Many individuals with problems of substance addiction become unable to base their drug-use decisions on the long-term outcome of their choices. We present here a neural framework that explains this ‘myopia' for future consequences. We suggest that addiction may be the product of an imbalance between two separate, but interacting, neural systems that subserve decision-making: A reactive system for signaling pain or pleasure of immediate prospects with the amygdala as a key structure, and a reflective system for signaling pain or pleasure of future prospects involving highly the prefrontal cortex. Through development, socialization, and individuals' learning of social rules, the reflective system gains control over the reactive system via several cognitive (e.g., response inhibition, shifting) and neural mechanisms (fronto-parietal network). However, this control is not absolute; hyperactivity within the reactive system can override the reflective system and the neurotoxicity of drugs could lead to the disruption in self-regulation. We propose that drugs can trigger bottom-up, involuntary signals originating from the amygdala that modulate, bias, or even hijack the goal-driven cognitive resources that are needed for the normal operation of the reflective system and for exercising the willpower to resist drugs. We finally develop the idea that different patterns of imbalance between reactive and reflective systems could lead to distinct patterns of clinical impulsivity involved in the vulnerability, the development, and the relapse to drugs.
Introduction
Researchers in cognitive neuroscience have started to investigate how people make decisions. From studies of individuals with focal brain damage, who show poor decision-making, and from functional neuroimaging studies of participants performing decision-making tasks, as well as studies using cellular recording techniques, new hypotheses about the neural and the cognitive underpinnings of decision-making have emerged. Interestingly, some of these strategies have been adopted in investigations of the decision-making mechanisms of individuals with substance dependence problems and addiction. These approaches have provided new insights about the vulnerability and the development of addictive behaviors, as well as the repeated relapse of many of these individuals to the compulsive use of addictive substances, even after periods of abstinence.
Brain Lesions and Poor Decision-Making
After injury to the ventromedial prefrontal cortex [VMPC; for the purposes of this piece, VMPC is defined as the ventral medial prefrontal cortex and the medial sector of the orbitofrontal cortex, thus encompassing Brodmann's areas (BA) 25, lower 24, 32, and medial aspect of 11, 12, and 10], patients tend to recover with normal intelligence, memory, speech, sensation, and movement but emotion and social behavior change completely.1 As a result, these patients begin to have difficulties planning their workday and choosing friends, partners, and activities. The actions they elect to pursue often lead to losses of diverse order, for example, financial losses, losses in social standing, and losses of family and friends. The choices they make are no longer advantageous; the patients often decide against their best interests and fail to learn from previous mistakes. These decisions are strikingly different from the kinds of choices these patients were known to make before their brain damage. These observations—normal intellect and abnormalities in decision-making, emotion, and feeling in VMPC patients—led Damasio1 to propose what has become an influential neural theory of decision-making, the Somatic Marker Hypothesis (SMH). The cardinal point of this theory is that emotion-related signals (somatic markers) assist cognitive processes in implementing decisions. When the VMPC syndrome was initially described,1 the decision-making deficit seen in these patients was puzzling because their poor decision-making and failure to learn from repeated mistakes was obvious in their everyday lives, but there was no laboratory probe to detect and measure their impairment. This challenge was overcome by the development of the Iowa Gambling Task (IGT).2 In this task, subjects choose from four decks of cards, each with a different potential payoff, to maximize their monetary gain. After each choice, subjects receive feedback telling them how much money they won or lost. Through this feedback, normal decision-makers learn to avoid decks that yield high immediate gains but larger future losses down the line. In contrast, patients with VMPC damage and drug addicts fail to make advantageous choices despite their intact ability to update expected reward values.3 As we will describe under the SMH, while normal individuals experience a state of arousal during the time of deliberation prior to making risky and disadvantageous choices, the insensitivity to future consequences (myopia for the future) seen in VMPC patients is thought to be derived from their failure to experience this affective state. This affective experience is normally accompanied by bodily signals and somatic states that have become associated with risky decisions; these bodily signals (or somatic markers) are derived from prior experiences with reward and punishment. A further aspect of this theory is that these somatic markers can be nonconscious: They can bias the response selection even when a person is unaware of them.1,4 A closer inspection of processes subserving decision-making in the IGT also reveals the critical importance of processes related to memory, including the maintenance of active representations of certain information for a short period (i.e., several 10s of seconds) in working memory or for a longer period of time in episodic memory.
From a neurocognitive perspective, the normal functioning of the VMPC is contingent upon the integrity of other following neural systems: (a) the insula/somatosensory cortices, especially on the right side, are thought to be critical for representing emotional states, and (b) the dorsolateral sector of the prefrontal cortex (DLPC), as well as the hippocampal system, are thought to be critical for working and episodic memory, respectively. Thus decision-making depends on systems for emotion/affect and memory. Damage to any of these systems compromises the ability to make advantageous decisions in the long run. The role of VMPC is to link these systems together. Therefore, when damaged, there are many manifestations, including alterations of emotional/affective experience, poor decision-making and impulse control, and abnormal social functioning.
Addiction and Poor Decision-Making
There are two main psychological and behavioral similarities between patients with ventromedial prefrontal cortex (VMPC) damage and drug addicts.1 Both often deny, or are not aware, that they have a problem.2 When faced with a choice that brings immediate reward, even at the risk of incurring future negative outcomes, including loss of reputation, job, and family, they appear oblivious to the consequences of their actions.
Besides, abnormalities in the VMPC region and related poor decisions on the IGT were observed in cocaine addicts.5 This linkage energized a new line of research aimed at understanding the relationship between substance abuse and poor decision making.2,6–9 This approach has highlighted the key role that choice plays in addiction. Our aim in this article is to present a broad conceptual framework that brings together several disparate lines of research on addiction. We present here the view that addiction is a condition in which the neural mechanisms that enable one to choose according to long-term outcomes are weakened, thus leading to an inability to control one's temptation and the loss of one's willpower to resist drugs. This complements previous proposals that disruption of the VMPC leads to loss of self-directed behavior in favor of more automatic sensory-driven behavior.5
A Neural System for Willpower
The somatic marker hypothesis is a systems-level neuroanatomical and cognitive framework for choosing according to long-term, rather than short-term, outcomes.1 The key idea of this hypothesis is that the process of decision-making depends in many important ways on neural substrates that regulate homeostasis, emotion and feeling.1 The term somatic refers to the collection of body- and brain-related responses that hallmark affective and emotional responses. Both the amygdale and VMPC are critical for triggering somatic states, but as we will explain shortly, the amygdala responds to events that occur in the environment, whereas the VMPC triggers somatic states from memories, knowledge, and cognition. In order for somatic signals to influence cognition and behavior, they must act on appropriate neural systems. We believe that the key mechanism through which somatic states modulate cognition and behavior is mediated by neurotransmitter systems, especially dopamine and serotonin. Through these neurotransmitter systems, somatic (affective) signals can then act on several target cortical and subcortical sites that are critical for cognition and behavior, thus exerting modulatory or biasing effects on these systems (Figure 1). More specifically, during the process of pondering decisions, the immediate and future prospects of an option may trigger numerous affective (somatic) responses that conflict with each other. The end result is that an overall positive or negative signal emerges. At this juncture, our proposal for this overall positive or negative signal is hypothetical in nature, and not supported by empirical evidence. However, it is consistent the neurophysiological properties of neurons, especially the triggering of excitatory (EPSP) or inhibitory (IPSP) post synaptic potentials, and the “all or none” firing principles of post-synaptic neurons. Thus our hypothesis is that over the course of pondering a decision, positive and negative signals that are strong are reinforced, and weak ones are eliminated. This process can be very fast, and ultimately a winner takes all: in other words, an overall, more dominant, pattern of affective signaling emerges, which then can act on appropriate neural systems to modulate cognition and behavior.
Figure 1.
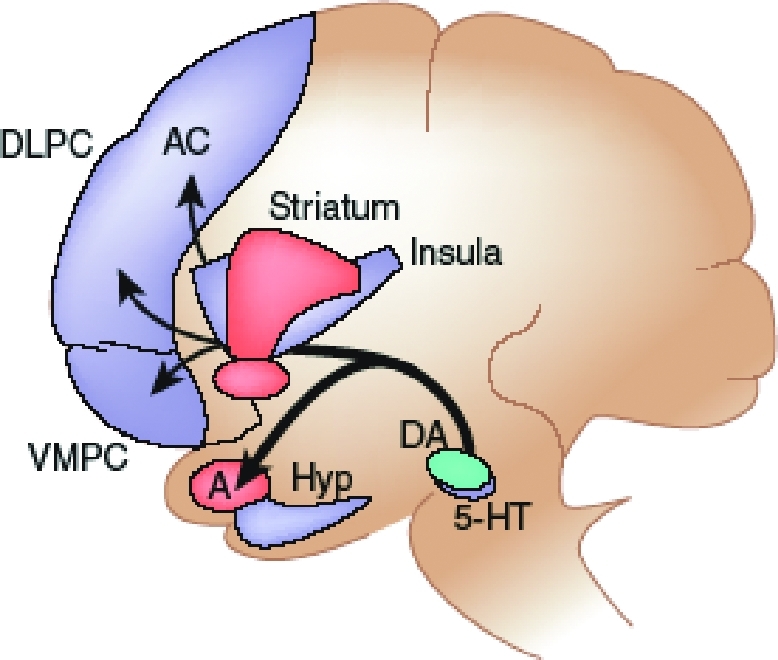
On this diagram, key structures belonging to the reactive system (red) and the reflective system (blue) are represented. An emergent dominant pattern of affective signaling can modulate activity of several components of the reactive and reflective systems. These include regions involved in (i) representing patterns of affective states (e.g., the insula and somatosensory cortices); (ii) triggering of affective states (e.g., amygdala (A) and VMPC); (iii) memory, impulse and attention control (e.g., lateral orbitofrontal, inferior frontal gyrus and dorso-lateral prefrontal (DLPC), hippocampus (Hip) and anterior cingulated (AC); and (iv) behavioral actions (e.g., striatum and supplementary motor area). 5-HT; serotonin; DA: dopamine. Reproduced with permission from Bechara A. Decision making, impulse control, and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience 2005;8(11):1458–63.
On the basis of this neural framework, we propose that willpower emerges from the dynamic interaction of two separate, but interacting, neural systems: a reactive system, in which the amygdala is a critical neural structure involved in triggering the affective/emotional signals of immediate outcomes, and a reflective system, in which the VMPC is a critical neural structure involved in triggering the affective/emotional signals of long-term outcomes (Figure 1).
This framework addresses one important question in drug addiction: Of the millions of people who drink alcohol or experiment with drugs, why do only about 3 to 10 percent become addicted? The view we present here challenges the old thinking that people may be equally vulnerable to addiction once drugs are made available, as drug use can invariably induce neuronal changes and homeostatic deregulation that lead to addiction. We argue that before one gets to the stage where a certain pattern of drug use can cause these severe changes to the brain, there is a decision by the person to keep using, or stop using, the drug. We argue that this mechanism protects most individuals who have experimented with drugs from getting to the point of losing their control over their drug use behavior and succumbing to severe addiction. For some individuals, however, this decision-making mechanism is relatively weak. Such individuals might be vulnerable to addiction because the process that enables one to inhibit actions elicited by the reactive system is dysfunctional. The source of this dysfunction, we will suggest, can be genetic or environmentally induced.
The Reactive Brain System
Physiological evidence suggests that responses triggered through the amygdala are short lived and habituate very quickly.11 Indeed, pleasant or aversive stimuli, such as encountering an object that induces fear (a ‘fear object', such as a snake) or a cue predictive of a fear object, trigger quick, automatic, and obligatory affective/emotional responses through the amygdala system. According to the somatic marker framework, the amygdala links the features of the stimulus to its affective/emotional attributes. More generally, it has been proposed that the amygdala is critically involved in relevance detection. Evaluation of relevance may then elicit responses in the emotional components, including enhanced sensory analysis and enhanced encoding into memory, as well as autonomic, motor, and cognitive effects.12
The affective/emotional response is particularly evoked through visceral motor structures, such as the hypothalamus and autonomic brainstem nuclei that produce changes in internal milieu and visceral structures, as well as through behavior-related structures such as the striatum, periaqueductal gray (PAG), and other brainstem nuclei that produce changes in facial expression and specific approach or withdrawal behaviors. Unlike food and water, money does not initially have affective properties, but acquires them with human learning. Because this learning becomes so robust, exposure to monetary reward triggers affective signals that are automatic and obligatory and which we believe are mediated through the amygdala system. Indeed, it has been shown that autonomic responses to large sums of monetary gains or losses depend on the integrity of the amygdala, as patients with bilateral amygdala damage fail to show such responses.13 This is consistent with research showing that the brain can encode the value of various options on a common scale,14 thus suggesting that there may be a common neural ‘currency' that encodes the value of different options, thus allowing the reward value of money to be compared with that of food, sex, or other rewards.
Similarly, drugs may acquire conditioned properties, that is to say, powerful affective and emotional properties. In addicts, fast, automatic and exaggerated autonomic responses are triggered by cues related to the substance they abuse, similar to the effects of monetary gains.3 These immediate responses to drugs (e.g., a beer) or to drug-related stimuli (e.g., a bar), which are associated with drug cues, are numerous. For instance, addicts presented with drug-related stimuli often display changes in patterns of autonomic responding (e.g., increased skin conductance).15,16 Several lines of direct and indirect behavioral evidence have supported the view that conditioned approach behavior to drug cues relates to abnormal activity in the amygdala-ventral striatum system, thereby resulting in exaggerated processing of the incentive values of substance-related cues.17 This ascribes a functional role of the striatum in the motivational and behavioral aspects of drug seeking, and it is consistent with the currently proposed framework of addiction.
The Reflective Brain System
Affective reactions can also be generated from recall of personal—or imagination of hypothetical—affective/emotional events. Affective state patterns are acquired in brainstem nuclei, such as the parabrachial nuclei, and in somatosensory cortices (e.g., insula, somatosensory, and posterior cingulate cortices) from prior experiences with reward and punishment.1 After an affective state has been experienced at least once, a neural pattern for this state is formed. Subsequent evocation of memories of a previous experience reactivates the pattern of affective state belonging to an original experience. Provided that representations of these affective state patterns develop normally, the VMPC is a critical substrate in the neural system necessary for triggering affective states from recall or from imagination.13 This hypothesis is based on cognitive and physiological evidence from patients with lesions in the VMPC.13 However, it is also reasonable to suggest based on this evidence that recalling the experience of a drug reactivates the pattern of affective state belonging to the actual previous encounter of that drug. This mechanism should also bring up the negative consequences associated with drug use. These negative consequences are not simply aversive experiences resulting from the actual consumption of the drug. Rather, they relate to social (such as trouble with the law, family, or finances) and psychological harms associated with drug use. The affective state patterns of these negative consequences become represented in the brain when individuals learn from parents or society about the dangers of drug use. Therefore, one does not need to use drugs in order to fear their consequences; these negative consequences should be there, even before experimenting with drugs. However, having poor mechanisms of decision-making renders individuals oblivious to these negative consequences, thus facilitating their initiation of drug misuse. We also conceive that the somatic markers' impairments may represent a risk factor to escalate from a social use of licit drugs (such as alcohol) to become a true addict. Indeed, drug users often encounter associated problems (such as hangover) generating negative affective patterns. It is hypothesized that in case of compromised neural systems for activations of somatic markers, further drug-related decisions will not be biased by this negative experience, thus rendering the individual more likely to escalate their drug use, despite the negative consequences, and to succumb to severe addiction.
Decision-making reflects a process in which a choice is made after reflecting on the consequences of that choice. The choice between another drug use episode and the potential of losing a job, family breakdown, and financial ruin down the line presents a dilemma to an addict, and a decision has to be made. Individuals with a weakness in this process (that is to say, those who do not reflect on the consequences of their decisions) may be similar to individuals with the personality trait of ‘nonplanning impulsivity,' a tendency to live for the moment with no regard for the future18 or the trait of ‘lack of premeditation,' an absence of the tendency to think and reflect on the consequences of an act before engaging in that act.19 Several tasks are now used to study this decision-making processes, including the IGT and the Cambridge Gamble and Risk Tasks.20,21 In line with this idea, in undergraduate students, the lack of premeditation, one of the four facets of impulsivity identified by Whiteside and Lynam,19 was especially linked to disadvantageous decisions on the IGT task.22 A critical neural region for this mechanism is the VMPC region, especially its more anterior sector (i.e., the one that spares the anterior cingulate), but other neural components outlined earlier are also important.13
Impairments in decision-making are evident in addicts, regardless of the type of drug they abuse, which suggests that poor decision-making may relate to addiction in general, rather than the effects of one specific type of drug. Alcohol, cannabis, cocaine, opioid, and methamphetamine abusers show impairments in decision-making on a variety of tasks.2,5,6,23 Although the differences in cognitive impairments brought on by the use of different drugs remains elusive, we have obtained preliminary evidence suggesting that chronic use of methamphetamine may be more harmful to decision-making than the use of other drugs.24 Direct comparison of the decision-making impairments in addicts on the IGT versus patients with VMPC damage showed that a significantly high proportion of addicts (63% vs. 27% of normal controls) performed within the range of VMPC patients, whereas the rest performed within the range of the majority of normal controls.3 Further characterization of these decision-making deficits, using skin conductance response (SCR) measures as indices of affective states during performance of the task, showed that this small minority of addicts (the 37% of addicts who performed normally) matched normal controls in all respects. However, the remainder of the addicts (the 63% who performed abnormally) had two profiles: one subgroup matched the VMPC patients in all respects (that is, they had abnormal SCRs when they pondered risky decisions), but another subgroup did not match the VMPC patients. This pattern of abnormal physiological responses when making risky decisions in addicts was also obtained with the Cambridge Gamble Task.25 A minority of normal controls performed like addicts and VMPC patients on the IGT, and with additional SCR measures, some of them matched the profile of VMPC patients, and some healthy participants were more like the addicts who did not match the VMPC patients.2,3 These studies suggest that decision-making deficits in addicts, and surprisingly, in some normal controls, are not uniform across all individuals. Our view is that attention to individual, as opposed to group, differences in these decision-making deficits is the key to understanding the nature of the addiction problem, its prognosis, and possible treatment. However, decision-making is not the only mechanism by which the reflective system exerts control over the impulsive system. There may be more than one mechanism for this control.
Normal functioning of the VMPC is contingent upon the integrity of other neural systems. One system involves the insula and other somatosensory cortices, especially on the right side, that are critical for representing patterns of emotional/affective states.1 Patients with right parietal damage (encompassing insula and somatosensory cortex) show impairments in decision-making;13 addicts show functional abnormalities in these parietal regions when performing decision-making tasks.8 The other system involves the dorsolateral sector of the prefrontal cortex and the hippocampus, which are critical for memory.13 Indeed, maintaining an active representation of memory over a delay period involves the dorsolateral sector of the prefrontal cortex, and patients with damage to this structure show compromised decision-making;20 addicts who have deficits in working memory also show compromised decision-making.26 Several voxel-brain-morphometry studies of brain scans of addicts found varying degrees of structural abnormalities in main components of the reflective system (Figure 1), including the VMPC, anterior cingulate, insular cortex,27 dorsolateral prefrontal cortex, and lateral orbitofrontal/inferior frontal gyrus.28 Abnormalities have also been detected in white matter pathways connecting these structures.29,30 Convergent results have also been obtained from functional neuroimaging studies.5,8,9 However, it is difficult to determine whether these abnormalities preceded or were the consequences of drug use. Our view is that a degree of abnormality pre-existed the addiction state, by facilitating the progress from experimentation to addiction. However, any subsequent excessive and chronic use of drugs can exacerbate these abnormalities.
Other Processes Subserving Decision-Making
Besides the involvement of somatic markers, working memory, and the episodic memory in decision-making, we recognize the importance of other processes, besides decision-making, that are involved in the control of behaviors. The first concerns the ability to deliberately suppress dominant, automatic, or prepotent responses.31 Poor performance on laboratory instruments, such as the Stop Signal task, the go/no-go paradigm, or the Hayling task, reflects the inhibition of dominant response. For instance, on the Hayling task,32 subjects were asked to give a word that made no sense at all in the context of a sentence in which the last very predictable word was missing. Interestingly, we recently showed that patients with an obsessive-compulsive disorder made significantly more errors (sentence-related responses) in the Hayling task than controls participants.33 In addition, the frequency of these errors specifically correlated with the compulsion symptoms, suggesting that the compulsions are related to a deficit affecting the inhibition of a prepotent response.33 In the same vein, recently detoxified alcoholics also gave more sentence-related words than controls subjects, thus indicating a response inhibition deficit.34 Disturbances in this inhibition mechanism may relate to the personality trait of motor impulsivity, or the trait of ‘urgency', the tendency to experience strong impulses, frequently under conditions of negative affect.19 Consistent with this idea, alcohol-dependent individuals known to exhibit dominant response inhibition deficits also scored higher in “urgency,” relative to healthy, non-substance abusing, control subjects.19
A critical neural region for the suppression of dominant/irrelevant response seems to be the right orbitofrontal gyrus and the right middle/superior frontal gyrus and also the posterior regions located in the left superior parietal gyrus and in the right intraparietal sulcus.35 Consistent with this observation, functional neuroimaging studies in addicts with inhibition deficits reveal diminished activity in frontoparietal systems involved in these inhibitory control mechanisms.7,9
Another mechanism of cognitive inhibitory control is the ability to resist to proactive interference.31 Resistance to proactive interference reflects the ability to resist the memory intrusion of information that was previously relevant but has since become irrelevant. Cognitive tasks in which interfering information is presented prior to the target information and was previously relevant to the task, such as the Brown-Peterson or AB-AC tasks investigate this type of inhibition. A critical neural region for this mechanism appears to be the left inferior frontal cortex.36 Disturbances in this mechanism may relate to the personality trait of ‘lack of perseverance,” the ability to remain focused on a task that may be boring or difficult.19 A deficit affecting the inhibition process should be specifically related to the occurrence of obsessions and intrusive thoughts about drugs.
Bottom-up Influence of the Reactive System
The reflective system may generate affective (somatic) states through top-down mechanisms, but then ascending signals from these affective states can exert bottom-up influence on cognition. Thus, when one is pondering a decision, numerous affective (somatic) signals that conflict with each other may be triggered simultaneously through both the reactive and reflective systems. The result is emergence of an overall positive or negative affective state. Ascending signals from this overall affective state can then modulate activity of several components of the reactive and reflective systems (Figure 1). We have previously proposed that the key mechanism by which these bottom-up signals modulate synaptic activity at telencephalic targets is pharmacological.37 The cell bodies of the neurotransmitters dopamine, serotonin, noradrenalin, and acetylcholine are located in the brainstem; the axon terminals of these neurotransmitter neurons make synapses on cells and/or terminals throughout the cortex. Anatomically, both the amygdala and VMPC have direct access to these neurotransmitter cell bodies in the brainstem. For affective states and homeostatic signals generated in the body, a number of channels can convey their signals to these neurotransmitter nuclei, but we have suggested that the vagus nerve is the most critical.13 Changes in neurotransmitter release can modulate synaptic activity in several components of the reactive and reflective systems. First, changes in representation of patterns of affective states (for example, in the insula and other somatosensory cortices) can lead to an increase in the reward utility of the drug. Second, changes in triggering of affective states (for example, in amygdala and VMPC) can lower the threshold for triggering subsequent affective signals related to drugs. Third, alterations in impulse control and the inhibition of unwanted memories or thoughts (for example, in lateral orbitofrontal, inferior frontal gyrus and dorsolateral prefrontal, hippocampus, and anterior cingulate) can strengthen thoughts about drugs and make shifting attention to other thoughts more difficult. Finally, changes in regions involved in behavior (striatum and supplementary motor area) can translate into motor routine actions that may lead to drug use (Figure 1).
The outline of these pharmacological systems given here is very simplistic, mainly because there are many excellent reviews that describe the molecular mechanisms by which neurotransmitters affect synaptic activity in addictive states and that explain how these activities influence cognitive systems such as memory.38,39 Other excellent lines of research have attempted to differentiate the specific roles of dopaminergic, serotonergic, or noradrenergic systems in decision-making, impulse control40,41 and time delay.42 Therefore, the main purpose here is not to detail the processes and mechanisms of any one specific pharmacological system. Rather, the goal is to illustrate (1) how one can relate molecular and pharmacological studies on drug addiction to neural systems concerned with mechanisms of affect and emotion and (2) the influence of drug addiction on cognition. The proposed arrangement provides a way for affective signals to exert a bottom-up influence on the reflective system. If, for instance, the signals triggered by the impulsive system were relatively strong, they would have the capacity to hijack the top-down, goal-driven cognitive resources needed for the normal operation of the reflective system and exercising the willpower to resist drugs.
Hyperactive Reactive System
Hyperactivity in bottom-up mechanisms of the reactive system can weaken control of the reflective system. Evidence suggests that conditions leading to hyperactivity in this system include hypersensitivity and attention bias to reward-related stimuli. Addicts trigger exaggerated autonomic responses to cues related to the substances they abuse.2,3,41 Although addicts show blunted affective responses to affective stimuli that are not drug related,43 we have shown that addicts trigger exaggerated autonomic responses when exposed to monetary reward in the IGT.2,3 Perhaps money represents a special case, in that it may be automatically linked to buying drugs. Using different versions of the IGT, combined with skin conductance responses (SCRs), we identified a subgroup of addicts that were different from both VMPC patients and the majority of normal controls. This subgroup of addicts was drawn to choices that yielded larger gains, irrespective of the losses that were encountered, and they generated exaggerated SCRs when they won money.2,3 Interestingly, direct autonomic responses to wins and losses are blocked in patients with bilateral amygdala damage. In contrast, in VMPC patients, the SCR defect is specific to the anticipatory phase when they are pondering which option to choose.13 This suggests that addicts suffer from the opposite condition of amygdala lesion patients—that is, their amygdala is over-responsive to reward. This is supported by functional neuroimaging studies showing increased amygdala activity in response to drug-related cues45,46 and that this exaggerated brain response generalizes to monetary reward.46
Besides, both psychoactive substance abusers and dependent patients also exhibit attentional changes—they showed a bias to attend to drug-related rather than drug-unrelated stimuli.47–49 For instance, when participants have to name the color of words with a certain emotional charge (i.e., the emotional Stroop task), alcohol-dependent individuals and heavy alcohol drinkers are slower than light and non-alcohol drinkers in naming the color of alcohol-related rather than non-alcohol-related words.50 In a task consisting of identifying transient changes in visual scenes, people drinking ‘heavily' detected substance-related changes more quickly than light and non-alcohol drinkers.51 In the dot probe task, participants are presented with a series of pairs of words or pictures on a computer screen, one above the other, followed by a visual probe shown in the location of one of the words/pictures.52 Participants are instructed to indicate, as quickly as possible, whether the probe appeared in the upper or lower position on the computer screen. The hypothesis was that probe detection would be faster when the probe replaces stimuli related to the emotionally salient stimuli, as compared to a neutral stimulus.52 As reviewed by Franken,47 drug users including opiate addicts, tobacco smokers, recreational cannabis users, and heavy social drinkers responded faster to probes that appeared in the location of drug-related stimuli (pictures), as opposed to neutral pictures, thus suggesting that their attention was preferentially allocated to the spatial location of drug-related cues.
Other studies using a variant of the go/no-go paradigm, requiring a response to targets and no response to distracters, showed that response inhibition and shifting deficits were more pronounced when alcohol-related words were the targets, thus suggesting that substance-related cues trigger bottom-up mechanisms in substance abusers, which influence top-down cognitive mechanisms, such as motor impulse and attention control.49 Another approach for studying these attention biases has been the use of cognitive models7 that deconstruct complex behavioral decisions, such as those made in the IGT, into simpler component processes of decision-making. One of the component processes is the tendency of a subject to pay more attention to gains or losses encountered on previous trials in order to make future decisions. Addicts show patterns of high attention to monetary gains, which are more frequent in men than in women,7 thus providing indirect evidence for the hypothesis that the amygdale system in addicts is hyperactive in response to monetary reward, which is presumably so closely linked to drug reward. As indicated earlier, in fact addicts may become less responsive to many forms of other rewards, but they are over-responsive to drug-related cues, and monetary reward could be one of most strongly associated cues with the ability to obtain a drug reward.
Modulating Factors
The control function of the reflective system is complex, and even under normal circumstances, several factors can modify the strength of affective signals triggered by the reflective system, thus influencing its control over the reactive system. Indeed, one of the fundamental questions in decision-making research is how humans assign value to options.
Several factors affect the value of a choice, and research has begun to explore the neural basis of these factors. We have proposed a neural framework for how factors that affect decision-making—such as time delay, the probability of the outcome, or the tangibility of the reward—could be implemented in the VMPC.38
We have suggested that information conveying immediacy (the near future) engages more posterior VMPC (including anterior cingulate, basal forebrain and nucleus accumbens), whereas information conveying delay (distant future) engages more anterior VMPC cortices (such as frontal pole). This is on the basis of the finding that major advancement in the size, complexity, and connectivity of the frontal lobes in humans has occurred in relation to Brodmann area (BA) 10 (the frontal pole).53 Furthermore, the more posterior areas of the VMPC (such as BA 25) are directly connected to brain structures involved in triggering (autonomic, neurotransmitter nuclei) or representing (sensory nuclei in the brainstem, and insular and somatosensory cortices) affective (somatic) states, whereas access of more anterior areas is polysynaptic and indirect.54 It follows that coupling of information to representations of affective states via posterior VMPC is associated with relatively fast, effortless, and strong affective signals, whereas the signaling via more anterior VMPC is relatively slowed, effortful, and weak.
This view is supported by recent functional imaging studies addressing how the perceived delay to receiving a reward modulates activity in reward-related brain areas.42 This discounting mechanism of time is also relevant to addiction as attested by performance of drug addicts on a delay discounting task where participants chose between a large delayed reward (US $1000) and smaller more immediate rewards (US $1–$999) across a range of delays (6 hours to 25 years). Results indicate that addicts tend to exhibit a higher temporal discounting rate than normal people; that is, they prefer smaller, sooner rewards over larger, later rewards.21 Thus, events that are more immediate in time (such as having the drug now as opposed to the delayed consequences) have a stronger capacity to influence decision-making, so strongly that it can potentially hijack cognition in the direction of short-term outcomes.
Similarly, we have suggested that information conveying higher certainty (or higher probability) engages posterior VMPC, whereas information conveying lower certainty engages anterior VMPC.37 Functional imaging studies implicating the parietal cortex and anterior cingulate cortex in computing the probability of outcomes on the basis of available options8 are supportive of this view. This mechanism for processing probabilities is also relevant to addiction, as cocaine addicts show abnormalities in the activity of neural structures critical for decision-making in proportion to the degree of certainty (or uncertainty) that they have about receiving their drug at the end of a brain scanning session.5
Finally, reward values are processed by the VMPC region, and representations of these values are modulated by homeostatic factors, such as hunger.55 Given the view that neural systems supporting drug reward have evolved to subserve natural motivational functions, such as feeding,56 drug withdrawal can be viewed like hunger57 in that once it is present, it increases the utility of drug reward, and, in doing so, it influences the decision to use drugs. This suggestion is consistent with the incentive motivational view of drug addiction proposing that although physical withdrawal signs are neither necessary nor sufficient for taking drugs, they exaggerate the incentive impact of drugs, thereby increasing the motivation to use drugs.56 Thus in the presence of withdrawal, the capacity of bottom-up homeostatic signals to hijack control mechanisms of the reflective system is increased.
Implications for Treatment and Directions for Future Research
Most addicts show behavioral signs of poor decision-making. However, when scrutinizing closer the profiles of their behavioral and physiological responses, only some addicts matched the VMPC patients in terms of their state of arousal during the time of pondering their decisions, i.e., prior to making risky and disadvantageous choices. The insensitivity to future consequences (“myopia for the future”) seen in these patients may reflect their failure to trigger these affective (somatic) states and to benefit from their influence. Such patients can be described as oblivious to the future consequences of their decisions, irrespective of having positive or negative consequences. Instead, the decisions of these patients can be described as primarily guided by the immediate prospects of the choice they are about to make. The profiles of other addicts only partially matched those of VMPC patients. In fact, their behavioral and physiological profiles suggested that they are hypersensitive to reward, so that the prospect of immediate drug reward outweighs the prospect of future negative consequences. These differences may have implications for prognosis, and they provide testable hypotheses that could be addressed in future research: addicts who match VMPC patients may have a harder time recovering from addiction and remaining abstinent, in comparison to addicts who partially match the VMPC patients. Indeed, we anticipate that a main aspect of drug relapse prevention consists of having the capacity to re-experience from thought, prior negative emotional states related to drug use. This phenomenon may increase the motivation to remain abstinent from drugs.
One intriguing aspect of the approach of characterizing the decision-making profiles of substance-dependent individuals was that a subgroup of addicts appeared normal and did not show behavioral or physiological signs of decision-making deficits.58 This suggests that not every drug user has impaired decision-making. We have suggested that these addicts should be described as ‘functional' addicts, because a closer inspection of their everyday lives has shown that they have suffered minimal social and psychological harm as a consequence of their drug use: for example, they have manage to keep their jobs.2 Nonetheless, this point should be explored further in future research. Nonetheless, our view is that poor decision-making in addiction is evident primarily when individuals persist in escalating their drug use in the face of rising adverse consequences.
According to this view, people described as addicted to coffee, sweets, the internet, and so on do not necessarily have impaired decision-making, unless their choices bring increasing social, physical, or psychological harms. However, an alternate possibility is that the lack of evidence for decision-making deficits in this subgroup of addicts is a limitation of the proposed affect-based (somatic marker) framework, in that it does not capture all instances of addiction.
Finally, the most intriguing aspect of this approach is that one subgroup of normal controls shows behavioral and physiological profiles that match those of VMPC patients. This raises the question of whether these individuals are predisposed, or at higher risk, for addiction than individuals with normal decision-making capacities. This suggestion is reasonable in light of the evidence that one predisposing factor to addiction is heredity, and genes can act in general fashion (such as the serotonin transporter gene) to predispose individuals to multiple, as opposed to specific drug addictions.59
Future research using functional imaging methods could focus on relationships between (1) genotypes related to specific neurotransmitter systems (for example, the serotonin transporter gene), (2) the level of neural activity in specific neural circuits, and (3) quality of choice, as shown by complex laboratory tasks of decision-making. This will reveal whether genetic factors lead to suboptimal function in specific neural systems and behaviors reflecting poor decision-making.
However, not all predisposing factors are necessarily genetic; other factors could be environmental (such as drug neurotoxicity or impaired child-parent interactions) or the product of gene-environment interactions. Although chronic misuse of most addictive drugs are neurotoxic (e.g., amphetamine,60 alcohol61), their use may still have a higher impact on brain and behaviors during adolescence. Indeed, evidence suggests that the functions of the prefrontal cortex may not develop fully until the age of 21, and until such a time, the development of neural connections that underlie decision-making, and the control over powerful temptations, is still taking place.62–64 Therefore, exposing the prefrontal cortex to drugs before its maturity could be harmful to decision-making, just like exposing the fetus to drugs during pregnancy. However, the fact remains that not every adolescent who tries drugs ends up addicted; it takes more than mere exposure to drugs to become addicted. Therefore, our hypothesis is that poor decision-making in addiction is not the product of drug use; rather, poor decision-making is what leads to addiction. Future systemic and longitudinal studies on decision-making in young adolescents should test this hypothesis and determine whether neurocognitive development can serve as a marker predictive of addictive disorders. This research should also take into consideration models of addiction that describe a progressive dysregulation of reward brain circuitry concomitant with a spiraling path from controlled drug use to addiction65 and should examine whether drug users undergo a slow and gradual hijacking of their willpower as they move from controlled use to addiction. However, not every individual who tries drugs ends up on this down-spiraling path; we hypothesize that those with poor decision-making capabilities are more vulnerable and those with normal decision-making capabilities are more resistant. These are testable hypotheses with clear predictions that can be addressed in future research.
Concluding Remarks
Numerous psychological theories have proposed that behavior is determined by the interplay of reactive (automatic) and reflexive (controlled) processing.66 More specifically, evidence67 suggests that valence constitutes a very basic and automatic form of core affect that derives from psychological process of evaluation (judging the environment in terms of whether it is good or bad, helpful or harmful, rewarding or threatening). Furthermore, people differ in their focus of valence, and this seems to be a stable characteristic of an individual that is related to valuation sensitivity. Nevertheless, the automatic incentive-responses tendencies resulting from the process of valuation are controlled or regulated by reflexive processes, allowing responses to be more flexible and coordinated to the context. Finally, neuropsychological, neuroimaging, and cognitive studies indicate that there are distinct, although related, controlled processes involving a network of multiple interacting brain regions in which the prefrontal cortex is just one component.35,68
These dual-process theories have been frequently used to identify distinct pathways to psychological disorders.69 In this framework, specific forms of psychopathology are the product of complex interactions between reactive, incentive-response tendencies and reflexive, regulatory processes. In the present paper, we applied this framework to addiction problems. However, it could be argued that this dual-process framework is an oversimplified view that does not take in account the specificity and complexity of the psychopathological states (and more specifically of substance abuse), and also which cannot really be tested. In fact, there exists a great deal of evidence suggesting that the comorbidity among externalizing disorders (such as antisocial disorders and substance abuse) is best modeled by an underlying normally distributed continuum of risk for multiple disorders within the externalizing psychopathology, including substance abuse.70 In addition, a dual-process model may support a formulation suggesting that substance abuse may be the product of multiple routes involving low effortful control (along with secondary problems in regulation of negative emotions), a strong approach tendency (or reward sensitivity), or a low withdrawal tendency (or punishment sensitivity). In the same vein, specific relationships can be hypothesized between distinct types of substance abuse-related symptoms and distinct controlled processes (such as between drug-related intrusive thoughts and the ability to resist to proactive interference and compulsive drug abusing and the ability to inhibit a prepotent response). Finally, it should be noted that a cognitive-motivational model relating basic motivational tendencies and controlled (decision making and working memory) processes has been proposed by Finn71 in the domain of alcoholism and some of its components have already been empirically tested.
Contributor Information
Xavier Noël, Dr. Noël is from the Clinic of Addictions, CHU-Brugmann, Université Libre de Bruxelles, Belgium.
Martial Van Der Linden, Dr. Van Der Linden is from the Department of Cognitive Psychophathology, University of Geneva, Switzerland.
Antoine Bechara, Dr. Bechara is from the Brain and Creativity Institute, University of Southern California, Los Angeles, California, and Department of Neurology, University of Iowa, Iowa City, Iowa..
References
- 1.Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York, NY: Grosset/Putnam; 1994. [Google Scholar]
- 2.Bechara A. Neurobiology of decision-making: Risk and reward. Semin Clin Neuropsychiatry. 2001;6:205–16. doi: 10.1053/scnp.2001.22927. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A. Risky business: Emotion, decision-making and addiction. J Gambling Studies. 2003;19:23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 4.Bierman D, Destrebecqz A, Cleeremans A. Intuitive decision making in complex situations: Somatic markers in an artificial grammar learning task. Cogn Affect Behav Neurosci. 2005;5:297–305. doi: 10.3758/cabn.5.3.297. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Rahman S, Sahakia B, Rudolph NC, et al. Decision-making and neuropsychiatry. Trends Cogn Sci. 2001;5:271–7. doi: 10.1016/s1364-6613(00)01650-8. [DOI] [PubMed] [Google Scholar]
- 7.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cog Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Ernst M, Paulus MP. Neurobiology of decision-making: A selective review from a neurocognitive and clinical perspective. Biologic Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–52. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nature Neuroscience. 2005;8:1458. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 11.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–57. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 12.Sander D, Grafman J, Zalla T. The human amygdale: An evolved system for relevance detection. Rev Neurosci. 2003;14:303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- 13.Bechara A. Disturbances of emotion regulation after focal brain lesions. Int Rev Neurobiol. 2004;62:159–93. doi: 10.1016/S0074-7742(04)62006-X. [DOI] [PubMed] [Google Scholar]
- 14.Montague PR, Berns GS. Neural economics and the biological substrates of valuation. Neuron. 2002;36:265–84. doi: 10.1016/s0896-6273(02)00974-1. [DOI] [PubMed] [Google Scholar]
- 15.Baker TB, Morse E, Sherman JE. The Nebraska Symposium on Motivation: Alcohol Use and Abuse. Lincoln: University of Nebraska Press; 1987. The motivation to use drugs: A psychobiological analysis of urges. In: Rivers PC (ed): pp. 257–323. [PubMed] [Google Scholar]
- 16.Rohnsenow DJ, Niaura RS, Childress AR, et al. Cue reactivity in addictive behaviors: Theoretical and treatment implications. Int J Addict. 1990;25:957–93. doi: 10.3109/10826089109071030. [DOI] [PubMed] [Google Scholar]
- 17.Everitt BJ, Parkinson JA, Olmstead MC, et al. Associative processes in addiction and reward: The role of amygdala and ventral striatal subsystems: the role of amygdala-ventral striatal subsystems. Ann NY Acad Sci. 1999;877:412–38. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 18.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Whiteside S, Lynam D. The five-factor model and impulsivity: Using a structural model of personality to understand impulsivity. Personality and individual differences. 2001;30:669–89. [Google Scholar]
- 20.Clark L, Cools R, Robbins T. The neuropsychology of ventral prefrontal cortex: decision-making and reversal learning. Brain Cogn. 2004;55:41–53. doi: 10.1016/S0278-2626(03)00284-7. [DOI] [PubMed] [Google Scholar]
- 21.Monterosso J, Ehrman R, Napier K, et al. Three decisionmaking tasks in cocaine-dependent patients: Do they measure the same construct? Addiction. 2001;96:1825–37. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- 22.Zermatten A, Van der Linden M, d'Acremont M, et al. Impulsivity and decision making. J Nerv Ment Dis. 2005;193:647–50. doi: 10.1097/01.nmd.0000180777.41295.65. [DOI] [PubMed] [Google Scholar]
- 23.Martin EM, Bechara A. Decision-making and drug of choice in substance-dependent individuals: a preliminary report. Biologic Psychiatry. 2003;53:97S. [Google Scholar]
- 24.Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: Preliminary observations. J Exp Clin Neuropsychol. doi: 10.1080/13803390600582446. (in press) [DOI] [PubMed] [Google Scholar]
- 25.Fishbein D, Hyde C, Eldreth D, et al. Cognitive performance and autonomic reactivity in abstinent drug abusers and nonusers. Exp Clin Psychopharmacol. 2005;13:25–40. doi: 10.1037/1064-1297.13.1.25. [DOI] [PubMed] [Google Scholar]
- 26.Bechara A, Martin E. Impaired decision-making related to working memory deficitsin substance addicts. Neuropsychology. 2004;18:152–62. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- 27.Franklin TR, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biologic Psychiatry. 2002;51:134–42. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 28.Matochik JA, London ED, Eldreth DA, et al. Frontal cortical tissue composition in abstinent cocaine abusers: A magnetic resonance imaging study. Neuroimage. 2003;19:1095–102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 29.Bartzokis G, Goldstein IB, Hance DB, et al. The incidence of T2-weighted MR imaging signal abnormalities in the brain of cocaine-dependent patients is age-related and region-specific. Am J Neuroradiol. 1999;20:1628–35. [PMC free article] [PubMed] [Google Scholar]
- 30.Lim KO, Choi SJ, Pomara N, et al. Reduced frontal white matter integrity in cocaine dependence: A controlled diffusion tensor imaging study. Biologic Psychiatry. 2002;51:890–5. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 31.Friedman NP, Miyake A. The relations among inhibition and interference control functions: A latent variable analysis. J Exp Psychol. 2004;133:101–35. doi: 10.1037/0096-3445.133.1.101. Gen. [DOI] [PubMed] [Google Scholar]
- 32.Burgess PW, Shallice T. Bizarre responses, rule detection, and frontal lobe lesions. Cortex. 1998;32:241–59. doi: 10.1016/s0010-9452(96)80049-9. [DOI] [PubMed] [Google Scholar]
- 33.Van der Linden M, Ceschi G, Zermatten A, et al. Investigation of response inhibition in obsessive-compulsive disorder using the Hayling task. J Int Neuropsychologic Soc. 2005;11:776–83. doi: 10.1017/S1355617705050927. [DOI] [PubMed] [Google Scholar]
- 34.Noël X, Van Der Linden M, Schmidt N, et al. Supervisory attentional system in non-amnesic male alcoholic subjects. Arch Gen Psychiatry. 2000;58:1152–8. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- 35.Collette F, Van der Linden M, Laureys S, et al. Exploring the unity and diversity of the neural substrates of executive functioning. Human Brain Mapping. 2005;25:409–23. doi: 10.1002/hbm.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jonides J, Nee DE. Brain mechanisms of proactive interference in working memory. Neuroscience. 2006;139:181–93. doi: 10.1016/j.neuroscience.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 37.Bechara A, Damasio AR. The somatic marker hypothesis: A neural theory of economic decision. Games Econ Behav. 2005;52:336–72. [Google Scholar]
- 38.Hyman SE. Addiction: A disease of learning and memory. Am J Psychiatry. 2005;162:1414–22. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- 39.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nature Reviews. Neuroscience. 2001;2:119–28. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 40.Clarke HF, Dalley JW, Crofts HS, et al. Cognitive inflexibility after prefrontal serotonin depletion. Science. 2004;304:878–80. doi: 10.1126/science.1094987. [DOI] [PubMed] [Google Scholar]
- 41.Rogers RD, Lancaster M, Wakeley J, Bhagwagar Z. Effects of beta-adrenoreceptor blockade on components of human decision-making. Psychopharmacology. 2004;172:157–64. doi: 10.1007/s00213-003-1641-5. [DOI] [PubMed] [Google Scholar]
- 42.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 43.Aguilar de Arcos F, Verdejo A, Peralta MI, Sanchez-Barrera M, Perez-Garcia M. Experience of emotions in substance abusers exposed to images containing neutral, positive, and negative affective stimuli. Drug Alcohol Depend. 78:159–67. doi: 10.1016/j.drugalcdep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 44.London ED, Ernst M, Grant S, et al. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–42. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 45.Childress AR, Mozley PD, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Breiter HC, Aharon I, Kahneman D, et al. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–39. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 47.Franken IHA. Drug craving and addiction: integration psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:563–79. doi: 10.1016/S0278-5846(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 48.Field M, Mogg K, Zetteler J, Bradley BP. Attention biases for alcohol cues in heavy and light social drinkers: The roles of initial orienting and maintained attention. Psychopharmacology. 2004;176:88. doi: 10.1007/s00213-004-1855-1. [DOI] [PubMed] [Google Scholar]
- 49.Noel X, Van Der Linden M, Verbanck P, et al. Deficits of inhibitory control and of shifting associated with cognitive bias in polysubstance abusers with alcoholism. Addiction. 2005;100:1302–9. doi: 10.1111/j.1360-0443.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnsen BH, Laberg JC, Cox WM, V, et al. Alcoholic subjects' attentional bias in the processing of alcohol-related words. Psychol Addict Behav. 1997;8:111–15. [Google Scholar]
- 51.Jones BT, Jones BC, Smith H, Copley N. A flicker paradigm for inducing change blindness reveals alcohol and cannabis information processing biases in social users. Addiction. 2003;98:235–44. doi: 10.1046/j.1360-0443.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 52.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Semendeferi K, Armstrong E, Schleicher A, et al. Prefrontal cortex in humans and apes: A comparative study of area 10. Am J Physic Anthropol. 2001;114:224–41. doi: 10.1002/1096-8644(200103)114:3<224::AID-AJPA1022>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 54.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex. 2000;10:206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 55.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychologic Rev. 1987;94:469–92. [PubMed] [Google Scholar]
- 57.Nader K, Bechara A, van der Kooy D. Neurobiological constraints on behavioral models of motivation. Annual Rev Psychol. 1997;48:85–114. doi: 10.1146/annurev.psych.48.1.85. [DOI] [PubMed] [Google Scholar]
- 58.Bechara A, Damasio H. Decision-making and addiction (part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–89. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 59.Goldman D, Bergen A. General and specific inheritance of substance abuse and alcoholism. Arch Gen Psychiatry. 1998;55:964–65. doi: 10.1001/archpsyc.55.11.964. [DOI] [PubMed] [Google Scholar]
- 60.Hanson GR, Rau KS, Fleckenstein E. The methamphetamine experience : A NIDA partnership. Neuropharmacol. 2004;47:92–100. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Krill JJ, Halliday GM. Brain shrinkage in alcoholics: A decade on and what have we learned? Prog Neurobiol. 1999;58:381–87. doi: 10.1016/s0301-0082(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 62.Eslinger PJ. Conceptualizing, describing, and measuring components of executive function. In: In: Lyon GR, Krasnegor NA, editors. Attention, Memory, and Executive Function. Baltimore, MD: Paul H Brooks; 1999. pp. 420–41. [Google Scholar]
- 63.Crone EA, Jennings JR, Van der Molen MW. Developmental change in feedback processing as reflected by phasic heart rate changes. Dev Psychol. 2004;40:1228–38. doi: 10.1037/0012-1649.40.6.1228. [DOI] [PubMed] [Google Scholar]
- 64.Overman WH, Frassrand K, Ansel S, et al. Performance on the IOWA card task by adolescents and adults. Neuropsychologia. 2004;42:1838–51. doi: 10.1016/j.neuropsychologia.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 65.Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 66.Feldman Barrett L, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of mind. Psychologic Bull. 2004;130:553–73. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feldman Barrett L. Valence is a building block of emotional life. J Res Personality. 2006;40:35–55. [Google Scholar]
- 68.Bechara A, Van der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–9. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- 69.Nigg JT. Temperament and developmental psychopathology. J Child Psychol Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- 70.Krueger RF, Markon KE, Patrick CJ, Iacono WG. Externalizing psychopathology in adulthood: A dimensional-spectrum conceptualization and its implications for DSM-V. J Abnorm Psychol. 2005;114:537–50. doi: 10.1037/0021-843X.114.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finn PR. Motivation, working memory, and decision making: A cognitive-motivational theory of personality vulnerability to alcoholism. Behav Cogn Neurosci Rev. 2002;1:183205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]


