Abstract
Background and Aims
The spatial distribution of cytotypes can provide valuable insights into evolutionary patterns of polyploid complexes. In a previous study the macro-scale distribution of the three main cytotypes in Senecio carniolicus (Asteraceae) within the Eastern Alps was characterized. Employing a roughly 12-fold extended sampling, the present study focuses on unravelling patterns of cytotype distribution on the meso- and microscale and on correlating those with ecological properties of the growing sites.
Methods
DAPI flow cytometry of dried samples was used to determine DNA ploidy level in 5033 individuals from 100 populations spread over the entire Eastern Alpine distribution area of S. carniolicus. Descriptors of microhabitats as well as spatial data were recorded in the field, and analysed with a mixed-effects ANOVA.
Key Results
Extensive variation in DNA ploidy levels (2x, 3x, 4x, 5x, 6x, 7x, 8x, 9x) was detected. Of the main cytotypes, diploids and hexaploids were widespread and had strongly overlapping distributions resulting in the frequent occurrence of cytotype mixtures (half of the investigated populations), whereas tetraploids were disjunctly distributed and occurred in the south-west and the east of the species' distribution area. In spite of the frequent co-occurrence of cytotypes, only 1 % of the samples belonged to secondary cytotypes (3x, 5x, 7x, 8x, 9x). Diploids, tetraploids and hexaploids were altitudinally segregated, but with broad overlap. Similarly, highly significant differences in vegetation and rock cover as well as microhabitat exposure were found between the main cytotypes.
Conclusions
Senecio carniolicus shows a remarkable diversity of cytotypes. The distribution of the three main cytotypes (2x, 4x, 6x) has been shaped by Pleistocene glaciations to different extents. Whereas tetraploids are nearly entirely restricted to refugia, hexaploids colonized areas that were extensively glaciated. Diploid and hexaploid individuals often co-occur in mixed populations, where they are spatially and ecologically segregated at both the meso-scale (altitudinal differentiation, exposure of the growing site) and the micro-scale (cover of vegetation and bare rock). With regard to the ecological parameters investigated, the tetraploid cytotype occupies an intermediate position. The rareness of secondary cytotypes suggests the presence of strong pre- or post-zygotic mating barriers.
Keywords: Contact zones, cytotype mixture, Eastern Alps, flow cytometry, habitat segregation, ploidy level, polyploidy, refugia, Senecio carniolicus
INTRODUCTION
Polyploidization, the multiplication of complete chromosome sets, has played a fundamental role in the evolution and diversification of angiosperms (Soltis et al., 2009). Estimates of its frequency have experienced a steady upward trend, although current discussions are more focused on how many rounds of polyploidization various lineages might have undergone rather than on estimating the percentage of polyploid angiosperm species (Soltis et al., 2003). Major radiations in the angiosperm tree of life have been temporally and perhaps causally linked to key polyploidization events (Fawcett et al., 2009; Soltis et al., 2009). Formerly believed to be ‘a hindrance to the evolutionary success of higher plants’ (Stebbins, 1971), polyploidy is now recognized as an important diversifying force in evolutionary history, and one of the important mechanisms of sympatric speciation in land plants (Otto and Whitton, 2000).
Successful long-term establishment of a newly arisen polyploid results in cytotype mixtures, which may either be a transitional state or stable through time. In the latter case, they may form narrow contact zones, eventually comprising only a few populations, as evidenced in Chamaerion angustifolium (Husband and Schemske, 2000), Knautia arvensis (Kolář et al., 2009), Melampodium (Stuessy et al., 2004) and Ranunculus adoneus (Baack, 2004). Cytotype mixtures extending over large areas have been less frequently reported, for example for Galax urceolata (Burton and Husband, 1999) or Solidago altissima (Halverson et al., 2008), and sometimes involved apomixis as in Arnica cordifolia (Kao, 2008). They are expected to be maintained by different means of reproductive isolation that prevent the minority cytotype from losing too many gametes in crosses with the majority cytotype, which eventually would lead to its extirpation (minority cytotype exclusion principle; Levin, 1975; Husband, 2000). Reproductive isolation is often conferred by the interaction of several mechanisms, which reduce or even inhibit gene flow between potentially interbreeding populations (Widmer et al., 2009). Pre-zygotic isolating mechanisms such as adaptation to different pollinators, flowering time divergence, ecological or habitat differentiation, as well as the predominance of selfing or apomixis prevent pollination or fertilization and thus the formation of hybrid zygotes (Petit et al., 1999). In contrast, post-zygotic isolating mechanisms concern the viability and reproductive success of hybrid offspring (Orr and Presgraves, 2000; Husband and Sabara, 2004; Rieseberg and Willis, 2007).
Theoretical considerations and empirical studies suggest that the role of inter-cytotype hybrids in mixed stands of different cytotypes is ambiguous. On the one hand, offspring from inter-cytotype crosses are often sterile, producing non-functional, unbalanced gametes, especially when hybridization results in odd ploidy levels (Levin, 1975). Additionally, backcrossing of polyploid plants with either parent most often leads to non-viable progeny due to endosperm malfunction (Köhler et al., 2010). This is often referred to as ‘triploid block’ (Felber, 1991), although comparable outcomes may also be expected for higher-ploidy level crosses. In this case hybridization weakens both parental lineages and potentially drives the rarer cytotype to extinction (Levin, 1975; Husband, 2000). On the other hand, odd-ploid hybrids may not only produce functional gametes with complete chromosome complements (Felber and Bever, 1997; Burton and Husband, 2001), but are also likely to generate an enhanced number of unreduced gametes. Backcrosses with diploid parental lineages may act as additional sources for the recurrent formation of polyploids (Burton and Husband, 2001). Under these conditions, hybrids may mediate the coexistence and stabilize the equilibrium between co-occurring cytotypes (Felber and Bever, 1997).
A good system for studying various aspects of stable cytotype mixtures and incipient speciation processes is Senecio carniolicus. This common and abundant high mountain species of the Eastern Alps and the Carpathians was long considered to be uniformly hexaploid, but recent investigations (Suda et al., 2007) revealed a complex pattern with three main cytotypes (di-, tetra- and hexaploid). Although a considerable number of the investigated sample sites contained cytotype mixtures, only very few odd-ploid individuals (pentaploids, heptaploids) were found, indicating that there are effective mechanisms preventing the formation of inter-cytotype hybrids. Further support for this hypothesis arises from the absence of tetraploids – the potential hybridogenic offspring – from many sample sites containing mixtures of both diploid and hexaploid individuals including Mt Hoher Sadnig, Austria, where roughly 500 individuals have been investigated (Schönswetter et al., 2007; Hülber et al., 2009). To date, mechanisms maintaining cytotype mixtures in S. carniolicus are not fully understood, but there is evidence for habitat segregation between diploid and hexaploid cytotypes. Whereas hexaploids are linked to communities with denser vegetation, diploids are growing in open, rocky habitats (Hülber et al., 2009), the greater abundance of which at higher altitudes is probably responsible for the previously found altitudinal separation of cytotypes (Schönswetter et al., 2007). As these hypotheses were based on data collected from a single mountain with diploids and hexaploids only, they need to be tested on a wider geographical scale and also including tetraploids, the third main cytotype.
Here, we explore the cytotype distribution of S. carniolicus sensu lato in the Eastern Alps on different spatial scales, i.e. macro-scale (entire Eastern Alps), meso-scale (within each of the 100 investigated single mountains) and micro-scale (immediate environment of each of the roughly 3000 individuals). Employing a moderately increased number of populations and a roughly 12-fold extended sampling of individuals as compared with Suda et al. (2007), we test for spatial segregation on all three spatial scales across the entire Eastern Alpine distribution area also including the tetraploid cytotype. Geographical and environmental descriptors representing the meso-scale position (altitude, geographical coordinates) and the ecological micro-site conditions (e.g. rock and vegetation cover) were recorded to address the following questions: (1) Does the macro-scale pattern of the distribution of the main cytotypes (2x, 4x, 6x) change with an increased number of investigated individuals, i.e. are areas which were previously thought to be cytologically uniform in fact heterogeneous? (2) Can the hypothesis of habitat segregation between diploids and hexaploids on the meso- (altitude) and on the micro-scale (dense vegetation and high rock cover, respectively), as established from an in-depth study on a single mountain (Schönswetter et al., 2007; Hülber et al., 2009), be corroborated on a range-wide scale? If at all, how are tetraploids segregated from diploids and hexaploids? (3) Which secondary cytotypes (3x, 5x, >6x) exist, how frequent are they and does the combination of associated main cytotypes give an indication for their origin (hybridogenic or via unreduced gametes)? The results presented form the basis for future molecular investigations exploring the evolution of the intricate polyploid complex of S. carniolicus.
MATERIAL AND METHODS
Study species
Senecio carniolicus Willd. (Asteraceae) is a common mountain species endemic to the Eastern European Alps and the Carpathians. It is a herbaceous perennial that inhabits a variety of habitats on siliceous bedrock, such as grasslands, dwarf shrub communities, stable screes, moraines, rock crevices and fellfields, ranging from the treeline up to altitudes of 3300 m a.s.l. (Reisigl and Pitschmann, 1958).
Plant material and recording of environmental descriptors
In summer 2008, 100 sampling sites were visited in Switzerland, Italy, Austria and Slovenia, covering the entire distribution of S. carniolicus in the Eastern Alps (Fig. 1, Appendix). The Carpathian distribution was not included because Suda et al. (2007) found exclusively hexaploid populations in that area. Leaves of approx. 30 individuals per sampling site were collected spanning the entire local altitudinal range and habitat types and were dried in silica gel. We aimed to collect samples as representative as possible for the population, i.e. areas with high densities of individuals were sampled more intensively than areas with less frequent occurrences. Additionally, we did not favour specific individuals (flowering or particularly big plants) but chose individuals at random. This strategy combined with our inability to distinguish cytotypes in the field at the time of collecting ensured an unbiased sample. Herbarium specimens for each sampling site were deposited in the herbarium of the University of Vienna (WU).
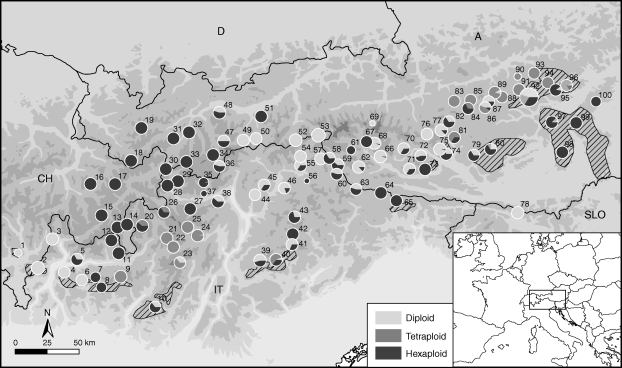
Fig. 1.
Distribution of the three main cytotypes of Senecio carniolicus in the Eastern Alps based on a sampling of 2914 individuals from 100 sample sites (labelled 1–100). The size of each pie chart is proportional to the number of sampled individuals. Hatched areas indicate presumed glacial refugia on siliceous bedrock (modified from Tribsch and Schönswetter, 2003 and Schönswetter et al., 2005). Details of the populations are given in the Appendix.
We recorded environmental parameters describing the micro-site, namely exposure and inclination, as well as percentage coverage of rock (>1 cm) and flowering plants (in the following termed vegetation cover) within a distance of 0·2 m from each sampled individual (in the following referred to as central individuals). Geographical coordinates were recorded with a GPS system, and GPS-corrected barometric calibration was used for altitude. Furthermore, we determined the number of additional S. carniolicus individuals in the same plot (referred to as additional individuals) and sampled – where available – two individuals for ploidy-level estimation.
Flow cytometry
DNA ploidy levels of silica-dried leaf tissue were determined using DAPI flow cytometry as described by Suda et al. (2007), but using Pisum sativum as the sole internal reference standard. Mean fluorescence values of standard and sample never differed more than 3·4-fold, thus being well within the range deemed acceptable by Suda and Leitch (2010). Vicia faba was not suitable for flow cytometric analysis of 9x plants because the similarity in genome size with the standard resulted in overlapping peaks. Pooled samples of three Senecio individuals were usually analysed. In the case of mixed-ploidy samples or low-quality histograms (i.e. coefficients of variation, CVs, of G0/G1 Senecio peaks >5 %, high background or a low number of intact nuclei forming the peaks), each individual was re-analysed separately. Analyses of all minority cytotypes were repeated 2–5 times on different days to minimize potential instrument instability.
Statistical analyses
The following environmental descriptors were used in the analyses: altitude, inclination, exposure, as well as cover of rock, flowering plants and cryptogams. Exposure was determined in degrees (0–360°) and subsequently transformed (‘Northing’, ‘Easting’) to values between 0 (North or East, respectively) and 1 (South or West, respectively). In order to account for different altitudinal ranges of the alpine and subnival zones in different parts of the Alps, relative altitude was used, which is defined as the altitudinal distance from the local tree line (extracted from topographical maps with scales of 1 : 25 000 or 1 : 50 000). Except for altitude, each descriptor was arc-sine transformed to approximate a normal distribution. To test for significant differences among the three main cytotypes, a mixed-effects ANOVA was applied separately to each environmental descriptor. We used linear fixed-effects models computed by the glmer-function included in the lme4 library (Bates and Sarkar, 2008) of R (R Development Core Team, 2008). We used the environmental descriptor as response, the main cytotypes as categorical predictor and the sampling site as grouping variable to calculate random intercepts. With this approach we accounted for potential spatial autocorrelation among individuals from the same sample site. For all models a Gaussian error distribution was assumed. Degrees of freedom of the models were defined as the number of observations minus the number of estimated parameters (i.e. 3) minus 1, assuming that all levels of the grouping variable consume one degree of freedom. Differences in the number of additional individuals among cytotypes were tested using the same model structure, but assuming a Poisson error distribution.
In order to evaluate potential clustering of cytotypes within sampling sites, pairwise Euclidean distances among individuals of each mixed population were calculated using GPS coordinates and altitude. The proportion of pairwise distances between plants of the same cytotype was computed relative to the total number of distances within each of the following distance classes: <10 m, 10–100 m, 100–1000 m and >1000 m. Finally, presence or absence of additional individuals (see above) was used as a criterion to define the lowest distance class (0–0·2 m).
RESULTS
Occurrence, distribution and frequency of cytotypes
DNA ploidy levels were estimated from 5033 plants (2914 central individuals and 2119 additional individuals; Appendix, and Supplementary Data Table S1, available online). Flow cytometric analyses mostly yielded high-resolution histograms, with average sample CV of 3·37 % (range 1·08–7·71 %) and average standard CV of 2·63 % (range 1·03–6·46 %). The arbitrary threshold of 5·0 % was achieved in 92·6 and 98·0 % of sample and standard runs, respectively. Only small between-day variation (4·1 % maximum) was observed when the same sample was re-analysed. Collectively, these measures of quality indicate that the recorded fluorescence values are stable and allow a reliable estimation of DNA ploidy levels.
Eight distinct groups of fluorescence intensities were obtained (see Supplementary Data Table S2, Figs S2 and S3). Most samples matched the fluorescence values reported by Suda et al. (2007) for karyologically counted diploid (2n = 2x = 40), tetraploid (2n = 4x = 80) and hexaploid (2n = 6x = 120) individuals. Five additional groups corresponded to DNA-pentaploids, DNA-heptaploids and previously unknown DNA-triploids, putative DNA-octoploids, and DNA-enneaploids. The relative monoploid genome size varied only weakly among the ploidy levels (see Supplementary Data Table S2). Slightly lower fluorescence values per monoploid genome observed in DNA-octoploids together with their rarity (only two individuals found in one population) leave room for some uncertainty in the inferred ploidy. Most plants belonged to DNA-hexaploids (49·6 %), whereas DNA-diploids and DNA-tetraploids made up 33·8 and 15·6 % of the samples, respectively. Secondary cytotypes were found in 27 sample sites: six DNA-triploids, 37 DNA-pentaploids, four DNA-heptaploids, two DNA-octoploids and five DNA-enneaploids, totalling 54 individuals (1·07 %). For simplicity, we will refer to the different cytotypes from here on without using the prefix ‘DNA-’.
The distribution of the three main cytotypes of S. carniolicus is complex, showing some degree of large-scale spatial segregation with large areas of overlap and hence frequent cytotype mixture (Fig. 1). Di- and hexaploid individuals are widespread throughout most of the distribution range. Hexaploids are absent from the western-most Hohe Tauern and from the south-western and south-eastern distribution margin around Lago di Como and in the Karawanken/Karavanke, respectively, where only diploids occur. The Central Alps around the upper Inn valley are exclusively populated by hexaploids and correspond to a large break in the distribution of diploids. Tetraploids were found in two disjunct areas, whose centres are located in the Niedere Tauern in the East and in the Ortler and Adamello massifs in the west. Roughly half of the sample sites (44 %) contained more than one main cytotype. All possible combinations of cytotypes were found with 2x/6x mixtures being most frequent (28 % of sample sites), whereas 2x/4x and 4x/6x sample sites were rare (3 and 5 %, respectively). In 8 % of the sample sites, all three main cytotypes occurred. Spatial co-occurrence patterns of cytotypes within selected mixed populations are shown in the Supplementary Data (Fig. S1).
Many sampled individuals, irrespective of ploidy level, had no conspecific neighbours within a 0·2-m radius, but in sample plots with open habitats or vegetation gaps numerous additional individuals were sometimes encountered. The number of additional individuals did not differ (P = 0·371) between tetraploids and hexaploids (both with median 0 additional individuals), whereas diploids had significantly more neighbours (1 individual as median) compared with other main cytotypes (P < 0·001 for both).
Secondary cytotypes were scattered over the distribution area both in mixed and in otherwise pure sample sites. Their occurrence depended on the composition of main cytotypes within sample sites (Table 1). Among secondary cytotypes, pentaploids were most frequent (37 individuals in 14 populations) and occurred with the exception of a single population (PLE; for details see Appendix) exclusively in populations comprising tetraploids. More than one-quarter of all pentaploids were found in otherwise purely tetraploid populations. Triploids were considerably rarer (six individuals in four populations) and were only found in sample sites where diploid individuals were present, either in pure diploid populations or in mixed populations comprising all three main cytotypes. Hepta-, octo- and enneaploid plants were exclusively found in association with hexaploids.
Table 1.
Abundance of secondary cytotype individuals of Senecio carniolicus in relation to the presence of di-, tetra- and hexaploid individuals within sampling sites
| No. of secondary cytotype individuals (no. of sampling sites) |
|||||||
|---|---|---|---|---|---|---|---|
| Main cytotypes within sampling sites | No. of sampling sites | No. of individuals | Triploid | Pentaploid | Heptaploid | Octoploid | Enneaploid |
| Diploid | 15 | 711 | 3 (3) | ||||
| Di-, tetraploid | 3 | 162 | 4 (1) | ||||
| Di-, tetra-, hexaploid | 8 | 467 | 3 (1) | 8 (4) | 1 (1) | ||
| Di-, hexaploid | 29 | 1595 | 3 (1) | 1 (1) | 2 (1) | 2 (2) | |
| Tetraploid | 10 | 403 | 10 (5) | ||||
| Tetra-, hexaploid | 5 | 229 | 12 (3) | ||||
| Hexaploid | 30 | 1466 | 2 (2) | 3 (3) | |||
| Total | 100 | 5033 | 6 (4) | 37 (14) | 4 (4) | 2 (1) | 5 (5) |
Close relationships of each secondary cytotype to a single main cytotype were also encountered on the micro-scale. Within a distance of 0·2 m, triploids were exclusively associated with diploids (three individuals) and pentaploids grew close to themselves (ten individuals) and/or to tetraploids (20 individuals) with the exception of a single individual located close to a diploid. Similarly, hepta-, octo- or enneaploids occurred next to individuals with a ploidy level equal to or higher than hexaploid (seven individuals).
Ecological differentiation among cytotypes
Cytotypes were spatially clustered within mixed sampling sites. While 99·98 % of all pairwise distances <0·2 m involved individuals of the same ploidy level (n = 2088 comparisons), this proportion decreased to 93·6 % (n = 513) in the distance class <10 m, 78·5 % (n = 2401) for 10–100 m, 58·3 % (n = 12 358) for 100–1000 m and 37·0 % (n = 4879) for distances >1000 m.
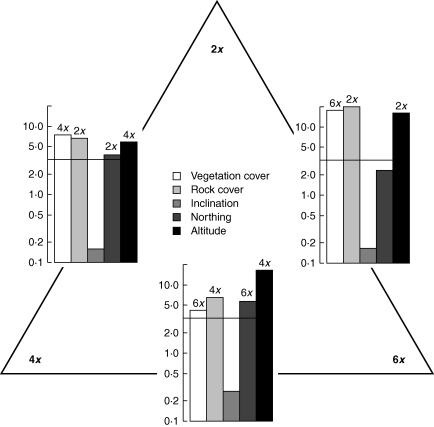
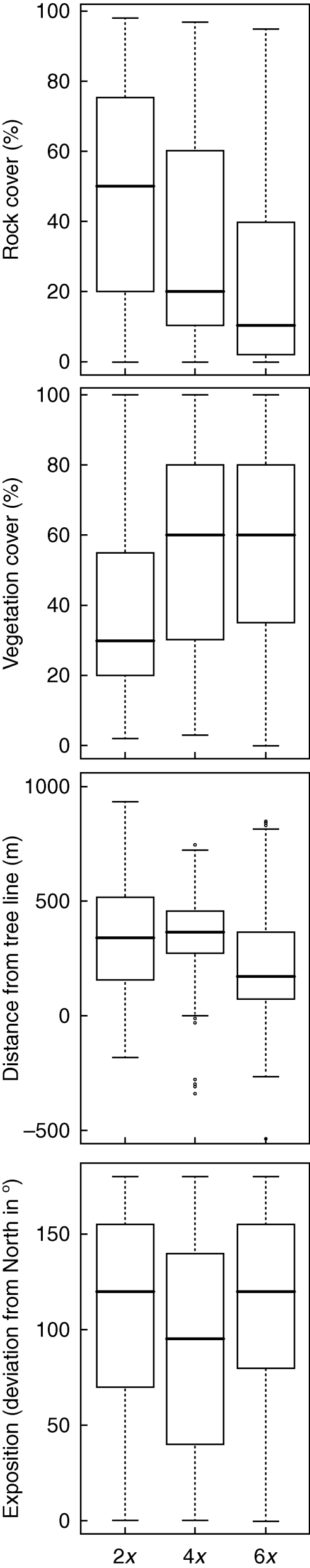
Pairwise comparisons among the three main cytotypes revealed highly significant differences for most of the environmental descriptors investigated (Fig. 2). Diploids, tetraploids and hexaploids differed significantly in vegetation and rock cover as well as in the relative altitude (P < 0·001 in all cases) while northing discriminated between tetraploids and the other cytotypes but not between diploids and hexaploids. Local inclination, cryptogam cover and East/West exposure had no significant explanatory value. Cover of rock and of vegetation displayed an inverse relationship and revealed largely clear differences between diploid and hexaploid individuals (Fig. 3). Most diploids occupied rocky micro-sites with low vegetation cover, whereas hexaploids were generally associated with high vegetation cover. Tetraploids displayed an intermediate behaviour with regard to rock cover, while vegetation cover was similar to those of hexaploids. Both diploids and hexaploids covered a large altitudinal range with hexaploids centred at lower altitudes than diploids which ascended to the highest relative altitudes sampled. Tetraploids showed a narrower vertical distribution but highest average values. Furthermore, tetraploids were more frequent on northerly exposed slopes, whereas di- and hexaploids appeared to be more indifferent with respect to exposure.
Fig. 2.
Pairwise comparisons of five environmental descriptors among the three main cytotypes of Senecio carniolicus. Bars represent the absolute value of the t-statistic derived from linear mixed-effects models as a measure of the difference between the cytotypes. Black lines correspond to a significance level of P = 0·001. Above the bars the cytotype with the significantly greater values of the descriptor is given.
Fig. 3.
Comparison of the main cytotypes of Senecio carniolicus with respect to rock cover, vegetation cover, altitudinal distance to the local tree line and north/south exposure of the microhabitat. Boxes span the range between the 25th and 75th percentile with indicated median, and whiskers extend to 1·5-fold the interquartile range. Outliers are represented by open circles.
DISCUSSION
Diploids, tetraploids and hexaploids, the main cytotypes encountered in Senecio carniolicus, are partially segregated on the macro-scale, i.e. throughout the whole Alpine range, but overlap strongly. The resulting frequent occurrence of cytotype mixtures enables us to explore patterns and mechanisms of spatial and ecological segregation within roughly half of the 100 investigated sample sites (meso-scale) as well as with respect to the immediate vicinity of about 3000 investigated individuals (micro-scale).
The macro-scale: cytogeography across the Eastern Alps
The distribution pattern of diploid, tetraploid and hexaploid cytotypes throughout the Eastern Alps was found to be remarkably complex (Suda et al., 2007). The approximately 12-fold increase of overall sample size here compared with the previous study resulted only in minor changes in the large-scale pattern of cytotype distribution (e.g. in the Niedere Tauern, thought to be dominated by tetraploids, diploids are widespread, but occur nearly exclusively south of the main chain), but the proportion of mixed sample sites, mostly di- and hexaploids, rose from about one-third to nearly a half (Fig. 1). Range disruption and survival of at least the last cold stage of the Pleistocene in disjoint refugia appear to have played an important role in shaping the current cytotype distribution. Areas previously suggested to be glacial refugia based on molecular data as well as on species distribution patterns (Tribsch and Schönswetter, 2003; Schönswetter et al., 2005) match regions with high main cytotype diversity. Additionally, the two main regions comprising only diploids (populations 1–4 and 6 from the south-western part and population 78 from Karawanken/Karavanke; Fig. 1) overlap with presumed refugia, a pattern in line with early concepts of presumably relictual diploids being mostly found in unglaciated areas (Ehrendorfer, 1958). The seemingly contradictory exclusive occurrence of hexaploids in the only weakly glaciated (van Husen, 1987) refugial area of the easternmost margin of the Alpine distribution (populations 98–100) fits well with the early recognized poverty of its alpine flora (Scharfetter, 1909) and is probably related to the current scarcity of suitable habitats for the high-altitude adapted (see below) diploid and tetraploid cytotypes, which might have become extirpated due to habitat loss as vegetation zones ascended after the last glaciation. Furthermore, both disjoint distribution areas of the tetraploid cytotype, separated by a gap of >180 km, correspond to putative glacial refugia (Schönswetter et al., 2003, 2005; Tribsch and Schönswetter, 2003) and are characterized by a large number of rare or endemic plant taxa (Schneeweiss and Schönswetter, 1999; Tribsch, 2004). Consequently, the distribution pattern of this cytotype was probably shaped by extensive habitat loss during cold stages of the Pleistocene and weak colonization ability after the last glacial period. This contradicts widespread assumptions about colonizing and competitive success of polyploids as compared with diploids (Stebbins, 1984, 1985; Otto and Whitton, 2000; Otto et al., 2007). It is unclear which traits of the tetraploids are limiting their success in dispersal and population establishment. Ongoing experiments suggest delayed germination and lower seedling viability of tetraploids (M. Sonnleitner and R. Flatscher, unpubl. res.), which may at least in part be responsible for their weaker colonizing ability. In contrast to tetraploids, the hexaploid cytotype is the most widespread and the one exclusively found in the north-western part of the distribution range, which was the most intensively glaciated area of the Eastern Alps during the Last Glacial Maximum (van Husen, 1987), in agreement with the above-mentioned hypotheses of polyploids being superior colonizers of novel habitats as compared with diploids. Underlying factors include greater plasticity and adaptation potential due to gene redundancy and the massive genomic restructuring processes triggered by whole-genome duplication (reviewed by Otto and Whitton, 2000; Wendel, 2000; Hegarty and Hiscock, 2008; Leitch and Leitch, 2008), but the precise nature of the factors responsible for the higher colonization ability of the hexaploids, such as better dispersal capabilities or higher competitiveness, remains to be established.
Segregation on meso- and microscales
The high frequency of mixed-cytotype populations offers the possibility to test for spatial patterns on smaller geographical scales. Previous studies within a single model population in the Austrian Alps have provided evidence for spatial segregation of diploid and hexaploid cytotypes on meso- as well as microscales (Schönswetter et al., 2007; Hülber et al., 2009). The present study allows a generalization of these patterns for the entire Eastern Alps and provides a first characterization of the habitat requirements of the tetraploid cytotype.
Individuals sharing the same cytotype are spatially strongly aggregated in mixed sampling sites. Whereas virtually no cytotype mixture was encountered within a radius of 0·2 m, variation increased with distance and in the distance class of >1000 m only slightly more than one-third of all pairwise distances involved individuals of the same ploidy level. This pattern may be explained by a correlation between spatial proximity and microhabitat similarity, i.e. neighbouring individuals are more likely to share a similar environment than more distant individuals. Alternatively, clustering of individuals may be caused by dispersal limitation. Although the fruits of S. carniolicus are anemochorous, dense flocks of seedlings around adult plants are frequently observed in the field (M. Sonnleitner and R. Flatscher, pers. obs.). This is in line with dispersal kernels of plant diaspores usually reaching their greatest density in the immediate surroundings of the mother plant (Nathan, 2006).
Di- and hexaploid cytotypes of S. carniolicus were suggested to be adapted to open, rocky microhabitats and to dense grass swards or dwarf shrub communities, respectively (Schönswetter et al., 2007; Hülber et al., 2009). Our results confirm this as a general pattern over the entire distribution area (Fig. 2). The low-growing diploids are mainly found in rocky habitats with sparse and prostrate vegetation or single grass tussocks and in open, heavily cryoperturbed fellfields (as described by Ellenberg, 1996). In contrast, the larger and potentially more competitive hexaploids populate habitats characterized by high vegetation and low rock cover. These habitat preferences correlate with distinct characteristics of the two cytotypes, especially differences in plant height and leaf length (R. Flatscher et al., unpubl. res.). Our results are in line with other case studies reporting higher productivity and competition ability of polyploids as compared with their diploid progenitors (Lumaret et al., 1987; Lindner and Garcia, 1997; Petit and Thompson, 1997). Consequently, polyploids were often shown to inhabit more nutrient-rich communities with dense vegetation, whereas diploids tend towards more open habitats (Ståhlberg, 2009) where competition for essential resources is reduced and abiotic stress becomes the limiting factor.
Diploid and hexaploid cytotypes are centred at different altitudes, albeit with considerable overlap (Figs 2 and 3) and, in accordance with previous findings (Schönswetter et al., 2007), diploids generally ascend higher than hexaploids (see next paragraph for a discussion on the tetraploids). The observed altitudinal differentiation could either be a direct consequence of altitude and related changes in abiotic parameters such as lower temperatures, lower CO2 partial pressure, higher irradiation and UV stress (Körner, 2003). Alternatively, it may reflect that, as a general rule, the extent of open habitats with sparse vegetation increases with altitude while dense grasslands and dwarf shrub vegetation decline (Körner, 2003). This correlation probably reconciles the two competing hypothesis for segregation of di- and hexaploid cytotypes of S. carniolicus, i.e. altitude (Schönswetter et al., 2007) and openness of the vegetation (Hülber et al., 2009).
Definition of the ecological niche of the tetraploids is not straightforward. Their altitudinal amplitude was narrower and significantly higher than those of the other two cytotypes (Figs 2 and 3). With regard to rock and to vegetation cover, tetraploid S. carniolicus occupies an intermediate position between di- and hexaploids. Vegetation cover is similarly high, but still statistically significantly smaller than that found in hexaploids, and rock cover is significantly lower than in diploids (Figs 2 and 3). However, tetraploids are more frequent on or sometimes even restricted to north-facing slopes, whereas di- and hexaploids seemed to be more indifferent towards exposure direction (Figs 2 and 3). Furthermore, tetraploids have a tendency towards slightly base-rich soils (P. Escobar García et al., pers. obs.) whereas diploids and hexaploids are strictly acidophilic. The single exception is the disjunct diploid population 78 in the Karawanken/Karavanke range, where S. carniolicus grows on dolomite bedrock, which may be a product of local adaptation processes facilitated by its isolated position.
Secondary cytotypes: occurrence and possible evolutionary implications
Secondary cytotypes, i.e. tri-, penta-, hepta-, octo- and enneaploids, were encountered in low frequencies only (approx. 1 % altogether). This number is comparable with previous results of Suda et al. (2007) but based here on a more than 12-fold increased sample size; tri-, octo- and enneaploids are reported here for the first time for S. carniolicus. It should be noted that ploidy levels in our study were inferred from nuclear DNA amounts (DNA ploidy levels; Suda et al., 2006) only because the rarity of secondary cytotypes in the field prevents their cytological investigation. S. carniolicus represents the most salient example of high intraspecific ploidy variation (occurrence of eight DNA ploidy levels) ever recorded in a sexually reproducing angiosperm with monocentric chromosomes. Disregarding aneuploid series (e.g. Lewis et al., 1967; Murray and Young, 2001), no more than six different euploid chromosomal levels have previously been observed for the same species (Marhold et al., 2010). Note, however, that the number of cytotypes found in a taxon is of course dependent on the adopted taxonomic framework. Future potential recognition of several taxa within S. carniolicus will reduce the number of intraspecific cytotypes. Regardless, the present data clearly demonstrate that ploidy diversity in natural populations is much higher than originally believed and that representative cytotype screening (most conveniently done using flow cytometry) can reshape our perception of the magnitude and dynamics of genome duplication in the wild.
Crosses involving unreduced gametes and hybridization between different cytotypes are commonly put forward as mechanisms to explain the formation of secondary cytotypes (Ramsey and Schemske, 1998). As in other groups (Ramsey, 2007), unreduced gametes are rarely formed in S. carniolicus (up to 0·6 % as judged from pollen diameter; Fössinger, 2010). Nevertheless, a significant role of unreduced gametes in the origin of secondary cytotypes is suggested by (1) the tight association of secondary cytotypes with their putative parental main cytotypes (3x with 2x; 5x with 4x; >6x with 6x) on the micro-scale (see Results), (2) the existence of 7x, 8x and 9x cytotypes as well as (3) the occurrence of triploids in geographically isolated purely diploid populations (populations 6, 49, 76) and of pentaploids in purely tetraploid populations (ten of 27 populations). The latter two cases may, however, also be the result of hybridization, involving a higher-ploid parent not sampled, no longer existing or growing on a different mountain massif (i.e. long-distance pollen transfer). Further data will be necessary to establish the relative contributions of these mechanisms to the origin of secondary cytotypes in S. carniolicus.
CONCLUSIONS AND OUTLOOK
Even if in S. carniolicus the 12-fold increase in the number of investigated individuals does not significantly affect the cytogeographical pattern described previously (Suda et al., 2007), only large data sets such as that presented here with a sampling scheme which is both intensive (many plants per site) and extensive (many sites throughout the whole distribution area; Halverson et al., 2008) allow for a synthetic approach addressing questions about polyploid origin, their morphological, ecological or reproductive differentiation as well as the mechanisms of polyploid speciation.
We have shown that the three main cytotypes of S. carniolicus (2x, 4x, 6x) exhibit a complex distribution pattern with an unusually high proportion of contact areas with varying spatial extent. As causes for this distribution pattern we suggest historical processes connected to Pleistocene climatic fluctuations on the macro-scale and ecological divergence on the meso- and micro-scales. As habitat segregation does not prevent the occurrence of different cytotypes in close spatial proximity, other pre- or post-zygotic isolating mechanisms need to be invoked to explain the obviously strong reproductive isolation. These are the subject of current ongoing research that combines molecular data, to enable the reconstruction of spatio-temporal evolutionary diversification of S. carniolicus, with experimental approaches, to explore the mechanisms maintaining cytotype mixtures.
SUPPLEMENTARY DATA
Supplementary Data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: Relative fluorescence intensities of DAPI-stained nuclei of 5033 silica gel-dried leaf samples of Senecio carniolicus from 100 sample localities. Table S2: Mean relative fluorescence intensities (per monoploid genome) of individual ploidy levels of Senecio carniolicus in the present study and in previous work (Suda et al., 2007). Fig. S1: Spatial distribution of individuals of Senecio carniolicus within selected mixed populations. Fig. S2: Mean relative fluorescence intensities of DNA-diploid, DNA-triploid, DNA-tetraploid, DNA-pentaploid and DNA-hexaploid samples of Senecio carniolicus. Fig. S3: Mean relative fluorescence intensities of DNA-hexaploid, DNA-heptaploid, DNA-octoploid and DNA-enneaploid samples of Senecio carniolicus.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Austrian Science Fund (P20736-B16 to P.S.). Flow cytometric analyses were supported by long-term research plans no. AV0Z60050516 (from the Academy of Sciences of the Czech Republic) and no. MSM0021620828 (from the Ministry of Education, Youth and Sports of the Czech Republic). The following authorities issued collecting permits: Austria: Land Steiermark (FA13C-53S7/65-2008), Land Salzburg (30503/253-1017/4-2008), Land Kärnten (15-NAT-805/2-2008), Land Vorarlberg (IVe-116·00); Italy: Autonome Provinz Bozen – Südtirol/Provincia Autonoma di Bolzano – Alto Adige (63·01·05/298143); Switzerland: Ufficio della natura e del paesaggio – Cantone Ticino (s.n., dated 26 March, 2008), Amt für Natur und Umwelt – Kanton Graubünden (La 7·73/5807). We thank Hanna Weiss-Schneeweiss for helpful discussions, and Daniela Stawik, Christian Gilli, Bozo Frajman and Manfred Schmucker for help with fieldwork.
APPENDIX
Overview of the 100 sample sites where material of Senecio carniolicus was collected for the present study. Geographical position (centroid of the individual coordinates) and altitudinal range of sampled individuals are given as well as DNA ploidy levels with the number of individuals per ploidy level (n) and the total number (N) of individuals investigated per sample site. Herbarium vouchers stored in WU are labelled with the population code. Country abbreviations: A, Austria; CH, Switzerland; IT, Italy; SLO, Slovenia.
| Geographical position |
n samples per DNA ploidy level |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Code | Locality (Country) | Latitude (°N) | Longitude (°E) | Altitudinal range (m a.s.l.) | 2x | 3x | 4x | 5x | 6x | 7x | 8x | 9x | N |
| 1 | Cima dell Uomo (CH) | 46·231 | 8·940 | 2249–2358 | 16 | 16 | |||||||
| 2 | Pizzo di Gino (IT) | 46·123 | 9·144 | 1864–2226 | 63 | 63 | |||||||
| 3 | Campanile di Val Marina (IT) | 46·333 | 9·294 | 2134–2495 | 49 | 49 | |||||||
| 4 | Monte Legnone (IT) | 46·093 | 9·417 | 2122–2591 | 47 | 47 | |||||||
| 5 | Passo Locino (IT) | 46·188 | 9·549 | 2110–2553 | 19 | 32 | 51 | ||||||
| 6 | Monte Verrobbio (IT) | 46·039 | 9·602 | 2044–2083 | 41 | 1 | 42 | ||||||
| 7 | Cima Cadelle (IT) | 46·059 | 9·743 | 2112–2482 | 43 | 43 | |||||||
| 8 | Laghi Gemelli (IT) | 45·986 | 9·805 | 2142–2330 | 21 | 21 | |||||||
| 9 | Pizzo di Coca (IT) | 46·064 | 10·003 | 2174–2724 | 52 | 1 | 53 | ||||||
| 10 | Monte Colombine (IT) | 45·845 | 10·359 | 2095–2212 | 22 | 39 | 61 | ||||||
| 11 | Bocchetta Vicima (IT) | 46·229 | 9·968 | 2242–2689 | 61 | 61 | |||||||
| 12 | Bocchetta della Forbici (IT) | 46·326 | 9·904 | 2175–2673 | 65 | 65 | |||||||
| 13 | Diavolezza, Munt Pers (CH) | 46·420 | 9·971 | 2127–3162 | 66 | 66 | |||||||
| 14 | Monte Vago (IT) | 46·439 | 10·070 | 2313–3055 | 71 | 1 | 72 | ||||||
| 15 | Piz Nair (CH) | 46·507 | 9·804 | 2355–2991 | 64 | 64 | |||||||
| 16 | Sandhubel (CH) | 46·732 | 9·687 | 2346–2697 | 68 | 68 | |||||||
| 17 | Flüela Schwarzhorn (CH) | 46·733 | 9·650 | 2347–3093 | 52 | 52 | |||||||
| 18 | Hohes Rad (AT) | 46·899 | 10·112 | 2120–2910 | 52 | 52 | |||||||
| 19 | Arlensattel (AT) | 47·137 | 10·221 | 2073–2162 | 60 | 60 | |||||||
| 20 | Monte Verva (IT) | 46·431 | 10·228 | 2098–2967 | 18 | 5 | 37 | 60 | |||||
| 21 | Monte di Gavia (IT) | 46·342 | 10·483 | 2404–3077 | 41 | 1 | 42 | ||||||
| 22 | Monte Serodine (IT) | 46·276 | 10·550 | 2476–2629 | 52 | 52 | |||||||
| 23 | Cresta del Belvedere (IT) | 46·164 | 10·615 | 2305–2581 | 14 | 29 | 43 | ||||||
| 24 | Cima Valletta (IT) | 46·356 | 10·803 | 2260–2821 | 1 | 58 | 4 | 63 | |||||
| 25 | Cima Cavaion (IT) | 46·420 | 10·701 | 2386–2826 | 56 | 1 | 57 | ||||||
| 26 | Stilfser Joch (IT) | 46·528 | 10·453 | 2539–3078 | 14 | 41 | 55 | ||||||
| 27 | Laaser Spitze (IT) | 46·550 | 10·729 | 2272–2850 | 59 | 59 | |||||||
| 28 | Watles (IT) | 46·719 | 10·494 | 2161–2551 | 60 | 60 | |||||||
| 29 | Steinmandlköpfl, Mittereck (IT) | 46·749 | 10·598 | 2347–2864 | 53 | 53 | |||||||
| 30 | Piz Lad (IT) | 46·840 | 10·475 | 2227–2740 | 67 | 1 | 1 | 69 | |||||
| 31 | Fisser Joch, Brunnenkopf (AT) | 47·061 | 10·562 | 2520–2670 | 53 | 1 | 54 | ||||||
| 32 | Hohe Aifnerspitze (AT) | 47·103 | 10·724 | 2265–2705 | 44 | 44 | |||||||
| 33 | Riffljoch (AT) | 46·891 | 10·694 | 2380–2950 | 54 | 54 | |||||||
| 34 | Gaislachkogel (AT) | 46·937 | 10·976 | 2290–2955 | 51 | 1 | 52 | ||||||
| 35 | Schröfwand (IT) | 46·739 | 10·874 | 2398–2730 | 2 | 42 | 44 | ||||||
| 36 | Festkogel (AT) | 46·855 | 11·041 | 2170–3040 | 25 | 24 | 49 | ||||||
| 37 | Vermoispitze (IT) | 46·659 | 10·875 | 2486–2875 | 9 | 9 | |||||||
| 38 | Naturnser Hochwart (IT) | 46·601 | 11·022 | 2201–2605 | 21 | 44 | 2 | 1 | 68 | ||||
| 39 | Monte Ziolera (IT) | 46·170 | 11·450 | 2035–2747 | 40 | 28 | 68 | ||||||
| 40 | Cima D'Asta (IT) | 46·172 | 11·616 | 1969–2847 | 4 | 42 | 2 | 22 | 70 | ||||
| 41 | Cavallazza Piccola (IT) | 46·282 | 11·782 | 2020–2316 | 26 | 19 | 45 | ||||||
| 42 | Col Margherita (IT) | 46·356 | 11·799 | 2091–2514 | 63 | 63 | |||||||
| 43 | Pre de Ciapel (IT) | 46·476 | 11·818 | 2359–2553 | 16 | 46 | 1 | 63 | |||||
| 44 | Sarner Scharte (IT) | 46·647 | 11·410 | 2035–2435 | 47 | 47 | |||||||
| 45 | Schrotthorn (IT) | 46·715 | 11·507 | 2252–2589 | 46 | 20 | 66 | ||||||
| 46 | Plose (IT) | 46·690 | 11·720 | 2134–2408 | 54 | 15 | 69 | ||||||
| 47 | Schrankogel (AT) | 47·035 | 11·091 | 2190–3135 | 26 | 31 | 57 | ||||||
| 48 | Rietzer Grießkogel (AT) | 47·242 | 11·052 | 2230–2795 | 24 | 35 | 59 | ||||||
| 49 | Habicht (AT) | 47·041 | 11·299 | 2600–2740 | 49 | 1 | 50 | ||||||
| 50 | Nößlachjoch, Eggersteller (AT) | 47·052 | 11·434 | 2180–2270 | 49 | 49 | |||||||
| 51 | Patscherkofel, Viggarspitze (AT) | 47·212 | 11·492 | 2000–2295 | 51 | 51 | |||||||
| 52 | Saurüssel (AT) | 47·033 | 11·839 | 2520–2885 | 63 | 63 | |||||||
| 53 | Rauchkofel (IT) | 47·069 | 12·090 | 2247–2797 | 68 | 68 | |||||||
| 54 | Speikboden (IT) | 46·915 | 11·896 | 2204–2513 | 53 | 53 | |||||||
| 55 | Sambock (IT) | 46·851 | 11·894 | 2175–2396 | 52 | 19 | 1 | 72 | |||||
| 56 | Kronplatz (IT) | 46·737 | 11·957 | 2255–2281 | 14 | 14 | |||||||
| 57 | Antholzer Scharte (IT) | 46·892 | 12·109 | 1970–2600 | 59 | 59 | |||||||
| 58 | Almerhorn (AT) | 46·896 | 12·198 | 2070–2810 | 24 | 38 | 62 | ||||||
| 59 | Riepenspitz (IT) | 46·846 | 12·288 | 2114–2771 | 19 | 41 | 60 | ||||||
| 60 | Toblacher Pfannhorn (IT) | 46·779 | 12·276 | 2147–2661 | 31 | 27 | 58 | ||||||
| 61 | Donnerstein (AT) | 46·948 | 12·431 | 2675–2685 | 22 | 22 | |||||||
| 62 | Gölbner (AT) | 46·826 | 12·505 | 2408–2946 | 44 | 6 | 50 | ||||||
| 63 | Col Quaternà (IT) | 46·665 | 12·471 | 2028–2506 | 32 | 30 | 62 | ||||||
| 64 | Monte Peralba (IT) | 46·633 | 12·729 | 2279–2383 | 74 | 74 | |||||||
| 65 | Monte Crostis (IT) | 46·571 | 12·888 | 2028–2283 | 53 | 53 | |||||||
| 66 | Schleinitz (AT) | 46·896 | 12·730 | 2193–2698 | 43 | 2 | 45 | ||||||
| 67 | Kalser Höhe (AT) | 47·006 | 12·599 | 2219–2363 | 33 | 33 | |||||||
| 68 | Schönleitenspitze (AT) | 46·992 | 12·680 | 2174–2759 | 32 | 4 | 36 | ||||||
| 69 | Kapruner Törl (AT) | 47·137 | 12·668 | 2346–2632 | 6 | 6 | |||||||
| 70 | Sadnig (AT) | 46·945 | 12·993 | 2202–2744 | 31 | 23 | 54 | ||||||
| 71 | Scharnik (AT) | 46·793 | 13·041 | 2418–2656 | 36 | 13 | 49 | ||||||
| 72 | Polinik (AT) | 46·889 | 13·167 | 1986–2767 | 35 | 24 | 59 | ||||||
| 73 | Dolzer, Gaugen (AT) | 46·789 | 13·203 | 2090–2195 | 50 | 50 | |||||||
| 74 | Gmeineck (AT) | 46·891 | 13·428 | 1419–2575 | 12 | 29 | 41 | ||||||
| 75 | Reiβeck (AT) | 46·932 | 13·364 | 2516–2743 | 28 | 2 | 30 | ||||||
| 76 | Ankogel (AT) | 47·045 | 13·238 | 2615–2868 | 45 | 1 | 46 | ||||||
| 77 | Großer Hafner (AT) | 47·066 | 13·393 | 2280–3007 | 29 | 3 | 32 | ||||||
| 78 | Belščica (AT / SLO) | 46·443 | 14·147 | 2000–2050 | 53 | 53 | |||||||
| 79 | Rosennock (AT) | 46·874 | 13·721 | 1879–2424 | 18 | 1 | 1 | 27 | 47 | ||||
| 80 | Bretthöhe (AT) | 46·910 | 13·902 | 1997–2312 | 12 | 5 | 21 | 1 | 39 | ||||
| 81 | Wandspitze (AT) | 47·010 | 13·526 | 2066–2605 | 17 | 3 | 8 | 28 | |||||
| 82 | Balonspitze (AT) | 47·123 | 13·480 | 2219–2828 | 17 | 36 | 53 | ||||||
| 83 | Seekarspitze (AT) | 47·272 | 13·544 | 2196–2410 | 39 | 39 | |||||||
| 84 | Zechnerkarspitze (AT) | 47·215 | 13·690 | 2120–2421 | 12 | 4 | 17 | 33 | |||||
| 85 | Trockenbrotscharte (AT) | 47·273 | 13·720 | 1919–2379 | 38 | 3 | 41 | ||||||
| 86 | Preber (AT) | 47·215 | 13·868 | 2293–2733 | 27 | 20 | 3 | 50 | |||||
| 87 | Predigstuhl (AT) | 47·257 | 13·912 | 2081–2540 | 30 | 14 | 6 | 50 | |||||
| 88 | Deneck (AT) | 47·284 | 14·053 | 2044–2426 | 36 | 36 | |||||||
| 89 | Großer Knallstein (AT) | 47·318 | 13·978 | 2193–2598 | 8 | 48 | 56 | ||||||
| 90 | Hochrettelstein (AT) | 47·424 | 14·232 | 2120–2227 | 12 | 12 | |||||||
| 91 | Hohenwart (AT) | 47·328 | 14·237 | 1875–2374 | 43 | 43 | |||||||
| 92 | Schießeck (AT) | 47·272 | 14·337 | 1997–2294 | 64 | 3 | 21 | 1 | 32 | 121 | |||
| 93 | Großer Bösenstein (AT) | 47·441 | 14·408 | 1953–2406 | 38 | 2 | 40 | ||||||
| 94 | Gamskogel (AT) | 47·366 | 14·546 | 1929–2329 | 38 | 3 | 41 | ||||||
| 95 | Großer Ringkogel (AT) | 47·311 | 14·628 | 1943–2277 | 16 | 3 | 40 | 59 | |||||
| 96 | Seckauer Zinken (AT) | 47·337 | 14·740 | 1903–2339 | 6 | 40 | 4 | 6 | 56 | ||||
| 97 | Zirbitzkogel (AT) | 47·080 | 14·565 | 1848–2223 | 4 | 2 | 28 | 34 | |||||
| 98 | Saualpe (AT) | 46·856 | 14·654 | 1948–2115 | 30 | 30 | |||||||
| 99 | Ameringkogel (AT) | 47·062 | 14·824 | 1891–2183 | 44 | 44 | |||||||
| 100 | Roβbachkogel (AT) | 47·207 | 15·045 | 1763–1877 | 27 | 27 | |||||||
LITERATURE CITED
- Baack EJ. Cytotype segregation on regional and microgeographic scales in snow buttercups (Ranunculus adoneus: Ranunculaceae) American Journal of Botany. 2004;91:1783–1788. doi: 10.3732/ajb.91.11.1783. [DOI] [PubMed] [Google Scholar]
- Bates D, Sarkar D. lme4: Linear mixed-effects models using S4 classes. 2008 http://cran.r-project.org/web/packages/lme4/index.html , R package version 0.999375-28 (accessed 16 March 2010) [Google Scholar]
- Burton TL, Husband BC. Population cytotype structure in the polyploid Galax urceolata (Diapensiaceae) Heredity. 1999;82:381–390. doi: 10.1038/sj.hdy.6884910. [DOI] [PubMed] [Google Scholar]
- Burton TL, Husband BC. Fecundity and offspring ploidy in matings among diploid, triploid and tetraploid Chamerion angustifolium (Onagraceae): consequences for tetraploid establishment. Heredity. 2001;87:573–582. doi: 10.1046/j.1365-2540.2001.00955.x. [DOI] [PubMed] [Google Scholar]
- Ehrendorfer F. Die geographische und ökologische Entfaltung des europäisch-alpinen Polyploidkomplexes Galium anisophyllum Vill. seit Beginn des Quartärs. Uppsala Universitets Årsskrift. 1958;6:176–181. [Google Scholar]
- Ellenberg H. Vegetation Mitteleuropas mit den Alpen in ökologischer, dynamischer und historischer Sicht. 5th edn. Stuttgart: Ulmer; 1996. [Google Scholar]
- Fawcett JA, Maere S, van de Peer Y. Plants with double genomes might have had a better chance to survive the Cretaceous–Tertiary extinction event. Proceedings of the National Academy of Sciences of the USA. 2009;106:5737–5742. doi: 10.1073/pnas.0900906106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felber F. Establishment of a tetraploid cytotype in a diploid population: effect of relative fitness of the cytotypes. Journal of Evolutionary Biology. 1991;4:195–207. [Google Scholar]
- Felber F, Bever JD. Effect of triploid fitness on the coexistence of diploids and tetraploids. Biological Journal of the Linnean Society. 1997;60:95–106. [Google Scholar]
- Fössinger S. Senecio carniolicus als Modellorganismus für Arten mit sympatrisch verbreiteten Zytotypen. 2010 Diploma thesis, Department of Biogeography and Botanical Garden, University of Vienna. [Google Scholar]
- Halverson K, Heard SB, Nason JD, Stireman JO. Origins, distribution, and local co-occurrence of polyploid cytotypes in Solidago altissima (Asteraceae) American Journal of Botany. 2008;95:50–58. doi: 10.3732/ajb.95.1.50. [DOI] [PubMed] [Google Scholar]
- Hegarty MJ, Hiscock SJ. Genomic clues to the evolutionary success of polyploid plants. Current Biology. 2008;18:435–444. doi: 10.1016/j.cub.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Hülber K, Sonnleitner M, Flatscher R, et al. Ecological segregation drives fine-scale cytotype distribution of Senecio carniolicus in the Eastern Alps. Preslia. 2009;81:309–319. [PMC free article] [PubMed] [Google Scholar]
- Husband BC. Constraints on polyploid evolution: a test of the minority cytotype exclusion principle. Proceedings of the Royal Society of London – Series B: Biological Sciences. 2000;267:217–223. doi: 10.1098/rspb.2000.0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husband BC, Sabara HA. Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae) New Phytologist. 2004;161:703–713. doi: 10.1046/j.1469-8137.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- Husband BC, Schemske DW. Ecological mechanisms of reproductive isolation between diploid and tetraploid Chamerion angustifolium. Journal of Ecology. 2000;88:689–701. [Google Scholar]
- van Husen D. Die Ostalpen in den Eiszeiten. Vienna: Geologische Bundesanstalt; 1987. [Google Scholar]
- Kao RH. Origins and widespread distribution of co-existing polyploids in Arnica cordifolia (Asteraceae) Annals of Botany. 2008;101:145–152. doi: 10.1093/aob/mcm271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Mittelsten Scheid O, Erilova A. The impact of the triploid block on the origin and evolution of polyploid plants. Trends in Genetics. 2010;26:142–148. doi: 10.1016/j.tig.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Kolář F, Štech M, Trávníček P, et al. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Annals of Botany. 2009;103:963–974. doi: 10.1093/aob/mcp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. Alpine plant life‐functional plant ecology of high mountain ecosystems. 2nd edn. Heidelberg: Springer; 2003. [Google Scholar]
- Leitch AR, Leitch IJ. Genomic plasticity and the diversity of polyploid plants. Science. 2008;320:481–483. doi: 10.1126/science.1153585. [DOI] [PubMed] [Google Scholar]
- Levin DA. Minority cytotype exclusion in local plant populations. Taxon. 1975;24:35–43. [Google Scholar]
- Lewis HL, Oliver RL, Suda Y. Cytogeography of Claytonia virginica and its allies. Annals of the Missouri Botanical Garden. 1967;54:153–171. [Google Scholar]
- Lindner R, Garcia A. Genetic differences between natural populations of diploid and tetraploid Dactylis glomerata ssp. izcoi. Grass and Forage Science. 1997;52:291–297. [Google Scholar]
- Lumaret R, Guillerm JL, Delay J, Loutfi AAL, Izco J, Jay M. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain) Oecologia. 1987;73:436–446. doi: 10.1007/BF00385262. [DOI] [PubMed] [Google Scholar]
- Marhold K, Kudoh H, Pak J-H, Watanabe K, Španiel S, Lihová J. Cytotype diversity and genome size variation in eastern Asian polyploid Cardamine (Brassicaceae) species. Annals of Botany. 2010;105:249–264. doi: 10.1093/aob/mcp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BG, Young AG. Widespread chromosome variation in the endangered grassland forb Rutidosis leptorrhynchoides F. Muell. (Asteraceae: Gnaphalieae) Annals of Botany. 2001;87:83–90. [Google Scholar]
- Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- Orr HA, Presgraves DC. Speciation by postzygotic isolation: forces, genes and molecules. Bioessays. 2000;22:1085–1094. doi: 10.1002/1521-1878(200012)22:12<1085::AID-BIES6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Otto CRV, Snodgrass JW, Forester DC, Mitchell JC, Miller RW. Climatic variation and the distribution of an amphibian polyploid complex. Journal of Animal Ecology. 2007;76:1053–1061. doi: 10.1111/j.1365-2656.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- Otto SP, Whitton J. Polyploid incidence and evolution. Annual Review of Genetics. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- Petit C, Thompson JD. Variation in phenotypic response to light availability between diploid and tetraploid populations of the perennial grass Arrhenatherum elatius from open and woodland sites. Journal of Ecology. 1997;85:657–667. [Google Scholar]
- Petit C, Bretagnolle F, Felber F. Evolutionary consequences of diploid–polyploid hybrid zones in wild species. Trends in Ecology and Evolution. 1999;14:306–311. doi: 10.1016/s0169-5347(99)01608-0. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Ramsey J. Unreduced gametes and neopolyploids in natural populations of Achillea borealis (Asteraceae) Heredity. 2007;98:143–150. doi: 10.1038/sj.hdy.6800912. [DOI] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics. 1998;29:467–501. [Google Scholar]
- Reisigl H, Pitschmann H. Obere Grenzen von Flora und Vegetation in der Nivalstufe der zentralen Ötztaler Alpen (Tirol) Plant Ecology. 1958;8:93–129. [Google Scholar]
- Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfetter R. Über die Artenarmut der ostalpinen Ausläufer der Zentralalpen. Österreichische Botanische Zeitschrift. 1909;59:215–221. [Google Scholar]
- Schneeweiss GM, Schönswetter P. Feinverbreitung, Ökologie und Gesellschaftsanschluss reliktischer Gefäßpflanzen der Niederen Tauern östlich des Sölkpasses (Steiermark, Österreich) Stapfia. 1999;61:1–242. [Google Scholar]
- Schönswetter P, Tribsch A, Schneeweiss GM, Niklfeld H. Disjunctions in relict alpine plants: phylogeography of Androsace brevis and A. wulfeniana (Primulaceae) Botanical Journal of the Linnean Society. 2003;141:437–446. [Google Scholar]
- Schönswetter P, Stehlik I, Hoolderegger R, Tribsch A. Molecular evidence for glacial refugia of mountain plants in the European Alps. Molecular Ecology. 2005;14:3547–3555. doi: 10.1111/j.1365-294X.2005.02683.x. [DOI] [PubMed] [Google Scholar]
- Schönswetter P, Lachmayer M, Lettner C, et al. Sympatric diploid and hexaploid cytotypes of Senecio carniolicus (Asteraceae) in the Eastern Alps are separated along an altitudinal gradient. Journal of Plant Research. 2007;120:721–725. doi: 10.1007/s10265-007-0108-x. [DOI] [PubMed] [Google Scholar]
- Soltis DE, Soltis PS, Tate JA. Advances in the study of polyploidy since Plant speciation. New Phytologist. 2003;161:173–191. [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, et al. Polyploidy and angiosperm diversification. American Journal of Botany. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Ståhlberg D. Habitat differentiation, hybridization and gene flow patterns in mixed populations of diploid and autotetraploid Dactylorhiza maculata s.l. (Orchidaceae) Evolutionary Ecology. 2009;23:295–328. [Google Scholar]
- Stebbins GL. Chromosomal evolution in higher plants. London: Edward Arnold; 1971. [Google Scholar]
- Stebbins GL. Polyploidy and the distribution of the arctic–alpine flora: new evidence and a new approach. Botanica Helvetica. 1984;94:1–13. [Google Scholar]
- Stebbins GL. Polyploidy, hybridization, and the invasion of new habitats. Annals of the Missouri Botanical Garden. 1985;72:824–832. [Google Scholar]
- Stuessy TF, Weiss-Schneeweiss H, Keil DJ. Diploid and polyploid cytotype distribution in Melampodium cinereum and M. leucanthum (Asteraceae, Heliantheae) American Journal of Botany. 2004;91:889–898. doi: 10.3732/ajb.91.6.889. [DOI] [PubMed] [Google Scholar]
- Suda J, Leitch IJ. The quest for suitable reference standards in genome size research. Cytometry Part A. 2010;77A:717–720. doi: 10.1002/cyto.a.20907. [DOI] [PubMed] [Google Scholar]
- Suda J, Krahulcová A, Trávníček P, Krahulec F. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GM, Trávníček P, Schönswetter P. Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plant Senecio carniolicus (Asteraceae) American Journal of Botany. 2007;94:1391–1401. doi: 10.3732/ajb.94.8.1391. [DOI] [PubMed] [Google Scholar]
- Tribsch A. Areas of endemism of vascular plants in the Eastern Alps in relation to Pleistocene glaciation. Journal of Biogeography. 2004;31:747–760. [Google Scholar]
- Tribsch A, Schönswetter P. Patterns of endemism and comparative phylogeography confirm palaeoenvironmental evidence for Pleistocene refugia in the Eastern Alps. Taxon. 2003;52:477–497. [Google Scholar]
- Wendel JF. Genome evolution in polyploids. Plant Molecular Biology. 2000;42:225–249. [PubMed] [Google Scholar]
- Widmer A, Lexer C, Cozzolino S. Evolution of reproductive isolation in plants. Heredity. 2009;102:31–38. doi: 10.1038/hdy.2008.69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.