Abstract
The function of glutamate receptors on oligodendrocytes and their precursor cells is poorly understood, with their only clear action being to damage these cells in pathological conditions. Here we review recent studies of glutamate signalling to oligodendrocyte lineage cells, and explore what its physiological function may be.
Introduction and context
Given the intimate association of neurons and glia in the central nervous system, it is not surprising that the main excitatory neurotransmitter, glutamate, is also recognized by a diverse group of resident glial cells. Oligodendrocyte lineage cells have long been known to respond to glutamate [1-3], but the only established effect of glutamate ‘signalling’ is the damage that these cells suffer in pathological conditions (for a review and relevant publications see [4]). For example, the extracellular glutamate level rises when glutamate transporters reverse in conditions such as stroke, or secondary ischaemia caused by blood vessel damage following spinal cord injury, or in development when inadequate blood flow reaches the white matter around the cerebral ventricles leading to cerebral palsy. Changes in the expression levels of the enzymes glutamate dehydrogenase, glutamine synthetase, and glutaminase also lead to a rise in extracellular glutamate concentration in multiple sclerosis lesions. An elevated glutamate level activates receptors that damage oligodendrocytes or, in the case of cerebral palsy, the precursor cells that will become oligodendrocytes. Are there, however, any positive aspects of glutamate signalling to oligodendrocyte lineage cells?
Major recent advances
A significant step forward came with the discovery that neurons send synaptic input to oligodendrocyte precursor cells (OPCs) in the grey matter [5], and also in the white matter [6-8]. These contacts have the ultrastructural and pharmacological features of bona fide excitatory synapses involving glial α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate (KA) receptors (or GABAA receptors: the intracellular [Cl-] is high in OPCs, so GABA is excitatory [9]). In the white matter this input was shown to occur from unmyelinated axons [7,8], prompting the notion that this could be a developmental signal from active axons, instructing nearby OPCs to stop proliferating and to start to myelinate the axons (Figure 1). Conceivably, such signalling could, with other known trophic factors, help to match the number of oligodendrocytes formed to the length of axon that needs to be myelinated. However, although previous work in culture showed that glutamate does inhibit the proliferation (and lineage progression) of OPCs [10], the demonstration that the synapses onto precursors are maintained through cell division [11,12] argues strongly against an immediate inhibitory effect of synaptic input on proliferation rate. Activation of AMPA receptors on OPCs might also promote OPC migration to sites of myelination [13], although it is not known whether glutamate released from neuronal synapses onto the OPCs can have this effect, and migration would seem to be incompatible with maintaining the presence of synapses from particular axons. AMPA/KA receptors on OPCs might also trigger metabolic interactions between axons and ensheathing glia [14]. As OPCs mature into myelinating oligodendrocytes, the synaptic input from axons is lost [15,16] (Figure 1).
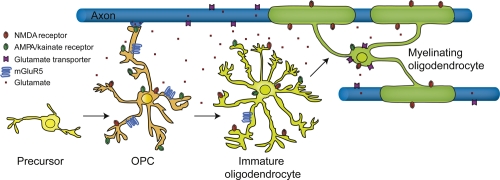
Figure 1. Glutamate receptor expression on oligodendrocyte lineage cells.
Schematic depiction of a myelinating oligodendrocyte (right) that has differentiated from a mitotic progenitor (oligodendrocyte precursor cell [OPC], left), which was in synaptic contact with an unmyelinated axon. OPCs, immature oligodendrocytes, and mature oligodendrocytes express glutamate receptors. Axonal and oligodendrocyte glutamate transporters cause a non-vesicular glutamate release in conditions of energy deprivation such as stroke and secondary ischaemia following spinal cord injury. Whether glutamatergic stimulation of OPCs regulates their differentiation and myelination awaits in vivo evidence. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, NMDA, N-methyl D-aspartate; mGluR, metabotropic glutamate receptor.
Amplification of the effect of excitatory synaptic input onto OPCs was recently suggested to occur because a subpopulation of OPCs (identified by their expression of the oligodendrocyte specific transcription factor Olig2 and the proteoglycan NG2) express voltage-gated Na+ channels that, at least in some rat OPCs, are present at a density sufficient to evoke action potentials [17]. In contrast, another subclass of OPCs neither received synaptic input nor fired action potentials [17]. Studies on mouse OPCs (identified by expression of dsRed driven by the NG2 promoter) reported no action potentials in Na+-channel-expressing OPCs [15], and it was suggested that the action-potential-generating cells were migrating interneurons rather than OPCs. However, this is hard to reconcile with their expression of Olig2 and NG2 and their lack of expression of NeuN [17]. Indeed, recent work on mouse OPCs (labelled with green fluorescent protein expressed under control of the Pdgfrα or Sox10 promoters) revealed that the size of the voltage-gated Na+ current in these cells was fivefold smaller than in rat OPCs [18], resulting in spike-like regenerative activity being much weaker [12,18,19]. Excitatory synaptic input will tend to activate the voltage-gated Na+ current, and thus depolarize the cell further, while simultaneously raising [Na+]i. These effects will promote a rise of [Ca2+]i [20], but whether this alters the migratory behaviour of OPCs, regulates differentiation, or leads OPCs to release some factor onto other cells currently remains obscure.
It is of increasing interest to define the receptor subtypes mediating glutamatergic signalling to oligodendrocyte lineage cells, and their subcellular location. It was originally believed that ischaemic damage to oligodendrocytes and their precursors resulted solely from the activation of AMPA/KA receptors, but increasing evidence implicates NMDA (N-methyl D-aspartate) receptors as well. Applying glutamate or NMDA activates NMDA receptor- as well as AMPA/KA receptor-mediated currents in the cells [6,15,17,21]. In ischaemia, activation of NMDA receptors is partly responsible for damage to developing OPCs [21] and to the myelinating processes of oligodendrocytes [22,23], and (along with KA receptor activation) contributes to retraction of the paranodal folds from the node of Ranvier [24], raising the question of whether glutamate receptors are preferentially localized at the paranodal folds of the myelin. However, the extent to which blocking NMDA receptors preserves the function of myelinated axons after ischaemia requires further investigation [25,26]. The NMDA receptors involved show less Mg2+-block than neuronal NMDA receptors [6], suggesting that they have an unusual subunit composition [6,22,23]. Surprisingly, given these results, transcriptome analysis [27] indicates that although NMDA receptors are expressed in OPCs, their expression at the mRNA level drops to low levels as the cells mature into myelinating oligodendrocytes, yet immunocytochemistry suggests that NMDA receptors are present both on the myelinating processes of oligodendrocytes [6,22,23] and on developing oligodendrocyte precursors [21]. A similar downregulation of AMPA receptors with development has also been reported [15,16].
NMDA receptors do not seem to contribute to synaptic currents evoked in OPCs by neuronal activity [7,8,15,16], raising the question of what their physiological function may be. One suggestion, based on experiments in which dorsal root ganglion cells are myelinated by OPCs in culture, is that the growth factor neuregulin produces a switch in the mode of myelination by OPCs: without neuregulin added to the culture medium myelination is independent of neuronal activity and glutamate release, while with neuregulin added myelination depends both on action potentials and on activation of NMDA receptors [28]. Myelination is generally thought to depend on action potentials [29], but myelination of fixed axons (which clearly have no action potentials) has also been reported [30] and this may correspond to the activity-independent mode of myelination seen in these studies.
In addition to ionotropic glutamate receptors, developing oligodendrocytes in culture and in vivo express metabotropic glutamate receptors (mGluRs), particularly mGluR5, which is downregulated as the cells mature [27,31-33]. The function of these receptors is unclear but, in cultured OPCs, activation of group 1 mGluRs (presumably mGluR5) raises [Ca2+]i [31], leads to the release of brain-derived neurotrophic factor [34] (which could promote myelin formation), and reduces both excitotoxic damage to the cells and apoptosis induced by staurosporine [32,33,35].
Future directions
Considerable work will be required to establish the true function of glutamatergic signalling to oligodendrocyte lineage cells. Genetic engineering in mice will provide some insight into the roles of particular glutamate receptor subtypes. However, for a phenomenon as important as myelination, it is likely that several mechanisms will operate in parallel, and with functional redundancy in place mutant phenotypes may not be informative. There are several crucial aspects of neuron-to-glia signalling that we need to establish. Is it only the glutamate that is released at synapses onto OPCs that is functionally relevant, or can tonic activation of high-affinity NMDA receptors also modulate cell function? What is the role of glutamate transporters, which are reported either to generate a large current in mature oligodendrocytes [15,16] or to greatly reduce the glutamate-evoked current [6]? What are the pathways downstream of glutamate receptors that control OPC development and myelination, and does this signalling contribute to determining whether OPCs develop into oligodendrocytes, or into astrocytes or neurons [36-38]? Do these pathways also function to maintain myelination in the adult animal? Are there functions of glutamatergic signalling other than regulation of oligodendrocyte development? Does glutamate signalling play a role in the formation of the glial scar in pathology, or in remyelination, since adult OPCs receive glutamatergic synaptic input as they migrate during remyelination [39]?
Acknowledgments
Research in the authors’ labs is supported by the Wellcome Trust, the EU (Leukotreat, Neuropromise, Neuron-Glia Interactions in Nerve Development and Disease [NGIDD]), the European Research Council, the German Federal Ministry of Education and Research (BMBF) (Leukonet), and the German Research Foundation (DFG) (Center for Molecular Physiology of the Brain [CMPB], SFB/TR43).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- KA
kainate
- mGluR
metabotropic glutamate receptor
- NMDA
N-methyl D-aspartate
- OPC
oligodendrocyte precursor cell
Competing Interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at: http://f1000.com/reports/b/2/57
References
- 1.Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–24. doi: 10.1016/0896-6273(90)90109-S. [DOI] [PubMed] [Google Scholar]
- 2.Berger T, Walz W, Schnitzer J, Kettenmann H. GABA- and glutamate-activated currents in glial cells of the mouse corpus callosum slice. J Neurosci Res. 1992;31:21–7. doi: 10.1002/jnr.490310104. [DOI] [PubMed] [Google Scholar]
- 3.Patneau DK, Wright PW, Winters C, Mayer ML, Gallo V. Glial cells of the oligodendrocyte lineage express both kainate- and AMPA-preferring subtypes of glutamate receptor. Neuron. 1994;12:357–71. doi: 10.1016/0896-6273(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 4.Káradóttir R, Attwell D. Neurotransmitter receptors in the life and death of oligodendrocytes. Neuroscience. 2006;145:1426–38. doi: 10.1016/j.neuroscience.2006.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–91. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 6.Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–6. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kukley M, Capetillo-Zarate E, Dietrich D. Vesicular glutamate release from axons in white matter. Nat Neurosci. 2007;10:311–20. doi: 10.1038/nn1850. [DOI] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Venkatesh Murthy 27 Apr 2007
- 8.Ziskin JL, Nishiyama A, Rubio M, Fukaya M, Bergles DE. Vesicular release of glutamate from unmyelinated axons in white matter. Nat Neurosci. 2007;10:321–30. doi: 10.1038/nn1854. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.5 Must ReadEvaluated by Elior Peles 19 Feb 2007, Klaus-Armin Nave 23 Feb 2007, Venkatesh Murthy 27 Apr 2007
- 9.Lin SC, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- 10.Yuan X, Eisen AM, McBain CJ, Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–14. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- 11.Kukley M, Kiladze M, Tognatta R, Hans M, Swandulla D, Schramm J, Dietrich D. Glial cells are born with synapses. FASEB J. 2008;22:2957–69. doi: 10.1096/fj.07-090985. [DOI] [PubMed] [Google Scholar]
- 12.Ge WP, Zhou W, Luo Q, Jan LY, Jan YN. Dividing glial cells maintain differentiated properties including complex morphology and functional synapses. Proc Natl Acad Sci U S A. 2008;106:328–33. doi: 10.1073/pnas.0811353106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26:2458–66. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nave KA. Myelination and the trophic support of long axons. Nat Rev Neurosci. 2010;11:275–83. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 15.De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci. 2010;30:3600–11. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kukley M, Nishiyama A, Dietrich D. The fate of synaptic input to NG2 cells: neurons specifically downregulate transmitter release onto differentiating oligodendroglial cells. J Neurosci. 2010;30:8320–31. doi: 10.1523/JNEUROSCI.0854-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Káradóttir R, Hamilton NB, Bakiri Y, Attwell D. Spiking and nonspiking classes of oligodendrocyte precursor glia in CNS white matter. Nat Neurosci. 2008;11:450–6. doi: 10.1038/nn2060. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Michael Fehlings 24 Apr 2008
- 18.Clarke LE, Hamilton NB, Kessaris N, Richardson WD, Attwell D. The distribution of two types of oligodendrocyte precursor cell in different brain areas [abstract] Glia. 2009;57:S83. [Google Scholar]
- 19.Chittajallu R, Aguirre A, Gallo V. NG2-positive cells in the mouse white and grey matter display distinct physiological properties. J Physiol. 2004;561:109–22. doi: 10.1113/jphysiol.2004.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong XP, Li XY, Zhou B, Shen W, Zhang ZJ, Xu TL, Duan S. Ca2+ signaling evoked by activation of Na+ channels and Na+/Ca2+ exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–28. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning SM, Talos DM, Zhou C, Selip DB, Park HK, Park CJ, Volpe JJ, Jensen FE. NMDA receptor blockade with memantine attenuates white matter injury in a rat model of periventricular leukomalacia. J Neurosci. 2008;28:6670–8. doi: 10.1523/JNEUROSCI.1702-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 23.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, Zamponi GW, Wang W, Stys PK. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–92. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]; F1000 Factor 3.0 RecommendedEvaluated by Elior Peles 08 May 2006
- 24.Fu Y, Sun W, Shi Y, Shi R, Cheng JX. Glutamate excitotoxicity inflicts paranodal myelin splitting and retraction. PLoS One. 2009;4:e6705. doi: 10.1371/journal.pone.0006705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakiri Y, Hamilton NB, Káradóttir R, Attwell D. Testing NMDA receptor block as a therapeutic strategy for reducing ischaemic damage to CNS white matter. Glia. 2008;56:233–40. doi: 10.1002/glia.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tekkök SB, Ye Z, Ransom BR. Excitotoxic mechanisms of ischemic injury in myelinated white matter. J Cereb Blood Flow Metab. 2007;27:1540–52. doi: 10.1038/sj.jcbfm.9600455. [DOI] [PubMed] [Google Scholar]
- 27.Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–78. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 4.9 Must ReadEvaluated by Valina Dawson 14 Jan 2008, Elior Peles 15 Jan 2008, Brian Popko 16 Jan 2008
- 28.Luzhynskaya A, Lundgaard I, Wang Z, ffrench-Constant C, Attwell D, Káradóttir RT. Neuregulin induces NMDA receptor dependent myelination by oligodendrocytes [abstract] Glia. 2009;57:S43. doi: 10.1371/journal.pbio.1001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc Natl Acad Sci U S A. 1996;93:9887–92. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SS, Kelland EE, Tokar E, De la Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2008;105:14662–7. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Factor 6.0 Must ReadEvaluated by Michael Sendtner 22 Oct 2008
- 31.Luyt K, Varadi A, Molnar E. Functional metabotropic glutamate receptors are expressed in oligodendrocyte precursor cells. J Neurochem. 2003;84:1452–64. doi: 10.1046/j.1471-4159.2003.01661.x. [DOI] [PubMed] [Google Scholar]
- 32.Deng W, Wang H, Rosenberg PA, Volpe JV, Jensen FE. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A. 2004;101:7751–6. doi: 10.1073/pnas.0307850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luyt K, Varadi A, Durant CF, Molnar E. Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J Neurochem. 2006;99:641–56. doi: 10.1111/j.1471-4159.2006.04103.x. [DOI] [PubMed] [Google Scholar]
- 34.Bagayogo IP, Dreyfus CF. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN Neuro. 2009;1:e00001. doi: 10.1042/AN20090006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelland EE, Toms NJ. Group 1 metabotropic glutamate receptors limit AMPA receptor-mediated oligodendrocyte progenitor cell death. Eur J Pharm. 2001;42:R3–R4. doi: 10.1016/s0014-2999(01)01157-8. [DOI] [PubMed] [Google Scholar]
- 36.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Götz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–42. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 38.Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFR alpha/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Etxeberria A, Mangin JM, Aguirre A, Gallo V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat Neurosci. 2010;13:287–9. doi: 10.1038/nn.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]