et al. Spontaneous human adult stem cell transformation. Cancer Res. 2005;65:3035–3039. doi: 10.1158/0008-5472.CAN-04-4194. [DOI] [PubMed] [Google Scholar]
- 92.Bernardo ME, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149. doi: 10.1158/0008-5472.CAN-06-4690. [DOI] [PubMed] [Google Scholar]
- 93.Sawada R, Ito T, Tsuchiya T. Changes in expression of genes related to cell proliferation in human mesenchymal stem cells during in vitro culture in comparison with cancer cells. J Artif Organs. 2006;9:179–184. doi: 10.1007/s10047-006-0338-z. [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, et al. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7:509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 95.Rubio D, et al. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS One. 2008;3:e1398. doi: 10.1371/journal.pone.0001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rubio D, et al. Human mesenchymal stem cell transformation is associated with a mesenchymal-epithelial transition. Exp Cell Res. 2008;314:691–698. doi: 10.1016/j.yexcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 97.Moioli EK, Hong L, Guardado J, Clark PA, Mao JJ. Sustained release of TGFbeta3 from PLGA microspheres and its effect on early osteogenic differentiation of human mesenchymal stem cells. Tissue Eng. 2006;12:537–546. doi: 10.1089/ten.2006.12.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sumanasinghe RD, Osborne JA, Loboa EG. Mesenchymal stem cell-seeded collagen matrices for bone repair: effects of cyclic tensile strain, cell density, and media conditions on matrix contraction in vitro. J Biomed Mater Res A. 2009;88:778–786. doi: 10.1002/jbm.a.31913. [DOI] [PubMed] [Google Scholar]
- 99.Schneider OD, et al. Cotton wool-like nanocomposite biomaterials prepared by electrospinning: in vitro bioactivity and osteogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res B Appl Biomater. 2008;84:350–362. doi: 10.1002/jbm.b.30878. [DOI] [PubMed] [Google Scholar]
- 100.Plotnikov AN, et al. Xenografted adult human mesenchymal stem cells provide a platform for sustained biological pacemaker function in canine heart. Circulation. 2007;116:706–713. doi: 10.1161/CIRCULATIONAHA.107.703231. [DOI] [PubMed] [Google Scholar]
- 101.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20:531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]