Abstract
Objective:
This study examines the prognostic significance of human papillomavirus (HPV) in patients with locally advanced oropharyngeal squamous cell carcinoma (SCC) treated primarily with surgery or definitive radiotherapy.
Methods:
One hundred and ninety-eight patients with Stage 3/4 SCC were followed up for recurrence in any form or death from any cause for between 1 and 235 months after diagnosis. HPV status was determined using HPV E6-targeted multiplex real-time PCR/p16 immunohistochemistry. Determinants of recurrence and mortality hazards were modelled using Cox's regression with censoring at follow-up dates.
Results:
Forty-two per cent of cancers were HPV-positive (87% type 16). HPV predicted loco-regional control, event-free survival and overall survival in multivariable analysis. Within the surgery with adjuvant radiotherapy (n=110), definitive radiotherapy-alone (n=24) and definitive radiotherapy with chemotherapy (n=47) groups, patients with HPV-positive cancers were one-third or less as likely to have loco-regional recurrence, an event or to die of any cause as those with HPV-negative cancers after adjusting for age, gender, tumour grade, AJCC stage and primary site. The 14 patients treated with surgery alone were considered too few for multivariable analysis.
Conclusion:
HPV status predicts better outcome in oropharyngeal cancer treated with surgery plus adjuvant radiotherapy as well as with definitive radiation therapy±chemotherapy.
Keywords: human papillomavirus, oropharyngeal SCC or oropharyngeal cancer, outcome, surgery, radiotherapy
It is now accepted that human papillomavirus (HPV) is an aetiological agent of up to 60% of oropharyngeal squamous cell carcinomas (SCCs) (Gillison et al, 2000; Vidal and Gillison, 2008) and there have been recent reports that the incidence of HPV-induced oropharyngeal cancer is increasing (Frisch et al, 2000; Hammarstedt et al, 2006; Hong et al, 2010c). Our studies and others have shown that the HPV-positive subset of cancers is biologically distinct (Andl et al, 1998; Li et al, 2003a; Weinberger et al, 2006; Fakhry et al, 2008; Vidal and Gillison, 2008; Lassen et al, 2009; Sedaghat et al, 2009; Hong et al, 2010a, 2010b). Most notable has been the association of HPV with a favourable clinical outcome.
Radiation therapy has an important role in the management of oropharyngeal cancers, either as definitive therapy or as an adjuvant therapy after surgery. Altered fractionation and concurrent chemotherapy have been developed to maximise disease control (Pignon et al, 2000; Bourhis et al, 2006). Recent studies have provided evidence that the favourable prognosis associated with HPV relates to a better response to radiation therapy used either alone (Lassen et al, 2009; Sedaghat et al, 2009) or in combination with chemotherapy (Kumar et al, 2007; Fakhry et al, 2008). There are, however, limited data on the relationship of HPV status to the outcome of oropharyngeal cancers treated with surgery with or without adjuvant radiation therapy (Licitra et al, 2006; Fischer et al, 2008, 2010).
There is increasing use of HPV status to guide treatment of oropharyngeal SCC (Shoushtari et al, 2010). This study examines the effect of HPV on outcomes after surgery with adjuvant radiation therapy or with definitive radiation therapy (with or without chemotherapy) in a large series of locally advanced oropharyngeal SCCs from the same geographic region.
Materials and methods
Study population
Investigations were carried out on 202 consecutive patients with AJCC Stage 3 and 4 oropharyngeal SCC treated with curative intent at hospitals in Sydney, Australia, between 1987 and 2006. One hundred and forty-six cases were from Royal Prince Alfred Hospital. Human papillomavirus status for two patients could not be determined (a 71-year-old man with a Stage 3 tonsillar SCC treated with definitive radiation therapy and a 46-year-old man with a Stage 4 oropharyngeal SCC treated with definitive radiation therapy and concurrent chemotherapy), and two patients were missing adequate follow-up data (a 46-year-old man with a Stage 4 tonsillar SCC and a 68-year-old man with Stage 4 tonsillar SCC), leaving 198 patients. The study was approved by the ethics committees of Sydney South West Area Health Service (Protocols X05-0308, CH62/6/2006-041, 2006/055). Most of the demographic and clinicopathological data were retrieved from the database of the Sydney Head and Neck Cancer Institute at Royal Prince Alfred Hospital, which spans the study period. Department of Radiation Oncology and Anatomical Pathology databases and hospital records were used to verify and input missing data as required.
Patients were followed up for the occurrence of an event, defined as recurrence in any form or death from any cause, for between 1 and 235 (median 26) months after diagnosis. Patients who did not experience an event before the end of follow-up were known at that date to be alive and free of recurrence.
Laboratory studies
HPV status of cancers
Evidence that HPV is transcriptionally active or localised to the nuclei of tumour cells is needed to establish causality in head and neck cancer (Vidal and Gillison, 2008). Overexpression of p16 resulting from downregulation of retinoblastoma protein by HPV E7 has been used as a surrogate marker of HPV E7 expression in several studies (Klussmann et al, 2003; Licitra et al, 2006). In our study, an HPV-positive cancer was defined as one testing positive for both HPV DNA and p16 (Smeets et al, 2007). The presence and type of HPV DNA were determined on two to six 4–5 μm sections of formalin-fixed paraffin-embedded cancer, using an HPV E6-based multiplex real-time PCR assay (MT-PCR) modified from the method of Stanley and Szewczuk (2005). This assay simultaneously detects and identifies 21 HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, 70, 73, 82, 53, 6, 11 and 26). The HPV 16 assay (Supplementary Figure 1) has a sensitivity of 400 viral genome copies per ml at the 95% confidence level (CI) and compares well with our nested PCR method, which was validated against previously published PCR methods (Brestovac et al, 2005). DNA was extracted using the RNA QIAmp RNA viral mini kit (Qiagen, Hilden, Germany). The first step involved incorporation of all primer pairs into two PCR mixes and amplification for 20 cycles in a conventional thermocycler. Products from each of these mixes were then passed into triplex TaqMan real-time PCR assays with probes having FAM, VIC or Cy5 labels and cycling performed for 40 cycles in a real-time thermocycler (RotorGene 6000, Corbett Research, Mortlake, NSW, Australia). A Corbett CAS 1200 robot was used to inoculate samples and to make the transfers from the initial PCR into the TaqMan PCR mixes. A measured amount of equine herpesvirus was introduced into the extraction lysis buffer and used to monitor the efficiency of DNA extraction and removal of PCR inhibitors. Stringent precautions were used to minimise the possibility of cross-contamination. Section cutting was carried out in an institution remote from that used for the PCR analyses; microtome blades were cleaned with xylene between blocks, and changed frequently. PCR procedures were carried out in a laboratory equipped with separate rooms for preparing reagents, extracting samples, handling PCR products and for thermocycling. Water blanks were included after every fifth sample. The presence of cancer cells in sections used for HPV testing was confirmed by a pathologist (CSL) in an H&E-stained section cut after those for HPV analysis. All discrepant HPV DNA/p16 results were confirmed by retesting for both HPV DNA and p16. No attempt was made to microdissect cancer from surrounding tissue.
Expression of p16 was carried out by semiquantitative immunohistochemistry. Antigen retrieval was carried out using Target Retrieval Solution pH9 (Dako Corporation, Carpinteria, CA, USA) in a microwave oven for 10 min on high setting. After cooling, slides were placed in an autostainer (Dako). Endogenous peroxidase was blocked using 0.3% hydrogen peroxide in Tris-buffered saline (TBS); sections were incubated with primary antibody (Clone JC2 Neomarkers, Fremont, CA, USA) (one out of 200) for 30 min, washed in TBS, treated with the EnVision Flex Dual Link horseradish peroxidase/DAB visualisation system (Dako) and then counterstained with haematoxylin. Staining was evaluated by three observers, including at least one pathologist (CSL). Typically, strong diffuse p16 staining was seen in the nucleus and cytoplasm of cancer cells with the proportion essentially all or none (Supplementary Figures 2a and 2b). Weak focal staining was recorded as negative. All researchers were blinded to clinical and other laboratory data until results were finalised. The grade for all cancers was reviewed by two study pathologists (CSL, JRK). Any variation from a previous report or between observers was resolved over a double-headed microscope.
Statistical analyses
Associations between HPV status and clinicopathological characteristics were assessed using a two-sample t-test for the continuous variable age and chi-squared tests for categorical variables. One-way analysis of variance and chi-squared tests were used to compare patient characteristics in four treatment groups: surgery alone; surgery with adjuvant radiation therapy; definitive radiation therapy alone; and definitive radiation therapy with chemotherapy.
Survival analyses were conducted for the outcomes of loco-regional recurrence, event-free survival and overall survival, with time to each outcome calculated from the date of diagnosis. An event was defined as recurrence in any form or death from any cause, with only the first event taken into account. Patients without events were censored at the date of last follow-up. Univariate associations between HPV status and time to loco-regional recurrence, an event or death from any cause, were summarised for all patients and separately for each treatment group using Cox's proportional hazards models. Unadjusted survival curves were obtained using Kaplan–Meier estimates.
Overall multivariable survival models were constructed with inclusion of HPV status and other known or potential correlates of outcome: age, gender, tumour grade, AJCC stage, primary site and treatment modality. Multivariable survival models were constructed separately for the three radiation therapy treatment groups, adjusting for HPV status, age, gender, tumour grade, AJCC stage and primary site where possible. Multivariable analyses were not carried out for the surgery-alone group because of the small numbers (14).
We assessed whether the effect of HPV differed by treatment type by using interaction terms between HPV status and treatment (categorised as surgery with adjuvant radiation therapy, definitive radiation therapy with chemotherapy and definitive radiation therapy alone), and adjusting only for age, tumour grade, tumour stage and site to avoid potential overfitting problems owing to small numbers. Adjusted survival curves by HPV status and treatment were obtained for the three treatment groups using Cox regression models, again adjusting only for age, tumour grade, stage and site. All analyses were conducted using the SAS System for Windows (SAS Institute, Cary, NC, USA) and Stata Statistical Software (Stata Corporation, College Station, TX, USA).
Results
HPV status and type distribution
Forty-two per cent (83 out of 198) of the cancers were HPV-positive. Human papillomavirus type 16 accounted for 87% (72 out of 83) of the HPV-positive cases. Human papillomavirus types 35 and 18 were the most common other HPV types. Five cancers positive for HPV type 16 also contained a second HPV type (two 35, one each of 33, 39 and 56). Twenty-one (11%) cancers tested HPV DNA positive/p16 negative and three (2%) cancers were HPV DNA negative/p16 positive (Supplementary Table 1). The 21 HPV DNA-positive/p16-negative samples were included in the HPV-negative group, as the molecular and phenotypic characteristics of this group resemble those of HPV-negative/p16-negative cancers (our unpublished data, Smeets et al, 2007; Weinberger et al, 2009). The three patients with HPV-negative/p16-positive cancers were excluded from further analyses because it was not known whether they were induced by an HPV type undetectable by the assay or were HPV negative (p16 upregulated through an HPV-unrelated pathway). They were too few to analyse separately. Thus, the total number of cancers in the final analysis was 195.
The patients’ characteristics are summarised in Table 1. Compared with patients with HPV-negative cancers, patients with HPV-positive cancers were younger (mean age 54.4 vs 62.6 years, P<0.0001), more likely to have primary disease in the tonsil (P=0.003) and had higher grade cancers (P=0.002). Patients with HPV-positive cancers were also more likely to have Stage 4 than Stage 3 cancers (P=0.03), and this was driven by more advanced nodal disease (P=0.02).
Table 1. Demographic and clinical characteristics of the study population.
| All patients (n=195) | HPV positive (n=83) | HPV negative (n=112) | HPV-positive vs HPV-negative P-value a | |
|---|---|---|---|---|
| Mean age at diagnosis (range) | 59.1 (range 34–84) | 54.4 (range 34–84) | 62.6 (range 44–84) | <0.0001 |
| Gender | ||||
| Males | 159 (82%) | 69 (83%) | 90 (80%) | 0.6 |
| Females | 36 (18%) | 14 (17%) | 22 (20%) | |
| Location | ||||
| Tonsil | 124 (64%) | 61 (73%) | 63 (56%) | 0.003 |
| Base of tongue | 36 (18%) | 16 (19%) | 20 (18%) | |
| Other subsites | 35 (18%) | 6 (7%) | 29 (26%) | |
| T classification b | ||||
| T1 | 21 (11%) | 13 (16%) | 8 (7%) | 0.03 |
| T2 | 60 (31%) | 31 (37%) | 29 (26%) | |
| T3 | 72 (37%) | 23 (28%) | 49 (44%) | |
| T4 | 41 (21%) | 16 (19%) | 25 (23%) | |
| N classification b | ||||
| N0 | 45 (23%) | 12 (14%) | 33 (30%) | 0.02 |
| N1 | 56 (29%) | 21 (25%) | 35 (32%) | |
| N2 | 74 (38%) | 39 (47%) | 35 (32%) | |
| N3 | 19 (10%) | 11 (13%) | 8 (7%) | |
| Stage | ||||
| 3 | 78 (40%) | 26 (31%) | 52 (46%) | 0.03 |
| 4 | 117 (60%) | 57 (69%) | 60 (54%) | |
| Grade | ||||
| 1, 2 | 118 (61%) | 40 (48%) | 78 (70%) | 0.002 |
| 3 | 77 (39%) | 43 (52%) | 34 (30%) | |
| Treatment | ||||
| Surgery alonec | 14 (7%) | 4 (5%) | 10 (9%) | 0.7 |
| Surgery and adjuvant RTd | 110 (56%) | 47 (57%) | 63 (56%) | |
| Definitive RT alone | 24 (12%) | 10 (12%) | 14 (13%) | |
| Definitive RT and CTe | 47 (24%) | 22 (27%) | 25 (22%) | |
Abbreviations: HPV=human papillomavirus; RT=radiation therapy; CT=chemotherapy.
Test for heterogeneity.
One missing observation.
Includes one patient who also received induction CT.
Includes 10 patients who also received CT (five induction and five concurrent).
Induction CT (n=12) and concurrent CT (n=35).
Treatment
The choice of treatment modality was based primarily on clinician and institution preference. Of the 195 patients analysed, 14 were treated with radical surgery alone (five declined adjuvant radiation therapy, one died of other than head and neck cancer before adjuvant radiation therapy and the remaining eight were not referred for adjuvant radiation therapy for unknown reasons). One hundred and ten patients were treated with surgery with adjuvant radiation therapy (five with concurrent chemotherapy and five with induction chemotherapy) (Table 1). Fourteen of the 17 patients with T3N0 tumours treated with surgery and adjuvant radiotherapy had elective neck dissections. Seventy-one patients were treated with definitive radiation therapy. Of these, 47 had chemoradiotherapy (35 concurrent, 12 induction) and 24 had radiation alone. Within the surgery with adjuvant radiotherapy group, separate analysis of the 10 patients who received chemotherapy was not carried out, and within the definitive radiotherapy group, patients receiving concurrent radiotherapy were grouped with those having induction chemotherapy because of small numbers.
The 35 patients who received concurrent chemotherapy and definitive radiation therapy (mainly 5-fluorouracil or 3-weekly cisplatin) were treated between 1990 and 2006; all but three of these patients were treated after 1996. These 35 patients were equally divided between the HPV-positive and -negative groups. Over the study period, there was a change in definitive radiation dose scheduling from 60 Gy in 30 fractions over 6 weeks in the early 1990s to 70 Gy in 35 fractions over 7 weeks from 1999. The dose of adjuvant radiation therapy also increased over the study period from 50 Gy in 25 fractions to 60 Gy in 30 fractions in more recent years. All patients were treated with multiple photon fields using standard fractionation. Patients receiving surgery alone were older than other patients and patients receiving definitive radiotherapy with chemotherapy were more likely to have tumours located in the base of tongue (Table 2).
Table 2. Characteristics of patients by treatment group.
| Surgery alone (n=14) | Surgery with adjuvant RT (n=110) | Definitive RT alone (n=24) | Definitive RT with CT (n=47) | P-value a | |
|---|---|---|---|---|---|
| Mean age at diagnosis (range) | 67.1 (44–84) | 58.2 (34–84) | 60.7 (38–84) | 58.2 (38–83) | 0.03 |
| Gender | |||||
| Males | 10 (71%) | 85 (77%) | 22 (92%) | 42 (89%) | 0.1 |
| Females | 4 (29%) | 25 (23%) | 2 (8%) | 5 (11%) | |
| Location | |||||
| Tonsil | 9 (65%) | 79 (72%) | 16 (67%) | 20 (43%) | 0.02 |
| Base of tongue | 2 (14%) | 16 (15%) | 2 (8%) | 16 (34%) | |
| Other subsites | 3 (21%) | 15 (14%) | 6 (25%) | 11 (23%) | |
| Stage | |||||
| 3 | 8 (57%) | 39 (35%) | 9 (38%) | 22 (47%) | 0.3 |
| 4 | 6 (43%) | 71 (65%) | 15 (63%) | 25 (53%) | |
| Grade | |||||
| 1, 2 | 10 (71%) | 62 (56%) | 15 (63%) | 31 (66%) | 0.5 |
| 3 | 4 (29%) | 48 (44%) | 9 (38%) | 16 (34%) | |
| HPV status | |||||
| Negative | 10 (71%) | 63 (57%) | 14 (58%) | 25 (53%) | 0.7 |
| Positive | 4 (29%) | 47 (43%) | 10 (42%) | 22 (47%) | |
Abbreviations: CT=chemotherapy; HPV=human papillomavirus; RT=radiation therapy.
Test for heterogeneity.
Outcome analyses
Effect of HPV status
One patient was missing loco-regional recurrence status, yielding 194 patients for analysis. Loco-regional recurrence occurred in 54 (28%) of 194 patients: in four of the 14 (29%) patients treated with surgery alone, 24 of the 109 (22%) patients treated with surgery and adjuvant radiation therapy, 10 of the 25 (40%) patients treated with definitive radiotherapy alone and 16 of the 47 (34%) patients treated with definitive radiation therapy with chemotherapy. Recurrence occurred at the primary site in 30 patients and in the regional nodal area (with disease controlled at the primary site) in 24 patients. Sixteen patients developed distant metastasis as the first site of recurrence, none of whom had simultaneous loco-regional recurrence.
Univariate analysis of data from all patients showed that HPV-positive cancers were less likely to recur loco-regionally than were HPV-negative cancers (Table 3). This association remained after adjustment for age, gender, grade, stage, primary site within the oropharynx and treatment group (hazard ratio (HR)=0.27; 95% CI: 0.13–0.52; Table 3).
Table 3. Associations between HPV status and risk of recurrence, event and/or death.
|
Treatment group
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
All (n=195)
|
Surgery alone (n=14)
|
Surgery with adjuvant radiotherapy (n=110)
|
Definitive radiotherapy alone (n=24)
|
Definitive radiotherapy and chemotherapy (n=47)
|
||||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Loco-regional recurrence | ||||||||||
| Univariate | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Positive | 0.34 (0.17, 0.61) | 0.0002 | 0.71 (0.04, 5.57) | 0.8 | 0.38 (0.14, 0.91) | 0.03 | 0.18 (0.03, 0.75) | 0.02 | 0.36 (0.10, 1.03) | 0.06 |
| Adjusted | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Positive | 0.27 (0.13, 0.52)a | <0.0001 | Not estimatedb | 0.30 (0.10, 0.82)c | 0.02 | 0.12 (0.01, 0.61)d | 0.009 | 0.25 (0.06, 0.79)c | 0.02 | |
| Event-free survival | ||||||||||
| Univariate | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Positive | 0.31 (0.19, 0.49) | <0.0001 | 0.50 (0.07, 2.10) | 0.4 | 0.28 (0.13, 0.53) | <0.0001 | 0.19 (0.04, 0.60) | 0.004 | 0.46 (0.19, 1.03) | 0.06 |
| Adjusted | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Positive | 0.23 (0.14, 0.39)a | <0.0001 | Not estimatedb | 0.19 (0.08, 0.40)c | <0.0001 | 0.14 (0.02, 0.54)d | 0.003 | 0.36 (0.14, 0.85)c | 0.02 | |
| Overall survival | ||||||||||
| Univariate | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| Positive | 0.29 (0.17, 0.48) | <0.0001 | 0.98 (0.14, 4.57) | 1.0 | 0.18 (0.07, 0.40) | <0.0001 | 0.24 (0.05, 0.82) | 0.02 | 0.43 (0.17, 0.99) | 0.05 |
| Adjusted | ||||||||||
| HPV | ||||||||||
| Negative | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| Positive | 0.24 (0.13, 0.42)a | <0.0001 | Not estimatedb | 0.11 (0.04, 0.28)c | <0.0001 | 0.11 (0.01, 0.52)d | 0.004 | 0.37 (0.13, 0.90)c | 0.03 | |
Abbreviations: HPV=human papillomavirus; HR=hazard ratio; CI=confidence interval; RT=radiation therapy; CT=chemotherapy.
Adjusted for age, gender, tumour grade, tumour stage, primary site and treatment.
Multivariable analyses not carried out owing to small numbers.
Adjusted for age, gender, tumour grade, tumour stage and primary site.
Adjusted for age, tumour grade and tumour stage; gender and primary site not adjusted owing to categories with low numbers of events.
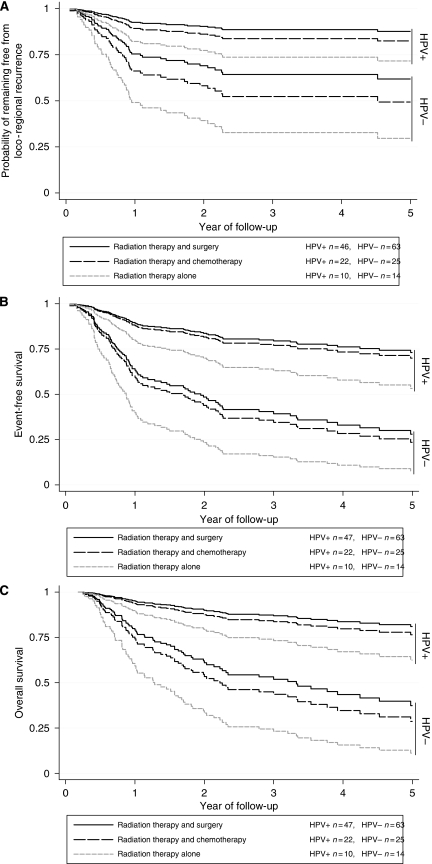
There was little evidence that the effect of HPV on loco-regional recurrence differed by treatment group (Figure 1A); P=0.7 for interaction between HPV status and treatment group in the adjusted analysis. After adjustment for age, tumour grade and tumour stage, the risk of loco-regional recurrence associated with HPV-positive cancers relative to HPV-negative cancers was about one-third (HR=0.30; 95% CI: 0.10–0.82) in the surgery with adjuvant radiation therapy group, one-eighth (HR=0.12; 95% CI: 0.01–0.61) in the definitive radiation therapy-alone group and one-quarter (HR=0.25; 95% CI: 0.06–0.79) in the definitive radiation therapy with chemotherapy group (Table 3).
Figure 1.
Probability of (A) remaining free from loco-regional recurrence, (B) survival free from an event and (C) overall survival by HPV status and type of radiation therapy, adjusted for age, tumour grade, stage and site.
There were 99 events involving nine of the 14 (64%) patients treated with surgery alone, 48 of the 110 patients (44%) treated with surgery and adjuvant radiation therapy, 16 of the 24 (67%) patients treated with definitive radiotherapy alone and 26 of the 47 (55%) patients treated with definitive radiation therapy and chemotherapy. Univariate analysis of data from all patients showed that patients with HPV-positive cancers were less likely to suffer an event than those with HPV-negative cancers (Table 3). This association remained after adjustment for age, gender, grade, stage, primary site within the oropharynx and treatment type (HR=0.23; 95% CI: 0.14–0.39).
There was little evidence that the effect of HPV on event-free survival differed by treatment group (Figure 1B); P=0.4 for interaction between HPV status and treatment group in the adjusted analysis. After adjustment for age, tumour grade and stage, the risk of an event associated with HPV-positive cancers relative to HPV-negative cancers was about one fifth (HR=0.19; 95% CI: 0.08–0.40) in the surgery with adjuvant radiation therapy group, about one-seventh (HR=0.14; 95% CI: 0.02–0.54) in the definitive radiation therapy-alone group and about one-third (HR=0.36; 95% CI: 0.14–0.85) in the definitive radiation therapy with chemotherapy group (Table 3).
There were 82 deaths from any cause involving seven of the 14 patients (50%) treated with surgery alone, 38 of the 110 patients (35%) receiving surgery with adjuvant radiation therapy, 13 of the 24 patients (54%) receiving definitive radiotherapy alone and 24 of the 47 patients (51%) receiving definitive radiation therapy with chemotherapy. Univariate analysis of data from all patients showed that patients with HPV-positive cancers were less likely to die of any cause than those with HPV-negative cancers (Table 3). This association remained after adjustment for age, gender, grade, stage, primary site within the oropharynx and treatment type (HR=0.24, 95% CI: 0.13–0.42).
There was little evidence that the effect of HPV on overall survival differed by treatment group (Figure 1C); P=0.3 for interaction between HPV status and treatment group in the adjusted analysis. After adjustment for age, tumour grade and stage, the risk of death from any cause associated with HPV-positive cancers relative to HPV-negative cancers was almost one-tenth (HR=0.11; 95% CI: 0.04–0.28) in the surgery with adjuvant radiation therapy group, almost one-tenth (HR=0.11; 95% CI: 0.01–0.52) in the definitive radiation therapy-alone group and about one-third (HR=0.37; 95% CI: 0.13–0.90) in the definitive radiation therapy with chemotherapy group (Table 3).
Effects of clinical variables
On the basis of multivariable analyses of all patients adjusting for HPV status, type of treatment was the only clinical characteristic that showed evidence of an association with loco-regional recurrence (P=0.06), with the surgery and adjuvant radiotherapy group having the lowest risk of loco-regional recurrence and the surgery-only group having the highest risk.
Multivariable predictors of event-free survival were identified as stage (P=0.0001), site of cancer (P=0.04) and type of treatment (P=0.0003) after adjusting for HPV status. Poorer outcomes were seen in Stage 4 patients, patients with cancer in sites other than tonsil and patients receiving surgery only.
After adjusting for HPV status, clinical characteristics that showed evidence of associations with overall survival were identified as stage (P<0.0001), site of cancer (P=0.001) and type of treatment (P=0.05). Again, poorest outcomes were seen in Stage 4 patients, patients with cancer in sites other than tonsil and patients receiving surgery only.
When stratified by HPV status, outcome-adjusted survival against each end point was consistently highest for surgery with adjuvant radiotherapy, intermediate for definitive radiotherapy with chemotherapy and lowest for definitive radiotherapy alone (Figure 1A–C). (Patients treated with surgery alone were too few to include in these comparisons.) These differences in outcome by treatment type should be interpreted with caution because they are not randomised comparisons and may be affected by confounding by indications for particular treatment types. For each outcome, there was little evidence that the effect of treatment type was modified by stage or by site of cancer (P for interaction >0.3 in each case).
Discussion
Recent studies have suggested that the favourable outcome associated with HPV in oropharyngeal cancer is owing to an increased sensitivity of virus-related cancers to radiation therapy or chemoradiotherapy (Fakhry et al, 2008; Kumar et al, 2008; Lassen et al, 2009; Sedaghat et al, 2009). Data from patients treated primarily with surgery (with or without adjuvant radiotherapy) are limited, but there have been recent reports of a survival advantage for those with HPV-positive cancers (Licitra et al, 2006; Fischer et al, 2010). Our findings show that HPV status is a strong predictor of loco-regional recurrence and survival in patients treated either by surgery with adjuvant radiotherapy or by an organ-preserving approach using definitive radiotherapy with or without chemotherapy. Mounting evidence that the effect of HPV on outcome in oropharyngeal cancer is independent of treatment modality is not inconsistent with theories that HPV-positive cancers are more radiosensitive than HPV-negative cancers, but rather suggests that other factors including immune surveillance to virus-specific tumour antigens and a lack of field cancerisation (Mellin et al, 2000; Lindel et al, 2001) may have an important contributing role.
As most recent studies have focused on HPV and outcome following definitive radiotherapy or chemoradiotherapy, the interest of this study centres on patients with surgery as primary treatment. Our results suggest that outcomes in patients with locally advanced HPV-positive cancers treated with surgery and adjuvant radiotherapy were as good as, and possibly better than, those treated with definitive radiotherapy with or without chemotherapy. This also appeared to be the case for HPV-negative cancers, and the effect of HPV status on outcome did not appear to differ materially between these treatment groups. In their study of patients with all stages of disease, Licitra et al (2006) reported an effect of HPV on outcome in patients treated with surgery alone as well as those receiving surgery with adjuvant radiotherapy. In our study, overall outcomes were poorer in patients treated with surgery alone than in other treatment groups and there was only weak evidence for a better outcome for HPV-positive cancers treated with surgery alone on univariate analysis. However, we only included patients with locally advanced disease who, in our centre, have long been routinely referred for adjuvant radiotherapy (six of the 14 patients did not have adjuvant radiation therapy as recommended). The numbers receiving surgery only were too few to adequately evaluate outcome by HPV status. It was, however, worth noting that this group of patients tended to be older and to have HPV-negative cancer.
The seemingly lower HRs for outcome of HPV-positive cancer relative to HPV-negative cancer in the definitive radiotherapy-only group than in the definitive radiotherapy with chemotherapy group were unexpected. However, the wide CIs about the HRs and the high P-values for interaction make any inference from this difference very uncertain. Moreover, we cannot exclude selection bias relating to performance status, co-morbidities, smoking and alcohol exposure that might affect the HRs (Hafkamp et al, 2008; Ang et al, 2010; Maxwell et al, 2010).
The heterogeneity of disease within the head and neck region is well recognised. Although oropharyngeal SCCs are regarded as a relatively homogeneous group, studies with the statistical power to examine site within the oropharynx have found that patients with base of tongue SCCs have the worst prognosis (Zhen et al, 2004; Sundaram et al, 2005). Our overall findings indicate that site within the oropharynx is an independent risk factor for survival with tonsillar cancers having the best outcome. This finding suggests that stratification of future trials of oropharyngeal cancer by subsite may be worthwhile.
The proportion of oropharyngeal cancers attributable to HPV has varied from 19% to more than 60% across different studies (Gillison et al, 2000; Vidal and Gillison, 2008). Our overall HPV positivity rate of 46% is lower than that reported in some recent studies, but this is explained by the 20-year period of the study. By 2005–2006, rates in our centre had risen to 66% (Hong et al, 2010c). This increase is consistent with trends in other western countries (Frisch et al, 2000; Hammarstedt et al, 2006; Chaturvedi et al, 2008). Variation in HPV-positivity rates across different studies is also attributable to differences in the specificity and sensitivity of the HPV detection assays. However, there do seem to be geographic or ethnic differences (Li et al, 2003b, 2007), which highlights the need for standardised procedures for determining HPV status (Braakhuis et al, 2009). The proportion of our cancers testing HPV DNA positive/p16 negative, indicating that the virus is not causal, is lower than that in some studies (van Houten et al, 2001; Wiest et al, 2002; Weinberger et al, 2006), despite the high sensitivity of our HPV DNA assay.
Testing for HPV status of oropharyngeal SCCs is increasing in clinical practice, but there is no level 1 or 2 evidence to guide treatment based on HPV status. We conclude that patients with HPV-positive locally advanced oropharyngeal SCC have a more favourable prognosis than HPV-negative cancers, regardless of whether they are treated with radical surgery plus adjuvant radiation therapy or by an organ-preserving approach using definitive radiation therapy with or without chemotherapy. The possibility that type of treatment, as well as HPV status, may influence outcome warrants investigation in randomised controlled trials.
Acknowledgments
This study was supported by grants from the Diagnostics and Technology Branch of the Australian Government Department of Health and Ageing with the support of Cancer Australia, The Cure Cancer Foundation Australia and Sydney Head and Neck Cancer Institute. We are grateful to Dr Dion Forstner, Dr Christine Loo, Dr Louise Hughes, Dr Jimin Fei, Dr Nham Tran, Dr Eric Lee, Ms Cecilia Ng, Ms Luiza Peculis, Ms Elise Jackson and Dr Andrew Milne for their contributions to the study.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
Supplementary Material
References
- Andl T, Kahn T, Pfuhl A, Nicola T, Erber R, Conradt C, Klein W, Helbig M, Dietz A, Weidauer H, Bosch FX (1998) Etiological involvement of oncogenic human papillomavirus in tonsillar squamous cell carcinomas lacking retinoblastoma cell cycle control. Cancer Res 58: 5–13 [PubMed] [Google Scholar]
- Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, Horiot JC, Le Maitre A, Pajak TF, Poulsen MG, O’Sullivan B, Dobrowsky W, Hliniak A, Skladowski K, Hay JH, Pinto LH, Fallai C, Fu KK, Sylvester R, Pignon JP (2006) Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 368: 843–854 [DOI] [PubMed] [Google Scholar]
- Braakhuis BJ, Brakenhoff RH, Meijer CJ, Snijders PJ, Leemans CR (2009) Human papilloma virus in head and neck cancer: the need for a standardised assay to assess the full clinical importance. Eur J Cancer 45: 2935–2939 [DOI] [PubMed] [Google Scholar]
- Brestovac B, Harnett GB, Smith DW, Frost F, Shellam GR (2005) Multiplex nested PCR (MNP) assay for the detection of 15 high risk genotypes of human papillomavirus. J Clin Virol 33: 116–122 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Engels EA, Anderson WF, Gillison ML (2008) Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26: 612–619 [DOI] [PubMed] [Google Scholar]
- Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML (2008) Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 100: 261–269 [DOI] [PubMed] [Google Scholar]
- Fischer CA, Zlobec I, Green E, Probst S, Storck C, Lugli A, Tornillo L, Wolfensberger M, Terracciano LM (2010) Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer 126: 1256–1262 [DOI] [PubMed] [Google Scholar]
- Fischer C, Zlobec I, Stockli E, Probst S, Storck C, Tornillo L, Lugli A, Wolfensberger M, Terracciano L (2008) Is immunohistochemical epidermal growth factor receptor expression overestimated as a prognostic factor in head-neck squamous cell carcinoma? A retrospective analysis based on a tissue microarray of 365 carcinomas. Hum Pathol 39: 1527–1534 [DOI] [PubMed] [Google Scholar]
- Frisch M, Hjalgrim H, Jaeger AB, Biggar RJ (2000) Changing patterns of tonsillar squamous cell carcinoma in the United States. Cancer Causes Control 11: 489–495 [DOI] [PubMed] [Google Scholar]
- Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709–720 [DOI] [PubMed] [Google Scholar]
- Hafkamp HC, Manni JJ, Haesevoets A, Voogd AC, Schepers M, Bot FJ, Hopman AH, Ramaekers FC, Speel EJ (2008) Marked differences in survival rate between smokers and nonsmokers with HPV 16-associated tonsillar carcinomas. Int J Cancer 122: 2656–2664 [DOI] [PubMed] [Google Scholar]
- Hammarstedt L, Lindquist D, Dahlstrand H, Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W, Dalianis T, Munck-Wikland E (2006) Human papillomavirus as a risk factor for the increase in incidence of tonsillar cancer. Int J Cancer 119: 2620–2623 [DOI] [PubMed] [Google Scholar]
- Hong A, Dobbins T, Lee CS, Jones D, Jackson E, Clark J, Armstrong B, Harnett G, Milross C, O’Brien C, Rose B (2010a) Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis in oropharyngeal cancer. Eur J Cancer 46(11): 2088–2096 [DOI] [PubMed] [Google Scholar]
- Hong AM, Dobbins TA, Lee CS, Jones D, Fei J, Clark JR, Armstrong BK, Harnett GB, Milross CG, Tran N, Peculis LD, Ng C, Milne AG, Loo C, Hughes LJ, Forstner DF, O’Brien CJ, Rose BR (2010b) Use of cyclin D1 in conjunction with human papillomavirus status to predict outcome in oropharyngeal cancer. Int J Cancer [e-pub ahead of print] [DOI] [PubMed]
- Hong AM, Grulich AE, Jones D, Lee CS, Garland SM, Dobbins TA, Clark JR, Harnett GB, Milross CG, O’Brien CJ, Rose BR (2010c) Squamous cell carcinoma of the oropharynx in Australian males induced by human papillomavirus vaccine targets. Vaccine 28: 3269–3272 [DOI] [PubMed] [Google Scholar]
- Klussmann JP, Gultekin E, Weissenborn SJ, Wieland U, Dries V, Dienes HP, Eckel HE, Pfister HJ, Fuchs PG (2003) Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol 162: 747–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Cordell KG, Lee JS, Prince ME, Tran HH, Wolf GT, Urba SG, Worden FP, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D’Silva NJ, Yang K, Kurnit DM, Bradford CR, Carey TE (2007) Response to therapy and outcomes in oropharyngeal cancer are associated with biomarkers including human papillomavirus, epidermal growth factor receptor, gender, and smoking. Int J Radiat Oncol Biol Phys 69: S109–S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, Wolf GT, Urba SG, Chepeha DB, Teknos TN, Eisbruch A, Tsien CI, Taylor JM, D’Silva NJ, Yang K, Kurnit DM, Bauer JA, Bradford CR, Carey TE (2008) EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol 26: 3128–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen P, Eriksen JG, Hamilton-Dutoit S, Tramm T, Alsner J, Overgaard J (2009) Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol 27: 1992–1998 [DOI] [PubMed] [Google Scholar]
- Li W, Thompson CH, O’Brien CJ, McNeil EB, Scolyer RA, Cossart YE, Veness MJ, Walker DM, Morgan GJ, Rose BR (2003a) Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer 106(4): 553–558 [DOI] [PubMed] [Google Scholar]
- Li W, Thompson CH, Xin D, Cossart YE, O’Brien CJ, McNeil EB, Gao K, Scolyer RA, Rose BR (2003b) Absence of human papillomavirus in tonsillar squamous cell carcinomas from Chinese patients. Am J Pathol 163: 2185–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tran N, Lee SC, O’Brien CJ, Tse GM, Scolyer RA, Hong A, Milross C, Yu KH, Rose BR (2007) New evidence for geographic variation in the role of human papillomavirus in tonsillar carcinogenesis. Pathology 39: 217–222 [DOI] [PubMed] [Google Scholar]
- Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantu G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630–5636 [DOI] [PubMed] [Google Scholar]
- Lindel K, Beer KT, Laissue J, Greiner RH, Aebersold DM (2001) Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer 92: 805–813 [DOI] [PubMed] [Google Scholar]
- Maxwell JH, Kumar B, Feng FY, Worden FP, Lee JS, Eisbruch A, Wolf GT, Prince ME, Moyer JS, Teknos TN, Chepeha DB, McHugh JB, Urba SG, Stoerker J, Walline HM, Kurnit DM, Cordell KG, Davis SJ, Ward PD, Bradford CR, Carey TE (2010) Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin Cancer Res 16: 1226–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin H, Friesland S, Lewensohn R, Dalianis T, Munck-Wikland E (2000) Human papillomavirus (HPV) DNA in tonsillar cancer: clinical correlates, risk of relapse, and survival. Int J Cancer 89: 300–304 [PubMed] [Google Scholar]
- Pignon JP, Bourhis J, Domenge C, Designe L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355: 949–955 [PubMed] [Google Scholar]
- Sedaghat AR, Zhang Z, Begum S, Palermo R, Best S, Ulmer KM, Levine M, Zinreich E, Messing BP, Gold D, Wu AA, Niparko KJ, Kowalski J, Hirata RM, Saunders JR, Westra WH, Pai SI (2009) Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope 119(8): 1542–1549 [DOI] [PubMed] [Google Scholar]
- Shoushtari AN, Rahimi NP, Schlesinger DJ, Read PW (2010) Survey on human papillomavirus/p16 screening use in oropharyngeal carcinoma patients in the United States. Cancer 116: 514–519 [DOI] [PubMed] [Google Scholar]
- Smeets SJ, Hesselink AT, Speel EJ, Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ, Leemans CR, Brakenhoff RH (2007) A novel algorithm for reliable detection of human papillomavirus in paraffin embedded head and neck cancer specimen. Int J Cancer 121: 2465–2472 [DOI] [PubMed] [Google Scholar]
- Stanley KK, Szewczuk E (2005) Multiplexed tandem PCR: gene profiling from small amounts of RNA using SYBR Green detection. Nucleic Acids Res 33: e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaram K, Schwartz J, Har-El G, Lucente F (2005) Carcinoma of the oropharynx: factors affecting outcome. Laryngoscope 115: 1536–1542 [DOI] [PubMed] [Google Scholar]
- van Houten VM, Snijders PJ, van den Brekel MW, Kummer JA, Meijer CJ, van Leeuwen B, Denkers F, Smeele LE, Snow GB, Brakenhoff RH (2001) Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int J Cancer 93: 232–235 [DOI] [PubMed] [Google Scholar]
- Vidal L, Gillison ML (2008) Human papillomavirus in HNSCC: recognition of a distinct disease type. Hematol Oncol Clin N Am 22: 1125–1142, vii [DOI] [PubMed] [Google Scholar]
- Weinberger PM, Yu Z, Haffty BG, Kowalski D, Harigopal M, Brandsma J, Sasaki C, Joe J, Camp RL, Rimm DL, Psyrri A (2006) Molecular classification identifies a subset of human papillomavirus – associated oropharyngeal cancers with favorable prognosis. J Clin Oncol 24: 736–747 [DOI] [PubMed] [Google Scholar]
- Weinberger PM, Yu Z, Kountourakis P, Sasaki C, Haffty BG, Kowalski D, Merkley MA, Rimm DL, Camp RL, Psyrri A (2009) Defining molecular phenotypes of human papillomavirus-associated oropharyngeal squamous cell carcinoma: validation of three-class hypothesis. Otolaryngol Head Neck Surg 141: 382–389 [DOI] [PubMed] [Google Scholar]
- Wiest T, Schwarz E, Enders C, Flechtenmacher C, Bosch FX (2002) Involvement of intact HPV16 E6/E7 gene expression in head and neck cancers with unaltered p53 status and perturbed pRb cell cycle control. Oncogene 21: 1510–1517 [DOI] [PubMed] [Google Scholar]
- Zhen W, Karnell LH, Hoffman HT, Funk GF, Buatti JM, Menck HR (2004) The National Cancer Data Base report on squamous cell carcinoma of the base of tongue. Head Neck 26: 660–674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.