Abstract
Background and Aims
Plant competition studies are restricted by the difficulty of quantifying root systems of competitors. Analyses are usually limited to above-ground traits. Here, a new approach to address this issue is reported.
Methods
Root system weights of competing plants can be estimated from: shoot weights of competitors; combined root weights of competitors; and slopes (scaling exponents, α) and intercepts (allometric coefficients, β) of ln-regressions of root weight on shoot weight of isolated plants. If competition induces no change in root : shoot growth, α and β values of competing and isolated plants will be equal. Measured combined root weight of competitors will equal that estimated allometrically from measured shoot weights of each competing plant. Combined root weights can be partitioned directly among competitors. If, as will be more usual, competition changes relative root and shoot growth, the competitors' combined root weight will not equal that estimated allometrically and cannot be partitioned directly. However, if the isolated-plant α and β values are adjusted until the estimated combined root weight of competitors matches the measured combined root weight, the latter can be partitioned among competitors using their new α and β values. The approach is illustrated using two herbaceous species, Dactylis glomerata and Plantago lanceolata.
Key Results
Allometric modelling revealed a large and continuous increase in the root : shoot ratio by Dactylis, but not Plantago, during competition. This was associated with a superior whole-plant dry weight increase in Dactylis, which was ultimately 2·5-fold greater than that of Plantago. Whole-plant growth dominance of Dactylis over Plantago, as deduced from allometric modelling, occurred 14–24 d earlier than suggested by shoot data alone.
Conclusion
Given reasonable assumptions, allometric modelling can analyse competitive interactions in any species mixture, and overcomes a long-standing problem in studies of competition.
Keywords: Allocation, allometry, competition, dry weight, Dactylis glomerata, growth, modelling, Plantago lanceolata, root, shoot, whole-plant
INTRODUCTION
Adjustments in root and shoot growth are often assumed to be a fundamental facet of a plant's phenotypic plasticity in response to its environment. Such adjustments can be simple ontogenetic correlates of size or growth rate and do not necessarily represent adaptive responses to compensate for resource limitations imposed on a plant by its environment, including by its neighbours (Reich, 2002). An alternative view is that relatively greater root growth in response to shortages of nutrients or water could maximize a plant's probability of capturing those resources, especially if a competitor fails to respond to a comparable extent. Such responses will, therefore, be associated ultimately with increased fitness; likewise, greater leaf and/or stem growth would be expected to occur if light or CO2 is limiting. This is the ‘functional equilibrium’ (Brouwer, 1962) or ‘balanced growth’ (Davidson, 1969) hypothesis, which is supported by some experiments (Shipley and Meziane, 2002; Berendse and Möller, 2009), but criticised elsewhere for being over-simplistic and failing to explain or predict many observed responses (Farrar and Gunn, 1998; Poorter and Nagel, 2000; Reich, 2002; Müller et al., 2000). Yet another theory (Enquist and Niklas, 2002) considers the global biophysical constraints on biomass partitioning by individual plants within which the responses of all species are expected to fall. But whatever its detail, the general idea of shifts in growth allocation patterns remains embedded in standard explanations of how different plant species can compete and coexist.
One difficulty faced by any explanatory framework attempting to integrate the responses of root and shoot growth with a plant's competitiveness is the mundane but intractable one of estimating the root growth of competing individuals growing in soil (Cahill, 2002; Zobel and Zobel, 2002). It is difficult to separate the entangled roots of neighbouring plants unless they are very small or only a small portion of each root system is sampled (Robinson et al., 1999). If C3 and C4 species are grown together, there is the possibility of using the distinct 13C natural abundances of those species to partition the combined root mass of the competitors once it has been extracted from the soil (Wong and Osmond, 1991). An inert, porous material such as Turface, supplemented by frequent additions of nutrients and water (Gurevitch et al., 1990), can act as a growth medium that minimizes entanglement of multiple root systems, allowing their relatively easy manual separation (J. Gurevitch, Stony Brook University, New York, pers. comm.). Species-specific biochemical (Roumet et al., 2006) and molecular (Mommer et al., 2008) markers have been used to quantify the relative amounts of material contributed by each species to multi-species root mixtures, but are expensive to apply routinely. Plant competition studies on soil-grown plants must otherwise restrict their analyses to above-ground data such as shoot growth or, where possible, seed production. Below-ground responses to competition, and their relationship to those above ground, have largely resisted detailed investigation.
Here, an allometric modelling approach is developed and used to estimate the root weights of competing individuals of two species. To do this, root and shoot growth of isolated plants is compared with that of competing plants. Measured species-specific root–shoot allometries of isolated plants are first applied to the competitors, the null hypothesis being that the presence of neighbours induces no allometric changes from those expressed when growing alone. If that null hypothesis is true, the combined root mass of the competing plants, which can be measured, will equal the combined root mass estimated allometrically from the separate shoot weights of each competitor, which can also be measured. Therefore, the combined root mass can then be partitioned directly among the competitors according to the allometries of the isolated plants.
If, however, the null hypothesis is rejected and the presence of neighbours causes shifts in relative root and shoot growth, the combined root mass of the competing plants will not equal that estimated allometrically from the shoot weights of the competitors and, obviously, the isolated-plant allometries cannot then be applied directly to competitors. The problem is then to estimate the allometries of the competing plants. This can be done by incrementally adjusting the isolated-plant allometries until the estimated combined root masses of competitors, obtained by again using their measured shoot weights, agrees with the measured combined root mass (hence ‘allometric modelling’). The resulting allometric parameters estimated for each competitor will then be consistent with the measured total amount of root produced. These parameters can then be used to explore the root–shoot interactions of the competitors.
This approach is illustrated using an experiment involving two herbaceous species common in the British Isles, Dactylis glomerata and Plantago lanceolata.
MATERIALS AND METHODS
Theory
For a young herbaceous plant, the allometric variation in its root weight (R) can be described as a simple power function of its shoot weight (S):
| (1) |
where α is the scaling exponent and β the allometric coefficient. A linear version of eqn (1) is obtained by ln-transformation:
| (2) |
(Niklas, 1994, p. 16). α is the ratio of root and shoot relative growth rates (Hunt and Nicholls, 1986). For isometric growth, α = 1, and root and shoot weights increase in the same proportion. When α > 1, root growth disproportionately exceeds that of the shoot, and when α < 1, the opposite is true. The allometric coefficient (β) has no clear physiological definition, but variations in β can cause differences in root : shoot ratio even if α is constant (Farrar and Gunn, 1998). Instantaneous root : shoot ratio depends, therefore, on both α and β.
If two plants, A and B, compete, each will express its own root : shoot allometry as manifested in the α and β values it attains, and which may not necessarily be the same as those when A and B are grown alone. When A and B compete, their combined root weight (RC) is the sum of their individual root weights (RA + RB) but, in practice, neither RA nor RB can be measured directly. But RC should be resolvable into its allometric components since, from eqn (1),
| (3) |
where the subscripts A and B denote the two species. SA and SB are the measured shoot weights of the species when competing. RC estimated by eqn (3) must equal the measured combined root weight (RM) of A and B when competing. ln RC and ln RM should therefore be related via a linear regression of the form
| (4) |
in which the slope (a) and intercept (b) should equal 1 and 0, respectively, and ε is the estimation error. The problem is to estimate α and β values of A and B to satisfy eqn (4) subject to this constraint. Once estimated, these α and β values can be used with measured values of SA and SB to derive corresponding estimates of RA and RB. The estimation procedure is described below under ‘Data analysis and allometric modelling’.
Experiment
Seeds of Dactylis glomerata and Plantago lanceolata were germinated in plug trays of John Innes seedling compost in a controlled environment chamber at 16 °C with 12 h of supplementary lighting per day. After 3 weeks, seedling plugs were transferred to 10-cm-diameter pots containing sieved, low-nutrient agricultural topsoil (sandy loam, pH 5·5). One individual plant or two (one of each species) were planted in each pot. Pots were placed under supplementary lighting (12 h per day) in a glasshouse maintained at 16 °C. All pots were watered with Phostrogen all-purpose general fertilizer at regular intervals. No attempt was made to impose conditions favouring either above- or below-ground competition. Leaf canopies were not screened; nor were root systems separated by barriers (cf. Donald, 1958). Twenty destructive harvests were taken at 3- or 4-d intervals from 10 to 76 d after sowing. Each plant combination was replicated three times. At each harvest, shoots were removed and roots washed from the soil. The intermingled roots of competing plants were not separated but treated as composite samples. All harvested material was oven-dried at 80 °C for 48 h prior to weighing.
Data analysis and allometric modelling
Reduced major axis (RMA) regressions (Niklas, 1994, p. 331) of ln-transformed root and shoot dry weights of isolated plants yielded estimates of α and β for Dactylis and Plantago when grown alone. (RMA, or Model II, regressions are identical to the first axis of a principal components analysis.) These values of α and β were substituted into eqn (3) along with corresponding measured shoot dry weights to derive initial estimates of RA, RB and, therefore, of RC for competing plants.
ln RC was regressed on ln RM (eqn 4) using data from all 60 plants. If the resulting slope (a) and intercept (b) of that regression differed from 1 and 0, respectively, the initial values of α and β (i.e. those estimated for isolated plants) for Dactylis, Plantago or both were adjusted incrementally and simultaneously until a = 1·0 ± 0·0001 and b = 0·0 ± 0·0001. The resulting α and β values of the two species were then, by necessity, consistent with those expressed by competing individuals of the two species in that the estimated combined root weights of the competitors (RC) matched those measured in the experiment (RM). Those allometries were then used to estimate the root dry weights of competitors at each harvest and, along with the measured shoot dry weights, the corresponding root : shoot ratios, using eqn (1).
RESULTS
The roots and shoots of both species grew approximately isometrically when in the absence of neighbours, increasing in dry weight by up to four orders of magnitude during the experiment. Confidence limits on the estimates of the scaling exponent (α) of Dactylis bracketed a value of 1, just below the lower 95 % confidence limit for the α estimate of Plantago (Table 1). Allometric coefficients (β) for the two species differed; β of Dactylis significantly exceeded that of Plantago (P < 0·05).
Table 1.
Root : shoot allometries of Dactylis glomerata and Plantago lanceolata when growing alone
| Species | α | β | n | R2 |
|---|---|---|---|---|
| Dactylis glomerata | 0·989 (0·948–1·10) | 0·544 (0·423–0·698) | 60 | 0·974 |
| Plantago lanceolata | 1·05 (1·01–1·09) | 0·349 (0·272–0·448) | 60 | 0·978 |
α and β are the scaling exponent and allometric coefficient, respectively (eqn 1) estimated from RMA regressions of ln root dry weight (mg) on ln shoot dry weight (mg).
Numbers in parenthesis are 95 % confidence limits on the estimates of α and β.
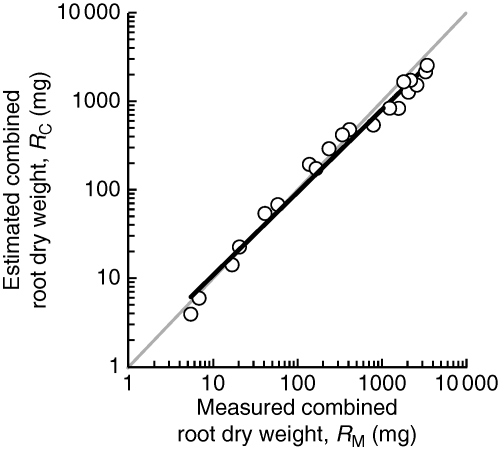
The linear regression of ln RC on ln RM had a slope (a) and intercept (b) significantly different from 1 and 0, respectively, these values falling outside the 95 % confidence limits of each parameter (Fig. 1). Therefore, the combined root dry weights of competitors could not be predicted simply by applying the allometric statistics obtained for Dactylis and Plantago growing alone. It follows that the root : shoot allometries of Dactylis, Plantago or both were not necessarily the same when these plants competed as when they grew alone.
Fig. 1.
Combined root dry weights (RC) of competing Dactylis glomerata and Plantago lanceolata estimated from the allometries obtained for isolated plants (Table 1) and the measured combined root weights of the two species when growing together (RM). Each symbol represents a separate harvest and is the mean of three replicates. The bold line is the linear regression of ln-transformed data (cf. eqn 4): slope = 0·935 (0·874–0·996, 95 % CL), intercept 1·26 (0·875–1·82), R2 = 0·982, n = 20. The grey line is the 1 : 1 relationship (slope = 1, intercept = 0).
The α and β estimates for competing Dactylis and Plantago were adjusted from their initially assumed values – those obtained from Table 1 for isolated plants – in three ways. (1) Only Dactylis was assumed to respond to competition, Plantago's α and β values remaining unchanged from those it expressed when growing alone. Consistency between RC and RM was possible if Dactylis increased its β value significantly in response to competition (Table 2); no significant change in α was necessary. (2) When only Plantago was assumed to respond to competition, a significant increase in its α and a significant decrease in β were required to match RC with RM. (3) When both species were assumed to respond allometrically to competition, the changes in α and β required for RC and RM to agree were all within the 95 % confidence limits of those of isolated plants (cf. Table 1). This assumption predicted the smallest allometric responses to competition and, on that basis, is the most conservative of the three. Therefore, attention is here confined to the implications of that assumption.
Table 2.
Root : shoot allometries of Dactylis glomerata and Plantago lanceolata when growing together
| Responding species | Species | α | β | n | R2 |
|---|---|---|---|---|---|
| Dactylis glomerata only | Dactylis glomerata | 1·027 | 0·822* | 60 | 0·977 |
| Plantago lanceolata only | Plantago lanceolata | 1·222* | 0·167* | 60 | 0·973 |
| Both | Dactylis glomerata | 1·077 (1·076–1·078) | 0·543 (0·540–0·545) | 60 | 0·976 |
| Plantago lanceolata | 1·035 (1·031–1·040) | 0·286 (0·283–0·290) |
α and β values are the adjusted scaling exponents and allometric coefficients, respectively (eqn 1). These were derived by adjusting α and β from their initial values measured in isolated plants (Table 1) until linear regressions of ln-transformed estimated combined root weights (RC, mg) on measured ln-transformed combined root weights (RM, mg) produced values of a = 1·0 ± 0·0001 and b = 0·0 ± 0·0001 (eqn 4); the resulting R2 values of those regressions are also shown.
Allometric responses to competition were assumed for Dactylis alone, Plantago alone, or both species. When no response was assumed, the α and β values were those shown in Table 1 for isolated plants.
Asterisked α and β values are those that differed significantly (P < 0·05) from those expressed by isolated plants (Table 1); other values were not significantly different from those in Table 1.
Numbers in parenthesis are the 95 % confidence limits of repeated (n = 10) independent estimates of α and β when both Dactylis and Plantago were assumed to respond, to illustrate the accuracy of the numerical estimation procedure.
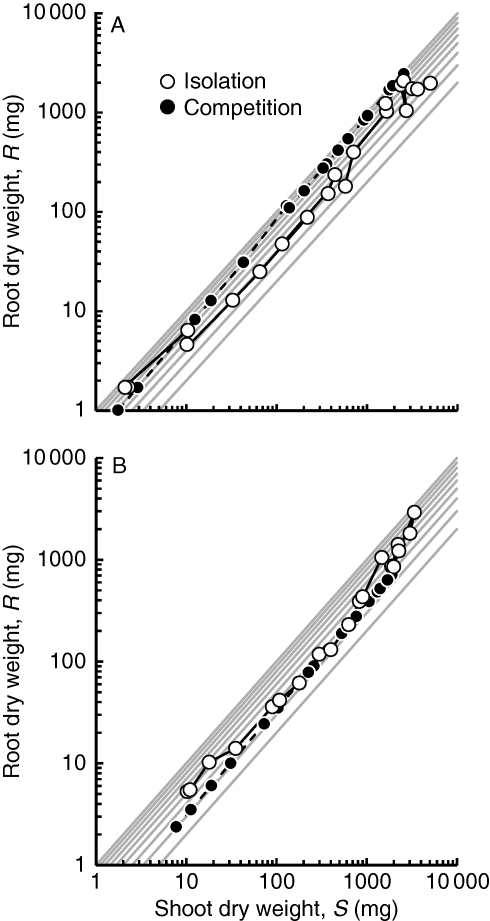
α and β values derived for competing plants (Table 2), together with measured shoot weights of those plants, were used to estimate root dry weights of plants at each harvest. From these, harvest-specific values of the root : shoot ratio (R:S) were derived. When grown in isolation, Dactylis maintained a mean measured R:S of 0·544 (0·471–0·618, 95 % CL) and Plantago of 0·504 (0·442–0·566). Although α and β values were not statistically different between isolated and competing plants (Table 2), they were nevertheless sufficient to generate contrasting R:S responses of the competitors as growth progressed (Fig. 2). The mean R:S of competing Dactylis was estimated to have been 0·846 (0·786–0·906, 95 % CL), whereas for Plantago the corresponding R:S was 0·343 (0·335–0·350). Dactylis adjusted its R:S from a value initially similar to that of isolated plants (approx. 0·5; Fig. 2), gradually increasing it to a maximum of approx. 1·0 by the end of the experiment. It achieved this by the most subtle of adjustments in α with hardly any detectable change in β from that it expressed when growing alone (Table 2). Competing Plantago, by contrast, barely changed its R:S during the experiment, maintaining a constant α value while reducing β only slightly.
Fig. 2.
Root and shoot dry weights of (A) Dactylis glomerata and (B) Plantago lanceolata grown in isolation or competing with each other, as indicated. Root weights of competitors were derived from the allometric modelling of combined root weights measured in the experiment, assuming that both species responded allometrically to competition (Table 2). Each symbol represents a separate harvest and is the mean of three replicates. α and β values for isolated plants are given in Table 1; those for competitors, in Table 2. Diagonal grey lines show constant root : shoot (R:S) ratios from R:S = 0·2 (the lowermost) to R:S = 1, in 0·1 increments.
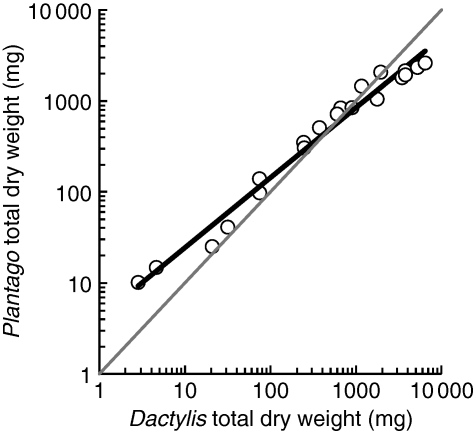
These adjustments (or the lack of them) in root : shoot allocation had implications for the outcome of competition in terms of dry matter production. The total dry weight of Plantago initially exceeded that of its competitor (Fig. 3). Above about 450 mg total dry weight (after growing for 38–41 d), however, the growth of Dactylis outstripped that of Plantago. By the end of the experiment, the total dry weight of Dactylis was 2·5-fold greater than that of Plantago. This superiority occurred after Dactylis had established a significantly larger R:S than that of Plantago (Fig. 2). Had only shoot weights been used to analyse growth, however, the apparent competitive dynamics of Dactylis and Plantago would have been substantially different. Although the initial shoot growth of Plantago was still greater than that of Dactylis, Dactylis did not become dominant above ground until its shoot dry weight exceeded 1104 mg, after growing for 55–62 d (data not shown). Including the total root dry weights derived from allometric modelling revealed that the growth superiority of Dactylis over Plantago occurred 14–24 d earlier than suggested by shoot data alone.
Fig. 3.
Total dry weights (i.e. the sum of shoot and root weights) of Dactylis glomerata and Plantago lanceolata when competing with one another. Shoot weights were measured. Root weights were derived from the allometric modelling of combined root weights measured in the experiment (Fig. 2). Each symbol represents a separate harvest and is the mean of three replicates. The bold line is the RMA regression of ln-transformed data: slope = 0·778 (0·724–0·832, 95 % CL), intercept 3·93 (2·79–5·53), R2 = 0·977, n = 20. The grey line is the 1 : 1 relationship (slope = 1, intercept = 0).
DISCUSSION
Allometric modelling allowed the otherwise unquantifiable root weights of competing plants to be estimated over an extended period of growth. The patterns of root growth derived from those estimates suggested that the eventual superiority of Dactylis over Plantago in terms of its total dry weight production (Fig. 3) was associated with a continual and potentially decisive increase in its root : shoot dry weight ratio (R:S; Fig. 2A). Plantago's considerable capacity to vary its R:S when growing alone (Fig. 2B) or when competing intraspecifically (Berendse and Möller, 2009) was apparently suppressed when in competition with Dactylis. This effect was perhaps exacerbated by eventual over-topping by the taller Dactylis leading to some growth inhibition by partial shading.
The large increases in R:S estimated for Dactylis when competing with Plantago, from 0·54 to 1·03, are physiologically unexceptional. In response to defined supplies of N or CO2, hydroponically grown Dactylis can vary its R:S from 0·3 to 1·5 (Harmens et al., 2000). In response to inter- and intraspecific neighbours and to different sizes of pots containing an inert rooting medium, Dactylis increased its R:S from 0·6 to 1·3 (Gurevitch et al., 1990). More surprising is the apparent lack of R:S response in Plantago when competing with Dactylis. In response to N and to changes in intraspecific neighbour density, Plantago can vary its R:S 4-fold, from 0·5 to 2·0 (Berendse and Möller, 2009). This scale of response was not evident in the present experiment when Plantago competed with Dactylis (Fig. 2). The root : shoot growth response of any species is regulated genetically and by local environmental conditions, such that R:S can vary widely but within limits set ultimately by biophysical constraints (Enquist and Niklas, 2002; Robinson, 2004). Nevertheless, it is difficult to predict precisely the R:S that a plant will express in a certain context, whether predictions are based primarily on physiological, environmental or ecological inputs (Hunt and Nicholls, 1986; Cheeseman, 1993; Reynolds and Pacala, 1992).
This is the first report of plant competition that is able to use total root system weights of individuals growing in the same volume of soil. Conclusions drawn from our analyses can, therefore, be based on the growth of whole plants, and are not limited to the responses of above-ground parts (Cahill, 2002). However, the growth responses of neighbours are outcomes of many processes, including competition. Growth responses are not, therefore, direct measures of ‘competition’, although they undoubtedly influence the future competitive potential of individuals. A better mechanistic understanding of competitive processes, and of their interactions with growth, will be possible by combining allometric modelling with direct measurements of competitive resource capture (C. Trinder et al., unpub. res.).
The experiment was not designed with the aim of isolating effects of above- and below-ground competition using barriers between canopies or root systems (Donald, 1958). The root : shoot responses of Dactylis and Plantago deduced from the allometric modelling of their growth patterns cannot be attributed unequivocally to a greater influence of conditions in the soil versus those above ground. The increase in R:S seen in Dactylis is, however, qualitatively consistent with the balanced growth hypothesis (Davidson, 1969): greater root growth when nutrients or water are in short supply relative to supplies of light or carbon. Hydroponically grown Dactylis increases its root : shoot scaling exponent (α) as the concentration of nitrate in the growth medium decreases (Harmens et al., 2000). But it is difficult to attribute definitively a given change in R:S to a given environmental stimulus, even when that stimulus is in the form of a well-defined experimental treatment (Poorter and Nagel, 2000).
The adjustments in root and shoot growth in response to competition inferred by allometric modelling were too small to be judged statistically different from those of isolated plants (Tables 1 and 2). Reliance on a purely allometric theory of plant allocation might have concluded, therefore, that root : shoot responses were unimportant to the interactions between Dactylis and Plantago. However, using the modelled allometries with the measured shoot weights of the competitors to derive their likely instantaneous root : shoot ratios revealed evidence for a strikingly divergent and functionally important response between the two species (Fig. 2). Contrasting approaches to analysing plant biomass allocation can generate substantial differences in the inferences that can be drawn from them, as highlighted previously by Poorter and Nagel (2000). While acknowledging the statistical problems of using ratios such as R:S [or its equivalent, root mass fraction, R/(R + S ); Reich, 2002] that are but snapshots of continually changing ontogenetic, size-dependent, processes (Hunt and Nicholls, 1986; Farrar and Gunn, 1998), they remain useful metrics with which to quantify plants' responses to their local environment, provided that they are estimated at multiple time-points and across wide ranges in plant size, as here.
Many values of α and β other than those reported in Table 2 would satisfy eqn (4). But using the α and β values measured in isolated plants as initial estimates significantly reduced the range of possible solutions to eqn (4) that were found; the values reported in Table 2 are effectively unique solutions, as judged by the narrow 95 % confidence limits of the α and β estimates. This approach also makes biological sense. When small, a plant is essentially isolated, at least if growing in an even-aged stand. It is safe then to assume that its initial root : shoot allometry matches that of a truly isolated plant growing in the same environment. When the effects of competition begin to be manifested and allometric responses initiated, plants would be expected to alter α and β from their initial values, and not from other arbitrary allometries that could nevertheless generate mathematically correct solutions to eqn (4). That assumption might not apply to a small plant growing with larger neighbours and the application of this approach to such circumstances should be done cautiously. As with all numerical procedures, it is advisable to confirm the apparent uniqueness of a solution for any dataset by conducting repeated, independent estimations.
The requirements for using this approach are to grow isolated plants as well as competitors, and to harvest plants frequently over a period sufficient to measure large dry weight changes from which reliable allometric statistics can be obtained for the isolated individuals. Its extension to more than two species would demand larger experiments. Including multiple competing species would also increase the number of ways in which allometric modelling could partition total combined root biomasses among the competitors. The latter issue could be handled using maximum likelihood techniques to generate probability distributions of α and β, the subsequent interpretation and selection of which would depend on applying species-specific information.
Extending the approach to the field is a more difficult step to envisage. Even if the required monoculture and mixed-species plots were established, or comparable areas of natural vegetation found, the perennial problem of quantitatively extracting roots from field soil would remain (Cahill, 2002; Zobel and Zobel, 2002). Underestimating root weight is inevitable in the field, especially for large plants (Robinson, 2004). If these practical problems can be accommodated, allometric modelling could be a powerful way to study the growth responses of neighbouring plants in the field. Including competitive root : shoot responses in trait-based analyses of community assembly (Shipley, 2010) could then be possible.
ACKNOWLEDGEMENTS
We are grateful to two reviewers for their helpful comments. This work was supported by the Natural Environment Research Council (NE/F004591/1).
LITERATURE CITED
- Berendse F, Möller F. Effects of competition on root-shoot allocation in Plantago lanceolata L.: adaptive plasticity or ontogenetic drift? Plant Ecology. 2009;201:567–573. [Google Scholar]
- Brouwer R. Distribution of dry matter in the plant. Netherlands Journal of Agricultural Sciences. 1962;10:399–408. [Google Scholar]
- Cahill JF. What evidence is necessary in studies which separate root and shoot competition along productivity gradient? Journal of Ecology. 2002;90:201–205. [Google Scholar]
- Cheeseman JM. Plant growth modelling without integrating mechanisms. Plant, Cell & Environment. 1993;16:137–147. [Google Scholar]
- Davidson RL. The effect of root/leaf temperature differentials on root/shoot ratios in some pasture grasses and clover. Annals of Botany. 1969;33:561–569. [Google Scholar]
- Donald CM. The interaction of competition for light and for nutrients. Australian Journal of Agricultural Research. 1958;9:421–425. [Google Scholar]
- Enquist BJ, Niklas KJ. Global allocation rules for patterns of biomass partitioning in seed plants. Science. 2002;295:1517–1520. doi: 10.1126/science.1066360. [DOI] [PubMed] [Google Scholar]
- Farrar J, Gunn S. Allocation: allometry, acclimation – and alchemy? In. In: Lambers H, Poorter H, Van Vuuren MMI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden: Backhuys Publishers; 1998. pp. 183–198. [Google Scholar]
- Gurevitch J, Wilson P, Stone JL, Teese P, Stoutenburgh RJ. Competition among old-field perennials at different levels of soil fertility and available space. Journal of Ecology. 1990;78:727–744. [Google Scholar]
- Harmens H, Stirling CM, Marshall C, Farrar JF. Is partitioning of dry weight and leaf area within Dactylis glomerata affected by N and CO2 enrichment? Annals of Botany. 2000;86:833–839. [Google Scholar]
- Hunt R, Nicholls AO. Stress and the coarse control of growth and root–shoot partitioning in herbaceous plants. Oikos. 1986;47:149–158. [Google Scholar]
- Mommer L, Wagemaker N, de Kroon H, Ouborg NJ. Unravelling belowground plant distributions: a real time PCR method for quantifying species proportions in mixed root samples. Molecular Ecology Notes. 2008;8:947–953. doi: 10.1111/j.1755-0998.2008.02130.x. [DOI] [PubMed] [Google Scholar]
- Müller I, Schmid B, Weiner J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:115–127. [Google Scholar]
- Niklas KJ. Plant allometry. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Poorter H, Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water. Australian Journal of Plant Physiology. 2000;27:595–607. [Google Scholar]
- Reich PB. Root–shoot relations: optimality in acclimation and adaptation or the “Emperor's New Clothes”? In. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. 3rd edn. New York, NY: Marcel Dekker; 2002. pp. 205–220. [Google Scholar]
- Reynolds HL, Pacala S. An analytical treatment of root-to-shoot ratio and plant competition for soil nutrient and light. American Naturalist. 1992;141:51–70. doi: 10.1086/285460. [DOI] [PubMed] [Google Scholar]
- Robinson D. Scaling the depths: below-ground allocation in plants, forests and biomes. Functional Ecology. 2004;18:290–295. [Google Scholar]
- Robinson D, Hodge A, Griffiths BS, Fitter AH. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proceedings of the Royal Society of London B–Biological Sciences. 1999;226:431–435. [Google Scholar]
- Roumet C, Picon-Cochard C, Dawson LA, et al. Quantifying species composition in root mixtures using two methods: near-infrared reflectance spectroscopy and plant wax markers. New Phytologist. 2006;170:631–638. doi: 10.1111/j.1469-8137.2006.01698.x. [DOI] [PubMed] [Google Scholar]
- Shipley B. From plant traits to vegetation structure: chance and selection in the assembly of ecological communities. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- Shipley B, Meziane D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Functional Ecology. 2002;16:326–331. [Google Scholar]
- Wong SC, Osmond CB. Elevated atmospheric partial pressure of CO2 and plant growth. III. Interactions between Triticum aestivum (C3) and Echinochloa frumentacea (C4) during growth in mixed culture under different CO2, N nutrition and irradiance treatments, with emphasis on below-ground responses estimated using the δ13C value of root biomass. Australian Journal of Plant Physiology. 1991;18:137–152. [Google Scholar]
- Zobel M, Zobel K. Studying plant competition: from root biomass to general aims. Journal of Ecology. 2002;90:578–580. [Google Scholar]