Abstract
The objective of this paper is to review recent studies on comorbidity and treatment of major depression (MD) and attention deficit/hyperactivity disorder (ADHD) in children and adolescents. Both ADHD and MD are commonly associated with other DSM-IV Axis I psychiatric disorders. ADHD is more commonly associated with oppositional defiant disorder and conduct disorder in children and adolescents. The literature on comorbidities of MD and ADHD suggests that when these two disorders occur together, they bring their own unique profiles, often including a number of other psychiatric disorders and severe symptoms. The guidelines for the use of first-line ADHD medications (psychostimulants and atomoxetine) and the use of antidepressants in patients with MD comorbid with ADHD (with and without psychostimulants) will also be reviewed. Recommendations for the sequencing of these medications in patients with comorbid MD and ADHD and other disorders (anxiety disorders, oppositional defiant disorder, conduct disorder) will also be made. The concept of “goodness of fit” as it applies to medication choices will also be outlined. Some antidepressants, such as imipramine, desipramine, and bupropion have been effective in treating major depression, anxiety disorders, and ADHD in adults. Tricyclic antidepressants have not been as effective in treating MD in children and adolescents; however, they can be used to treat adults with ADHD and MD. Some of the SSRIs are proven to be effective and safe in children and adolescents and can be considered in patients with comorbid MD and ADHD.
Keywords: Child and Adolescent, Depression, Attention Deficit/Hyperactivity Disorder, ADHD
Depression is a chronic disorder for many patients, as is attention deficit/ hyperactivity disorder (ADHD). Most studies indicate prevalence rates of 9 to 38 percent for depressive disorders in children with ADHD.1–3 The treatment of these patients presents major challenges to clinicians. Major depression (MD) often exacerbates the symptoms and dysfunctions of ADHD. Patients who have ADHD and who later develop MD may present with more severe dysfunctions than those who have MD alone; this may also worsen treatment outcome.4
Treatment and recovery require a full understanding of the comorbid symptoms and disorders commonly associated with MD and ADHD in children and adolescents. As recent clinical studies on the comorbidity of MD in children and adolescents indicate, approximately 1 in 5 children and adolescents with MD suffer only from MD, while the other 4 out of the 5 have other associated Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Axis I psychiatric disorders. Over half of the patients with MD have at least two other comorbid disorders.5
Anxiety disorders (AD), ADHD, and oppositional defiant disorders (ODD) are highly comorbid with MD. Recent comorbidity studies of MD and ADHD indicate that ADHD is frequently associated with MD, dysthymic disorder (DD), and bipolar disorder (BP).6–9 In many cases, “internalizing” and “externalizing” disorders are not entirely separate but closely related. Many patients with ADHD also suffer from “demoralization,” or a low/sad mood and chronic unhappiness. The inability to live up to their potential creates a sense of loss, and many experience “the inner pain.”10
A clinical study11 reported that among comorbid disorders, AD, ODD, and conduct disorder (CD) were the most common comorbid conditions in children and adolescents with MD (Table 1). The sample consisted of 188 (51.5%) boys and 177 (48.5%) girls (up to age 18) with MD. Each patient was assessed by a child and adolescent psychiatrist and a mental health team, including a child psychologist and social worker. The DSM-IV12 was used in diagnosis. Gadow and Sprafkin Early Childhood, Child, and Adolescent Symptom Inventories were completed to aid the diagnosis and differential diagnosis.13–15 Turgay Mood Disorders Structured Interview Guidelines and Rating Scales,16 which covers all DSM-IV diagnostic criteria items for MD and DD, was also completed by the patients and parents, and clinical interviews with the patients and their families were completed as part of the diagnostic process. Of the patients screened, 104 patients (28.4%) had a diagnosis of MD alone, while the remaining had comorbid disorders. ADHD was the most common comorbidity; 125 (34.24%) patients were diagnosed as ADHD (65% were combined type) as well. CD was diagnosed in 45 patients (12.32% of all patients); 62.32% were boys. A total of 112 patients (30.68% of the total) had generalized anxiety disorder, half of whom were boys. It was concluded that MD in a sample of children and adolescents was commonly associated with other serious psychiatric disorders in the majority of the patients. With the use of structured interviews and screening/rating scales, these disorders need to be carefully assessed and identified in order to offer the most effective and disorder-specific treatment alternatives.
Table 1.
Comorbidity differences in major depression and dysthymic disorder in children and adolescents
| Major Depression Turgay, et al.11 N=365 | Dysthymic Disorder Turgay, et al.17 N=240 | |
|---|---|---|
| Major Depression or Dysthymic Disorder only | 28.4% | 8.72 % |
| Attention Deficit/Hyperactivity Disorder | 34.24% | 62.9% |
| Oppositional Defiant Disorder | 26.84% | 41.7% |
| Generalized Anxiety Disorder | 30.68% | 28.3% |
| Conduct Disorder | 12.32% | 29.2% |
In some cases, DD is associated with MD. The presence of DD in patients with MD complicates the clinical picture further as DD increases the risk for other comorbid disorders.
A clinical study assessed a sample of 240 children and adolescents (ages 4–18 years) with DD and reported similar comorbidity profiles. The findings of both studies are outlined in Table 1.
Approximately 70 percent of children and adolescents with DD will eventually develop MD, resulting in the presence of both disorders; this is referred to as a “double depression” and is often associated with a protracted course.18
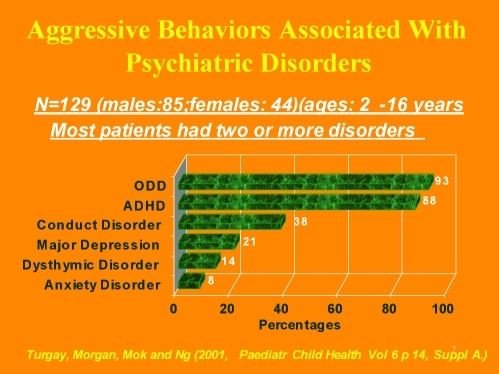
Whether it is due to the common comorbidity of ADHD and other disruptive behavior disorders in children and adolescents with MD, aggression is also a problem behavior in some patients with DD or MD. Figure 1 focuses on the relationship between aggression and psychopathology and outlines the percentages of different psychiatric disorders in a clinical population with severe aggression.19
Figure 1.
Aggressive behaviors associated with psychiatric disorders
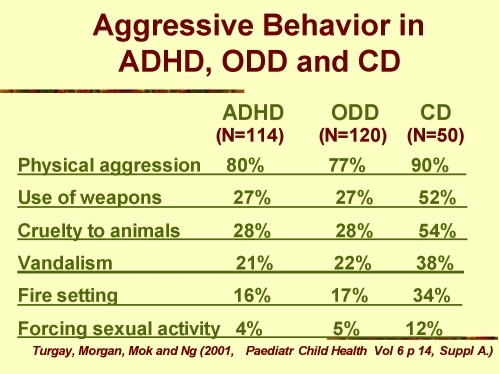
The association between ADHD, ODD, and CD is common; when ADHD becomes part of the clinical picture with MD, verbal and physical aggression also become serious problems. Figure 2 illustrates a clinical study that focused on the frequency and nature of aggressive behavior in MD/ADHD comorbidities.19
Figure 2.
Aggressive behavior in ADHD, ODD, and CD
In the presence of ADHD in children and adolescents with MD, clinicians must also screen and assess aggressive behaviors in many patients with ADHD, since these patients demonstrate these symptoms when they become depressed. The presence of the multiple internalizing and externalizing behavior disorders will contribute to making the differential diagnosis and treatment quite complicated. In working with patients with multiple comorbid disorders, Gadow and Sprafkin age-specific symptom checklists covering all DSM-IV disorders will be very useful (www.Checkmateplus.com).
ADHD in children and adolescents
Clinicians who treat children and adolescents with depression and comorbid ADHD must be familiar with the numerous target symptoms across both disorders and the associated comorbid disorders in order to accurately monitor and treat their patients.
ADHD is a common disorder in children, adolescents, and adults with good treatment responses (i.e., medication, psychosocial, and educational interventions). Approximately 8 to 10 percent of boys and 3 to 4 percent of girls under the age of 18 have ADHD, and about 80 percent of children with ADHD continue meeting the diagnostic criteria for ADHD into adolescence; approximately 60 percent will maintain some core symptoms of ADHD into adulthood. The nature of the presentation and symptomatology is different for the various age groups. Although there are some differences in symptomatology and comorbidity between male and female subjects, the impact of the disorder on patients and their families is similar.4
The symptoms and subtypes of ADHD and associated comorbid disorders change significantly throughout the life cycle. Hyperactivity and impulsivity often decrease as patients get older; however, the demands on attention and other cognitive skills may increase. The ratios of ADHD/ADD cases will get smaller as ADHD predominantly inattentive type is the most common subtype in adulthood.4
The most common comorbid psychiatric disorder in early childhood is ODD. The risk for the development of CD in children with both ADHD and ODD is 2 to 3 times greater than in children with ADHD without ODD. The presence of ODD in children with ADHD should be regarded seriously, as ODD can often serve as a transition to the more serious pathology CD. Language disorders and enuresis are also common. In approximately 50 to 70 percent of children with ADHD, there are either general or specific learning disabilities. ADHD is 2 to 3 times more common in children with developmental disabilities or borderline and mental retardation. In the school age years, symptoms of anxiety may also be observed, while symptoms of mood disorders may be more prevalent in early adolescence. For many patients with ADHD symptoms, tics and Tourette syndrome are also common in childhood. In adulthood, the most common comorbid disorders are anxiety and mood disorders.20 Tics in these patients need to be differentiated from extrapyramidal symptoms that may be side effects of medication. The multiple disorders in MD/ADHD patients need close monitoring to maximize a positive treatment outcome and minimize side effects.21
As ADHD often has a number of comorbid disorders associated with it, clinicians must be familiar with the wide range of symptoms that may occur and impede treatment. The following section outlines the main features of ADHD comorbidities.
A clinical study22 with a large sample (n=2902) focused on ADHD comorbidities, age, and gender relationships. In the diagnosis of ADHD and comorbid disorders, DSM-IV diagnostic criteria, DuPaul ADHD Rating Scale, Gadow-Sprafkin Child Symptom Inventory IV, and Offord and Boyle Ontario Child Health Study were used. As reported in Table 2, there was no incidence of MD in the 2- to 5-year-old group, but the frequency of DD and MD increased with age. In adults, 35.71 percent of men and 54.02 percent of women with ADHD had MD also. Table 2 outlines age, gender, and comorbidity relationships with ADHD. The prevalence of mood and anxiety disorders increases by age. Disruptive behavior disorders (ODD and CD) are more common in male subjects, while DD, MD, ADs are more common in female subjects. Table 2 supports the hypothesis that in MD/ADHD comorbidities, MD is added to the long-standing presence of ADHD with its comorbid disorders. Figure 1 indicates that ADHD, ODD, and CD are present in early life but the occurrence of DD, MD, and AD increases with age.
Table 2.
ADHD Comorbidity, age and gender relations22
| Age: 2 to 5 years | Males | Females | Total |
|---|---|---|---|
| ODD | 62.21% | 52.63% | 60.67% |
| CD | 22.74% | 17.54% | 21.91% |
| DD | 1.00% | 0.00% | 0.84% |
| MD | 0.00% | 0.00% | 0.00% |
| AD | 3.68% | 7.02% | 4.21% |
| PDDs | 20.07% | 24.56% | 20.79% |
| ADHD only | 10.37% | 7.02% | 9.83% |
| Total | 299 | 57 | 356 |
| Age: 6 to 12 years | Males | Females | Total |
|---|---|---|---|
| ODD | 62.44% | 52.23% | 60.36% |
| CD | 20.49% | 14.54% | 19.27% |
| AD | 10.77% | 13.35% | 11.30% |
| DD | 3.79% | 5.04% | 4.05% |
| PDDs | 3.72% | 2.67% | 3.50% |
| MD | 2.43% | 1.78% | 2.30% |
| ADHD only | 21.32% | 25.22% | 22.11% |
| Total | 1318 | 337 | 1655 |
| Age: 13 to 18 years | Males | Females | Total |
|---|---|---|---|
| ODD | 61.97% | 60.54% | 61.62% |
| CD | 30.87% | 27.89% | 30.13% |
| AD | 14.32% | 15.65% | 14.65% |
| MD | 10.29% | 25.17% | 13.97% |
| DD | 12.53% | 17.01% | 13.64% |
| PDDs | 2.46% | 2.04% | 2.36% |
| ADHD only | 17.23% | 12.24% | 15.99% |
| Total | 447 | 147 | 594 |
| Age: >18 years | Males | Females | Total |
|---|---|---|---|
| MD | 35.71% | 54.02% | 41.08% |
| AD | 14.76% | 27.59% | 18.52% |
| DD | 12.86% | 16.09% | 13.80% |
| ODD | 5.24% | 3.45% | 4.71% |
| CD | 0.48% | 3.45% | 1.35% |
| PDDs | 0.00% | 0.00% | 0.00% |
| ADHD only | 36.19% | 22.99% | 32.32% |
| Total | 210 | 87 | 297 |
KEY—
- OCD:
obsessive-compulsive disorder
- CD:
conduct disorder
- AD:
anxiety disorders
- MD:
major depression
- DD:
dysthymic disorder
- PDDs:
pervasive developmental disorders
- ADHD:
attention deficit/hyperactivity disorder
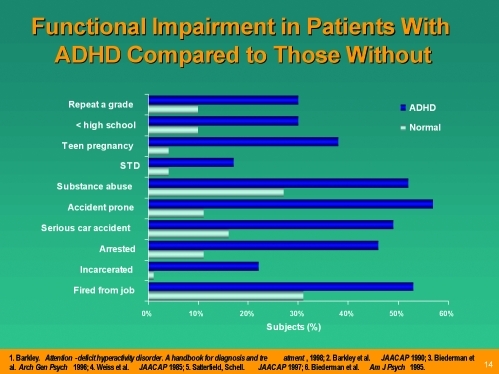
Figure 3 demonstrates the functional impairments that are often associated with ADHD in adolescents. The rate of self-injurious behavior and suicide is 2 to 3 times higher in children and adolescents with ADHD. The presence of impulsivity and aggression in patients with ADHD may significantly increase the rates of suicide in children and adolescents with MD and ADHD.
Figure 3.
Functional impairment in patient with ADHD compared to those without
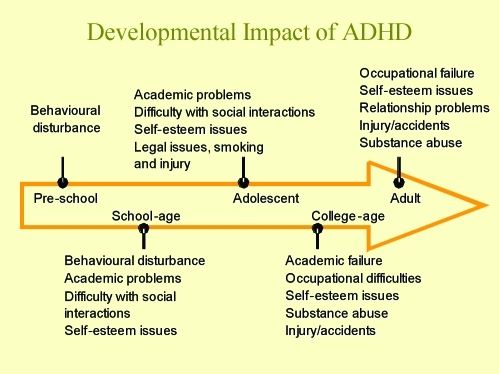
Figure 4 outlines the impact of ADHD at different developmental stages. It is apparent that the presence of MD will increase the negative impact in these areas. Most patients with an early childhood presentation of ADHD also experience severe functional, behavioral, and cognitive and executive dysfunctions. These factors will contribute to poorer performance and quality of life.22
Figure 4.
Developmental impact of ADHD
The negative impact of ADHD throughout the life cycle often interferes with each developmental stage and presents multiple challenges in adolescence and early adulthood. During these times, anxiety and mood disorders often increase in prevalence and further complicate the clinical picture for adolescents and young adults. Recovery, remission, and prevention from further major depressive episodes may not be possible for many patients with ADHD.
Many children and adolescents with ADHD describe the presence of depressive symptoms even when they do not meet the full diagnostic criteria for MD. The sense of “chronic unhappiness,” a common complaint from individuals with ADHD, may reach the severity level of a DD. The patients with comorbid ADHD and MD often present a major challenge in terms of treatment. The rate of suicide is much higher in patients with both ADHD and MD as compared with patients with MD alone. Both disorders may aggravate the symptoms of the other. An adolescent with a long history of inattentiveness and overactivity demonstrates some worsening of these symptoms with the establishment of MD. The addition of sleep disorder, particularly insomnia associated with MD, further contributes to problems associated with inattention.4,5,20
Pharmacological Treatment of MD With ADHD
Although seriousness of the combination of MD and ADHD was recognized by many clinicians for many years, there has not been any major focus on the systematic, randomized, clinical medication trials for treatment. No studies have compared stimulant responsiveness in ADHD and ADHD/MD subgroups, nor have there been any studies of differential response of these groups to psychotherapy. It is widely assumed that antidepressants might have a particular role in treating ADHD/MD subgroup, given the ability of these drugs to treat the symptoms of both ADHD and MD.23 Desipramine, however, was found to treat ADHD alone and ADHD with depression equally well, at least in terms of ADHD symptoms.24 It has been shown that fluoxetine and tricyclic antidepressants can be safely combined with stimulants. No unusual side effects were observed in any of these studies.25,26
Canadian ADHD Practice Guidelines27 recommend that both psychostimulants and atomoxetine are first-line medications and suggest that when possible, long-acting medications should be preferred to short-acting medications. Recent studies indicated that treatment outcome, remission rates, and patient, family, and teacher preference show that long-acting medications are superior to short-acting medications.27
Although the frequent comorbidity between MD and ADHD have been identified for many years, the evidence-based, systematic research on the effectiveness and safety of single or multiple medication use in this high-risk patient group lagged behind. In one of the small sample size clinical studies,28 fluoxetine and methylphenidate were combined for treatment of ADHD and comorbid depressive disorder, and effectiveness and safety of this combination were reported. The patients were taking 17 to 60mg of methylphenidate daily and had had at least a partial response to it. Patients were placed on fluoxetine in doses ranging from 2.5 to 20mg/day, with 60 percent of the subjects receiving the maximum dose. Significant improvements in attention, conduct, mood, and school work were observed with no usual side effects. The sample size was small, and it is hoped that this will be replicated in a larger study.
In June of 1998, a consensus conference was held in Dallas, Texas. US experts in child and adolescent mood disorders presented reviews of recent research on childhood depression and participated in developing the consensus algorithms.29 The Texas Children's Medication Algorithm Group reported on the consensus on medication treatment of childhood major depressive disorder with other specific comorbid disorders. The consensus was reached for the following general principles:
It is recognized that MD presents across the life span and is, in many cases, recurrent.
Psychoeducation is a critical element of medication treatment.
Parents and children should be informed about non-medication treatment alternatives.
The decision whether to try medication versus psychotherapy is left to clinician judgment with patient and family involvement in the decision process.
Child-specific assessments and outcome measures are important.
Treatment decisions are based on science and expert clinical consensus, not cost.
The panel emphasized that the diagnostic criteria are essentially the same as for adult MD except for inclusion of irritable mood, but the diagnosis process is different, requiring synthesizing information from both parents and children separately. It was suggested that when MD is identified to be with sufficient severity and the decision is made to use antidepressant medication, a monotherapy with a SSRI is initiated. It was noted that nonspecific therapies (supportive, psychodynamic, family therapy, etc.) are often essential in addition to medication as part of an individual treatment plan.
From the group of SSRIs, fluoxetine was recommended as a first-line treatment because of the safety and efficacy data and approval by the FDA.30 In open trials, sertraline appeared to be effective in treating adolescent outpatients with depression.31 A fluoxetine open-label trial was positive for fluoxetine.32 The positive effects of fluoxetine in treatment of depression in children and adolescents seemed to be more impressive than TCAs and other nontricyclic antidepressants (e.g., bupropion, nefazadone, venlafaxine, and mirtazepine) due to lack of supporting randomized, controlled studies of these medications against placebo demonstrating efficacy and effectiveness in childhood and adolescent depression, although data exists for adults.29,33
In another clinical study, paroxetine was compared to imipramine in adolescents with MD and was found to be effective and safe.31 It was noted that no noradrenergic antidepressants or TCAs metabolized to primarily noradrenergic metabolite (imipramine) have been shown to be effective in children and adolescents in the treatment of MD.34 In addition, TCAs have a relatively less favorable side effect profiles, higher patient attrition rate in the acute phase as compared with SSRIs, and high risk of toxicity, including lethality in overdose.
The Texas Children's Medication Algorithm Group recommended a second trial with SSRIs if the depressed child or adolescent did not respond well to the first SSRI trial. The consensus was reached by the Texas group to consider augmenting the antidepressants with lithium, thyroid, low-dose bupropion, and stimulants.29 For the patients who did not show sufficient recovery with SSRIs, it was suggested to try TCAs, venlafaxine, nefazodone, bupropion, or mirtazepine, although systematic placebo-controlled studies with large samples are still not available for these medications.
For the patients with MD and ADHD, the Texas group recommended a sufficient treatment with psychostimulants for at least two weeks and SSRI addition if the depressive symptoms did not improve sufficiently. For some suicidal patients with major depression, inpatient and/or residential treatment can be considered when the suicide risk is high, and it may not be wise to start only with psychostimulants and wait for an antidepressant effect. An immediate start with an SSRI with well-proven effectiveness (e.g., fluoxetine) that can also be combined with psychostimulants can be considered when the risk of suicide and/or serious self injurious behavior is found to be high. Many strategies and tactics are recommended to maximize the treatment effectiveness in complicated cases of MD that do not respond well to treatment.35
In the presence of both ADHD and MD in patients who are not on any medication, researchers with extensive experience in working with ADHD, mood disorders, and multiple comorbidities in children and adolescents36 presented quite useful directions for treatment actions. When depressive symptoms are found to be more predominant, accounting for 50 percent or more of the global clinical severity with marked loss of appetite, weight loss, severe insomnia, a well-planned suicide attempt and/or strong suicidal intent, and a past history of nonresponse or significant side effects to stimulants, starting with an antidepressant was recommended. The same authors recommended starting the treatment with psychostimulants when ADHD symptoms are more prominent, accounting for 50 percent or more of the global clinical severity, with minimal neurovegetative signs of depression, suicidal ideation but no intent or plan, and when history of ADHD precedes MD by more than a year. It was strongly recommended that one should never use a combination of a stimulant and an antidepressant as first-line treatment, since “one can never tell for sure when the depressive symptoms are secondary to frustration with the ADHD symptoms,” in which case it is possible that the depression will remit in response to successful treatment of the ADHD.” It was suggested that when the mood disorder completely remits after psychostimulant therapy alone, one might even question the validity of the MD diagnosis in that patient. The authors also stressed that MD should not be viewed as a contraindication to stimulant treatment. It has been suggested that once the first drug has been titrated to appropriate levels, the clinician should carefully examine the response rate to the first drug. Did the antidepressant improve the ADHD symptoms in addition to improving the mood? Did the stimulant affect the mood symptoms as well as the ADHD symptoms? The Texas group proposed a clinically meaningful decision tree in treating children and adolescents with ADHD and MD. They suggested leaving the patient on stimulant medication if the ADHD and depressive symptoms improve. If the ADHD and/or depressive symptoms worsen, the stimulant should be discontinued.
For the patients showing improvement in ADHD symptoms but no change in depressive symptoms, an addition of an antidepressant is suggested. Patients who were started on antidepressants should continue with antidepressants if both depressive and ADHD symptoms are improved. When the depressive symptoms are not improved, switching to a different class of antidepressant is recommended. When the depressive symptoms are improved with antidepressants but ADHD symptoms remain serious, adding psychostimulants is recommended. In children with serious depression, the Texas group recommends the use of bupropion because of its effectiveness as an antidepressant with positive effects in controlling ADHD. They also suggest that the clinician should try at least two different SSRIs if a patient is not responding to bupropion after four weeks of adequate dose. These guidelines are frequently quoted in many publications and can be supported as reliable guidelines until further evidence-based information becomes available through more extensive research in the treatment of ADHD/MD.
A recent clinical study37 assessed the tolerability and safety of atomoxetine combined with fluoxetine as well as the value of atomoxetine as a monotherapy for ADHD in the presence of depression or anxiety. Patients were randomized to treatment with fluoxetine (n=127) or placebo (n=46) under double-blind conditions for eight weeks, with concomitant atomoxetine use the last five weeks. At the end point, reductions in ADHD, depressive, and anxiety symptoms were marked for both treatment groups. Some differences between treatment groups for depressive symptoms were significant, but the magnitude of the differences were small and likely of limited clinical importance. The combination group had greater increases in blood pressure and pulse than did the monotherapy group. It was concluded that in pediatric patients with ADHD and comorbid symptoms of depression or anxiety, atomoxetine monotherapy appears to be effective for treating ADHD. Anxiety and depressive symptoms also improved, but the authors cautioned that the absence of a placebo-only arm did not allow them to conclude that these were specifically the result of treatment with atomoxetine. They also reported that combined atomoxetine/ fluoxetine therapy was well tolerated.
During the last five years, the clinicians struggling with children and adolescents with MD and multiple comorbidities observed the development of clinical guidelines by national professional organizations. In the absence of clear research evidence, the development of a consensus based on clinical experience provided useful guidelines for the clinicians in the front line. The American Academy of Child and Adolescent Psychiatry published their Practice Parameter for the use of stimulant medications in the treatment of children, adolescents, and adults and suggested that MD should be treated with antidepressants with or without psychostimulants for the patients with MD and comorbid ADHD.38
Canadian ADHD Practice Guidelines recognized the frequent comorbidity of MD and ADHD and made recommendations first to determine the severity of the disorders and then to treat the most disabling disorder first. The guidelines also recognized the presence of mild antidepressant effects of psychostimulants.27 The guidelines also noted that some patients may develop dysphoric symptoms while they are on psychostimulants and suggested that these patients be switched to non-stimulant medications if dose adjustment does not improve the depressive feelings.
Suicidal behavior has been reported in children and adolescents who are prescribed antidepressants, both in case reports and in clinical trials. One difficulty in interpreting suicidal behavior in depression studies is that suicide attempts and suicidal ideation are common symptoms of depression. If a suicide attempt is made during the course of treatment, it is difficult to identify the cause or causes of the event. It could be a lack of improvement or worsening of depressive symptoms, increased activation (increased energy either from improvement in the mood disorder or due to the medication), or it could be directly linked to the medication. The use of a placebo-control group helps to answer some but not all of these questions.
For all indications, the relative risk of suicide-related events was significantly increased among subjects given medication. The same was true in the trials of therapy for major depressive disorder. The average risk of such events among patients receiving antidepressants was four percent, twice that among patients given placebo (2%). Because of the relative rarity of these events (97 among more than 4200 children and adolescents included in the trials), the difference was only significant when data from all of the trials were pooled. Except for venlafaxine, individual medications were not statistically more likely than other medications to lead to suicidal behavior. Although there was increased risk of suicidal behavior associated with antidepressants, this risk included suicide attempt, preparatory acts, and suicidal ideation. Suicidal ideation accounted for the majority of the events, and there were no completed suicides among the 4400 youths included in the trials. There is strong support to continue treating children and adolescents with MD and ADHD with these medications, if and when indicated while monitoring these patients closely for any development of suicidal ideation and behavior. FDA recommended weekly follow-up during the dose adjustment stage.
Clinical Guidelines in Medication Selection for the Treatment of ADHD and Other Comorbid Disorders
There is an ample support in the literature based on a long, extensive research history for the use of ADHD medication in the treatment of ADHD alone or ADHD with other comorbid disorders. The clinicians are struggling with the decision on which medication(s) to use. This part of the article will attempt to provide some guidelines in medication choices. For the dosages to be used, the readers are referred to clinical guidelines recently developed by national organizations and product monographs of these medications.27,38
Until the FDA approval of atomoxetine, psychostimulants were the only approved medications for ADHD treatment. With the formal approval of atomoxetine by the FDA and other regulatory bodies in other parts of the world, a debate was started about what medications should be considered as first-line treatment agents. The following three schools of thought arose:
Those who supported the use of psychostimulants as first-line treatment
Those who supported the use of atomoxetine as the first-line treatment
Those who supported the use of both medications as the first-line treatment, as well as considering patient characteristics to determine which group of medication should be considered first.
We support the view that either of the two groups of medications approved for ADHD treatment cannot be selected one above the other since the effect size of these medications are about the same. There seems to be differences in the desired effect and side effect profiles. The selection process cannot be overly simplified and then generalized to all ADHD patients without considering each patient's comorbidities, symptom profiles, and different needs in terms of duration of effect. The following clinical dimensions are listed as possible clinical guidelines in deciding which medications should be considered first. This is a complicated clinical process requiring in-depth knowledge about the characteristics of the medications and individual differences in patient profiles. The process of matching these characteristics and determining the best match between the medication and the patient requires finding “the best fit.” The following clinical guidelines may be useful in the selection of the best medication for each patient:
1. Effective response to ADHD medications. The presence of a good response, insufficient response, or non-response to one kind of medication provides a clinically useful guide in medication selection for a patient. The patient who sufficiently responds to one kind of medication without any or with minimal side effects should be given this medication again rather than going through unnecessary trials. The patient who shows an insufficient response or no response at all to what the clinician considers a sufficient trial of medication should be given a different medication from a different class. In the determination of “sufficient response,” the new concepts of “normalization” or “remission” should be taken into consideration. In the past, when clinicians saw a 50-percent drop in symptoms based on the rating scales, they were satisfied with the effect of the medication. Recently, with the availability of evidence-based information on ADHD, clinicians are now able to decrease the symptom severity and symptom frequency so that patients' ratings can be within the range of the normative patient population. Patients who have ADHD symptoms throughout the day until bedtime may benefit from atomoxetine rather than psychostimulants because of atomoxetine's very long duration of effect. However, the aggression controlling effect is better with psychostimulants than with atomoxetine; therefore, the patient with serious noncompliant and/or aggressive behavior, who is at risk for suspension from school, or at risk for placement with social agencies may benefit more from a psychostimulant as opposed to the slower dose adjustment of atomoxetine.
2. Side effect profiles. A careful history of a patient's variety and severity of side effects with medications, symptom change with the continuation of treatment or dose adjustment, and symptom response to different strategies should be recorded to determine the best medication that will cause no or minor side effects. The negative impact of the side effects on the patient's overall functions should be carefully considered in the medication selection process.
3. Obtainability and affordability of the medication. The new generation ADHD medications are 2 to 3 times more expensive than the older, short-acting medications. Some of the new generation ADHD medications are not accepted by the third-party payers and government drug plans. The families who are not able to afford these drugs may need the most effective use of the medications that are reimbursed for them or that they can afford to pay out of pocket. This should be discussed openly by the family and clinician. The economic burden created by certain medications may play an important role in early termination and medication adherence.
4. Response of the selected medication for associated serious problems and/or comorbid disorders. It has been well established that most patients with ADHD are comorbid for other disorders, and many suffer from serious problems impairing their day-to-day functions, such as sleep and social behavior.5 Patients presenting with sleep initiation and maintenance problems may do better with atomoxetine than psychostimulants. Atomoxetine can be chosen for patients with ADHD,comorbid tic disorders, and ADs because of the evidence provided so far that these disorders respond to atomoxetine better than psychostimulants.
5. Patient, family, and clinician biases toward some medications. The biases of the patients, their families, and the clinician may play important roles in the acceptance of the medication treatment and adherence. If medication outcome is similar between different medications, the patient and family preferences regarding the choice of the medication should be taken into account. The patients and families who are comfortable with a medication may be more adherent.
6. Aversion and abuse. Although it has been well documented that the treatment of ADHD with psychostimulants may reduce the risk of substance abuse, there are still some patients who will abuse psychostimulants. Earlier substance abuse and history of substance abuse in the family may lead to choosing atomoxetine rather than psychostimulants since the abuse potential for atomoxetine seems to be lower than psychostimulants.39
Directions for Future Research
The integration of psychoactive medication and different psychosocial interventions in treating MD with ADHD and other associated comorbid disorders need to be rigorously and systematically studied. A clear need exists for developing consistent, evidence-based strategies in treating MD when comorbid with ADHD. Prospectively designed studies are needed to compare the safety and efficacy of antidepressants used with or without ADHD medications in order to gain further insight into the comparative effectiveness and side effect profiles for these treatments.
Conclusions
Both MD and ADHD are frequently comorbid with each other and with many other psychiatric disorders. The combination of MD and ADHD usually presents as complex and multiple psychiatric disorders. MD may be comorbid with dysthymic disorder—the “double depression” from which many patients suffer. MD is also frequently comorbid with ADs. Most depressed patients suffer from primary sleep disorders or disturbed sleep. ADHD is frequently comorbid with disruptive behavior disorders, such as ODD and CD, and substance use disorders. In many cases, chronic anger management problems and aggressive behavior are present as well. Hence, most patients who suffer from both MD and ADHD also suffer from their respective comorbidities, creating a very complex clinical profile. As a result, many patients in treatment will require more than one medication and the combination of psychosocial interventions.
Contributor Information
Atilla Turgay, Dr. Turgay is from the University of Toronto and the Scarborough Hospital.
Rubaba Ansari, Rubaba Ansari is a research associate at the Scarborough Hospital ADHD Clinic, Training and Research Institute, Ontario, Canada.
References
- 1.Bird HR, Canino G, Rubio-Stipec M. Estimates of prevalence of psychiatric maladjustment in a community survey in Puerto Rico. Arch Gen Psychiatry. 1988;45:1120–6. doi: 10.1001/archpsyc.1988.01800360068010. [DOI] [PubMed] [Google Scholar]
- 2.Anderson JC, Williams S, McGee R, et al. DSM-III disorders in preadolescent children: Prevalence in a large community sample. Arch Gen Psychiatry. 1998;44:69–76. doi: 10.1001/archpsyc.1987.01800130081010. [DOI] [PubMed] [Google Scholar]
- 3.Biederman J, Newcorn J, Sprich S. Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry. 1991;148:564–77. doi: 10.1176/ajp.148.5.564. [DOI] [PubMed] [Google Scholar]
- 4.Turgay A. Diagnosing and treating ADHD in adults. Can J CME. 2001;2:182–90. [Google Scholar]
- 5.Turgay A. Treatment of comorbidity in conduct disorder with attention deficit hyperactivity disorder (ADHD) (Special Report) Essential Psychopharmacol. 2005;6:277–90. [PubMed] [Google Scholar]
- 6.Ansari R, Turgay A, Ng D, Joseph L, et al. ADHD in child and adolescent psychiatric disorders: Gender and subtype relationships. Poster. New York, NY: Annual Scientific Meeting of the American Pyschiatric Association, 2005:88.
- 7.Ansari R, Turgay A, Ng D, Joseph L, Schwartz M, Chaudry N. ADHD comorbidity in psychiatric disorders: Subtype and gender relationships. Poster. New York, NY: Annual Scientific Meeting of the American Psychiatric Association, 2004:89.
- 8.Pliszka SR. Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorders: An overview. J Clin Psychiatry. 1998;59(suppl 7):50–8. [PubMed] [Google Scholar]
- 9.Biederman J. Resolved: Mania is frequently mistaken for ADHD in prepubertal children, affirmative. J Am Acad Child Adolesc Psychiatry. 1998;37:1091–3. doi: 10.1097/00004583-199810000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Weiss L. Attention Deficit Disorder in Adults. Dallas, TX: Taylor Publishing Co.; 1992. [Google Scholar]
- 11.Turgay A, Blanchard J, Ng D, et al. Comorbidities in child and adolescent major depression. San Francisco, CA: Annual Meeting of the American Psychiatric Association, 2003:270.
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Press Inc.; 1994. [Google Scholar]
- 13.Gadow KD, Sprafkin J. Early Childhood Inventories Manual. Stoneybrook, NY: Checkmate Plus; 1997. [Google Scholar]
- 14.Gadow KD, Sprafkin J. 1997. Child Symptom Inventories Manual. Checkmate Plus: Stonnybrook, NY.
- 15.Gadow KD, Sprafkin J. Adolescent Symptom Inventory-4 Manual. Stonnybrook, NY: Checkmate Plus; 1997. [Google Scholar]
- 16.Turgay A. Child and Adolescent Mood Disorders Structured Interviews, Screening, and Rating Scales. InstituteWest Bloomfield, MI: Integrative Therapy; 1995. [Google Scholar]
- 17.Turgay A, Ansari R, Mesci A, N, et al. Comorbidities of dysthymic disorder in children and adolescents. Poster. New York, NY: Annual Scientific Meeting of the American Psychiatric Association, 2004:173.
- 18.Kovacs M, Akiskal S, Gatsonis C, Parrone PL. Childhood onset dysthymic disorder. Arch Gen Psychiatry. 1994;51:365–74. doi: 10.1001/archpsyc.1994.03950050025003. [DOI] [PubMed] [Google Scholar]
- 19.Turgay A, Morgan A, Mok L, Ng D. Chronic and serious aggressive behavior in children and adolescents are commonly associated with psychiatric disorders. Paediatr Child Health. 2001;6(Suppl A):14. [Google Scholar]
- 20.Weiss M, Hechtman LT, Weiss G. ADHD in Adulthood: A Guide to Current Theory, Diagnosis and Treatment. Baltimore, MD: The Johns Hopkins University Press; 1999. [Google Scholar]
- 21.Turgay A. The aggressive and impulsive child: Innovations in the assessment and treatment of attention deficit hyperactivity disorder and disruptive behaviour disorders. Paediatr Child Health. 2004;9(Suppl B):38. [Google Scholar]
- 22.Turgay A, Ansari R, Schwartz M, et al. 2005. pp. 66–7. Comorbitiy differences in ADHD throughout the life cycle. Paper. Scientific and Clinical Report Session. Atlanta, GA: American Psychiatric Association Annual Scientific Meeting.
- 23.Pliszka SR. Comorbidity of attention-deficit/hyperactivity disorder with psychiatric disorders: An overview. J Clin Psychiatry. 1998;59(Suppl 7):50–8. [PubMed] [Google Scholar]
- 24.Biederman J, Baldessarini RJ, Wright V, et al. A double-blind placebo-controlled study of desipramine in the treatment of ADD, III: Lack of impact of comorbidity and family history factors on clinical response. J Am Acad Child Adolesc Psychiatry. 1993;32:199–204. doi: 10.1097/00004583-199301000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Rapport MD, Carlson GA, Kelly KL, et al. Methylphenidate and desipramine in hospitalized children, I: Separate and combined effects on cognitive function. J Am Acad Child Adolesc Psychiatry. 1993;32:333–42. doi: 10.1097/00004583-199303000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Pataki CS, Carlson GA, Kelly KL, et al. Side effects of methlphenidate and desipramine alone and in combination in children. J Am Acad Child Adolesc Psychiatry. 1993;32:1065–72. doi: 10.1097/00004583-199309000-00028. [DOI] [PubMed] [Google Scholar]
- 27.Canadian ADHD Resource Alliance (CADDRA) Canadian ADHD Practice Guidelines. Toronto, Canada: CADDRA Publication; 2006. [Google Scholar]
- 28.Gammon GD, Brown TE. Fluoxetine and methylphenidate in combination for treatment of ADHD and comorbid depressive disorder. J Child Adolesc Psychopharmacol. 1993;5:191–204. doi: 10.1089/cap.1993.3.1. [DOI] [PubMed] [Google Scholar]
- 29.Hughes CW, Emslie GJ, Crismon ML, et al. The Texas Children's Medication Algorithm Project: Report of the Texas consensus conference panel on medication treatment of childhood major depressive disorder. J Am Acad Child Adolesc Psychiatry. 1999;38:1442–54. doi: 10.1097/00004583-199911000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Emslie GJ, Rush AJ, Weinberg WA, et al. A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry. 1997;54:1031–7. doi: 10.1001/archpsyc.1997.01830230069010. [DOI] [PubMed] [Google Scholar]
- 31.Keller MB, Ryan ND, Birmaher B, et al. Paroxetine and imipramine in the treatment of adolescent depression. Abstract. Toronto: Annual Meeting of the American Psychiatric Association, 1998:123.
- 32.Ambrossini PJ, Wagner KD, Biederman J, et al. Multicenter, open-label sertraline study in adolescent outpatients with major depression. J Amer Acad Child Adolesc Psychiatry. 1999;38:566–72. doi: 10.1097/00004583-199905000-00018. [DOI] [PubMed] [Google Scholar]
- 33.Strober M, DeAntonio M, Schmidt-Lackner S, et al. The pharmacotherapy of depressive illness in adolescents: An open-label comparison of fluoxetine with imipramine treated historical controls. J Clin Psychiatry. 1999;60:164–9. doi: 10.4088/jcp.v60n0303. [DOI] [PubMed] [Google Scholar]
- 34.Green WH. Child and Adolescent Clinical Psychopharmacology. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 35.Rush AJ, Kupfer DK. Strategies and tactics in the treatment of depression. In: Gabbard GO, editor. Treatment of Psychiatric Disorders, Second Edition. Washington, DC: American Psychiatric Press, Inc.; 1995. pp. 1349–68. [Google Scholar]
- 36.Pliszka SR, Carlson CL, Swanson JM. ADHD with Comorbid Disorders: Clinical Assessment and Management. New York, NY: The Guilford Press; 1999. [Google Scholar]
- 37.Kratochvil CJ, Newcorn JH, Arnold LE, et al. Atomoxetine alone or combined with fluoxetine for treating ADHD with comorbid depressive or anxiety symptoms. J Am Acad Child Adolesc Psychiatry. 2005;44:915–24. doi: 10.1097/01.chi.0000169012.81536.38. [DOI] [PubMed] [Google Scholar]
- 38.American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2002;41(Suppl 2) doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- 39.Turgay A. Atomoxetine in the treatment of children, adolescents and adults with ADHD. Future Drugs. 2006;3(1):19–38. [Google Scholar]