Abstract
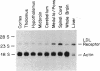
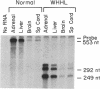
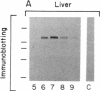
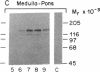
Hybridization studies with [32P]cDNA probes revealed detectable amounts of mRNA for the low density lipoprotein (LDL) receptor in the central nervous system (CNS) of rabbits. mRNA levels were highest in the medulla/pons and spinal cord, which were the most heavily myelinated regions that were studied. Lower, but detectable levels were present in cerebral cortex, hypothalamus, thalamus, midbrain, and cerebellum. In the medulla/pons and spinal cord, the levels of receptor mRNA were in a range comparable to that detected in the liver. The levels of receptor mRNA in whole brain were constant from 3 days of age to adulthood and, thus, did not vary in proportion to the rate of myelin synthesis. LDL receptor mRNA in the CNS was produced by the same gene that produced the liver and adrenal mRNA as revealed by the demonstration of a deletion in the neural mRNA of Watanabe-heritable hyperlipidemic (WHHL) rabbits identical to the deletion in the LDL receptor gene of these mutant animals. Using antibodies directed against the bovine LDL receptor, we showed that LDL receptor protein is present in the medulla/pons of adult cows. The cell types that express LDL receptors in the CNS and the functions of these receptors are unknown.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisiegel U., Schneider W. J., Brown M. S., Goldstein J. L. Immunoblot analysis of low density lipoprotein receptors in fibroblasts from subjects with familial hypercholesterolemia. J Biol Chem. 1982 Nov 10;257(21):13150–13156. [PubMed] [Google Scholar]
- Boyles J. K., Pitas R. E., Wilson E., Mahley R. W., Taylor J. M. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985 Oct;76(4):1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert J. A., Lipsky P. E. Modulation of human lymphocyte responses by low density lipoproteins (LDL): enhancement but not immunosuppression is mediated by LDL receptors. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4539–4543. doi: 10.1073/pnas.81.14.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOBBINGJ THE ENTRY OF CHOLESTEROL INTO RAT BRAIN DURING DEVELOPMENT. J Neurochem. 1963 Oct;10:739–742. doi: 10.1111/j.1471-4159.1963.tb08930.x. [DOI] [PubMed] [Google Scholar]
- Dalal K. B., Einstein E. R. Biochemical maturation of the central nervous system. I. Lipid changes. Brain Res. 1969 Dec;16(2):441–451. doi: 10.1016/0006-8993(69)90237-6. [DOI] [PubMed] [Google Scholar]
- Elshourbagy N. A., Liao W. S., Mahley R. W., Taylor J. M. Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):203–207. doi: 10.1073/pnas.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil G., Goldstein J. L., Slaughter C. A., Brown M. S. Cytoplasmic 3-hydroxy-3-methylglutaryl coenzyme A synthase from the hamster. I. Isolation and sequencing of a full-length cDNA. J Biol Chem. 1986 Mar 15;261(8):3710–3716. [PubMed] [Google Scholar]
- Gil G., Smith J. R., Goldstein J. L., Brown M. S. Optional exon in the 5'-untranslated region of 3-hydroxy-3-methylglutaryl coenzyme A synthase gene: conserved sequence and splicing pattern in humans and hamsters. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1863–1866. doi: 10.1073/pnas.84.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius M. J., Shooter E. M., Pitas R. E., Mahley R. W. Lipoprotein uptake by neuronal growth cones in vitro. Science. 1987 May 22;236(4804):959–962. doi: 10.1126/science.3576212. [DOI] [PubMed] [Google Scholar]
- Kabara J. J. A critical review of brain cholesterol metabolism. Prog Brain Res. 1973;40(0):363–382. doi: 10.1016/S0079-6123(08)60700-1. [DOI] [PubMed] [Google Scholar]
- Kandutsch A. A., Saucier S. E. Regulation of sterol synthesis in developing brains of normal and jimpy mice. Arch Biochem Biophys. 1969 Dec;135(1):201–208. doi: 10.1016/0003-9861(69)90531-1. [DOI] [PubMed] [Google Scholar]
- Ma P. T., Gil G., Südhof T. C., Bilheimer D. W., Goldstein J. L., Brown M. S. Mevinolin, an inhibitor of cholesterol synthesis, induces mRNA for low density lipoprotein receptor in livers of hamsters and rabbits. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8370–8374. doi: 10.1073/pnas.83.21.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma P. T., Yamamoto T., Goldstein J. L., Brown M. S. Increased mRNA for low density lipoprotein receptor in livers of rabbits treated with 17 alpha-ethinyl estradiol. Proc Natl Acad Sci U S A. 1986 Feb;83(3):792–796. doi: 10.1073/pnas.83.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P. S., Carey M., Forte T., Vega G. L. Apolipoproteins in human cerebrospinal fluid. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4646–4649. doi: 10.1073/pnas.76.9.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Schneider W. J., Yamamoto T., Luskey K. L., Brown M. S., Goldstein J. L. Domain map of the LDL receptor: sequence homology with the epidermal growth factor precursor. Cell. 1984 Jun;37(2):577–585. doi: 10.1016/0092-8674(84)90388-x. [DOI] [PubMed] [Google Scholar]
- Schneider W. J., Beisiegel U., Goldstein J. L., Brown M. S. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J Biol Chem. 1982 Mar 10;257(5):2664–2673. [PubMed] [Google Scholar]
- Serougne C., Lefevre C., Chevallier F. Cholesterol transfer between brain and plasma in the rat: a model for the turnover of cerebral cholesterol. Exp Neurol. 1976 Apr;51(1):229–240. doi: 10.1016/0014-4886(76)90066-2. [DOI] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yamamoto T., Bishop R. W., Brown M. S., Goldstein J. L., Russell D. W. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986 Jun 6;232(4755):1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilversmit D. B., Hughes L. B. Incorporation in vivo of labeled plasma cholesterol into aortas of young and old rabbits. Atherosclerosis. 1973 Jul-Aug;18(1):141–152. doi: 10.1016/0021-9150(73)90125-1. [DOI] [PubMed] [Google Scholar]