Abstract
Background and Aims
With the advent of transgenic crops, genetically modified, herbicide-resistant Brassica napus has become a model system for examining the risks and potential ecological consequences of escape of transgenes from cultivation into wild compatible species. Escaped transgenic feral B. napus and hybrids with compatible weedy species have been identified outside of agriculture and without the apparent selection for herbicide resistance. However, herbicide (glyphosate) exposure can extend beyond crop field boundaries, and a drift-level of herbicide could function as a selective agent contributing to increased persistence of transgenes in the environment.
Methods
The effects of a drift level (0·1 × the field application rate) of glyphosate herbicide and varied levels of plant competition were examined on plant fitness-associated traits and gene flow in a simulated field plot, common garden experiment. Plants included transgenic, glyphosate-resistant B. napus, its weedy ancestor B. rapa, and hybrid and advanced generations derived from them.
Key Results
The results of this experiment demonstrate reductions in reproductive fitness for non-transgenic genotypes and a contrasting increase in plant fitness for transgenic genotypes as a result of glyphosate-drift treatments. Results also suggest that a drift level of glyphosate spray may influence the movement of transgenes among transgenic crops and weeds and alter the processes of hybridization and introgression in non-agronomic habitats by impacting flowering phenology and pollen availability within the community.
Conclusions
The results of this study demonstrate the potential for persistence of glyphosate resistance transgenes in weedy plant communities due to the effect of glyphosate spray drift on plant fitness. Additionally, glyphosate drift has the potential to change the gene-flow dynamics between compatible transgenic crops and weeds, simultaneously reducing direct introgression into weedy species while contributing to an increase in the transgenic seed bank.
Keywords: Gene flow, canola, herbicide drift, transgene escape, plant ecology, mesocosms, Brassica napus, Brassica rapa
INTRODUCTION
The escape of transgenic crops and crop alleles from agriculture into wild and weedy populations is an ecological concern that has received heightened attention in recent years. Hybridization and gene flow between closely related plant species is not inherently ecologically hazardous as crops and weeds have exchanged genes since the domestication of crops species (Ellstrand, 2001). However, the potential effects of hybridization and introgression on plant communities, such as genetic extinction and assimilation of wild species (Levin et al., 1996; Rhymer and Simberloff, 1996; Wolf et al., 2001), expansion into new habitats (Rieseberg et al., 2007) or increased weediness (Ellstrand et al., 1999; Ellstrand, 2001; Lu and Snow, 2005) suggest that crop gene escape and persistence outside of cultivation may result in adverse ecological consequences. As transgenes are designed to confer new traits to crops (e.g. herbicide resistance, insect resistance), introgression of these traits into compatible weeds also has the potential to contribute to the ecological consequences mentioned above.
Research examining transgenic gene-flow risks and consequences has utilized a number of agricultural crops. In many cases, the genetically modified (GM) crop is cultivated in geographic isolation from wild or weedy compatible relatives, and therefore poses little risk of cross pollination and transgene escape (e.g. cotton, maize and soybean in the United States). However, in cases where crop and compatible weed distributions overlap, as is the case for canola (Brassica napus) and its weedy relatives, transgenes have been shown to escape and persist in non-agronomic habitats (Jørgensen and Andersen, 1994; Warwick et al., 2008).
Brassica napus is an allotetraploid (2n = 38 = AACC) derived naturally from hybridization between the diploid species B. rapa (2n = 20 = AA) and B. oleracea (2n = 18 = CC) (UN, 1935). Brassica napus can spontaneously hybridize with B. rapa in greenhouse and field experiments (Jørgensen and Andersen, 1994; Landbo et al., 1996; Mikkelsen et al., 1996; Halfhill et al., 2002, 2004; Warwick et al., 2003; Wilkinson et al., 2003; Simard et al., 2006;). Both vegetative and reproductive measures indicate that F1 hybrids between these two species can have moderately high, albeit variable, fitness (Hauser et al., 1998a; Warwick et al., 2003). Early-generation backcrosses (BC1) between F1 hybrids and B. napus and B. rapa typically suffer reductions in fitness (Hauser et al., 1998b) but later-generation backcrosses (BC2, BC3) often recover fully (Snow et al., 1999).
In addition to inter-specific hybrids, intra-specific hybridization with weedy feral populations of escaped B. napus can occur in crop fields and field margins after canola is cultivated due to seed loss during the harvest process or transportation to processing plants (Gulden et al., 2003; Knispel et al., 2008). Should volunteer plants germinate and grow in the following years, they may serve as transgene reservoirs, acting as pollen donors to sexually compatible weeds, or as recipients of weedy pollen from neighbouring wild compatible species (Beckie et al., 2003; Pilson and Prendeville, 2004). Considering the examples above, the escape of crop alleles, both non-transgenic and transgenic, is inevitable wherever B. napus and B. rapa occur in sympatry (Warwick et al., 2003; Légère, 2005).
In the case of transgenic glyphosate-resistant B. napus, the CP4 EPSPS transgene is believed to be selectively neutral in the absence of herbicide application (Hancock, 2003). Despite observed fitness decreases of early backcross generations, transgenic, herbicide-resistant, introgressed B. rapa has been found to persist in abandoned fields and waste places in Canada (Warwick et al., 2003; Simard et al., 2006; Warwick et al., 2008) and Europe (Jørgensen and Andersen, 1994; Hansen et al., 2001). How transgenic hybrids and introgressed individuals are able to persist in environments where the transgene does not confer a selective advantage is integral to understanding the long-term consequences of transgene escape in weedy plant communities.
The selective potential of herbicide extends beyond the boundaries of agricultural fields and roadside applications as some herbicides are carried by variable winds and airflow. The lower selection provided by herbicide drift on field boundary communities could be an important factor contributing to the escape and introgression of transgenes into weedy communities. While hybridization and introgression could occur within fields where herbicide selection is high, the rotational practices of farmers would limit the ability of introgressed weeds to escape out of crop fields. However, compatible weeds (or feral crops) in field boundaries may be overlooked, providing the time necessary for successful introgression. Additionally, the higher concentration of herbicides used within fields (relative to drift concentrations) would limit the ability of weedy species to survive and flower. Studies on herbicide drift have indicated the concentration of herbicides can range from 0·1 × to 0·01 × the strength of field applications between 5 and 400 m from spray boundaries (Yates et al., 1978; Ganzelmeier and Rautmann, 2000). The effects of occasional, sub-lethal herbicide spray on different crop and weed species are likely to vary; however, reductions in plant fitness can be expected. Additionally, the effects of herbicide drift may impact gene-flow processes between crops and adjacent weeds by altering the fitness of sexually compatible weedy species.
In the presence of glyphosate drift, transgenic hybrid and introgressed glyphosate-resistant plants are likely to have greater fitness in the plant community relative to non-resistant plants. It is hypothesized that alleles conferring glyphosate resistance are beneficial outside of cultivation, e.g. when glyphosate herbicide is present along field margins and roadsides, i.e. under the selective pressure created by glyphosate drift, feral GM plants and hybrid or backcross progeny of crop and compatible wild species would benefit from the expression of glyphosate resistance transgenes.
The objectives of this research were to examine whether glyphosate drift could contribute to the persistence of glyphosate resistance transgenes outside of agriculture, and to evaluate the ecological consequences of CP4 EPSPS transgene escape to compatible recipients. In this study, the fitness of parental, hybrid and introgressed genotypes of transgenic B. napus and weedy B. rapa and the gene-flow rate of CP4 EPSPS to B. rapa and F1 hybrid plants under the selective pressure of drift-level glyphosate spray are measured.
MATERIALS AND METHODS
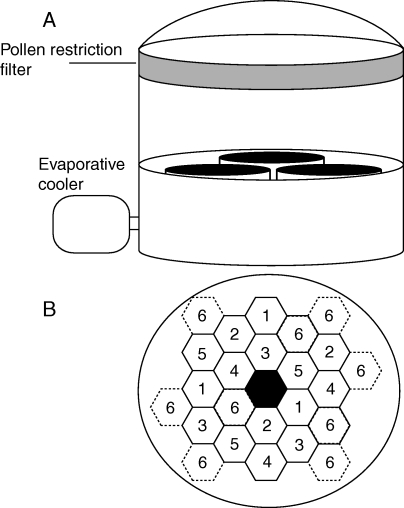
Experiments were conducted at the US-EPA Western Ecology Division facilities in Corvallis, Oregon, USA. Experiments were performed within replicated outdoor mesocosms (Heagle et al., 1973; Watrud et al., 2010) equipped with pollen filters and insect netting to prevent environmental escape of Brassica pollen and seeds, and to prevent movement of pollinators between mesocosms. These mesocosms are enclosed with 8-mil polyvinyl chloride (PVC) film (Livingston Coating, Charlotte, NC, USA), exposed to ambient light, and are temperature modified by evaporative coolers to approximate ambient temperature. Mesocosms were separated by approx. 3 m and did not have any connectivity (Fig. 1A). Each mesocosm is 3·1 m wide and 3·3 m tall, and contains three large tubs, each with 1·2 m2 of soil surface area and a depth of 0·6 m. Each tub contained a mixed sandy loam soil (Rexius Transportation Northwest, Eugene, OR, USA). Each tub is watered with centrally located metered irrigation. A spatially balanced planting design was utilized in each tub to create equal interaction between experimental plants (Fig. 1B). Experiments were performed in each of two years (2006, 2007) for a standard growth season (early spring to summer). Seedlings germinated in greenhouses were transplanted and marked individually for data recording. Plants were grown from mid-April to mid-July in 2006, and from mid-March to mid-July in 2007.
Fig. 1.
Schematic of sunlit mesocosm units and the planting grid used for spatial arrangement of plant replicates within each mesocosm. (A) Mesocosms are independent units enclosed within clear 8-mil PVC film, and include an evaporative cooler to assist in temperature control, a pollen restriction filter and insect netting to contain pollen and pollinators. Mesocosms contain three replicate soil tubs with 1·2 m2 of soil surface and 0·6 m depth each. Each mesocosm tub was planted with one of three randomly assigned competition treatments. (B) Within each mesocosm tub, an 18-cell planting grid is utilized to arrange the experimental genotypes in a spatially balanced pattern. Competitor plants are added at position 6. The central cell is the location of each tub's independent irrigation system.
Plants
Five different species/genotype lines of Brassica were used in these experiments. The two parental lines included transgenic B. napus and non-transgenic, weedy B. rapa. The remaining three genotypes in the study consisted of hybrid and backcross generations: F1 hybrid; B. napus backcross 1 (hereafter, BC1–Bn); and B. rapa backcross 1 (hereafter, BC1–Br). Backcross generations to B. rapa are a necessary step for stable introgression of CP4 EPSPS into weedy B. rapa populations while backcross generations to B. napus are an equally important direction for introgression as weedy B. napus could serve as a reservoir for the transgene and for persistent introgression into B. rapa.
The B. napus parent in the experiments (n = 72) was a glyphosate-resistant cultivar, RaideRR (Monsanto Co., GT73) characterized as a homozygous line with a single transgene event that carries the CP4 EPSPS gene for glyphosate resistance (Monsanto Co., St Louis, MO, USA) and the linked glyphosate oxidase gene, GOX. Examination of the segregation pattern resulting from hybridization tests and introgression into B. rapa suggests that the CP4EPSPS–GOX transgene insert lies in the A nuclear genome and segregates as a dominant trait (Anonymous, 2002, 2005). As a local source of B. rapa was not available, the B. rapa parent in the experiment (n = 72) was obtained from the USDA-GRIN plant germplasm facility (PI 633155). Brassica rapa is a self-incompatible diploid species which carries the A genome. Hybrid (F1) and introgressed (BC1) genotypes were formed prior to this experiment via manual pollination under greenhouse conditions. The F1 generation (n = 72) was formed with paternal B. napus and maternal B. rapa. The BC1 genotypes were formed by reciprocal backcrossing from the F1 genotype (paternal) to each of the original parental lines. The F1 hybrid lines were doubly screened for transgene presence by surviving an application of glyphosate herbicide at twice the field application rate (f.a.r.) for canola glyphosate-resistant canola cultivation (2 × f.a.r = 4·68 L ha−1). Using the 1× rate of glyphosate can sometimes allow sensitive plants to survive. Twice the normal rate of glyphosate was chosen for screening the hybrid plants in the greenhouse to discriminate clearly between sensitive and resistant plants. Surviving plants were also quality checked with lateral flow test strips which test for presence of the transgenic CP4 EPSPS protein (TraitChek; Strategic Diagnostics, Newark, DE, USA). The hybrid status of a subset of individuals was verified with flow cytometry (data not shown; Halfhill et al., 2003). All of the BC1–Bn plants (n = 72) used were verified as positive for the transgenic CP4EPSPS protein and represented a mixed population of homozygous and hemizygous plants for transgene presence. The BC1–Br genotype seeds generated for use in this study included both negatively and positively transgenic individual plants due to low recovery of progeny from the manual F1 × B. rapa hybridizations. Reductions in BC1–Br progeny viability has been reported previously (Hauser et al., 1998a). Thus, both BC1–Br plants expressing glyphosate resistance [BC1–Br (+) (n = 40], and sibling BC1–Br plants without glyphosate resistance [BC1–Br (–) (n = 32], were used in this experiment. Response variables for the BC1–Br genotypes were analysed separately for positive and negative plants.
Treatments
Eight mesocosm chambers were utilized in our experiment in each year. Four randomly selected mesocosms received no treatment, designed to mimic communities without exposure to glyphosate-drift. The remaining four mesocosms received a single dose of 0·1 × the f.a.r. of glyphosate herbicide (0·1 × =0·234 L ha−1) used for transgenic canola cultivation. Glyphosate-drift rates can range from 0·1 × to 0·01 × f.a.r from 5 to 400 m from crop fields (Yates et al., 1978; Ganzelmeier and Rautmann, 2000), but can vary due to many factors including wind speed, humidity and local topography. Additionally, the potential for out-crossing to occur between crop B. napus and weeds (e.g. feral B. napus and B. rapa) is predicted to be higher at low distances (Staniland et al., 2000; Jenczewski et al., 2003). Thus a single concentration of 0·1 × f.a.r. of glyphosate herbicide was chosen to simulate glyphosate-drift exposure for weedy communities adjacent to crop fields where natural hybridization and introgression between B. napus and B. rapa is likely to occur. Glyphosate was applied using a flat fan spray nozzle (TeeJet 80015VS, Spraying Systems Co., Wheaton, IL, USA) held at 45 cm above the plant canopy to mimic the effects of drift from application of glyphosate from a nearby crop field or weed management target (Al-Khatib and Peterson, 1999; Bird et al., 2002). Glyphosate-drift treatments were applied inside the mesocosms when transplants were established and the majority of plants had reached the bolting stage of development (24 May in 2006, and 10 May in 2007).
Within each mesocosm, competition treatments were applied using one of three different plant compositions to a tub. Competition treatments included a control treatment, in which three replicates of each of the five Brassica genotypes were present (tub n = 15, n = 3 per genotype; Fig. 1B). The second competition treatment was designed to examine the competition effects of non-Brassica weedy species. It was applied by adding to the control treatment planting grid three replicate plants of each of the common weedy ruderal species Panicum capillare (witchgrass), Achillea millefolium (common yarrow) and Lapsana communis (nipplewort; tub n = 24) planted within and around the original planting grid (Fig. 1B). These three weedy species were chosen to represent broadly distributed and common weeds of disturbed soils such as fallow fields and roadsides. A final competition treatment, to examine intra-generic competition effects, was planted with the control planting grid plus an additional nine plants of weedy B. rapa (tub n = 24) planted within and around the original planting grid (Fig. 1B). Pollinators (Musca domestica) were added to all mesocosms (1000 pupae per mesocosm) after herbicide treatment application to facilitate pollination (Halfhill et al., 2003) and gene flow between genotypes and, in particular, to B. rapa, a self-incompatible species.
As mentioned above, the plants used to fill the BC1–Br genotype slots within the mesocosm tubs comprised a mixture of both transgenic [BC1–Br (+) n = 40] and non-transgenic [BC1–Br (–) n = 32] plants. The planting pattern used for this genotype included two positive plants in the control and Brassica competition tubs and a single positive plant in the ruderal competition tub. Negative plants filled the remaining positions.
Data collection and analysis
Individual plants were harvested at the end of the growing season after the conclusion of flowering and siliques (seed pods) had filled. Plants were harvested by hand by collecting above-ground tissues and separating vegetative and reproductive tissues to minimize shatter and loss of seeds. Competitor plants were excluded from data collection and analysis. Fitness-associated traits were measured for every individual plant within the experiment and included vegetative biomass (VBM), seed biomass (SBM) and estimated seed number. Although fitness can depend on many factors, for annual plants, vegetative growth and reproductive success, or seed production, are good correlates of plant fitness. Vegetative biomass was dried in a forced-air oven at 60 °C for 10 d and was measured as total above-ground biomass (minus seeds) in grams. Brassica siliques were collected in separate envelopes and dried at ambient temperatures. Following harvest, seeds were removed from siliques and recorded as total SBM in grams while silique mass was included in VBM measurements. Seed number was estimated at the plant level by determining individual seed weight from 100-seed subsamples (when 100 seeds were available) multiplied by total SBM. Seed number is a more appropriate measurement than SBM for comparing genotypes as the genotypes in this study produced seeds of different average weights (e.g. B. napus > B. rapa) (data not shown). In examining the reproductive fitness measurements, both seed biomass and seed number were similarly affected by treatments (data not shown). For brevity, only the treatment effects on seed number are reported. Gene-flow rates, and estimated number of transgenic seeds produced via gene flow were also measured from the B. rapa and the F1 hybrid genotype. Transgene gene flow was measured by screening 100 seed subsamples for each individual B. rapa and F1 hybrid plant with a 2 × f.a.r of glyphosate (4·68 L ha−1). As mentioned above, the 2× rate of glyphosate was used to screen seedlings as 1× applications can sometimes allow sensitive plants to survive. Due to logistical constraints (e.g. thousands of seedlings) it was not possible to molecularly characterize the screened seedlings. Resistance of seedlings to glyphosate spray was confirmed by using lateral flow TraitChek strips which detect the expression of the transgenic protein CP4 EPSPS (TraitChek) (Warwick et al., 2008). To control for potential false positive and false negative screening results, a qualitative assessment was performed to identify the weakest surviving plant and strongest affected plant followed by verification of transgene expression (or lack of expression) using the lateral flow strips. The proportion of transgenic seeds per 100 seeds was calculated to estimate gene flow to each plant. This measurement of gene flow was then multiplied by the total seed number values to estimate the total number of transgenic seeds produced by B. rapa and F1 hybrid genotypes. Gene-flow rate measurements to the F1 hybrid are more complicated than for B. rapa due to the transgene hemizygosity of this genotype. The F1 hybrid is functionally heterozygous for the dominant glyphosate-resistant transgene; thus 50 % of the progeny are expected to segregate as glyphosate resistant if pollinated by a non-transgenic pollen source (e.g. B. rapa), 75 % should be resistant if self-pollinated and 100 % should be resistant if pollinated by transgenic B. napus.
Vegetative biomass and estimated seed numbers produced by the different plant genotypes for the two years of the study were combined and analysed by multivariate analysis of variance (MANOVA). While measurements were taken from individual plants, the effects of glyphosate treatment, year, and their interaction were analysed at the mesocosm level as between-subject factors using the whole-plot error term for a split-plot design. The effects of competition, genotype and their interactions with year and glyphosate treatment were tested as within-subject factors at the tub level using the split-plot error term. When there were significant interactions, further analysis was performed as on data subsets (ANOVA) (Snedecor and Cochran, 1980; Looney and Stanley, 1989). Since estimated seed number ranged over several magnitudes between genotypes, data were transformed prior to statistical analysis using the natural log transformation and values were back-transformed for reporting the results. Additionally, gene-flow data are measured as a proportion; thus values were transformed prior to statistical analysis using the arcsin transformation and values were back-transformed for reporting results. The MANOVA and ANOVA analyses were generated using SAS/STAT software (PROC GLM). To test for differences in the distribution of transgene gene flow to B. rapa plants, the Wilcoxon rank sum test (exact) was employed using mesocosm averages with SAS/STAT software (PROC NPAR1WAY), Version 9·1 of the SAS System for Windows.
RESULTS
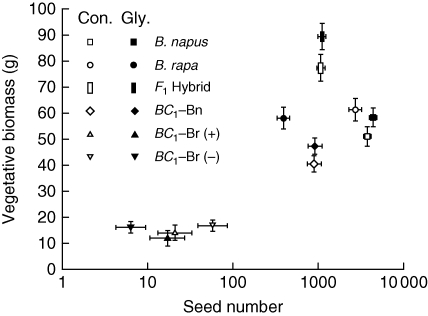
In the experiments, B. rapa and B. napus plants had roughly equivalent VBM in control mesocosms, whereas the F1 hybrid displayed a transgressive phenotype of high VBM, exceeding that of all other genotypes. In contrast, both backcross genotypes, BC1–Bn and BC1–Br, displayed reductions in plant size relative to the F1 parent and were the least vigorous plants. The BC1–Br plants, (+) and (–), were the smallest genotypes in the study (Fig. 2).
Fig. 2.
Relationship between vegetative and reproductive measurements in control and glyphosate-drift treatments. Points indicate genotypes averaged at the mesocosm level over both years. Open symbols represent control treatment (Con.), closed symbols represent glyphosate-drift treatment (Gly.). Error bars indicate s.e.
In control mesocosms, Brassica napus produced the greatest number of seeds (approx. 4000) followed by B. rapa (approx. 3000). The F1 hybrid and BC1–Bn produced a much lower number of seeds (approx. 1000) than the parental genotypes, and the BC1–Br positive and negative plants produced very few seeds (approx. 20–60) (Fig. 2). Plant response to glyphosate and competition treatments was genotype specific and depended on the plant fitness trait that was measured. Significant interactions were detected between the main effects of genotype glyphosate-drift treatment, competition and year (Table 1). Significant interactions were dominated by the effects of genotype (P < 0·001); thus the datasets were examined independently for each genotype (Table 2).
Table 1.
MANOVA results for combined dataset of main effects and interaction effects for vegetative and reproductive measures
| d.f. | Vegetative biomass | Seed no. | |
|---|---|---|---|
| Whole plot | |||
| Glyphosate drift (T) | 1 | 0·272 | 0·001 |
| Year (Y) | 1 | 0·427 | 0·881 |
| T × Y | 1 | 0·063 | 0·926 |
| Split plot | |||
| Competition (.C) | 2 | 0·032 | <0·001 |
| C × T | 2 | 0·535 | 0·183 |
| Y × C | 2 | 0·027 | 0·417 |
| Genotype (G) | 5 | <0·001 | <0·001 |
| G × T | 4 | 0·003 | <0·001 |
| G × C | 10 | <0·001 | <0·001 |
| G × C × T | 10 | 0·041 | 0·368 |
| G × Y | 5 | <0·001 | <0·001 |
| G × Y × T | 5 | <0·001 | 0·739 |
| Y × C × T | 4 | 0·867 | 0·155 |
| G × Y × C | 10 | 0·013 | 0·012 |
| G × Y × C × T | 10 | 0·098 | 0·303 |
Values in bold type represent P-values with significant values (P < 0·05).
Table 2.
ANOVA results for effects of glyphosate drift, year, and competition treatments by genotype
|
BC1–Br |
|||||||
|---|---|---|---|---|---|---|---|
| d.f. | B. rapa | B. napus | F1 hybrid | pos (+) | neg (–) | BC1–Bn | |
| VBM | |||||||
| Glyphosate drift (T) | 1 | 0·544 | 0·129 | 0·015 | 0·404 | 0·842 | 0·116 |
| Year (Y) | 1 | 0·033 | 0·239 | 0·003 | <0·001 | 0·816 | 0·767 |
| T × Y | 1 | 0·769 | 0·042 | 0·003 | 0·702 | 0·651 | 0·047 |
| Competition (.C) | 2 | 0·019 | 0·047 | 0·003 | 0·002 | 0·020 | 0·632 |
| T × C | 2 | 0·232 | 0·895 | 0·230 | 0·881 | 0·974 | 0·270 |
| Y × C | 2 | 0·149 | 0·225 | 0·042 | 0·336 | 0·987 | 0·434 |
| Y × C × T | 2 | 0·507 | 0·482 | 0·339 | 0·892 | 0·551 | 0·423 |
| Seed number | |||||||
| Glyphosate drift (T) | 1 | <0·001 | 0·195 | 0·882 | 0·753 | <0·001 | 0·924 |
| Year (Y) | 1 | 0·002 | 0·009 | 0·536 | 0·162 | 0·012 | 0·008 |
| T × Y | 1 | 0·073 | 0·536 | 0·412 | 0·899 | 0·443 | 0·442 |
| Competition (.C) | 2 | 0·136 | 0·144 | 0·489 | 0·005 | 0·045 | 0·731 |
| T × C | 2 | 0·519 | 0·257 | 0·666 | 0·907 | 0·093 | 0·284 |
| Y × C | 2 | 0·302 | 0·483 | 0·712 | 0·390 | 0·044 | 0·524 |
| Y × C × T | 2 | 0·158 | 0·988 | 0·250 | 0·372 | 0·440 | 0·154 |
Values in bold type represent P-values with significant changes from control measurements (P < 0·05).
Non-transgenic B. rapa and BC1–Br (–)
Both vegetative and reproductive fitness measurements for B. rapa were significantly different between the two years of this study (Table 2). In 2006, B. rapa plants were smaller and produced significantly less VBM when compared with measurements in 2007. Despite lower VBM in 2006, B. rapa produced a higher average number of seeds in 2006 than in 2007 (data not shown).
Application of drift-level glyphosate to sensitive plants was expected to reduce VBM or result in plant death. However, there was no significant effect of glyphosate-drift on VBM for B. rapa (Table 2 and Fig. 2). Instead it was observed that drift-level glyphosate exposure temporarily halted normal vegetative growth of B. rapa and developing flower buds were stunted or killed. After a recovery period (approx. 7–20 d, pers. obs.), the glyphosate-drift treated B. rapa plants produced prolific new growth from axillary meristems and an equivalent amount of VBM compared with control plants. A similar lack of apparent glyphosate-drift effect on BC1–Br (–) VBM was also observed, though due to small stature, a developmental pause for this genotype would be difficult to assess. After the vegetative recovery, B. rapa plants were seen to develop new buds, resume flowering and successfully set seeds, though significantly less than B. rapa in the control mesocosms. The BC1–Br (–) plants also produced significantly fewer seeds under glyphosate-drift treatment (Table 2 and Fig. 2).
Transgenic genotypes [B. napus, F1 hybrid, BC1–Bn and BC1–Br (+)]
Transgenic genotypes typically benefitted from carrying the glyphosat-resistance transgene or were unaffected by glyphosate drift (Table 2). Significant interactions between glyphosate-drift treatment and year were observed for VBM in B. napus, the F1 hybrid, and the BC1–Bn genotypes (Table 2). In all three cases, the interaction was due to a significant increase in biomass in 2006 in glyphosate-drift mesocosms, an increase not observed in 2007. The BC1–Br (+) genotype displayed less vigorous vegetative growth overall, and did not significantly differ in glyphosate-drift treatments from control treatments in either year.
For measurements of seed number, no interactions between year and treatment were observed (Table 2), though there were significant differences between the two years of the study. Both B. napus and the BC1–Bn genotype had greater seed numbers produced in 2007 (data not shown). None of the transgenic genotypes produced greater seed numbers in glyphosate-drift mesocosms as a result of glyphosate resistance (Table 2).
Competition effects
Competition generally contributed to reduced VBM in B. rapa, B. napus and BC1–Br genotypes, but produced inconsistent effects in the F1 hybrid and BC1–Bn genotypes (data not shown). The competition treatments in the experiment had little effect on seed numbers though competition treatments did slightly increase the seed number produced on BC1–Br (+) plants. Competition effects on fitness were inconsistent across the two years of the study and for each genotype. Additionally, there was a lack of significant interaction between competition and glyphosate-drift treatments and no consistent effects of either the intra-generic or ruderal competition was observed. Thus, the predictive ability of the data and effects of the two competition treatments on the fitness of the parental and hybrid genotypes in this experiment are inconclusive.
Gene flow and transgenic seed number
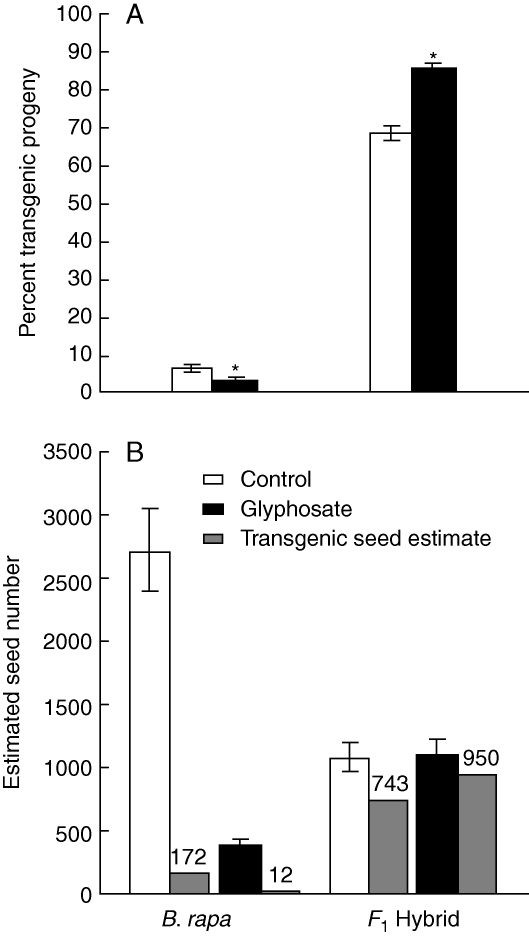
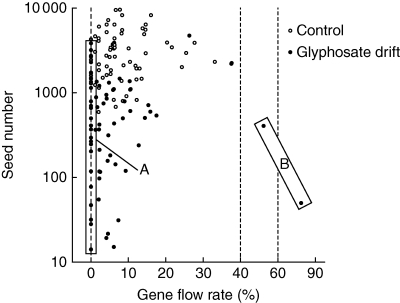
Gene-flow rates to B. rapa plants in control mesocosms were not significantly different between the two years of the study but gene-flow rates were significantly lower due to glyphosate-drift treatments (Table 3 and Fig. 3A). Additionally, the distribution of gene flow differed between control and glyphosate-drift treated mesocosms (Wilcoxon exact P = 0·021; Fig. 4). The pattern of transgene gene flow to B. rapa in glyphosate-treated mesocosms was skewed toward a large number of B. rapa individuals with zero gene flow, while control mesocosms had a different distribution, with the majority of B. rapa individuals receiving 5–10 % gene flow (Fig. 4). The estimated number of transgenic seeds (total seed number × gene-flow rate) produced on B. rapa plants was fewer in glyphosate drift-treated mesocosms than in control mesocosms in both years (Fig. 3B), reflective of the reduced level of gene flow observed for drift-treated plants and the significant reduction in total seeds produced by B. rapa in glyphosate drift-treated mesocosms. The gene-flow rate to the F1 hybrid was greater in 2007 than in 2006 (data not shown) and also greater in glyphosate-drift mesocosms relative to control mesocosms (Table 3 and Fig. 3A). As a result of higher gene-flow rates and no change in seed production, there was a concurrent increase in the estimated number of transgenic seeds produced on F1 hybrid plants in glyphosate-drift mesocosms (Fig. 3B).
Table 3.
ANOVA results for effects of year and glyphosate treatment on total seed number and gene-flow rate for Brassica rapa and the F1 hybrid genotype
| d.f. | Seed no. | Gene flow | |
|---|---|---|---|
| Brassica rapa | |||
| Glyphosate drift | 1 | <0·001 | 0·036 |
| Year | 1 | 0·002 | 0·074 |
| Y × T | 1 | 0·073 | 0·864 |
| F1 hybrid | |||
| Glyphosate drift | 1 | 0·882 | <0·001 |
| Year | 1 | 0·536 | 0·005 |
| Y × T | 1 | 0·412 | 0·180 |
Values in bold type represent P-values with significant changes from control measurements (P < 0·05).
Fig. 3.
Gene flow and transgenic seed number estimates for Brassica rapa and the F1 hybrid genotype. Error bars indicate standard error measurements. * Significant deviation from control at P < 0·05. Gene flow expressed as percentage of transgenic progeny produced by B. rapa and F1 hybrid genotypes (A) and transgenic seed number estimates (B).
Fig. 4.
Effect of glyphosate-drift on seed number and gene flow to B. rapa plants. The graph depicts individual plant measurements for seed number and gene flow. (A) Skew of observations of zero gene flow with low-to-average seed production indicating reduced available transgenic pollen; (B) a few observations of very high gene flow indicating plant recovery with a greater proportion of transgenic pollen available. Note that the x-axis is broken at 40 % and 65 %.
DISCUSSION
The measurements for vegetative and reproductive fitness in this experiment are largely in agreement with previous research documenting the fitness differences between the crop (B. napus) and weed (B. rapa), as well as the transgressive fitness of F1 hybrids and the depressed fitness of early backcross generations (Hauser et al., 1998a, b). The application of drift-level glyphosate did not substantially affect the relative fitness relationship between the parental, hybrid, and early backcross generations beyond expected reductions due to glyphosate damage in sensitive genotypes. A greater, though slight, reproductive fitness of the BC1–Br (+) genotype compared with the BC1–Br (–) genotype under glyphosate-drift treatments was observed. Additionally, there was a lower sample size for the BC1–Br genotypes and further studies should be conducted to validate this observation. This slight increase in fitness would suggest that the CP4 EPSPS transgene may increase the fitness of BC1–Br plants in areas exposed to glyphosate drift. The seed production values are very low in this genotype but introgression of transgenes into wild B. rapa depends on successful introgression through the BC1–Br generation. Moreover, introgressed, transgenic B. rapa has been observed in weedy habitats, demonstrating that this apparent fitness bottleneck can be overcome (Warwick et al., 2008). Although more research is needed to validate these observations under field conditions, the results demonstrate that glyphosate-drift pressure coupled with transgenic BC1–Br plants may increase the likelihood of passing this fitness bottleneck.
Gene flow between B. napus and B. rapa has been previously examined in both greenhouse and field studies (Jørgensen and Andersen, 1994; Landbo et al., 1996; Mikkelsen et al., 1996; Halfhill et al., 2002, 2004; Warwick et al., 2003; Wilkinson et al., 2003; Allainguillaume et al., 2006; Simard et al., 2006). However, this study is unique in that it examines gene-flow rates under selective pressure, and compares the competition of transgenic and non-transgenic pollen inputs on hybrid generations. Glyphosate drift had two different but associated effects on pollen-mediated gene-flow processes within the experiment.
First, simulated glyphosate drift directly affected gene flow to B. rapa by reducing the number of viable flowers available for pollination, observed as the developmental flowering time delay in glyphosate-drift mesocosms. This flowering shift appears to have moved the majority of B. rapa plants beyond the normal flowering time of the transgenic genotypes in the experiment, resulting in a significant reduction in the rate of gene flow to B. rapa and in the formation of transgenic seeds. A few B. rapa plants appear to have recovered while B. napus was still flowering and would have had a different composition of available pollen (low B. rapa, high B. napus), more likely serving as maternal parents to transgenic pollen and experiencing high transgene gene-flow rates. Brassica rapa plants that recovered after the pollen/flower output of B. napus was in decline, however, would be preferentially pollinated by the other recovering B. rapa in the mesocosm, producing only non-transgenic seeds (zero gene flow).
The effects of glyphosate drift on B. rapa gene-flow rates and flowering phenology in this experiment were measured from a single B. rapa genotype. In naturally weedy communities, B. rapa has a flowering phenology that can overlap with the flowering time of B. napus (Johannessen et al., 2006). In cases of early-flowering B. rapa, a delay in flowering phenology due to drift-level concentrations of glyphosate at a field boundary may encourage transgene flow by synchronizing flowering phenologies of both the crop and weed. However, later-flowering genotypes of B. rapa could also be desynchronized from B. napus flowering when exposed to glyphosate drift, reducing the potential for transgene gene flow. There are many other factors which could be affected by flowering time changes in a natural setting that cannot be feasibly tested within mesocosms. For example, alterations in flowering time due to glyphosate-drift affects could potentially alter seed predation rates and seed bank inputs as well as change the dynamics of disease and herbivore pressure.
It is likely that the developmental stage of B. rapa and hybrid plants is important in determining the extent of flower phenology changes and the degree of the impact that glyphosate drift could have on gene flow. Exposure at an earlier or later developmental stage could alter the magnitude of changes observed in the experiments. Glyphosate treatments in this experiment were intended to simulate the effects of herbicide drift near field edges or roadsides, not within crop fields. The application of glyphosate in canola agriculture occurs after germination and up to the six-leaf stage of plant growth (Anonymous, 2009). However, the timing and dosage of exposure to herbicide drift in weedy communities likely depends on the types of adjacent crop fields (i.e. soybeans or corn) or differences in weed control practices (for example, see Froese et al., 2005) at field boundaries and roadsides. Escaped or volunteer canola and associated compatible weeds and hybrids can also have variable secondary dormancy and germination (López-Granados and Lutman, 1998) and could be exposed to more temporal variability in glyphosate application than weeds growing within crop fields. Future research quantifying flowering time changes and plant recovery rates of different B. rapa genotypes and other weedy compatible species after exposure to varied drift-level herbicide treatments and at varied developmental times would be useful to understand better the potentially broader influence of glyphosate drift on transgene escape.
The second effect of glyphosate drift in the mesocosm community was to alter the ratio of transgenic and non-transgenic pollen in the mesocosms available for gene flow to the F1 hybrid. While F1 hybrids have been reported to be largely self-incompatible (Warwick et al., 2003), some selfing of F1 hybrids is possible and the lowest expected proportion of transgenic seeds obtained from F1 hybrids should be between 50 and 75 %. Within control mesocosms, gene-flow rates to the F1 hybrid were between 50 % and 75 % (69 %), suggesting a relatively balanced mix of non-transgenic [B. rapa, BC1–Br (–)], and transgenic [F1 hybrid, BC1–Bn, BC1–Br (+), and B. napus] pollen in competition for F1 ovules. However, in glyphosate-drift mesocosms, B. rapa flowering was suppressed and the proportion of available pollen for gene flow in the mesocosms was altered in favour of transgenic pollen. Consequently, the results show that F1 hybrid plants were preferentially pollinated with transgenic pollen, resulting in the greater gene-flow rates (86 %) and greater number of estimated transgenic seeds on F1 plants. The consequence of this pollen availability shift is a bias for F1 introgression with transgenic B. napus, enriching for BC1–Bn seeds in the next generation. This result would seem to suggest that glyphosate drift could initially slow introgression of transgenes into wild B. rapa populations. However, if this phenomenon occurs in weedy habitats, preferential pollination of F1 hybrids by transgenic pollen could produce a persistent and more fit population of transgenic donors (BC1–Bn vs. BC1–Br) that would be present and available as a pollen source for future introgression into B. rapa once glyphosate-drift pressure had eased. Additionally, successful introgression and stabilization of glyphosate resistance into B. rapa could also contribute to gene flow as a transgene reservoir in weedy populations.
In summary, the potential for changes in the movement and persistence of glyphosate-resistance transgenes in non-agronomic habitats has been identified by examining gene-flow and fitness traits under the pressure of glyphosate drift. These experiments were performed in outdoor, sunlit mesocosms and were not performed as a field experiment. Future field-based studies should be undertaken where transgenic canola has or is already in cultivation to validate these observations and to test for other environmental factors that influence the gene-flow dynamics of weedy populations exposed to glyphosate drift.
From the present experiments it can be inferred that a drift rate (0·1 × f.a.r.) of glyphosate has the potential to significantly reduce the total number of B. rapa seeds that enter the seed bank in the following year, as well as reduce the total number of transgenic seeds produced on B. rapa plants. However, glyphosate-drift pressure on mixed B. napus, B. rapa and hybrid weed communities could also contribute to increased transgenic seeds produced on first-generation hybrids by changing the dynamics of pollen availability and competition. Glyphosate-drift application exerted a selective pressure that resulted in a dramatic reduction in reproductive fitness of sensitive genotypes while resistant genotypes were able to take advantage of glyphosate resistance and maintain or increase their reproductive fitness. The fitness and gene-flow results may help explain reports of transgene escape and persistence of transgenes in B. rapa beyond crop fields and in waste places (Warwick et al., 2008) despite the lack of obvious herbicide applications. The present results demonstrate that sub-lethal glyphosate applications may be sufficient to alter the fitness and gene-flow dynamics of transgenes outside of agriculture and within weedy communities.
ACKNOWLEDGEMENTS
We would like to acknowledge horticultural and technical support provided by George King, Milt Plocher, Marjorie Storm, Gail Heine and Fred Senecal (Dynamac Corporation). The information in this document has been funded wholly (or in part) by the US Environmental Protection Agency. It has been subjected to review by the National Health and Environmental Effects Research Laboratory's Western Ecology Division and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
LITERATURE CITED
- Al-Khatib K, Peterson D. Soybean (Glycine max) response to simulated drift from selected sulfonylurea herbicides, dicamba, glyphosate, and glufosinate. Weed Technology. 1999;13:264–270. [Google Scholar]
- Allainguillaume J, Alexander M, Bullock JM, et al. Fitness of hybrids between rapeseed (Brassica napus) and wild Brassica rapa in natural habitats. Molecular Ecology. 2006;15:1175–1184. doi: 10.1111/j.1365-294X.2006.02856.x. [DOI] [PubMed] [Google Scholar]
- Anonymous. Safety Assessment of Roundup Ready Canola Event GT73. St Louis, MO: Monsanto Co; 2002. pp. 1–35. http://www.monsanto.com/monsanto/content/products/productivity/roundup/canola_es.pdf. (accessed 17 August 2010) [Google Scholar]
- Anonymous. MON-ØØØ73-7 (GT73, RT73) AGBIOS. 2005 http://www.agbios.com. (accessed 17 August 2010) [Google Scholar]
- Anonymous. Roundup Ready® Canola. St Louis, MO: Monsanto Co; 2009. http://www.monsanto.com/monsanto/ag_products/input_traits/canola.asp. (accessed 17 August 2010) [Google Scholar]
- Beckie HJ, Warwick SI, Nair H, Seguin-Swartz G. Gene flow in commercial fields of herbicide-resistant canola (Brassica napus) Ecological Applications. 2003;13:1276–1294. [Google Scholar]
- Bird S, Perry S, Ray S, Teske M. Evaluation of the AgDISP aerial spray algorithms in the AgDRIFT model. Environmental Toxicology and Chemistry. 2002;21:672–681. doi: 10.1897/1551-5028(2002)021<0672:eotaas>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Ellstrand NC. When transgenes wander, should we worry? Plant Physiology. 2001;125:1543–1545. doi: 10.1104/pp.125.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellstrand NC, Prentice HC, Hancock JF. Gene flow and introgression from domesticated plants into their wild relatives. Annual Review of Ecology and Systematics. 1999;30:539–563. [Google Scholar]
- Froese NT, Acker RCV, Friesen LF. Influence of spring tillage and glyphosate treatment on dandelion (Taraxacum officinale) control in glyphosate-resistant canola. Weed Technology. 2005;19:283–292. [Google Scholar]
- Ganzelmeier H, Rautmann D. Drift, drift reducing sprayers and sprayer testing. Aspects of Applied Biology. 2000;57:1–10. [Google Scholar]
- Gulden RH, Shirtliffe SJ, Thomas AG. Harvest losses of canola (Brassica napus) cause large seedbank inputs. Weed Science. 2003;51:83–86. [Google Scholar]
- Halfhill M, Raymer P, Stewart C., Jr Bt-transgenic oilseed rape hybridization with its weedy relative, Brassica rapa. Environmental Biosafety Research. 2002;1:19–28. doi: 10.1051/ebr:2002002. [DOI] [PubMed] [Google Scholar]
- Halfhill M, Millwood R, Weissinger A, Warwick S, Stewart C. Additive transgene expression and genetic introgression in multiple green-fluorescent protein transgenic crop× weed hybrid generations. Theoretical and Applied Genetics. 2003;107:1533–1540. doi: 10.1007/s00122-003-1397-7. [DOI] [PubMed] [Google Scholar]
- Halfhill M, Zhu B, Warwick S, et al. Hybridization and backcrossing between transgenic oilseed rape and two related weed species under field conditions. Environmental Biosafety Research. 2004;3:73–81. doi: 10.1051/ebr:2004007. [DOI] [PubMed] [Google Scholar]
- Hancock J. A framework for assessing the risk of transgenic crops. BioScience. 2003;53:512–519. [Google Scholar]
- Hansen L, Siegismund H, Jørgensen R. Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genetic Resources and Crop Evolution. 2001;48:621–627. [Google Scholar]
- Hauser T, Shaw R, Østergard H. Fitness of Fl hybrids between weedy Brassica rapa and oilseed rape (B. napus) Heredity. 1998a;81:429–435. [Google Scholar]
- Hauser T, Jørgensen R, Østergard H. Fitness of backcross and F2 hybrids between weedy Brassica rapa and oilseed rape (B. napus) Heredity. 1998b;81:436–443. [Google Scholar]
- Heagle A, Body D, Heck W. An open-top field chamber to assess the impact of air pollution on plants. Journal of Environmental Quality. 1973;2:365. [Google Scholar]
- Jenczewski E, Ronfort J, Chèvre AM. Crop-to-wild gene flow, introgression and possible fitness effects of transgenes. Environmental Biosafety Research. 2003;2:9–24. doi: 10.1051/ebr:2003001. [DOI] [PubMed] [Google Scholar]
- Johannessen M, Andersen B, Jørgensen R. Competition affects gene flow from oilseed rape (<♀>) to Brassica rapa (<♂>) Heredity. 2006;96:360–367. doi: 10.1038/sj.hdy.6800796. [DOI] [PubMed] [Google Scholar]
- Jørgensen RB, Andersen B. Spontaneous hybridization between oilseed rape (Brassica napus) and weedy Brassica campestris: a risk of growing genetically modified oilseed rape. American Journal of Botany. 1994;81:1169–1175. [Google Scholar]
- Knispel A, McLachlan S, Van Acker R, Friesen L. Gene flow and multiple herbicide resistance in escaped canola populations. Weed Science. 2008;56:72–80. [Google Scholar]
- Landbo L, Andersen B, Jørgensen R. Natural hybridisation between oilseed rape and a wild relative: hybrids among seeds from weedy B. campestris. Hereditas. 1996;125:89–91. [Google Scholar]
- Légère A. Risks and consequences of gene flow from herbicide-resistant crops: canola Brassica napus L. as a case study. Pest Management Science. 2005;61:292–300. doi: 10.1002/ps.975. [DOI] [PubMed] [Google Scholar]
- Levin D, Francisco-Ortega J, Jansen R. Hybridization and the extinction of rare plant species. Conservation Biology. 1996;10:10–16. [Google Scholar]
- Looney S, Stanley W. Exploratory repeated measures analysis for two or more groups: review and update. The American Statistician. 1989;43:220–225. [Google Scholar]
- López-Granados F, Lutman P. Effect of environmental conditions on the dormancy and germination of volunteer oilseed rape seed (Brassica napus) Weed Science. 1998;46:419–423. [Google Scholar]
- Lu B, Snow A. Gene flow from genetically modified rice and its environmental consequences. BioScience. 2005;55:669–678. [Google Scholar]
- Mikkelsen T, Andersen B, Jørgensen R. The risk of crop transgene spread. Nature. 1996;380:31. [Google Scholar]
- Pilson D, Prendeville HR. Ecological effects of transgenic crops and the escape of transgenes into wild populations. Annual Review of Ecology, Evolution, and Systematics. 2004;35:149–174. [Google Scholar]
- Rhymer JM, Simberloff D. Extinction by hybridization and introgression. Annual Review of Ecology and Systematics. 1996;27:83–109. [Google Scholar]
- Rieseberg L, Kim S, Randell R, et al. Hybridization and the colonization of novel habitats by annual sunflowers. Genetica. 2007;129:149–165. doi: 10.1007/s10709-006-9011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M-J, Légère A, Warwick S. Transgenic Brassica napus fields and Brassica rapa weeds in Quebec: sympatry and weed-crop in situ hybridization. Botany. 2006;84:1842–1851. [Google Scholar]
- Snedecor G, Cochran W. Statistical methods. 7th edn. Ames, IA: The Iowa State University Press; 1980. [Google Scholar]
- Snow AA, Andersen B, Jørgensen RB. Costs of transgenic herbicide resistance introgressed from Brassica napus into weedy Brassica rapa. Molecular Ecology. 1999;8:605–615. [Google Scholar]
- Staniland BK, McVetty PBE, Friesen LF, Yarrow S, Freyssinet G, Freyssinet M. Effectiveness of border areas in confining the spread of transgenic Brassica napus pollen. Canadian Journal of Plant Science. 2000;80:521–526. [Google Scholar]
- UN. Genome-analysis in Brassica with special reference to the experimental formation of B. napus and peculiar mode of fertilization. Japanese Journal of Botany. 1935;7:389–452. [Google Scholar]
- Warwick S, Légère A, Simard M-J, James T. Do escaped transgenes persist in nature? The case of an herbicide resistance transgene in a weedy Brassica rapa population. Molecular Ecology. 2008;17:1387. doi: 10.1111/j.1365-294X.2007.03567.x. [DOI] [PubMed] [Google Scholar]
- Warwick SI, Simard M-J, Légère A, et al. Hybridization between transgenic Brassica napus L. and its wild relatives: B. rapa L., Raphanus raphanistrum L., Sinapis arvensis L., and Erucastrum gallicum (Willd.) O. E. Schulz. Theoretical and Applied Genetics. 2003;107:528–539. doi: 10.1007/s00122-003-1278-0. [DOI] [PubMed] [Google Scholar]
- Watrud LS, King G, Londo JP, Colasanti R, Smith BM, Waschmann RS, Lee EH. Changes in constructed Brassica communities treated with glyphosate drift. Ecological Applications. 2010 doi: 10.1890/09-2366.1. (in press) [DOI] [PubMed] [Google Scholar]
- Wilkinson MJ, Elliot LJ, Allainguillaume J, et al. Hybridization between Brassica napus and B. rapa on a national scale in the United Kingdom. Science. 2003;302:457–459. doi: 10.1126/science.1088200. [DOI] [PubMed] [Google Scholar]
- Wolf D, Takebayashi N, Rieseberg L. Predicting the risk of extinction through hybridization. Conservation Biology. 2001;15:1039–1053. [Google Scholar]
- Yates WE, Akesson NB, Bayer DE. Drift of glyphosate sprays applied with aerial and ground equipment. Weed Science. 1978;26(6):597–604. [Google Scholar]