Neuromodulation appears to be emerging as a new therapeutic field in psychiatric treatment. For decades, electroconvulsive therapy (ECT) was the lone device-based intervention routinely used in clinical psychiatric practice in the United States. However, in 2005, vagus nerve stimulation (VNS) was granted Food and Drug Administration approval for the treatment of severe, recurrent unipolar and bipolar depression. Other device-based interventions are currently being studied in an effort to expand the therapeutic options for treating a range of psychiatric conditions. Transcranial magnetic stimulation (TMS) is a medical device that may also ultimately be added to the therapeutic armentarium of neuropsychiatric devices. Over the past decade, a substantial body of literature supports antidepressant effects of TMS. Additionally, there are studies that indicate potential efficacy of TMS in treating a number of other psychiatric and nonpsychiatric disorders, such as posttraumatic stress disorder, obsessive compulsive disorder, auditory hallucinations in schizophrenia, and pain syndromes. In this review, we examine a number of topics of interest to clinicians regarding TMS, including its history; an overview of clinical administration, safety and adverse events; insight into neurobiological effects; similarities and differences as compared to ECT and VNS; a review of the literature examining its efficacy in the treatment of depression; and a brief overview of its possible role in treating a number of other psychiatric and nonpsychiatric conditions.
Neuromodulation and Therapeutics Overview
The therapeutics of affective disorders in psychiatry stand at an exciting crossroad. They may be on the verge of a paradigm change whereby the therapeutic target for the clinician is shifting from the neuronal synapse principally (as has been the case with our monoamine-based antidepressant therapies) to the neural circuitry of mood, which is the primary target of the emerging device-based treatments, such as vagus nerve stimulation (VNS), transcranial magnetic stimulation (TMS), and deep brain stimulation (DBS). For almost seven decades, psychiatry has had a sole medical device as an adjunct to pharmacotherapy and psychotherapy, namely electroconvulsive therapy (ECT). This solitary archetype changed in the United States in 2005 with VNS becoming the first 21st century medical device to gain official sanction by the Food and Drug Administration (FDA) as a valid therapeutic option for severe unipolar and bipolar depression.
In other fields in medicine, most notably cardiology, medical devices and procedures make a contribution to successful treatment on a par with drugs. It is possible that the range of devices now emerging in psychiatry (VNS, TMS, DBS), taken along with ECT, which collectively comprise a therapeutic field termed neuromodulation, will significantly broaden our range of therapeutic options. Medical devices may, in this regard, assume a role for the clinician comparable to that of pharmacotherapy and psychotherapy when selecting the appropriate treatment for the individual patient. This review will focus on one of these novel devices, TMS, and examine its emerging role in the treatment of major depression and perhaps a number of other psychiatric disorders.
A Brief History of TMS
The basic mechanism underlying TMS dates back to the work of Michael Faraday, who in 1839 discovered that a magnetic field can produce an electrical current in a conductive substance.1 It was not until 1985, however, that Barker and colleagues applied this principle to the direct stimulation of the human brain and the modern era of TMS began.2 Shortly afterward, TMS was first suggested as a possible treatment for depression. To date, the majority of work with TMS has focused on mood disorders, particularly unipolar major depression. In 1998, with some evidence emerging to support the use of TMS in treating depressive symptoms, an international workshop was held in Bethesda, Maryland, that developed the first consensus on safe and ethical procedures for the use of TMS in research studies.3
Over the past decade, a growing body of literature supports antidepressant effects of TMS in depression beyond simply that of “sham” or placebo TMS, but the magnitude and clinical relevance of these antidepressant effects remains a matter of some controversy.4 In addition to depression, there is active research investigating the utility of TMS in a number of other neuropsychiatric disorders including schizophrenia, bipolar disorder, posttraumatic stress disorder, and obsessive compulsive disorder.
Overview of TMS Administration
During TMS, a small insulated electromagnetic coil is placed on the scalp. A bank of capacitors is then rapidly discharged into the coil. The electromagnet converts the electrical activity into a pulsed magnetic field that then passes through the cranium with minimal impedance. The magnetic field, based on the counter-current electrophysiological principle, induces an electrical field in the underlying cerebral cortex. Upon delivery of TMS to the targeted area, if the induced electrical field is of sufficient intensity, the cortical neurons depolarize and action potentials are generated. Currently employed technology generates a magnetic field of approximately 1.5 Tesla (comparable to that of a standard MRI), and penetrates the field approximately 3cm in depth from the coil surface.
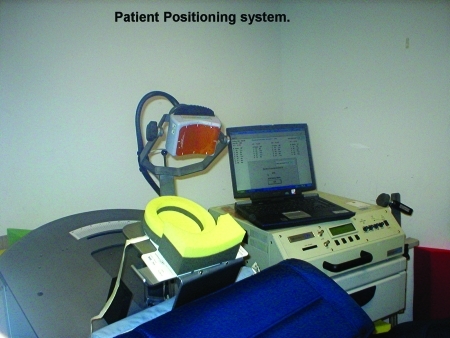
The frequency of pulsing of the field and whether the underlying neurons activated are excitatory or inhibitory in function together determine whether the ultimate effects on neural circuitry are excitatory or inhibitory. In general terms, TMS at frequencies of less than or equal to 1Hz is termed slow TMS and tends to be inhibitory. TMS at frequencies greater than 1Hz is termed fast TMS and tends to be excitatory. The pulses administered can be single, paired, or in a series (also called a “train,” which in turn can vary in its duration). When TMS is delivered in a series of pulses, or a train, this is termed repetitive TMS (rTMS). Generally, single and paired pulse-type TMS are used for neurodiagnostic purposes, whereas rTMS is the modality that is believed to have therapeutic potential in psychiatric disorders. The shape of coils also varies. Figures 1 and 2 illustrate two different commercially available rTMS devices.
Figure 1.
A MagStim TMS device with figure-eight shaped coil that is held flat against the scalp
Figure 2.
Neuronetics TMS device with oval-shaped magnet in which positioning is recorded in three dimensions
Motor Threshold
The minimal amount of energy required to activate the motor strip of a particular individual is called the motor threshold (MT) and is determined by titrating the amount of energy from the TMS device (expressed as a percentage of the device's available output) until a visible movement of the contralateral thumb is reliably produced following single pulses of TMS. By way of example, in the treatment of depression, determination of the motor threshold (on the left motor cortex) guides the dosing for the power of treatment delivered (expressed as a percent of motor threshold, usually in the 80–120% range of the derived MT). The site of the point of the optimal derived MT on the scalp also guides the anatomical placement of the coil for treatment. The coil is moved 5cm anteriorly in a parasagital plane from the site of motor threshold determination to the scalp overlying the left dorsolateral prefrontal cortex (DLPFC).
Stimulation Parameters
Other treatment variables include the inter-train interval (the time in between trains of stimulation when no stimulation is occurring—an important safety parameter), frequency of pulsing of the magnetic field (expressed in Hz), number of trains per session, and the duration of the session. As an example, the dose of rTMS for an individual patient per session who is being treated for depression might be expressed as 50 trains at 10Hz, five-second trains, with a 25-second inter-train interval at 120 percent of MT. This would then translate into a dose of 50 pulses per train, for a total of 2,500 pulses per session at 120-percent MT over a session length of 25 minutes.
Generally, a single session is conducted per treatment day, with five sessions (Monday to Friday) per treatment week, at least for acute treatment. The total number of treatment sessions in clinical trials has ranged from about 10 in the earlier studies to more recently 15 to 20 sessions over 3 to 4 weeks. Patients are awake, alert, and non-sedated during the rTMS treatment and can ordinarily leave immediately afterwards without a recovery period being necessary, as would be the case with ECT. The clinical administration and logistics of rTMS differ significantly from that of the other neuromodulation interventions, ECT and VNS. In Table 1, an overview is presented contrasting these three device-based interventions.
Table 1.
Comparison of rTMS with VNS and ECT treatment modalities
| rTMS | VNS | ECT | |
|---|---|---|---|
| Setting | Office-based | OR for implant, office-based dosing | Hospital-based |
| Intervention | Pulsed magnetic fields | Pulsed electrical stimulus of vagus nerve | Scalp electrical stimulus |
| Site | Left or right prefrontal cortex | Left vagus nerve afferents in neck region | Unilateral or bilateral hemispheric |
| Anesthesia | No | Implant only | Yes |
| Seizure | No | No | Yes |
| Session | 10–30 mins | Implant—60 mins Office—30 mins | 30 mins |
| Course | 15–20 sessions | 6–12 month trial | 6–12 sessions |
| Duration | 3–4 weeks | Minimum 3 months | 2–4 weeks |
| Efficacy | Depression likely | Generally accepted | Strong |
| Other uses | Possibly mania, schizophrenia, PTSD, OCD, catatonia, pain | Possible rapid cycling bipolar, bulimia | Mania, catatonia, NMS, schizoaffective |
| Cost | ? | ~ $25,000 for implant | ~ $15,000 per course |
| Side effects | Headache (5–10%), dizziness, slight seizure risk (<1/1,000) | Hoarseness (25-50%), occasional dyspnea, neck discomfort | Memory impairment, headache, confusion, myalgias |
Neurobiological Effects of TMS
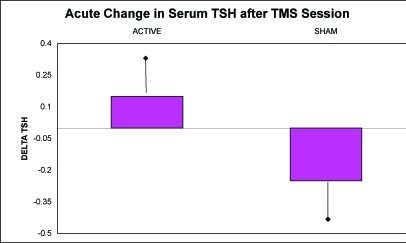
Animal models have been used to study the neurobiological effects of rTMS. It has been shown both that forebrain serotonin output is enhanced and that serotonin receptor function is modulated.5,6 The ability to apply both excitatory and inhibitory stimulation is of significant potential clinical utility, given that neuroimaging studies in neuropsychiatric disorders suggest that certain neuroanatomic regions may be hypoactive and others may be hyperactive. In addition, it is now known that rTMS has the ability to produce changes in regional brain activity both locally and in more distal neuroanatomic locations. For instance, one study demonstrated that rTMS stimulation to the left DLPFC produces changes both in prefrontal cortex and in paralimbic blood flow.7 Similarly, effects of prefrontal rTMS on limbic blood flow have been demonstrated.8 In human studies, Szuba, et al., found an increased output of thyroid stimulating hormone (TSH) in subjects with major depression in response to single sessions of rTMS at 10Hz applied to the left DLPFC when compared to sham sessions of rTMS at the same locale, pulse frequency and session duration (Figure 3).9 In addition, acute mood improvement was demonstrated in this investigation with real but not with control/sham sessions of rTMS. Activation of the hypothalamic-pituitary-axis in this fashion has been associated with relief of depressive symptomatology following sessions of ECT. Finally, normalization of the dexamethasone suppression test with rTMS has been reported.10
Figure 3.
Changes in TSH levels from pre- to post-sessions with active rTMS and control “sham” rTMS procedures (p=0.03)
rTMS in Depression—Does it Really Work?
Early studies. During the 10 years since the first case reports suggested a useful role for rTMS in the treatment of depression, a considerable amount of research has been done. However, interpretation of the studies conducted has ultimately been constrained by small sample sizes, short duration of rTMS courses (usually 1–2 weeks), variability of cortical site of stimulation (right vs. left DLPFC), whether treatment was administered medication-free or in combination with antidepressants, and finally, by a wide range of variability of dosing relative to motor threshold (80–130% MT). In general, it appears from the studies performed during the 1990s that rTMS appears to be of some benefit in reducing the symptoms of depression when compared to a sham rTMS procedure, but the magnitude of this antidepressant effect appears to be relatively modest. Thus, while these studies often demonstrated a subset of robust responders to rTMS, the overall reduction in symptom severity in the actively treated group rarely approached 50 percent, as reflected in the overall reduction in score on the Hamilton Depression Rating Scale.
rTMS versus ECT in depression. The difficulty in interpreting the efficacy of rTMS has decreased somewhat in the last few years as total number of pulses and sessions administered, as well as the duration of the clinical trials themselves have increased. Early trials with rTMS in depression likely set an unattainable standard by seeking clear efficacy with the procedure in largely treatment-resistant populations of depressed patients in a matter of 1 to 2 weeks. This would require rTMS to have a level of efficacy comparable to the most robust forms of ECT and superior to that of standard antidepressant drugs. The accompanying graphic by Gershon, et al., from their review of rTMS strongly suggests that rTMS should be administered at higher dosing levels for pulses, MT, and course duration to achieve the levels of efficacy that would be meaningful for patients and clinicians.11
More recent studies, particularly the head-to-head comparative studies with ECT, provide support for rTMS as an effective treatment. These studies generally involve longer treatment courses than those found in the early studies of rTMS. Typically, they entail 20 sessions of rTMS administered over a four-week period vs. a full course of ECT, which was usually right unilateral, but with the option to switch to bilateral if needed, or bilateral only from initiation of the ECT course. In all, there have now been five head-to-head trials comparing ECT and rTMS in severe depression that have been published.12–16 All of these studies were randomized so patients had to be ill enough to accept randomization to either condition. In each of the comparisons, rTMS was equal to ECT in efficacy but rTMS clearly demonstrated a better tolerability profile. One study did find ECT to be clearly superior to rTMS in the psychotic subgroup of depressed subjects with a 100-percent response rate (10/10 subjects responding) to ECT vs. only a 20-percent response rate with rTMS (2 out of 10 subjects responding).12
Interpreting comparison studies of rTMS and ECT. It is important to acknowledge that the failure to find a difference between ECT and rTMS does not mean for certain that rTMS is an effective modality of treatment as there was no placebo control in these studies. In addition, the absence of a difference between ECT and rTMS may reflect the relatively small sample sizes in the studies and thus a false negative or type II error. It might also be argued by some that the ECT response rates in these studies were generally lower than is classically expected, but nevertheless, they are consistent with the findings in patients with treatment-resistant depression in the recent ECT literature. In summary, the results of trials comparing rTMS to ECT are of particular interest as they demonstrate a novel modality of treatment that is able to furnish a level of efficacy comparable to ECT, or at the very least, certain forms of it. This must be regarded as promising clinically because if such efficacy with rTMS is in fact realized, it comes with quite a low side-effect burden compared to ECT as well as avoidance of the need for general anesthesia or a post-ictal recovery period.
Meta-analyses of rTMS efficacy in depression. Notwithstanding the favorable results of rTMS when compared with ECT, rTMS does not have to be equivalent in efficacy to ECT for it to be a worthwhile clinical treatment. Rather, rTMS must meet the same standard asked of other potential antidepressant interventions, namely to demonstrate clear efficacy when compared to a control or placebo procedure. Since a published large scale, multicenter, definitive trial of rTMS is currently not available, the best alternative approach in evaluating its utility is the technique of meta-analysis. To date, there are six published meta-analyses of rTMS efficacy in treating depression.17–21 Most, but not all, of the meta-analyses have concluded that rTMS exerts meaningful antidepressant effects and that the overall effect size is comparable to that found with antidepressants. For instance, in the meta-analysis conducted by Kozel and George, which examined left DLPFC form of rTMS only, the mean effect size detected was 0.53, which is very comparable to that of antidepressant medications. Additional analyses indicated that it would require at least 20 negative studies with rTMS to override this result and make it nonsignificant.19
Maintenance treatment with rTMS. If rTMS becomes accepted as a valid treatment option in the acute treatment of major depression, one important consideration for clinicians will be how to maintain the acute effects long term. Investigation of the maintenance potential of rTMS in depression has been very understudied to date. Grunhaus, et al., were able to demonstrate that rTMS was comparable to ECT in sustaining acute antidepressant effects over the subsequent six months when responders were transitioned from ECT or rTMS to medication only in the continuation phase.12 How often rTMS sessions would need to be administered to maintain efficacy following five sessions per week of acute treatment is an important practical question for which very little data are available. Results from our own center for a cohort of 10 subjects with major depression who are followed prospectively for between six months and six years are promising. We found that a schedule of 1 to 2 sessions per week is generally effective and that longer-term administration of rTMS could also be safe. Significantly, some subjects received close to 500 sessions of rTMS (equivalent to approximately 1 million rTMS pulses) with no evidence of any significant adverse events or toxicity. Furthermore, no seizures were noted in 1,831 sessions administered.22
Efficacy conclusions. When considered in totality, the preponderance of rTMS treatment studies conducted in depression are positive in outcome, but we are not yet able to state that this has been proven beyond a reasonable doubt. Nevertheless, from a clinical availability standpoint, several regulatory authorities worldwide have drawn a similar positive conclusion regarding the efficacy of rTMS for depression. Repetitive TMS is now clinically available and in use in Canada, Australia, Europe, and Israel, but it is not FDA-approved for use in the United States, where it remains an experimental intervention. To address this issue, a large, placebo-controlled, multicenter trial is underway in the US in an attempt to determine the status of rTMS as a treatment for unipolar major depression. With an enrollment goal of more than 300 subjects and a longer treatment course compared to that administered in rTMS studies published to date, it is anticipated that much will be learned from this trial about the ultimate efficacy and safety of rTMS in the treatment of major depression.
Safety of rTMS
Seizure risk. In general, rTMS seems to be both safe and well tolerated. The most significant risk factor with rTMS is the inadvertent induction of a seizure. Remaining within the recommended stimulation parameters, however, confers a margin of safety that should be combined with careful screening for underlying organic brain disease.3 Individuals with a personal history of nonfebrile seizures or a family history of epilepsy in a first-degree relative should not receive rTMS. Overall, the risk of an unwanted seizure appears to be less than 1 per 1000 rTMS sessions and compares favorably to the risk of seizures with marketed antidepressants, such as bupropion and tricyclic antidepressants. The administration of a self-reported safety questionnaire, such as the TMS Adult Safety Screen (TASS), is an additional useful safety-screening device.23
Other adverse events. Post-treatment headaches may affect about 10 percent of patients but are generally mild, brief, and easily managed with simple analgesia (such as ibuprofen or acetaminophen). Scalp pain at the site of stimulation during the treatment session also tends to be mild and limited to the time of stimulation during the treatment session. Because the rTMS device emits clicking sounds with each train of magnetic pulses, there is the potential for TMS devices to have adverse effects on hearing. Mild but transient and clinically insignificant shifts in auditory thresholds have been found in studies that evaluated hearing in subjects exposed to rTMS.24,25 To minimize any auditory risks, patients should wear ear plugs during the procedure. Induction of mania is not a widely recognized side effect of TMS; however, case reports of switching into mania have been described when rTMS was applied to depressed bipolar patients.26 In general, improvement in neuropsychological functioning has been reported following rTMS administration for major depression, but it has not proved possible to clearly separate this effect from the observed improvements in mood.16 Overall, the burden of side effects associated with rTMS is low and contrasts favorably with the weight gain and sexual dysfunction typical of many medications and with the negative cognitive effects of ECT.
TMS in Other Psychiatric Disorders
In addition to the growing body of literature evaluating the use of rTMS in the treatment of depression, there is emerging interest in evaluating its role in the treatment of other psychiatric disorders. Below is a brief description of some of this work.
Schizophrenia. In schizophrenia, at least 10 controlled trials have been published. Of these, six concluded that there may be a role for adjunctive rTMS in the treatment of schizophrenia,27–32 while four concluded that there is no clear evidence for benefit.33–36
In schizophrenia, rTMS has been used largely to target treatment-refractory hallucinations. Repetitive TMS is generally applied at 1Hz frequency over the left temporoparietal cortex with the intent of inhibiting dysfunctional auditory processing pathways in Wernicke's area that in turn may be linked to the production of auditory hallucinations. Further studies are needed to clarify the role of rTMS in this application.
Bipolar mania. At least three trials have studied the potential role of rTMS in the treatment of bipolar mania.37–39
The three trials were of small sample sizes (16–25 patients each). Two trials showed that rTMS may be of benefit in the treatment of bipolar mania using right DLPFC stimulation. However, one of the studies failed to find a difference between active and sham stimulation in bipolar mania.
Posttraumatic stress disorder (PTSD). Additionally, at least three studies have looked at rTMS for the treatment of PTSD including one double blind placebo-controlled trial and two open label trials.40–42 Work to date in this area is supportive of a possible role for rTMS in the treatment of PTSD.
Obsessive compulsive disorder (OCD). A minimum of three trials have been published looking at a potential role for rTMS in the treatment of OCD.43–45 Again, results from two of these trials indicate that rTMS may be of potential use in the treatment of OCD. However, in a recent systematic review by researchers from the Cochrane Database, it was concluded that there was insufficient evidence at this juncture of benefit from rTMS in the treatment of OCD.46
Catatonia. Two case reports reflecting the use of rTMS in the treatment of catatonia produced positive results.47,48 However, there are no published clinical trials to date on the use of rTMS in catatonia.
Clearly, there is a growing interest in evaluating the potential role of rTMS in the treatment of a range of psychiatric disorders. However, much work remains to be done to determine whether rTMS is truly efficacious in the treatment of any of these disorders and, if so, what the optimal treatment parameters should be.
TMS in NonPsychiatric Disorders
Interest in rTMS is not confined only to the treatment of psychiatric disorders. Researchers have been looking at rTMS in a variety of nonpsychiatric conditions as well. A number of studies have examined a potential role for rTMS in the treatment of pain disorders.49–55 From these studies, there is evidence that rTMS can be effective in treating both chronic and acute pain related to an assortment of conditions. Additionally, some investigators have looked at a role for rTMS in the treatment of Parkinson's disease and other movement disorders.56–62 The results of this early work are conflicting, however, in regards to the usefulness of rTMS in the treatment of Parkinson disease. Finally, rTMS has been evaluated as a potential intervention for the treatment for seizure disorders. These studies indicate that rTMS may have some role in the treatment of seizure disorders.63–68
In summary, there is growing interest in evaluating the potential role of rTMS in the treatment of a range of nonpsychiatric disorders. However, this work is at an early stage and further studies will be needed to determine if rTMS is truly efficacious in the treatment of any of these disorders and, if so, what the optimal treatment parameters might be.
Summary and Conclusions
Over the past decade, the field of rTMS has advanced in knowledge and clinical application. Clearly, rTMS shows promise in the treatment of depression, although a definitive answer on efficacy is still awaited. Repetitive TMS may also be useful in the treatment of a variety of other psychiatric and nonpsychiatric disorders. If rTMS secures FDA approval for major depression, then it would join VNS as the second neuromodulatory device to be approved in the US for treatment of psychiatric disorders within a short period of time. Additionally, other devices, such as DBS, are also under investigation for the treatment of psychiatric disorders. Since the advent of ECT, further therapeutic advances in psychiatry have been restricted to psychopharmacology and psychotherapy. Now, after a long hiatus, new devices to stimulate the brain are showing real promise and may assume a place in the therapeutic armamentarium of the psychiatrist alongside psychopharmacology, psychotherapy, and ECT.
References
- 1.Faraday M. Experimental research in electricity. Quartich. 1839 (B) [Google Scholar]
- 2.Barker AT, Jalinous R, Freeston IL. Noninvasive magnetic stimulation of the human motor cortex. Lancet. 1985;1(8437):1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- 3.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: Report and suggested guidelines. International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1988;108(1):1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 4.Sackeim HA. Repetitive transcranial magnetic stimulation: What are the next steps? Biol Psychiatry. 2000;48(10):959–61. doi: 10.1016/s0006-3223(00)01064-7. [DOI] [PubMed] [Google Scholar]
- 5.Juckel G, Mendlin A, Jacobs BL. Electrical stimulation of rat medial prefrontal cortex enhances forebrain serotonin output: implications for electroconvulsive therapy and transcranial magnetic stimulation in depression. Neuropsychopharmacology. 1999;21(3):391–8. doi: 10.1016/S0893-133X(98)00097-9. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Shachar D, Belmaker RH, Grisaru N, Klein E. Transcranial magnetic stimulation induces alterations in brain monoamines. J Neural Transm. 1997;104(2-3):191–7. doi: 10.1007/BF01273180. [DOI] [PubMed] [Google Scholar]
- 7.Teneback CC, Nahas Z, Speer AM, et al. Changes in prefrontal cortex and paralimbic activity in depression following two weeks of daily left prefrontal TMS. J Neuropsychiatry Clin Neurosci. 1999;11(4):426–35. doi: 10.1176/jnp.11.4.426. [DOI] [PubMed] [Google Scholar]
- 8.Nahas Z, Lomarev M, Roberts DR, et al. Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry. 2001;50(9):712–20. doi: 10.1016/s0006-3223(01)01199-4. [DOI] [PubMed] [Google Scholar]
- 9.Szuba MP, O'Reardon J, Rai AS, et al. Acute mood and thyroid stimulating hormone effects of transcranial magnetic stimulation in major depression. Biol Psychiatry. 2001;50(1):22–7. doi: 10.1016/s0006-3223(00)01118-5. [DOI] [PubMed] [Google Scholar]
- 10.Pridmore S. Rapid transcranial magnetic stimulation and normalization of the dexamethasone suppression test. Psychiatry Clin Neurosci. 1999;53(1):33–7. doi: 10.1046/j.1440-1819.1999.00467.x. [DOI] [PubMed] [Google Scholar]
- 11.Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160(5):835–45. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- 12.Grunhaus L, Dannon PN, Schreiber S, et al. Repetitive transcranial magnetic stimulation is as effective as electroconvulsive therapy in the treatment of nondelusional major depressive disorder: an open study. Biol Psychiatry. 2000;47(4):314–24. doi: 10.1016/s0006-3223(99)00254-1. [DOI] [PubMed] [Google Scholar]
- 13.Grunhaus L, Schreiber S, Dolberg OT, et al. A randomized controlled comparison of electroconvulsive therapy and repetitive transcranial magnetic stimulation in severe and resistant nonpsychotic major depression. Biol Psychiatry. 2003;53(4):324–31. doi: 10.1016/s0006-3223(02)01499-3. [DOI] [PubMed] [Google Scholar]
- 14.Janicak PG, Dowd SM, Martis B, et al. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: Preliminary results of a randomized trial. Biol Psychiatry. 2002;51(8):659–67. doi: 10.1016/s0006-3223(01)01354-3. [see comment] [DOI] [PubMed] [Google Scholar]
- 15.Pridmore S. Substitution of rapid transcranial magnetic stimulation treatments for electroconvulsive therapy treatments in a course of electroconvulsive therapy. Depress Anxiety. 2000;12(3):118–23. doi: 10.1002/1520-6394(2000)12:3<118::AID-DA2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 16.Schulze-Rauschenbach SC, Harms U, Schlaepfer TE, et al. Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. Br J Psychiatry. 2005;186:410–6. doi: 10.1192/bjp.186.5.410. [DOI] [PubMed] [Google Scholar]
- 17.McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med. 2001;31(7):1141–6. doi: 10.1017/s0033291701004378. [see comment] [DOI] [PubMed] [Google Scholar]
- 18.Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. 2002;5(1):73–103. doi: 10.1017/S1461145702002791. [DOI] [PubMed] [Google Scholar]
- 19.Kozel A, George M. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J Psychiatr Pract. 2002;(8):270–5. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Martin JL, Barbanoj MJ, Schlaepfer TE, et al. Repetitive transcranial magnetic stimulation for the treatment of depression. Systematic review and meta-analysis. Br J Psychiatry. 2003;182:480–91. doi: 10.1192/bjp.182.6.480. [see comment] [DOI] [PubMed] [Google Scholar]
- 21.Couturier JL. Efficacy of rapid-rate repetitive transcranial magnetic stimulation in the treatment of depression: a systematic review and meta-analysis. J Psychiatry Neurosci. 2005;30(2):83–90. [see comment] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Reardon JP, Blumner KH, Peshek A, et al. Long-term maintenance therapy for major depression with left prefrontal repetitive transcranial magnetic stimulation (rTMS) J Clinical Psychiatry. 2005;66:1524–1528. doi: 10.4088/jcp.v66n1205. [DOI] [PubMed] [Google Scholar]
- 23.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. 2001;112(4):720. doi: 10.1016/s1388-2457(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Cohen LG, Shotland LI, et al. No evidence of hearing loss in humans due to transcranial magnetic stimulation. Neurology. 1992;42(3 Pt 1):647–51. doi: 10.1212/wnl.42.3.647. [DOI] [PubMed] [Google Scholar]
- 25.Loo C, Sachdev P, Elsayed H, et al. Effects of a 2- to 4-week course of repetitive transcranial magnetic stimulation (rTMS) on neuropsychologic functioning, electroencephalogram, and auditory threshold in depressed patients. Biol Psychiatry. 2001;49(7):615–23. doi: 10.1016/s0006-3223(00)00996-3. [DOI] [PubMed] [Google Scholar]
- 26.Dolberg OT, Schreiber S, Grunhaus L. Transcranial magnetic stimulation-induced switch into mania: A report of two cases. Biol Psychiatry. 2001;49(5):468–70. doi: 10.1016/s0006-3223(00)01086-6. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman RE, Guerguieva R, Hawkins KA, et al. Temporoparietal transcranial magnetic stimulation for auditory hallucinations: Safety, efficacy, and moderators in 50-patient sample. Biologic Psychiatry. 2005;58:97–104. doi: 10.1016/j.biopsych.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman RE, Boutros NN, Hu S, et al. Transcranial magnetic stimulation and auditory hallucinations in schizophrenia. Lancet. 2000;355(9209):1073–5. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman RE, Boutros NN, Berman RM, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated “voices.”. Biol Psychiatry. 1999;46(1):130–2. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- 30.Rollnik JD, Huber TJ, Mogk H, et al. High frequency repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex in schizophrenic patients. Neuroreport. 2000;11(18):4013–5. doi: 10.1097/00001756-200012180-00022. [DOI] [PubMed] [Google Scholar]
- 31.d'Alfonso AA, Aleman A, Kessels RP, et al. Transcranial magnetic stimulation of left auditory cortex in patients with schizophrenia: Effects on hallucinations and neurocognition. J Neuropsychiatry Clin Neurosci. 2002;14(1):77–9. doi: 10.1176/jnp.14.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman RE, Hawkins KA, Gueorguieva R, et al. Transcranial magnetic stimulation of left temporoparietal cortex and medication-resistant auditory hallucinations. Arch Gen Psychiatry. 2003;60(1):49–56. doi: 10.1001/archpsyc.60.1.49. [DOI] [PubMed] [Google Scholar]
- 33.Klein E, Kolsky Y, Puyerovsky M, et al. Right prefrontal slow repetitive transcranial magnetic stimulation in schizophrenia: a double-blind sham-controlled pilot study. Biol Psychiatry. 1999;46(10):1451–4. doi: 10.1016/s0006-3223(99)00182-1. [DOI] [PubMed] [Google Scholar]
- 34.Holi MM, Eronen M, Toivonen K, et al. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr Bull. 2004;30(2):429–34. doi: 10.1093/oxfordjournals.schbul.a007089. [DOI] [PubMed] [Google Scholar]
- 35.Schonfeldt-Lecuona C, Gron G, Walter H, et al. Stereotaxic rTMS for the treatment of auditory hallucinations in schizophrenia. Neuroreport. 2004;15(10):1669–73. doi: 10.1097/01.wnr.0000126504.89983.ec. [DOI] [PubMed] [Google Scholar]
- 36.McIntosh AM, Semple D, Tasker K, et al. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2004;127(1-2):9–17. doi: 10.1016/j.psychres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Grisaru N, Chudakov B, Yaroslavsky Y, Belmaker RH. Transcranial magnetic stimulation in mania: a controlled study. Am J Psychiatry. 1998;155(11):1608–10. doi: 10.1176/ajp.155.11.1608. [DOI] [PubMed] [Google Scholar]
- 38.Kaptsan A, Yaroslavsky Y, Applebaum J, et al. Right prefrontal TMS versus sham treatment of mania: A controlled study. Bipolar Disord. 2003;5(1):36–9. doi: 10.1034/j.1399-5618.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 39.Michael N, Erfurth A. Treatment of bipolar mania with right prefrontal rapid transcranial magnetic stimulation. J Affect Dis. 2004;78:253–7. doi: 10.1016/S0165-0327(02)00308-7. [DOI] [PubMed] [Google Scholar]
- 40.Grisaru N, Amir M, Cohen H, Kaplan Z. Effect of transcranial magnetic stimulation in posttraumatic stress disorder: A preliminary study. Biol Psychiatry. 1998;44(1):52–5. doi: 10.1016/s0006-3223(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg PB, Mehndiratta RB, Mehndiratta YP, et al. Repetitive transcranial magnetic stimulation treatment of comorbid posttraumatic stress disorder and major depression. J Neuropsychiatry Clin Neurosci. 2002;14(3):270–6. doi: 10.1176/jnp.14.3.270. [DOI] [PubMed] [Google Scholar]
- 42.Cohen H, Kaplan Z, Kotler M, et al. Repetitive transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in posttraumatic stress disorder: A double-blind, placebo-controlled study. Am J Psychiatry. 2004;161(3):515–24. doi: 10.1176/appi.ajp.161.3.515. [DOI] [PubMed] [Google Scholar]
- 43.Alonso P, Pujol J, Cardoner N, et al. Right prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a double-blind, placebo-controlled study. Am J Psychiatry. 2001;158(7):1143–5. doi: 10.1176/appi.ajp.158.7.1143. [DOI] [PubMed] [Google Scholar]
- 44.Greenberg BD, George MS, Martin JD, et al. Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry. 1997;154(6):867–9. doi: 10.1176/ajp.154.6.867. [DOI] [PubMed] [Google Scholar]
- 45.Sachdev PS, McBride R, Loo CK, et al. Right versus left prefrontal transcranial magnetic stimulation for obsessive-compulsive disorder: a preliminary investigation. J Clin Psychiatry. 2001;62(12):981–4. doi: 10.4088/jcp.v62n1211. [DOI] [PubMed] [Google Scholar]
- 46.Martin JL, Barbanoj MJ, Perez V, Sacristan M. Transcranial magnetic stimulation for the treatment of obsessive-compulsive disorder. Cochrane Database Syst Rev. 2003:3. doi: 10.1002/14651858.CD003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grisaru N, Chudakov B, Yaroslavsky Y, Belmaker RH. Catatonia treated with transcranial magnetic stimulation. Am J Psychiatry. 1998;155(11):1630. [PubMed] [Google Scholar]
- 48.Saba G, Rocamora JF, Kalalou K, et al. Catatonia and transcranial magnetic stimulation. Am J Psychiatry. 2002;159(10):1794. doi: 10.1176/appi.ajp.159.10.1794. [DOI] [PubMed] [Google Scholar]
- 49.Lefaucheur JP, Drouot X, Keravel Y, et al. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport. 2001;12(13):2963–5. doi: 10.1097/00001756-200109170-00041. [DOI] [PubMed] [Google Scholar]
- 50.Canavero S, Bonicalzi V, Dotta M, et al. Transcranial magnetic cortical stimulation relieves central pain. Stereotact Funct Neurosurg. 2002;78(3-4):192–6. doi: 10.1159/000068965. [DOI] [PubMed] [Google Scholar]
- 51.Rollnik JD, Wustefeld S, Dauper J, et al. Repetitive transcranial magnetic stimulation for the treatment of chronic pain: A pilot study. Eur Neurol. 2002;48(1):6–10. doi: 10.1159/000064950. [DOI] [PubMed] [Google Scholar]
- 52.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. J Neurol Neurosurg Psychiatry. 2004;75(4):612–6. doi: 10.1136/jnnp.2003.022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pleger B, Janssen F, Schwenkreis P, et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neurosci Lett. 2004;356(2):87–90. doi: 10.1016/j.neulet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 54.Summers J, Johnson S, Pridmore S, Oberoi G. Changes to cold detection and pain thresholds following low and high frequency transcranial magnetic stimulation of the motor cortex. Neurosci Lett. 2004;368(2):197–200. doi: 10.1016/j.neulet.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Tamura Y, Okabe S, Ohnishi T, et al. Effects of 1-Hz repetitive transcranial magnetic stimulation on acute pain induced by capsaicin. Pain. 2004;107(1-2):107–15. doi: 10.1016/j.pain.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 56.Wassermann EM, Lisanby SH. Therapeutic application of repetitive transcranial magnetic stimulation: a review. Clin Neurophysiol. 2001;112(8):1367–77. doi: 10.1016/s1388-2457(01)00585-5. [DOI] [PubMed] [Google Scholar]
- 57.Siebner HR, Tormos JM, Ceballos-Baumann AO, et al. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer's cramp. Neurology. 1999;52(3):529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- 58.Shimamoto H, Takasaki K, Shigemori M, et al. Therapeutic effect and mechanism of repetitive transcranial magnetic stimulation in Parkinson's disease. J Neurol. 2001;248(Suppl 3):III48–52. doi: 10.1007/pl00007826. [DOI] [PubMed] [Google Scholar]
- 59.Sommer M, Kamm T, Tergau F, et al. Repetitive paired-pulse transcranial magnetic stimulation affects corticospinal excitability and finger tapping in Parkinson's disease. Clin Neurophysiol. 2002;113(6):944–50. doi: 10.1016/s1388-2457(02)00061-5. [DOI] [PubMed] [Google Scholar]
- 60.Okabe S, Ugawa Y, Kanazawa I. Effectiveness of rTMS on Parkinson's Disease Study Group. 0.2-Hz repetitive transcranial magnetic stimulation has no add-on effects as compared to a realistic sham stimulation in Parkinson's disease. Mov Disord. 2003;18(4):382–8. doi: 10.1002/mds.10370. [DOI] [PubMed] [Google Scholar]
- 61.Tsuji S, Akamatsu N. Does transcranial magnetic stimulation improve the motor symptoms of Parkinson disease? J Neurol. 2003;250(Suppl 3):III47–50. doi: 10.1007/s00415-003-1309-4. [DOI] [PubMed] [Google Scholar]
- 62.Huang YZ, Edwards MJ, Bhatia KP, Rothwell JC. One-Hz repetitive transcranial magnetic stimulation of the premotor cortex alters reciprocal inhibition in DYT1 dystonia. Mov Disord. 2004;19(1):54–9. doi: 10.1002/mds.10627. [DOI] [PubMed] [Google Scholar]
- 63.Tassinari CA, Cincotta M, Zaccara G, Michelucci R. Transcranial magnetic stimulation and epilepsy. Clin Neurophysiol. 2003;114(5):777–98. doi: 10.1016/s1388-2457(03)00004-x. [DOI] [PubMed] [Google Scholar]
- 64.Tergau F, Naumann U, Paulus W, Steinhoff BJ. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353(9171):2209. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- 65.Menkes DL, Gruenthal M. Slow-frequency repetitive transcranial magnetic stimulation in a patient with focal cortical dysplasia. Epilepsia. 2000;41(2):240–2. doi: 10.1111/j.1528-1157.2000.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 66.Theodore WH, Hunter K, Chen R, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology. 2002;59(4):560–2. doi: 10.1212/wnl.59.4.560. [DOI] [PubMed] [Google Scholar]
- 67.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, et al. rTMS reduces focal brain hyperperfusion in two patients with EPC. Acta Neurol Scand. 2004;109(4):290–6. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 68.Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3(2):111–8. doi: 10.1016/s1474-4422(03)00664-1. [erratum appears in Lancet Neurol 2004;3(6):332] [DOI] [PubMed] [Google Scholar]