Abstract
Background: Practitioners are increasingly presented with data procured from studies employing advanced neuroimaging techniques. The central role that neuroimaging occupies in contemporary psychiatric research highlights the need for practitioner familiarity with the neuroimaging technology and its clinical translation.
Methods: We conducted a PubMed search of all English-language articles published between January 1964 – October 2005. The search words were major depressive disorder, bipolar disorder, functional magnetic resonance imaging (fMRI), single-photon-emission computed tomography (SPECT), positron emission tomography (PET), voxel-based morphometry (VBM), region of interest (ROI), blood-oxygen-level-dependent (BOLD), glucose metabolism, blood flow, statistical parametric mapping (SPM), magnetic resonance spectroscopy (MRS), and diffusion-tensor imaging (DTI). The search was supplemented with a manual review of relevant references. The authors organize the review by addressing frequently asked questions on the topic of neuroimaging by mental healthcare providers.
Results: The localization of regional brain volumetric abnormalities with CT is enhanced with MRI techniques that allow for a separate assay of white and gray matter pathology (segmentation), cellular metabolism (MRS), and neurocircuitry (DTI). Positron emission tomography permits the quantification of brain glucose metabolism, regional blood flow, and receptor/transporter localization and function. Rapid changes in regional oxygen consumption may also be quantified with fMRI.
Conclusions: Neuroimaging technology has helped refine pathophysiological models of disease activity in mood disorders and illuminate mechanisms of drug activity. A priority research vista in mood disorders is the integration of neuroimaging investigations with other research methods (e.g., genetics, endocrinology, etc.)
Keywords: neuroimaging, major depressive disorder, bipolar disorder, magnetic resonance imaging, positron-emission tomography, single-photon emission-computed tomography, magnetic resonance spectroscopy, diffusion tensor imaging, statistical parametric mapping, blood-oxygen level dependent, region of interest, voxel-based morphometry
Introduction
Mood disorders are highly prevalent, often chronic medical disorders largely diagnosed and treated in primary care settings.1 Currently, mood disorders are a leading cause of disability globally, and there is increasing evidence that they are important risk factors for the development of major medical disorders such as coronary artery disease (CAD).2,3 The development of more effective therapeutic regimens for depression has been identified as a national health priority in the United States and elsewhere.4
Contemporary models describing the pathophysiology of mood disorders have been purposefully informed by neuroimaging technology.5,6 For example, it is currently posited that aberrant communication between limbic structures (e.g., cingulate cortex) and prefrontal regions may be a biomarker of depressive illness.7–10 This observation has provided the impetus for novel, hypothesis-driven treatments (e.g., deep brain stimulation) as an alternative therapeutic modality for treatment-resistant mood disorders.11
Routine laboratory investigations in mood disorders often include measurement of hematological and hormonal indices (e.g., thyroid).12 The role of neuroimaging in the diagnostic workup and management of a depressed subpopulation (e.g., depression secondary to a neurological condition) is well established.13 Nevertheless, it remains uncertain how neuroimaging assays may inform decision making in the clinical environment.
Practitioners are increasingly presented with data procured from studies employing advanced neuroimaging techniques, inviting the need for practitioner familiarity with neuroimaging tools and their potential clinical application.
Herein, we aim to provide the practicing clinician with a review of neuroimaging modalities employed in contemporary psychiatric research. Towards this aim, the authors review commonly asked questions on the topic.
Methods
We conducted a PubMed search of all English-language articles published between January, 1964, through October, 2005. The search words were major depressive disorder, bipolar disorder, functional magnetic resonance imaging (fMRI), single-photon-emission computed tomography (SPECT), positron emission tomography (PET), voxel-based morphometry (VBM), region of interest (ROI), blood-oxygen-level-dependent (BOLD), glucose metabolism, blood flow, statistical parametric mapping (SPM), magnetic resonance spectroscopy (MRS), and diffusion-tensor imaging (DTI). The search was supplemented with a manual review of relevant references. The authors organized the review by addressing frequently asked questions on the topic of neuroimaging by mental health-care providers.
1. What methodologies exist for evaluating brain structure? Structural, or volumetric, neuroimaging investigations aim to test the hypothesis that the pathophysiology of mood disorders is associated with quantifiable changes in global or regional neuronanatomy. Volumetric studies scrutinizing patients with mood disorders have consistently identified several anatomical abnormalities in brain regions that putatively subserve affect regulation and emotional expression.14,15 Over the past decade, computed tomography (CT) approaches have been largely supplanted by magnetic resonance imaging (MRI) as the structural neuroimaging modality of choice.16
Computed tomography offered an advance over conventional radiological techniques (two-dimensional x-rays) with improved spatial resolution, visual access across multiple dimensions, and accessibility to deep brain structures (e.g., basal ganglia).17 Inside the CT scanner, a rotating gantry equipped with an x-ray tube and arc-shaped detector encircle the patient. For each complete rotation, a thin section, (slice) of brain structure data is acquired. Successive sections are later reconstructed by a dedicated computer into two-dimensional representations of the scanned region with a resolution approaching 2 x 2mm.18
The advent of CT scanning permitted researchers to document volumetric and morphological (i.e., variations in shape) changes in brain structures. For example, in bipolar subjects, increased ventricular size, ventricular-brain ratios, and smaller cerebellar volumes15,19 have been documented with this neuroimaging modality.
The notable limitations of CT technology include exposure to ionizing radiation, spatial resolution, and inability to distinguish white and gray matter.17
Magnetic resonance imaging (MRI) generates anatomic images through the differential magnetic properties of hydrogen across different tissues.99 Current techniques in MRI image reconstruction offer a spatial resolution of less than 1mm,3 affording the possibility of visualizing and quantifying even small brain structures. Additional advantages over CT include the absence of ionizing radiation, and with three-dimensional MRI acquisition technology, the opportunity for more careful scrutiny of brain regions of interest (e.g., cingulate).20 These clinical and methodological advantages notwithstanding, a certain arbitrariness is applied when attempting to define anatomical boundaries in brain regions that are not well circumscribed (most notably the lateral thalamus), introducing a potential source of error in region of interest analyses (see question 7).14
Volumetric neuroimaging investigations in mood disorders have noted both similarities and differences between BD and MDD, and more frequently, between subjects with mood disorder (MDD or BD) and a psychiatrically unaffected control group.14,21,22 For example, compared to unaffected control groups, diminished prefrontal cortical volumes have been reported in both BD and MDD,23,24 while structural differences in subcortical regions have been observed between BD and MDD.14,25 In MDD, the basal ganglia are relatively smaller than in age-matched healthy controls,26–32 while increased striatal volumes have been observed in BD.33–36
The single unit of MRI resolution (1mm3) still encompasses a large cell number (>10,000 neurons), rendering the detection of a smaller, but clinically significant, cellular loss difficult. Other pragmatic limitations of MRI include the prohibition of any intracranial or intra-abdominal metallic implants, devices, or clips. Clinical deficits may also be observed independent of any anatomical abnormalities. In light of these inherent limitations of structural imaging, the identification of abnormalities in patients with mood disorders has provided a basis for refining neuroanatomical models of disease pathophysiology.23,37 Moreover, simultaneous analyses of functional and structural neuroimaging findings, in addition to longitudinal analysis in the depressed and remitted states, represent promising research vistas.38,39
2. What methods are used to evaluate brain function? Functional neuroimaging offers a dynamic output of disparate brain activities in contrast to the static snapshot of neuroanatomy afforded by volumetric investigations. Broadly sketched, brain function can be evaluated at the regional level through blood perfusion analyses, at the cellular level through indices of metabolism, and at the intracellular level through ligand-occupancy studies. A constellation of perfusion, metabolic, and cell-surface abnormalities has been documented in limbic and prefrontal structures in mood disorders.
3. What are the different applications of PET? Positron emission tomography (PET) relies on the localization of an injected radioactive neurotransmitter-derivative to produces three-dimensional images of the brain. These synthetic ligands are labeled with a rapidly decaying radioactive atom, usually Carbon-11, Fluorine-18, Oxygen-15, or Nitrogen-13. A supine subject is injected with a radioactive tracer that incrementally progresses through the PET camera. A gamma ray detector array captures the gamma rays produced at the collision site between a positron (emitted from the radioactive substance) and an electron in the tissue. As in CT scanning, the process is repeated, producing a series of thin two-dimensional slices of the brain that are later converted to a three-dimensional representation.
A PET investigation can provide data on blood flow (i.e., hypo/hyperperfusions) or other biochemical functions, depending on the identity of radioactively tagged molecule. Most neuronal glucose is consumed by ion pumps and the glutamate-glutamine recycling processes. Group differences in neuronal glucose metabolism can be evaluated via injection of a fluorine-tagged, non-hydrolyzable form of glucose, 18F-2-fluoro-2-deoxyglucose (FDG) to depressed and nondepressed cohorts. Early investigations employing FDG methodology in mood disorders initially examined brain activity in the resting state, or during a basic neuropsychological attention task in depressed and non-depressed cohorts.40–42 Later, investigations employed various pharmacological, affective, or cognitive challenges in conjunction with a glucose metabolism scan9,43–45 as a mechanism of activating regions of interest.
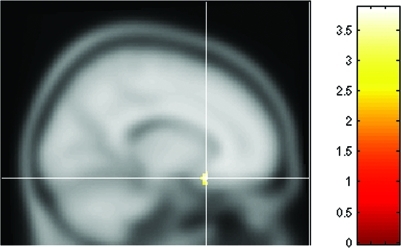
Decreased regional cerebral metabolic glucose rates in the prefrontal cortex (PFC) of depressed subjects have been a consistent finding in mood disorders,46–49 although relative hypermetabolism in distinct regions of the PFC have also been reported.50,51 Preclinical animal models and case reports also implicate PFC dysfunction with impairments in emotional perception and experience.52,53 Specifically, the subgenual region of the cortex (Figure 1) has been associated with baseline hypoactivity,23 which is further depressed when probed with a serotonergic depletion challenge43 and increased after recovery from depression.54 Investigations in other clinical samples have noted hyperactivity in this brain region that normalizes with response to antidepressant therapy.102,103 Although challenge studies utilizing blood flow as an outcome measure have greater temporal sensitivity, FDG has the advantage of being decoupled from the direct effects of pharmacological agents on cerebral circulation.
Figure 1.
The subgenual cingulate cortex
Radioactively labeled water (15O-H2O) provides an elegant technique of evaluating regional brain differences in cerebral blood flow. The relatively short half-life of 15O (~2min) provides an opportunity to administer a new bolus every 12 to 15 minutes and to acquire a new snapshot of blood flow within the same scanning session. Soon after the tracer enters the smaller vessels in the brain, data acquisition can begin and usually lasts 60 to 90 seconds. The acquisition of multiple data points during a single scanning session allows the possibility of provocation paradigms, and the exposition of aberrant neurocircuitry underlying dysfunctional attitudes that are not apparent under resting baseline conditions.
Changes in glucose metabolism and blood flow are thought to comprise an aggregate of chemical and hemodynamic processes involved in neural activity, which are the neurobiological signature of terminal field synaptic transmission. In a typical provocation experiment, changes in blood flow or glucose metabolism data acquired during the execution of a neuropsychologic task are compared with images obtained within the subject during a control condition. Regional increases in blood flow or glucose metabolism are interestingly increased as synaptic transmission in the experimental condition, relative to the control condition. Tacitly implied, reductions in these parameters are believed to underlie decreasing afferent transmission.5
Test-retest investigations suggest that relative hypometabolism normalizes after treatment pari passu with the patient's mood. Major depressive disorder has also been associated with abnormal activation of key limbic and paralimbic structures, including regionally distinct frontal and temporal lobes, the amygdalo-hippocampus complex, and the cingulate gyrus (Figure 2).
Figure 2.
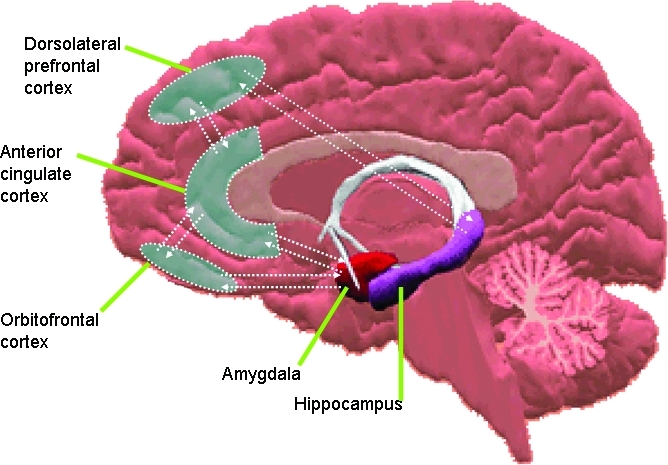
The neurocircuitry of mood disorders
4. Is SPECT different from PET? Single-photon emission computerized tomography (SPECT) is a technique similar to PET, providing information on blood flow and the distribution of radioactively labeled substances in the brain. The differences lie in the radioactive nuclei used in SPECT (Xenon-133, Technetium-99, Iodine-123) which have a longer half-life than those used in PET, and emit single instead of double gamma rays. Generally, SPECT offers less sensitivity and resolution than PET images, but the SPECT technique is also less expensive than PET. Finally, because SPECT scanners do not have to be located near a particle accelerator centers they are usually more accessible than PET centers.
Analysis of regional blood flow with SPECT has generally been replaced by 15O-H2O-PET or functional MRI (fMRI). Early investigations employing SPECT technology analyses reported a correlation between depression severity and frontal hypometabolism in depressed subjects.55
5. What are PET/SPECT ligands used for? A ligand is a molecule (a key) with an affinity for a unique biological target (a lock), most often a protein receptor or transporter. Developments in PET and SPECT methodologies incorporating ligands provide an opportunity to carefully scrutinize the pharmacodynamic effect of psychotropic medications.56–58 Ligands provide a surrogate marker for drug activity by measuring the ratio of ligand-receptor occupancy, versus drug occupancy. Several radiotracers have been developed for human imaging studies, targeting disparate neurotransmitters (i.e., cholinergic,59–61 glutamatergic,62 dopaminergic,63,64 and serotonergic.65–70
Both pre-synaptic and post-synaptic neuronal sites can be labeled with a radiotracer. Pre-synaptic sites can be involved in the regulation of neurotransmitter release from nerve terminals, while post-synaptic sites are at the beginning of the cascade of molecular events that will lead to the biological response.71 Therefore, the binding of different radiotracers pre- or post-synaptically may reveal different stages in diseases involving these systems.
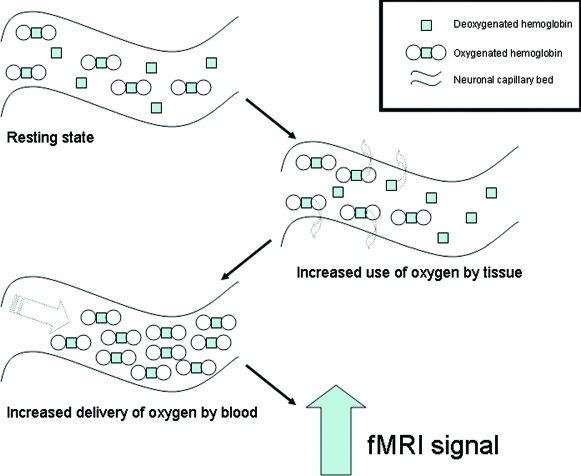
6. How is fMRI different from MRI? Recently, it has become possible to modify a conventional MRI scanner to allow study of dynamic brain function. Functional magnetic resonance imaging (fMRI), subsumes several related techniques. For example, the most commonly used fMRI technique (blood-oxygen-level-dependent or BOLD fMRI) evaluates changes in blood oxygenation with spatial (3–4mm) and temporal resolution (<1s) unmatched by PET or SPECT.
Here, the MRI scanner is tuned to resonate and image hydrogen atoms as in conventional MRI; however, a different component of the hydrogen atoms is measured which reflects the ferromagnetic nature of deoxygenated vs. oxygenated blood. Thus, an area with more oxygenated blood will appear as more intense, compared to when there is less oxygenated blood around. The activated areas initially experience a relative decrease in oxygenated blood as oxygen is extracted by the active regional neurons. Afterward, the amount of blood flowing to the area far outweighs the amount of oxygen that is extracted so that oxygenated blood is now higher (Figure 3).
Figure 3.
The neurobiology of the fMRI signal
This means that transient cognitive events can potentially be imaged and their impact on small structures like the amygdala can be resolved. Moreover, unlike PET and SPECT, most fMRI techniques are noninvasive and radiation-free, enabling repeat scans through different disease states (e.g., imaging a bipolar patient in manic, depressive, and euthymic states). The use of fMRI also permits the comparison of mental states within an individual in a single session versus the group means that are usually compared with PET or SPECT analyses.
BOLD fMRI may be used to compare images taken during one mental state to another in the same patient. Ideally, these states would differ in only one aspect, so that everything is controlled for except the behavior in question. For example, Fu, et al., scanned MDD patients and healthy controls during the presentation of facial stimuli morphed to express discriminable intensities of sadness.72
BOLD-fMRI paradigms generally have several periods of rest alternating with several periods of activation. The images collected during the active phase are then compared with the periods at rest (Figure 4). With current technology, fMRI-BOLD is best used for studying processes that can be purposefully modulated (e.g., language, vision, movement, hearing, and memory).
Figure 4.
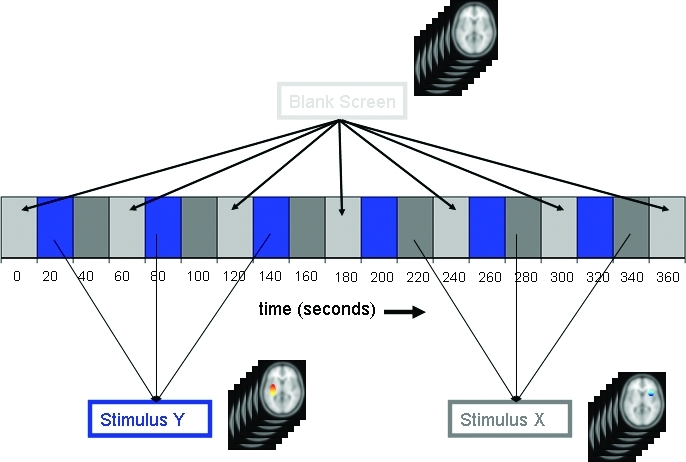
Analyzing fMRI data from a block design
Limitations of the BOLD-fMRI technique include its sensitivity to movement, partially limiting the available tasks to those without head movement (e.g., speaking). Moreover, artifacts can often be found in brain regions neighboring air (i.e., sinuses), potentially complicating the examination of regions at the base of the brain such as the orbitofrontal and medial temporal cortices.
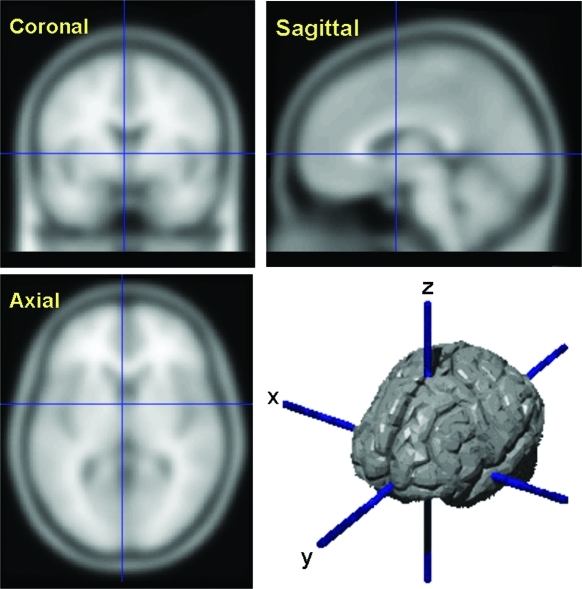
7. How are neuroimaging data analyzed? Neuroimaging analysis begins by projecting the acquired brain data onto a three-dimensional (-x -y -z) coordinate (Figure 5). The smallest unit of measurement is referred to as a voxel, or volume element (i.e., mm3). Thereafter, the choice of image analysis technique employed is driven by the investigators' specific questions, sensitivity to detect small differences in a specific locus, or the ability to survey the entire brain volume for statistically significant differences.
Figure 5.
Neuroimaging coordinates
When an affected brain region can be delimited in MRI scans, region of interest (ROI) analyses offer the greatest sensitivity for detecting abnormalities. Most ROI approaches involve overlaying the PET/SPECT/fMRI functional data on an anatomic MRI image, and manually demarcating the region. The inherent variability in ROI criteria between studies, however, and the absence of ROI validation provides the impetus for an approach that avoids the problems of not validated ROIs through an unbiased survey of the brain at the voxel level.
Statistical parametric mapping (SPM) is a technique that evaluates the whole brain volume independent of distinct neuroanatomical regions and produces a parametric map containing an average value for each voxel. The statistical value, usually a derivative of the t-test, evaluates the hypothesis that the particular voxel is differentially activated between the two groups or conditions.73 Before analysis, all brains are ‘transformed' to fit into a standardized template, corresponding to an average of 305 brains.104 The images are further blurred to minimize the impact of misalignment error and anatomical differences; however, the process inevitably leads to a loss of spatial resolution. Consequently, the procedure offers relatively decreased sensitivity for detecting abnormalities in small structures (e.g., amygdala) or areas characterized by high anatomic variability (e.g., orbital cortex). This decrease in sensitivity, however, is offset by the confidence that activations in brain regions outside an ROI are not ignored.
Voxel-based morphometry (VBM) is an adaptation of the SPM technique to volumetric (structural) analyses. In addition to its unbiased and fully automated whole brain analysis, VBM also permits an evaluation of segmented grey and white matter voxel concentrations.74,75 As in SPM, macroscopic within-group differences are minimized, allowing between-group differences in local tissue composition to be explored without employing unvalidated regions of interest.76 Abnormalities in gray matter distribution have been reported with VBM in subjects with MDD38,77–80 and in other mood disorders, particularly BD.76,81–85
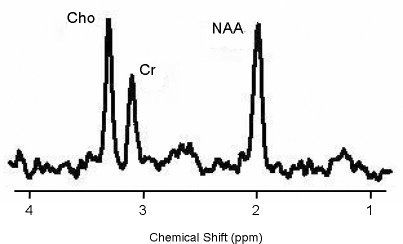
8. Can neuroimaging examine cellular structure? While the MRI technique provides cross-sectional anatomic images based on the tissue water content, magnetic resonance spectroscopy (MRS) is a technique that measures the concentration of in-vivo brain biochemical metabolites. MRS employs a magnetic field and a resonant radio-frequency (RF) pulse to observe the signal from a specific nucleus (e.g., proton [1H] or phosphorus [31P]) in the sample of interest). An MRS “spectrum” is produced with a frequency axis in parts per million (ppm) and a signal amplitude axis. Specific nuclei produce uniquely positioned single or multiple peaks along the frequency axis (Figure 6). The peak position is known as the chemical shift; while the signal amplitude of a peak, which is quantified, is directly related to the concentration of that assigned metabolite.86,100
Figure 6.
The MRS spectrum of neuronal markers
The MRS technique can be tweaked to evaluate disparate intracellular hypotheses through the selection of a particular nucleus of interest, a specific MR field strength, and select data-acquisition parameters. The specific nuclei that are selected will determine what biochemical information can be assessed and the spatial resolution of the signal. The MR signal sensitivity of the more frequently employed 1H spectroscopy is about 15 times greater than that of 31P spectroscopy.
With 1H spectroscopy, one can assess the viability of neurons, glutamate-glutamine neurotransmission cycling, the γ-aminobutyric acid (GABA) neuronal system, and the second messenger metabolism by measuring the metabolite levels of N-acetylaspartate (NAA), glutamate, glutamine, GABA, and myo-inositol, respectively. Spectroscopy using the 31P nuclei enables one to quantify metabolite levels of adenosine triphosphate (ATP), phosphocreatinine (PCr), and inorganic orthophosphate (Pi), which are associated with high- energy phosphate metabolism. Membrane phospholipid (MPL) synthesis and membrane degradation can also be assessed by measuring the freely mobile, water-soluble phosphomonoesters (free-PME, PC, PE) and phosphodiesters (free-PDE, GPC, GPE).
A survey of the MRS studies in bipolar disorder supports frontotemporal abnormalities, with the basal ganglia and thalamic regions showing additional alterations.87 The bioavailability neuronal concentration of psychotropic agents, such as lithium, can also be captured with the MRS technology.88 This information may be useful in determining optimal patient dosing.
MRS evaluations in MDD could be described as preliminary. However, the increased MPL precursor levels in MDD are consistent with findings in depressed bipolar samples, suggesting that a common altered mechanism may underlie the depressive phase of both disorders. Limitations of MRS are spatial resolution, relative paucity of nuclei, and its absence at most centers.
9. Are we only concerned about gray matter? Gray matter primarily consists of nerve cell bodies, dendrites, and unmyelinated axons, while white matter predominantly contains myelinated nerve fibers. These nerve fibers have a creamy white appearance due to myelin, a whitish insulating sheath that functions as an electrical insulator and ensures efficient nerve conduction. The breakdown, destruction, or loss of myelin (demyelination), such as seen in certain neurodegenerative diseases, results in impaired nerve impulse transmission.
Aberrant neurocircuitry has been postulated to underlie the pathophysiology of MDD10 and BD.89 Moreover, the finding that white matter hyperintensities (WMHs) are found at higher rates in patients with mood disorders, particularly late-life or treatment-resistant disorders, implicate white matter abnormalities in mood disorder etiology.90
Diffusion tensor imaging (DTI) measures are thought to be representative of brain tissue microstructure and are particularly useful for examining organized brain regions, such as white matter tract areas. Diffusion tensor imaging measures the water diffusion tensor using diffusion-weighted pulse sequences sensitive to microscopic random water motion. The resultant diffusion-weighted images (DWI) display allows for quantification of water diffusion along axes or diffusion-encoding directions. White matter may exhibit remarkable differences in diffusion, depending on which direction the diffusion sensitizing gradients are applied to and also on the direction of the white matter tracts that are imaged.91
Preliminary DTI investigations indicate impairment in neural connectivity in schizophrenia. Regions specifically identified as having diffusion abnormalities include the corpus callosum and distinct regions of the frontal cortex supporting theories of frontotemporal and frontoparietal disconnectivity in schizophrenia.
Investigators are also beginning to examine DTI alterations in late-life depression and bipolar disorder, conditions complicated by their association with cerebrovascular disease and other neurodegenerative processes.92,101
10. Should neuroimaging techniques be part of the diagnostic workup and management of the patient with mood disorders? As psychiatric symptoms are frequently the earliest signs of CNS pathology, Weinberger conducted a cost-benefit analysis and prescribed the following indications for CT scanning of psychiatric patients: 1) confusion and/or dementia of unknown cause, 2) first episode of a psychosis of unknown etiology, 3) movement disorder of unknown etiology, 4) anorexia nervosa, 5) prolonged catatonia, and 6) first episode of major affective disorder or personality change after age 50.93 A similar cost analysis for structural MRI application in pschiatry has not yet be conducted.
Currently, there are no indications for BOLD-fMRI in clinical psychiatry, although this technique holds considerable promise for unraveling the neuroanatomic basis of psychiatric disease. Structural imaging techniques are most useful for ruling out medical etiologies of mental status disturbances, functional neuroimaging techniques currently have an adjunctive role in the evaluation of dementia and seizure disorders and show promise for the evaluation of primary psychiatric disorders in the future.94 If receptor occupancy is integral in the pharmacotherapy of mood disorders, then PET ligand studies could possibly guide medication dosing.95
Neurologists and neurosurgeons are beginning to use fMRI in the clinical setting to noninvasively map language, motor, and memory function in patients undergoing neurosurgery. Thus, fMRI may be important in understanding how a given individual's brain functions and perhaps, in the future, making psychiatric diagnoses and treatment recommendations.
Discussion
Future of radiotracer development. There is a growing collection of PET and SPECT radiotracers, which are currently being used to investigate numerous neurological targets in psychiatric disorders. As PET technology becomes more widely available, there is potential for growth in the field, with more radiotracers becoming available, targeting a variety of biological sites. The increasing use of PET/SPECT imaging techniques by pharmaceutical companies, as part of drug development strategies, is likely to increase the array of radiotracers available for clinical research.
Integration with clinical measures. MacQueen and colleagues compared hippocampal function, as assessed by performance on hippocampal-dependent recollection memory tests, and hippocampal volumes with MRI, in depressed subjects.96 Although both first- and multiple-episode depressed groups had hippocampal dysfunction apparent on several tests of recollection memory; only depressed subjects with multiple depressive episodes had reduced hippocampal volume. Advanced curve-fitting analysis revealed a significant logarithmic association between illness duration and hippocampal volume. The investigators hypothesized that reductions in hippocampal volume may not antedate illness onset, but may decrease at the greatest rate in the early years after illness onset.
Integration with genetic risk. A common regulatory variant (5-HTTLPR) in the human serotonin transporter gene has been associated with vulnerability for affective disorders, including anxiety and depression. Hariri, et al., employed fMRI to explore the effects of 5-HTTLPR genotype on amygdala reactivity to harm avoidance in a large cohort of volunteers carefully screened for the absence of past and present medical or psychiatric illness.97 The investigators reported that 5-HTTLPR short allele individuals had a significantly greater activation of the amygdala in response to threatening stimuli. As the experiment was conducted in psychiatrically unaffected volunteers, the 5-HTTLPR may represent a classic susceptibility factor for affective disorders by biasing the functional reactivity of the amygdala.
Integration with measure of neuroimmunology. Interferon (IFN)-alpha is notorious for causing behavioral symptoms, including depression, fatigue, and cognitive dysfunction. Capuron and colleagues assessed the effects of low-dose IFN-alpha on brain activity, using functional magnetic resonance imaging during a task of visuospatial attention in patients infected with hepatitis C virus (HCV).98 Despite endorsing symptoms of impaired concentration and fatigue, IFN-alpha-treated patients exhibited task performance and activation of parietal and occipital brain regions similar to that seen in HCV-infected control subjects. In contradistinction, however, the IFN-alpha-treated patients exhibited significantly greater activation in the dorsal part of the anterior cingulate cortex (ACC), which was highly correlated with the number of task-related errors. Consistent with the role of the ACC in conflict monitoring, ACC activation during IFN-alpha administration suggests that neuoroimaging analyses hold the potential to capture cognitive impairments undetectable by conventional neuropsychological exams.
Surgical intervention. Treatment-resistant depression is a severely disabling disorder with no proven treatment options once multiple medications, psychotherapy, and electroconvulsive therapy have failed. Based on consistent observations of hyperactivity in the subgenual cingulate region (Brodmann area 25) in treatment-resistant depression, Mayberg, et al., investigated whether the application of chronic deep brain stimulation to modulate BA25 could reduce this elevated activity and produce clinical benefit in six patients with refractory depression.11 Chronic stimulation of white matter tracts adjacent to the subgenual cingulate gyrus was associated with a striking and sustained remission of depression in four of six patients. Antidepressant effects were associated with a marked reduction in local cerebral blood flow as well as changes in downstream limbic and cortical sites, measured using positron emission tomography. These encouraging results suggest that disrupting focal pathological activity in limbic-cortical circuits using electrical stimulation of brain regions identified by neuroimaging can effectively reverse symptoms in otherwise treatment-resistant depression.
Conclusions
Neuroimaging technology has helped refine pathophysiological models of disease activity in mood disorders and illuminate mechanisms of drug activity. A priority research vista in mood disorders is how to integrate neuroimaging investigations with other research methods (e.g., genetics, endocrinology, etc).
References
- 1.Von Korff M, Katon W, Unutzer J, et al. Improving depression care: Barriers, solutions, and research needs. J Fam Pract. 2001;50(6):E1. [PubMed] [Google Scholar]
- 2.Glassman AH, Shapiro PA. Depression and the course of coronary artery disease. Am J Psychiatry. 1998;155(1):4–11. doi: 10.1176/ajp.155.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients. Results from the Medical Outcomes Study. JAMA. 1989;262(7):914–9. [PubMed] [Google Scholar]
- 4.Demyttenaere K, Bruffaerts R, Posada-Villa J, et al. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291(21):2581–90. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 5.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48(8):813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 6.Soares JC. Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder. Int J Neuropsychopharmacol. 2003;6(2):171–80. doi: 10.1017/S1461145703003390. [DOI] [PubMed] [Google Scholar]
- 7.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8(4):1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 8.Rauch SL. Neuroimaging and neurocircuitry models pertaining to the neurosurgical treatment of psychiatric disorders. Neurosurg Clin N Am. 2003;14(2):213–viii. doi: 10.1016/s1042-3680(02)00114-6. [DOI] [PubMed] [Google Scholar]
- 9.Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 10.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22(1):409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Ordas DM, Labbate LA. Routine screening of thyroid function in patients hospitalized for major depression or dysthymia? Ann Clin Psychiatry. 1995;7(4):161–5. doi: 10.3109/10401239509149621. [DOI] [PubMed] [Google Scholar]
- 13.Erhart SM, Young AS, Marder SR, Mintz J. Clinical utility of magnetic resonance imaging radiographs for suspected organic syndromes in adult psychiatry. J Clin Psychiatry. 2005;66(8):968–73. doi: 10.4088/jcp.v66n0802. [DOI] [PubMed] [Google Scholar]
- 14.Strakowski SM, Adler CM, DelBello MP. Volumetric MRI studies of mood disorders: Do they distinguish unipolar and bipolar disorder? Bipolar Disord. 2002;4(2):80–8. doi: 10.1034/j.1399-5618.2002.01160.x. [DOI] [PubMed] [Google Scholar]
- 15.Konarski JZ, McIntyre RS, Rafi-Tari S, et al. Do volumetric neuroimaging investigations distinguish bipolar disorder and major depressive disorder? [Under Review] [DOI] [PubMed]
- 16.Na C, Doraiswamy PM, Lee KH, Krishnan KR. Magnetic resonance imaging in biological psychiatry. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15(5):581–93. doi: 10.1016/0278-5846(91)90048-6. [DOI] [PubMed] [Google Scholar]
- 17.Salazar OM, VanHoutte P, Plassche WM, Jr, Keller BE. The role of computed tomography in the diagnosis and management of brain tumors. J Comput Tomogr. 1981;5(3):256–67. doi: 10.1016/0149-936x(81)90041-2. [DOI] [PubMed] [Google Scholar]
- 18.Brooks RA, Di CG. Principles of computer assisted tomography (CAT) in radiographic and radioisotopic imaging. Phys Med Biol. 1976;21(5):689–732. doi: 10.1088/0031-9155/21/5/001. [DOI] [PubMed] [Google Scholar]
- 19.Stoll AL, Renshaw PF, Yurgelun-Todd DA, Cohen BM. Neuroimaging in bipolar disorder: What have we learned? Biol Psychiatry. 2000;48(6):505–17. doi: 10.1016/s0006-3223(00)00982-3. [DOI] [PubMed] [Google Scholar]
- 20.Maravilla KR, Sory WC. Magnetic resonance imaging of brain tumors. Semin Neurol. 1986;6(1):33–42. doi: 10.1055/s-2008-1041445. [DOI] [PubMed] [Google Scholar]
- 21.Sheline YI, Barch DM, Donnelly JM, et al. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: An fMRI study. Biol Psychiatry. 2001;50(9):651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- 22.Konarski JZ, McIntyre RS, Grupp LA, Kennedy SH. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders. J Psychiatry Neurosci. 2005;30(3):178–86. [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 24.Ongur D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95(22):13290–5. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konarski JZ, McIntyre RS, Kennedy SH, et al. Review of volumetric neuroimaging findings in bipolar disorders and major depressive disorder. [Under Review] [DOI] [PubMed]
- 26.Husain MM, McDonald WM, Doraiswamy PM, et al. A magnetic resonance imaging study of putamen nuclei in major depression. Psychiatry Res. 1991;40(2):95–9. doi: 10.1016/0925-4927(91)90001-7. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan KR, McDonald WM, Escalona PR, et al. Magnetic resonance imaging of the caudate nuclei in depression. Preliminary observations. Arch Gen Psychiatry. 1992;49(7):553–7. doi: 10.1001/archpsyc.1992.01820070047007. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan KR, McDonald WM, Doraiswamy PM, et al. Neuroanatomical substrates of depression in the elderly. Eur Arch Psychiatry Clin Neurosci. 1993;243(1):41–6. doi: 10.1007/BF02191522. [DOI] [PubMed] [Google Scholar]
- 29.Greenwald BS, Kramer-Ginsberg E, Bogerts B, et al. Qualitative magnetic resonance imaging findings in geriatric depression. Possible link between later-onset depression and Alzheimer's disease? Psychol Med. 1997;27(2):421–31. doi: 10.1017/s0033291796004576. [DOI] [PubMed] [Google Scholar]
- 30.Pillay SS, Renshaw PF, Bonello CM, et al. A quantitative magnetic resonance imaging study of caudate and lenticular nucleus gray matter volume in primary unipolar major depression: relationship to treatment response and clinical severity. Psychiatry Res. 1998;84(2-3):61–74. doi: 10.1016/s0925-4927(98)00048-1. [DOI] [PubMed] [Google Scholar]
- 31.Parashos SA, Tupler LA, Blitchington T, Krishnan KR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Res. 1998;84(1):7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- 32.Naismith S, Hickie I, Ward PB, et al. Caudate nucleus volumes and genetic determinants of homocysteine metabolism in the prediction of psychomotor speed in older persons with depression. Am J Psychiatry. 2002;159(12):2096–8. doi: 10.1176/appi.ajp.159.12.2096. [DOI] [PubMed] [Google Scholar]
- 33.Aylward EH, Roberts-Twillie JV, Barta PE. Basal ganglia Volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry. 1994;151(5):687–93. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- 34.Strakowski SM, DelBello MP, Sax KW, et al. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry. 1999;56(3):254–60. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- 35.Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Res. 2001;106(1):25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 36.Strakowski SM, DelBello MP, Zimmerman ME, et al. Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry. 2002;159(11):1841–7. doi: 10.1176/appi.ajp.159.11.1841. [DOI] [PubMed] [Google Scholar]
- 37.Sheline YI, Gado MH, Kraemer HC. Untreated depression and hippocampal volume loss. Am J Psychiatry. 2003;160(8):1516–8. doi: 10.1176/appi.ajp.160.8.1516. [DOI] [PubMed] [Google Scholar]
- 38.Pizzagalli DA, Oakes TR, Fox AS, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(4):325, 393–25, 405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 39.Videbech P, Ravnkilde B, Gammelgaard L, et al. The Danish PET/depression project: Performance on Stroop's test linked to white matter lesions in the brain. Psychiatry Res. 2004;130(2):117–30. doi: 10.1016/j.pscychresns.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Buchsbaum MS, Wu J, DeLisi LE, et al. Frontal cortex and basal ganglia metabolic rates assessed by positron emission tomography with [18F]2-deoxyglucose in affective illness. J Affect Disord. 1986;10(2):137–52. doi: 10.1016/0165-0327(86)90036-4. [DOI] [PubMed] [Google Scholar]
- 41.Buchsbaum MS, Kesslak JP, Lynch G, et al. Temporal and hippocampal metabolic rate during an olfactory memory task assessed by positron emission tomography in patients with dementia of the Alzheimer type and controls. Preliminary studies. Arch Gen Psychiatry. 1991;48(9):840–7. doi: 10.1001/archpsyc.1991.01810330064010. [DOI] [PubMed] [Google Scholar]
- 42.Post RM, DeLisi LE, Holcomb HH, et al. Glucose utilization in the temporal cortex of affectively ill patients: positron emission tomography. Biol Psychiatry. 1987;22(5):545–53. doi: 10.1016/0006-3223(87)90182-x. [DOI] [PubMed] [Google Scholar]
- 43.Mann JJ, Malone KM, Diehl DJ, et al. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153(2):174–82. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 44.Delgado PL, Price LH, Miller HL, et al. Serotonin and the neurobiology of depression. Effects of tryptophan depletion in drug-free depressed patients. Arch Gen Psychiatry. 1994;51(11):865–74. doi: 10.1001/archpsyc.1994.03950110025005. [DOI] [PubMed] [Google Scholar]
- 45.Berman KF, Doran AR, Pickar D, Weinberger DR. Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? Regional cerebral blood flow during cognitive activation. Br J Psychiatry. 1993;162:183–92. doi: 10.1192/bjp.162.2.183. [DOI] [PubMed] [Google Scholar]
- 46.Kennedy SH, Javanmard M, Vaccarino FJ. A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry. 1997;42(5):467–75. doi: 10.1177/070674379704200502. [DOI] [PubMed] [Google Scholar]
- 47.Parsey RV, Mann JJ. Applications of positron emission tomography in psychiatry. Semin Nucl Med. 2003;33(2):129–35. doi: 10.1053/snuc.2003.127302. [DOI] [PubMed] [Google Scholar]
- 48.Soares JC, Mann JJ. The functional neuroanatomy of mood disorders. J Psychiatr Res. 1997;31(4):393–432. doi: 10.1016/s0022-3956(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 49.Mayberg HS. Positron emission tomography imaging in depression: A neural systems perspective. Neuroimaging Clin N Am. 2003;13(4):805–15. doi: 10.1016/s1052-5149(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 50.Ketter TA, Kimbrell TA, George MS, et al. Baseline cerebral hypermetabolism associated with carbamazepine response, and hypometabolism with nimodipine response in mood disorders. Biol Psychiatry. 199915;46(10):1364–74. doi: 10.1016/s0006-3223(99)00210-3. [DOI] [PubMed] [Google Scholar]
- 51.Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann N Y Acad Sci. 1999;877:614–37. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 52.Clark L, Manes F. Social and emotional decision-making following frontal lobe injury. Neurocase. 2004;10(5):398–403. doi: 10.1080/13554790490882799. [DOI] [PubMed] [Google Scholar]
- 53.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 54.Nobler MS, Oquendo MA, Kegeles LS, et al. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001 February;158(2):305–8. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 55.George MS, Ketter TA, Post RM. SPECT and PET imaging in mood disorders. J Clin Psychiatry. 1993;54(Suppl):6–13. [PubMed] [Google Scholar]
- 56.Eckelman WC, Reba RC, Rzeszotarski WJ, et al. External imaging of cerebral muscarinic acetylcholine receptors. Science. 1984;223(4633):291–3. doi: 10.1126/science.6608148. [DOI] [PubMed] [Google Scholar]
- 57.Wagner HN, Jr, Burns HD, Dannals RF, et al. Imaging dopamine receptors in the human brain by positron tomography. Science. 1983;221(4617):1264–6. doi: 10.1126/science.6604315. [DOI] [PubMed] [Google Scholar]
- 58.Smith GS, Koppel J, Goldberg S. Applications of neuroreceptor imaging to psychiatry research. Psychopharmacol Bull. 2003;37(4):26–65. [PubMed] [Google Scholar]
- 59.Kuhl DE, Koeppe RA, Fessler JA, et al. In-vivo. mapping of cholinergic neurons in the human brain using SPECT and IBVM. J Nucl Med. 1994;35(3):405–10. [PubMed] [Google Scholar]
- 60.Iyo M, Namba H, Fukushi K, et al. Measurement of acetylcholinesterase by positron emission tomography in the brains of healthy controls and patients with Alzheimer's disease. Lancet. 1997;349(9068):1805–9. doi: 10.1016/S0140-6736(96)09124-6. [DOI] [PubMed] [Google Scholar]
- 61.Podruchny TA, Connolly C, Bokde A, et al. In-vivo. muscarinic 2 receptor imaging in cognitively normal young and older volunteers. Synapse. 2003;48(1):39–44. doi: 10.1002/syn.10165. [DOI] [PubMed] [Google Scholar]
- 62.Erlandsson K, Bressan RA, Mulligan RS, et al. Kinetic modelling of [123I]CNS 1261—A potential SPET tracer for the NMDA receptor. Nucl Med Biol. 2003;30(4):441–54. doi: 10.1016/s0969-8051(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 63.Farde L, Hall H, Ehrin E, Sedvall G. Quantitative analysis of D2 dopamine receptor binding in the living human brain by PET. Science. 1986;231(4735):258–61. doi: 10.1126/science.2867601. [DOI] [PubMed] [Google Scholar]
- 64.Costa DC, Verhoeff NP, Cullum ID, Ell PJ, Syed GM, Barrett J, Palazidou E, Toone B, Van RE, Bobeldijk M. In-vivo. characterisation of 3-iodo-6-methoxybenzamide 123I in humans. Eur J Nucl Med. 1990;16(11):813–6. doi: 10.1007/BF00833017. [DOI] [PubMed] [Google Scholar]
- 65.Wong DF, Wagner HN, Jr, Dannals RF, et al. Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain. Science. 1984;226(4681):1393–6. doi: 10.1126/science.6334363. [DOI] [PubMed] [Google Scholar]
- 66.Passchier J, van WA, Pieterman RM, et al. In-vivo. delineation of 5-HT1A receptors in human brain with [18F]MPPF. J Nucl Med. 2000;41(11):1830–5. [PubMed] [Google Scholar]
- 67.Szabo Z, Kao PF, Scheffel U, et al. Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652. Synapse. 1995;20(1):37–43. doi: 10.1002/syn.890200107. [DOI] [PubMed] [Google Scholar]
- 68.Houle S, Ginovart N, Hussey D, et al. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C]DAPP and [11C]DASB. Eur J Nucl Med. 2000;27(11):1719–22. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- 69.Meyer JH, Wilson AA, Ginovart N, et al. Occupancy of serotonin transporters by paroxetine and citalopram during treatment of depression: A [(11)C]DASB PET imaging study. Am J Psychiatry. 2001;158(11):1843–9. doi: 10.1176/appi.ajp.158.11.1843. [DOI] [PubMed] [Google Scholar]
- 70.Tauscher J, Verhoeff NP, Christensen BK, et al. Serotonin 5-HT1A receptor binding potential declines with age as measured by [11C]WAY-100635 and PET. Neuropsychopharmacology. 2001;24(5):522–30. doi: 10.1016/S0893-133X(00)00227-X. [DOI] [PubMed] [Google Scholar]
- 71.Maziere M. Cholinergic neurotransmission studied in vivo using positron emission tomography or single photon emission computerized tomography. Pharmacol Ther. 1995;66(1):83–101. doi: 10.1016/0163-7258(95)00003-y. [DOI] [PubMed] [Google Scholar]
- 72.Fu CH, Williams SC, Cleare AJ, et al. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61(9):877–89. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- 73.Friston KJ. Statistical parametric mapping. In: Thatcher RW, Hallett M, et al., editors. Functional Neuroimaging: Technical Foundations. San Diego, CA: Academic Press; 1994. pp. 79–83. [Google Scholar]
- 74.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 76.Bruno SD, Barker GJ, Cercignani M, et al. A study of bipolar disorder using magnetization transfer imaging and voxel-based morphometry. Brain. 2004;127(Pt 11):2433–40. doi: 10.1093/brain/awh274. [DOI] [PubMed] [Google Scholar]
- 77.Shah PJ, Ebmeier KP, Glabus MF, Goodwin GM. Cortical grey matter reductions associated with treatment-resistant chronic unipolar depression. Controlled magnetic resonance imaging study. Br J Psychiatry. 1998;172:527–32. doi: 10.1192/bjp.172.6.527. [DOI] [PubMed] [Google Scholar]
- 78.Bell-McGinty S, Butters MA, Meltzer CC, et al. Brain morphometric abnormalities in geriatric depression: Long-term neurobiological effects of illness duration. Am J Psychiatry. 2002;159(8):1424–7. doi: 10.1176/appi.ajp.159.8.1424. [DOI] [PubMed] [Google Scholar]
- 79.Ballmaier M, Toga AW, Blanton RE, et al. Anterior cingulate, gyrus rectus, and orbitofrontal abnormalities in elderly depressed patients: an MRI-based parcellation of the prefrontal cortex. Am J Psychiatry. 2004;161(1):99–108. doi: 10.1176/appi.ajp.161.1.99. [DOI] [PubMed] [Google Scholar]
- 80.Shah PJ, Glabus MF, Goodwin GM, Ebmeier KP. Chronic, treatment-resistant depression and right fronto-striatal atrophy. Br J Psychiatry. 2002;180:434–40. doi: 10.1192/bjp.180.5.434. [DOI] [PubMed] [Google Scholar]
- 81.Frangou S, Hadjulis M, Chitnis D, et al. The Maudsley Bipolar Disorder Project: Brain structural changes in bipolar 1 disorder. Bipolar Disord. 2002;4(S1):123–4. [Google Scholar]
- 82.Wilke M, Kowatch RA, DelBello MP, et al. Voxel-based morphometry in adolescents with bipolar disorder: First results. Psychiatry Res. 2004;131(1):57–69. doi: 10.1016/j.pscychresns.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 83.Lyoo IK, Kim MJ, Stoll AL, et al. Frontal lobe gray matter density decreases in bipolar I disorder. Biol Psychiatry. 2004;55(6):648–51. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 84.McIntosh AM, Job DE, Moorhead TW, et al. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biol Psychiatry. 2004;56(8):544–52. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 85.Lochhead RA, Parsey RV, Oquendo MA, Mann JJ. Regional brain gray matter volume differences in patients with bipolar disorder as assessed by optimized voxel-based morphometry. Biol Psychiatry. 2004;55(12):1154–62. doi: 10.1016/j.biopsych.2004.02.026. [DOI] [PubMed] [Google Scholar]
- 86.Stanley JA. In-vivo. magnetic resonance spectroscopy and its application to neuropsychiatric disorders. Can J Psychiatry. 2002;47(4):315–26. doi: 10.1177/070674370204700402. [DOI] [PubMed] [Google Scholar]
- 87.Silverstone PH, McGrath BM, Kim H. Bipolar disorder and myo-inositol: a review of the magnetic resonance spectroscopy findings. Bipolar Disord. 2005;7(1):1–10. doi: 10.1111/j.1399-5618.2004.00174.x. [DOI] [PubMed] [Google Scholar]
- 88.Soares JC, Boada F, Spencer S, et al. Brain lithium concentrations in bipolar disorder patients: Preliminary (7)Li magnetic resonance studies at 3 T. Biol Psychiatry. 2001;49(5):437–43. doi: 10.1016/s0006-3223(00)00985-9. [DOI] [PubMed] [Google Scholar]
- 89.Haldane M, Frangou S. New insights help define the pathophysiology of bipolar affective disorder: Neuroimaging and neuropathology findings. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(6):943–60. doi: 10.1016/j.pnpbp.2004.05.040. [DOI] [PubMed] [Google Scholar]
- 90.Regenold WT, Hisley KC, Obuchowski A, et al. Relationship of white matter hyperintensities to cerebrospinal fluid glucose polyol pathway metabolites: A pilot study in treatment-resistant affective disorder patients. J Affect Disord. 2005;85(3):341–50. doi: 10.1016/j.jad.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 91.Taylor WD, Hsu E, Krishnan KR, MacFall JR. Diffusion tensor imaging: Background, potential, and utility in psychiatric research. Biol Psychiatry. 2004;55(3):201–7. doi: 10.1016/j.biopsych.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Alexopoulos GS, Kiosses DN, Choi SJ, et al. Frontal white matter microstructure and treatment response of late-life depression: A preliminary study. Am J Psychiatry. 2002;159(11):1929–32. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 93.Weinberger DR. Brain disease and psychiatric illness: When should a psychiatrist order a CAT scan? Am J Psychiatry. 1984;141(12):1521–7. doi: 10.1176/ajp.141.12.1521. [DOI] [PubMed] [Google Scholar]
- 94.Rauch SL, Renshaw PF. Clinical neuroimaging in psychiatry. Harv Rev Psychiatry. 1995;2(6):297–312. doi: 10.3109/10673229509017151. [DOI] [PubMed] [Google Scholar]
- 95.Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? A new hypothesis. Am J Psychiatry. 2001;158(3):360–9. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- 96.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100(3):1387–92. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hariri AR, Drabant EM, Munoz KE, et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62(2):146–52. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 98.Capuron L, Miller AH. Cytokines and psychopathology: Lessons from interferon-alpha. Biol Psychiatry. 2004;56(11):819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Paushter DM, Madic MT, Borkowoki GP, et al. Magnetic resonance: Principles and applications. Med Clin North Am. 1984;68(6):1393–421. doi: 10.1016/s0025-7125(16)31069-0. [DOI] [PubMed] [Google Scholar]
- 100.Moore GJ, Galloway MP. Magnetic resonance spectroscopy: Neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36(2):5–23. [PubMed] [Google Scholar]
- 101.Beyer JL, Taylor WD, Thecfall JR, et al. Corticol white matter miscrostructural abnormalities in bipolar disorder. Neuropsychopharmacology. 2005;30(12):2225–9. doi: 10.1038/sj.npp.1300802. [DOI] [PubMed] [Google Scholar]
- 102.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48(8):830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 103.Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158(6):899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- 104.Mazziotta JC, Toga AW, Evans A. etal. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2(2):89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]