Abstract
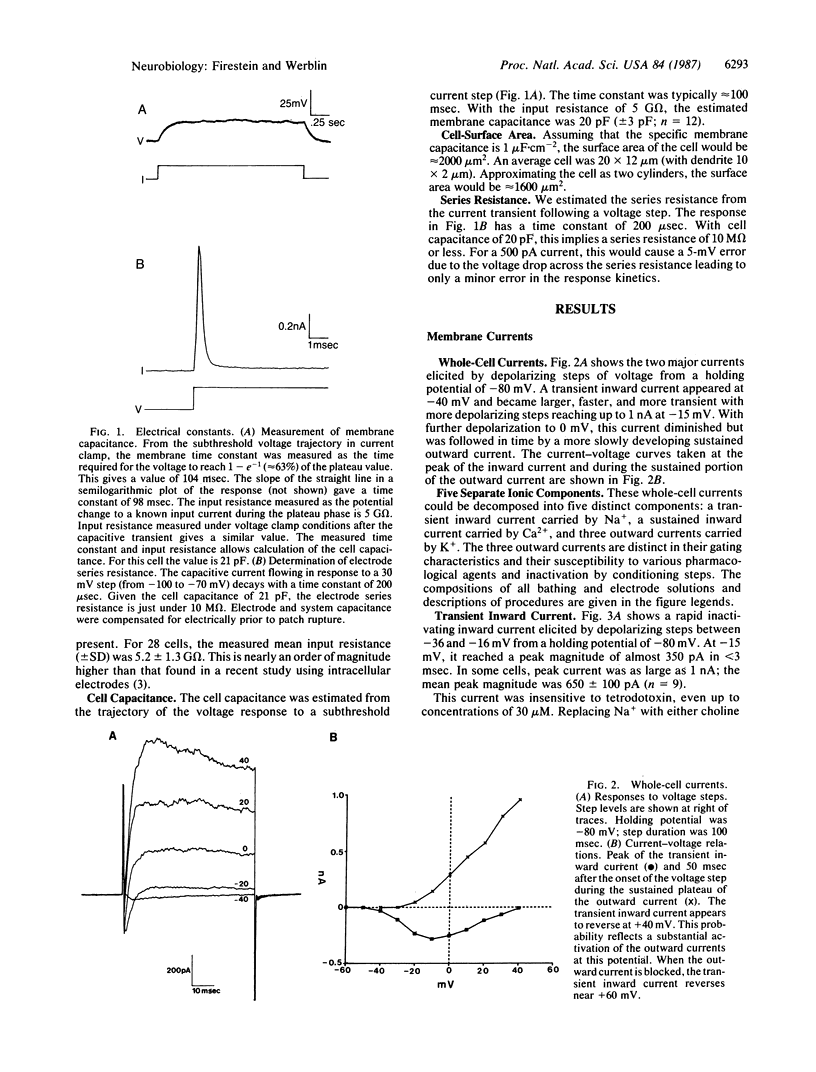
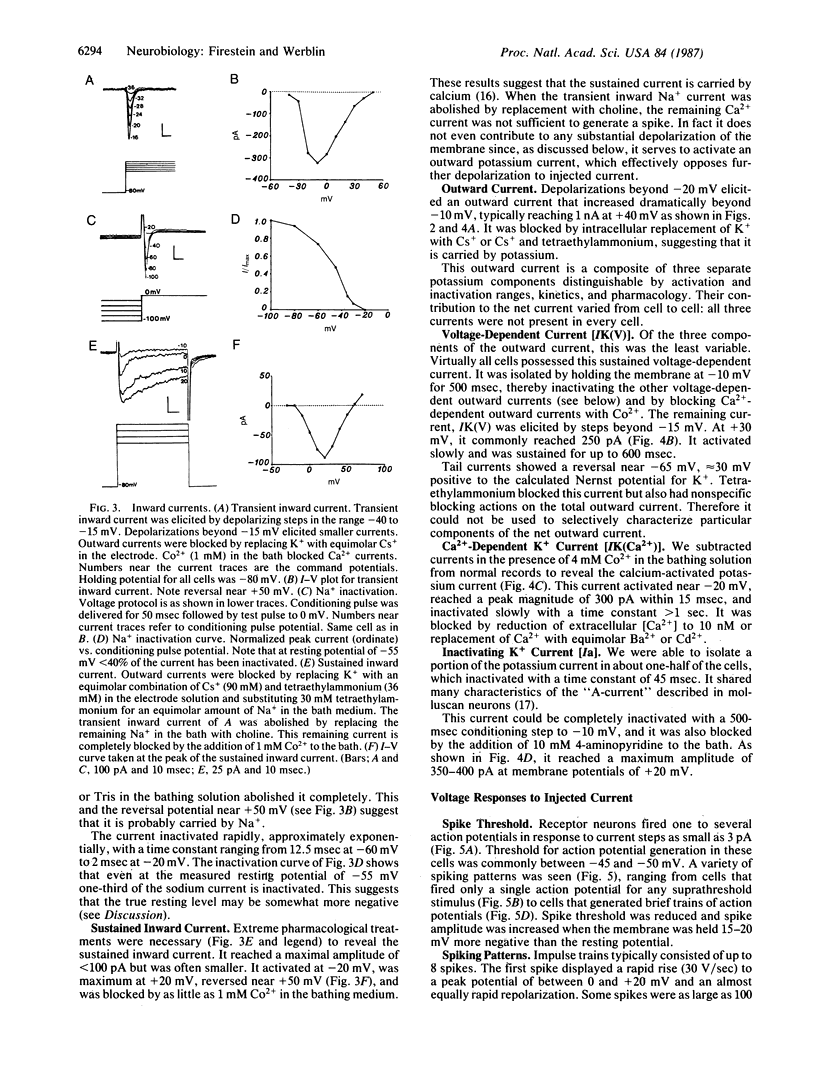
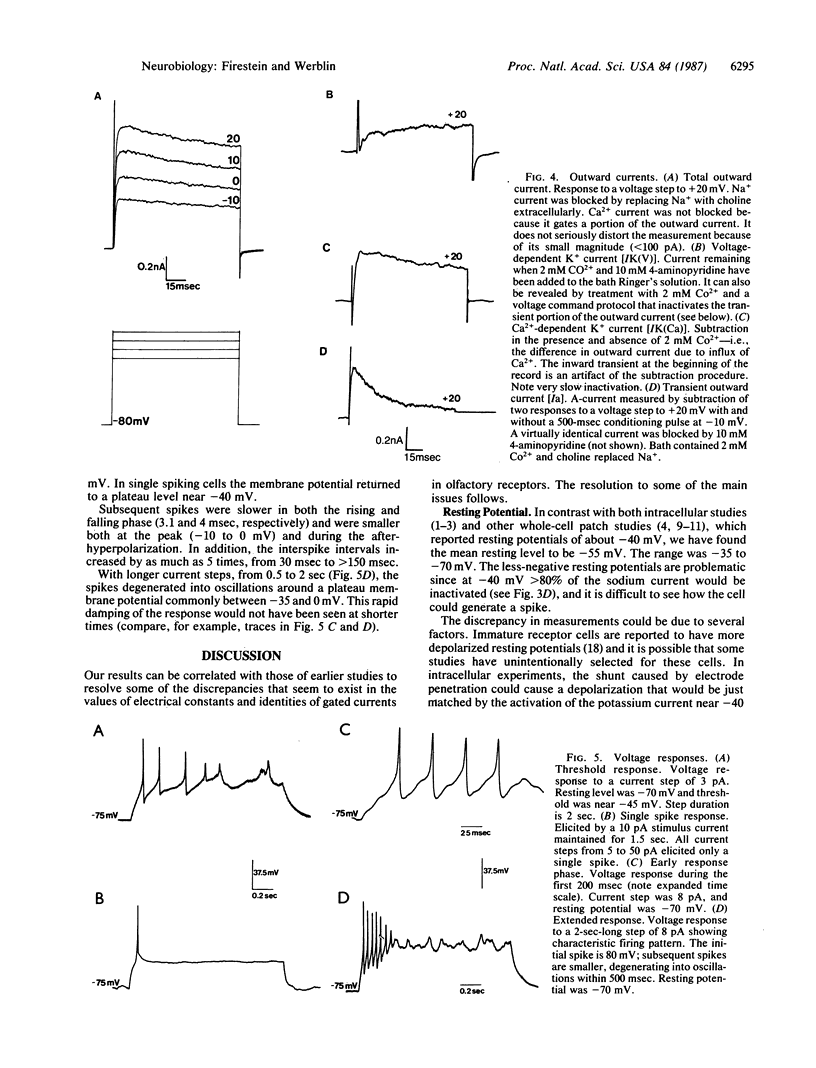
The electrical properties of enzymatically isolated olfactory receptor cells were studied with whole-cell patch clamp. Voltage-dependent currents could be separated into three ionic components: a transient inward sodium current, a sustained inward calcium current, and an outward potassium current. Three components of the outward current could be identified by their gating and kinetics: a calcium-dependent potassium current [IK(Ca)], a voltage-dependent potassium current [IK(V)], and a transient potassium current (Ia). Typical resting potentials were near -54 mV, and typical input resistance was 3-6 G omega. Thus, only 3 pA of injected current was required to depolarize the cell to spike threshold near -45 mV. The response to a current step consisted of either a single spike regardless of stimulus strength, or a train of less than 8 spikes, decrementing in amplitude and frequency over approximately equal to 250 msec. Thus, the receptor response cannot be finely graded with stimulus intensity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell T. V. Analysis of intracellular recordings from salamander olfactory epithelium. Brain Res. 1977 Mar 11;123(2):275–286. doi: 10.1016/0006-8993(77)90479-6. [DOI] [PubMed] [Google Scholar]
- Getchell T. V. Functional properties of vertebrate olfactory receptor neurons. Physiol Rev. 1986 Jul;66(3):772–818. doi: 10.1152/physrev.1986.66.3.772. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Masukawa L. M., Hedlund B., Shepherd G. M. Changes in the electrical properties of olfactory epithelial cells in the tiger salamander after olfactory nerve transection. J Neurosci. 1985 Jan;5(1):136–141. doi: 10.1523/JNEUROSCI.05-01-00136.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa L. M., Hedlund B., Shepherd G. M. Electrophysiological properties of identified cells in the in vitro olfactory epithelium of the tiger salamander. J Neurosci. 1985 Jan;5(1):128–135. doi: 10.1523/JNEUROSCI.05-01-00128.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafols J. A., Getchell T. V. Morphological relations between the receptor neurons, sustentacular cells and Schwann cells in the olfactory mucosa of the salamander. Anat Rec. 1983 May;206(1):87–101. doi: 10.1002/ar.1092060111. [DOI] [PubMed] [Google Scholar]
- Trotier D. A patch-clamp analysis of membrane currents in salamander olfactory receptor cells. Pflugers Arch. 1986 Dec;407(6):589–595. doi: 10.1007/BF00582636. [DOI] [PubMed] [Google Scholar]
- Trotier D., MacLeod P. Intracellular recordings from salamander olfactory receptor cells. Brain Res. 1983 Jun 6;268(2):225–237. doi: 10.1016/0006-8993(83)90488-2. [DOI] [PubMed] [Google Scholar]



