Abstract
Background and Aims
Plant functional traits are assumed to be adaptive. As selection acts on individuals and not on traits, interpreting the adaptive value of a trait not may be straightforward. For example, productive leaves are associated with fertile environments. However, it is not clear if productive leaves confer an advantage in these habitats, or if they are an advantage as part of a suite of coordinated traits.
Methods
Genotypes of Arabidopsis thaliana were grown in high and low nutrient treatments and low, neutral and high pH treatments. Nutrient availability is reduced in acidic or basic soils relative to neutral pH soils. pH treatments were used to alter the availability of resources rather than the amount of resources.
Key Results
Leaf function (specific leaf area, SLA) and life history (size at reproduction, age at reproduction) were variable across genotypes and were plastic. High nutrient availability induced higher SLA and larger size at reproduction. Genotypes that reproduced at large size in high nutrient conditions at neutral pH had the greatest fruit production. SLA was only indirectly related to fruit production through a causal relationship with rosette size; in high nutrient conditions, plants with high SLA were large at reproduction and had higher fruit production. In high nutrient and high pH treatments, plants were large at reproduction, but large size at reproduction was associated with low fecundity. This suggests that large size is adaptive under high nutrient availability.
Conclusions
Interpreting the adaptive value of functional traits will sometimes only be possible when these traits are considered as a suite of correlated and coordinated traits. Leaf functional traits may be important in defining adaptive strategies in A. thaliana but only through how they affect plant life history. Finally, manipulating soil pH can be a valuable tool in assessing adaptive plasticity on nutrient gradients.
Keywords: Adaptive plasticity, age at reproduction, Arabidopsis thaliana, functional traits, soil pH, rosette growth form, size at reproduction, SLA
INTRODUCTION
Functional traits are defined by their impact on fitness through their effects on plant growth, survival and reproduction (Violle et al., 2007). The identification of plant functional traits has been an important advance in defining ecological strategies and understanding how plants adapt to environmental variability (e.g. Grime, 1977; Bonser and Aarssen, 1996; Westoby et al., 2002; Reich et al., 2003). Suites of functional traits contributing to ecological strategies have been identified across species (e.g. Westoby et al., 2002). However, ecological strategies evolve in response to selection within species, and the expression of functional traits should be correlated with fitness in a given environment if they are to be considered part of an adaptive strategy (Reich et al., 2003). Although it is often assumed that functional traits have adaptive value, the link between function and fitness is not often tested (Ackerly and Monson, 2003; but see Geber and Griffen, 2003).
Understanding the factors contributing to functional trait expression across environments is dependent on interpreting how these traits can evolve (Bonser, 2006). The identification of leaf functional traits in plants is an example of a gap between documenting trait strategies across species and understanding how these strategies evolve within species. Leaf functional strategies are based on a series of correlations and trade-offs between functional traits. Specific leaf area (SLA – leaf area per unit mass), rate of photosynthesis and leaf nitrogen are positively correlated, and these traits are negatively related to leaf life span (e.g. Reich et al., 1997; Wright et al., 2004). These relationships are observed across species along an axis of highly productive short-lived leaves to unproductive long-lived leaves. Leaf functional traits could be important in defining plant strategies as they have been demonstrated to vary predictably across some environmental gradients. For example, SLA and leaf productivity tend to increase in habitats of increasing fertility (e.g. Cunningham et al., 1999; Wright et al., 2004; Hoffmann et al., 2005). Presumably, the differential expression of leaf traits allows plants to maximize growth rate where resources are abundant but minimize loss of leaf tissue where resources are limited and plants have a reduced capacity to replace lost tissue (Turner, 1994; Brunt et al., 2006). Selection to maximize nutrient retention and long leaf life spans in low nutrient environments should favour increased leaf toughness and lower SLA (e.g. Westoby et al., 2002; Read et al., 2009).
Could other selection scenarios also produce the observed leaf trait strategies? Leaves function within individual plants and the adaptive value of leaf functional traits must be measured in terms of individual fitness (Bonser, 2006). Fitness in plants is often related to size at reproduction (Wesselingh et al., 1997; Metcalf et al., 2003). In rosette-forming semelparous species, rosette size at reproduction is highly correlated with plant fitness (Kuss et al., 2008; Bonser and Aarssen, 2009). Large rosette size (area) could be achieved by extending the time for rosette growth (delaying reproduction) and/or by the rapid construction of inexpensive leaves. Under this scenario, the expression of SLA (and other correlated leaf functional traits) would evolve as a by-product of selection on life histories (i.e. the size at reproduction) rather than directly on leaf function. The evolution of leaf physiological traits has been demonstrated to be correlated to some plant traits such as leaf size (e.g. Geber and Dawson, 1990; Arntz and Delph, 2001). However, alternative hypotheses addressing how selection on correlated traits in integrated plant phenotypes can impact the evolution of leaf form function have rarely been addressed (Forster and Bonser, 2009). Examining how functional traits contribute to fitness is important if we wish to understand variation in fitness within species and the diversity of ecological strategies observed across species within and between habitats.
In this study, we examined functional traits and fitness across soil fertility treatments in genotypes of the annual plant Arabidopsis thaliana. Two approaches were employed to test for the adaptive value of functional traits. First, we tested for correlations and causal relationships (through path analysis) between functional traits and fitness in different nutrient treatments. Second, we tested for adaptive plasticity in functional traits through using soil pH treatments. Adaptive plasticity is most effectively assessed through the framework of the adaptive plasticity hypothesis (Dudley and Schmitt, 1996; Schmitt et al., 1999). Under this hypothesis, plasticity in a trait is adaptive if the expression of that trait confers high fitness in some environments but low fitness in other environments. Variability in pH provides an opportunity to test adaptive plasticity on nutrient gradients. High and low pH soils (relative to neutral pH soils) tend to limit the capacity for plants to acquire nutrients, but do not necessarily affect the abundance of nutrients (Foth, 1990; Brady and Weil, 2002; Mauseth, 2003; Vonlanthen et al., 2006). Protons in the soil are required to free many nutrient cations from particles of soil or organic matter. In acidic soils (high proton concentration), nutrients may be freed faster than they can be used by plants and they are leached from the soil. In basic soils (low proton concentration), many nutrients may remain bound to soil particles but unavailable to plants (see Mauseth, 2003). Thus, high or low pH soils may be perceived as fertile by the plant, and plants in these environments may produce an inappropriate phenotype. For example, plants may respond to high nutrient supply by producing a phenotype appropriate for a high nutrient environment, a maladapted phenotype for acidic or basic soils where nutrients are not readily available to plants. If a high nutrient phenotype (e.g. large size at reproduction, high SLA) yields high fitness where nutrients are available (neutral pH) but low fitness where nutrients are not readily available (low or high pH), then plasticity in size at reproduction and SLA is adaptive across a nutrient gradient. Alternatively, plants in these environments could produce a phenotype more suitable to a lower nutrient environment (an appropriate phenotype for that environment), demonstrating trait plasticity, but not providing evidence for adaptive plasticity.
We tested the following predictions. (1) Functional traits will vary across nutrient treatments, and variability in functional traits will be associated with fecundity (a measure of fitness) within nutrient treatments. For example, we predict plants in the high nutrient treatment will have large rosettes and high SLA relative to plants within the low nutrient treatment. Furthermore, large size at reproduction and high SLA will be related to high fecundity in the high nutrient treatment. (2) Plasticity in functional traits is adaptive. We test this prediction using pH treatments to induce plants to express an inappropriate phenotype in a nutrient treatment.
MATERIALS AND METHODS
Study species
We tested our predictions using inbred lines (genotypes) of the short-lived model plant species Arabidopsis thaliana, which develops as a basal rosette of leaves with flowering stems initiated from apical and axillary meristems of rosette leaves. Genotypes were obtained as seed from The Arabidopsis Information Resource (stable URL: http://www.arabidopsis.org). Eight target genotypes were used (cs1184, cs1284, cs1628, cs6011, cs6905, cs22548, cs22626, cs6041) and were selected as early flowering individual lines derived from natural populations. Two additional genotypes to be included in the experiment had very poor germination and were removed.
Experimental design
Seeds for each genotype were collected from single maternal individuals grown in high nutrient, neutral pH soil (see below). Seeds were placed on Petri dishes on moist filter paper and cold treated (4 °C) for 72 h. Petri dishes were then placed in the glasshouses of the University of New South Wales in full sunlight with temperature maintained at 20–25 °C to initiate germination. Seedlings were transplanted to pots at emergence of the cotyledons (approximately 10 d). Seven replicate pots of each genotype for each treatment combination (see below) were transplanted. Pots were 5 × 5 cm, 20 cm deep and filled with approximately 100 g of potting mix (a mixture of 50 % perlite and 50 % coconut fibre). Multiple seedlings were transplanted into each pot and thinned to one per pot 2 weeks after transplanting. During this establishment phase, all pots were watered with a dilute nutrient solution at neutral pH. The experiment was started 16 d after the seedlings were transplanted.
The experiment consisted of two nutrient treatments (high and low) and three pH treatments (low, neutral and high) in a full factorial design. The high nutrient treatment was established with a full-strength commercial hydroponic nutrient solution (Canna terra, Canna Corp., Toronto Canada). The low nutrient treatment was a 10 % dilution of this nutrient solution. The low pH treatment was established through the addition of phosphoric acid to the nutrient solutions, and the high pH treatment was established through the addition of potassium hydroxide (commercially available as ‘pH-down’ and ‘pH-up’, Holland Forge, Victoria, Australia). The neutral pH treatment was established by using tap water. This water was slightly alkaline; we neutralized the pH through the addition of pH-down (as above). The low pH treatment was maintained between pH 3 and 4, the neutral pH treatment was maintained between pH 6 and 7, and the high pH treatment was maintained between pH 8 and 9. We periodically checked the pH using a portable pH meter (Eutech Instruments, Singapore) from the nutrient solutions and the water runoff from the pots to ensure the desired treatment combinations were being maintained. The six different hydroponic solutions (three levels of pH × two levels of fertility) were made up weekly in individual 30-L reservoirs and plants were watered daily with the relevant solution. All pots were flushed with pure water fortnightly and then re-saturated with the relevant hydroponic solution to prevent build up of solutes in the potting mix. Due to the complicated nature of the nutritional regimes imposed, we grouped all plants that shared a common nutritional regime within a single block. We randomized the placement of the eight genotypes within each block and periodically (about once per week) randomly reorganized the experimental blocks on the glasshouse benches.
Arabidopsis thaliana has a distinct rosette juvenile phase during which most of the leaf material on the plant is produced. Unfortunately, the rosette senesces (and often decomposes) prior to the end of reproduction. In order to take measurements requiring destructive harvests of both vegetative and reproductive traits, we conducted two harvests: one at the age of first reproduction, and the other at final development, when fruit production had ceased. Thus, our experiment consisted of 672 individual plants (eight genotypes × two nutrient treatments × three pH treatments × two harvest dates × seven replicates per treatment combination and harvest date).
The following traits were measured at first harvest: rosette size (total leaf area), SLA (cm2 g−1) and age at first flower (from the start of the experiment). Leaf area was measured by using image analysis software (provided by the unit of comparative ecology, University of Sheffield). Leaves were then dried to constant biomass (70 °C for 3–4 d) and weighed. SLA was taken as an average value of all leaves. At the second harvest, we counted the total fruit number produced on each plant. Fruit number is closely correlated to seed production (and fitness) in A. thaliana (e.g. Pigliucci and Schmitt, 1999; Callahan and Pigliucci, 2002).
Data analysis
We performed a mixed-model multi-factor ANOVA to assess the effects of genotype, pH and nutrient availability on variability in fitness (fruit number), leaf function (SLA) and life-history traits (size and age at reproduction). The following factors were examined: genotype (G – random effect), pH (fixed effect), nutrient (N – fixed effect), G × pH (random effect), G × N (random effect), pH × N (fixed effect) and G × pH × N (random effect). Variables were log-transformed, where necessary, to meet the assumptions of statistical analysis. ANOVAs were conducted using Systat version 11 (Systat, Chicago, IL, USA). We used Pearson product moment correlations (JMP version 5, JMP, Cary, NC, USA) were used to assess significant (P < 0·05) pairwise correlations between traits. We used paired t-tests to test for differences in genotypic response in trait expression between neutral and low pH treatments and neutral and high pH treatments in both high and low nutrient addition treatments.
Path analysis
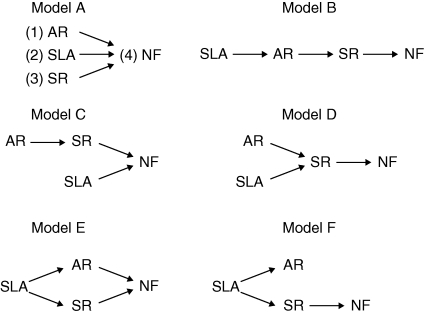
Finally, to examine how the relationships between leaf form and plant life histories influence fitness, we used a form of confirmatory path analysis known as d-sep tests (Shipley, 2000, 2004). For this analysis we compared a set of six a-priori path models (Fig. 1) of different causal relationships between leaf form (SLA), plant life histories and fitness in both the low and high nutrient treatments. For simplicity, we examined these causal relationships only in the neutral pH treatment. The high and low pH treatments were included to induce inappropriate phenotypes, making any path model more difficult to interpret. Each candidate path model tests a biologically plausible prediction regarding how leaf form, age at reproduction and rosette size at reproduction interact to influence plant fitness in terms of the number of fruit produced. Each model included represents a possible path of relationships between traits, including the predicted paths that leaf form directly affects fruit production and the alternative path that leaf form affects fruit production only through its influence on size at reproduction.
Fig. 1.
Six alternative hypotheses (models A–F) describing the causal structure linking plant growth and form to fitness in low and high nutrient treatments at neutral pH. Attributes include age at reproduction (AR), specific leaf area (SLA), rosette size at reproduction (SR) and the number of fruit (FN). Arrows indicate the direction of causality assumed in the models. Error terms are omitted for simplicity.
To identify the path model that best fits the data in the two treatments of interest, we first obtained the predicted conditional independence constraints that must apply if the hypothesized causal relationships are true. For each candidate model we estimated the basis set (BU), which is the list of pairs of variables in each model that are predicted to be probabilistically independent (not directly related by a causal pathway), conditional on any causal parents of the pair. We then tested each predicted independence claim against the data using Pearson's partial correlation coefficient. Following Shipley (2000), for example, if traits X and Y were predicted to be independent conditional on a set of variables Q = {A, B, C, … }, then we regressed X and Y separately on the variable set Q and estimated the correlation between the residuals. Lastly, we combined each separate test of conditional independence claim into a combined test for the overall fit of the entire model using Fisher's C statistic, C = –2Σln(pi), where the number k of null probabilities (pi) associated with the basis set follows a χ2 distribution with 2k degrees of freedom. A large value of C will indicate a significant difference between the observed and predicted patterns of conditional independence and is evidence against a particular candidate model. Finally, we estimated the path coefficients of the best-fitting models as the standardized regression coefficients of a least-squares regression. Regression models were conducted in JMP version 5.
RESULTS
Variation in functional and life-history traits across resource treatments
Functional, life-history and fitness traits were variable across the ANOVA main effects (Table 1). Nutrient availability explained a highly significant portion of the variation in fruit number, SLA and rosette size at reproduction (these traits were plastic across nutrient treatments). Similarly, pH explained significant variation in fruit number, rosette size and age at reproduction (these traits were plastic across pH treatments). Genotype explained significant variation in all traits (Table 1). We found significant genetic variation in plasticity across nutrient treatments (G × N) for SLA, and rosette size and age at reproduction. We also found significant genetic variation for plasticity in fruit number across pH gradients (G × pH), which was also nutrient dependent (G × N × pH) (Table 1).
Table 1.
Three-factor mixed-model ANOVA models for fruit number, specific leaf area (SLA), rosette size and age at reproduction for genotypes (G) of Arabidopsis thaliana across nutrient (N) and pH treatments
| Fruit number |
SLA |
Rosette size |
Age at reproduction |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of variation | d.f. | ms | P | d.f. | ms | P | d.f. | ms | P | d.f. | ms | P |
| Genotype | 7 | 0·278 | <0·0001 | 7 | 0·053 | <0·0001 | 7 | 0·04 | <0·0001 | 7 | 665·7 | <0·0001 |
| pH | 2 | 0·467 | 0·002 | 2 | 0·004 | 0·39 | 2 | 0·01 | 0·002 | 2 | 61·3 | 0·01 |
| Nutrient | 1 | 70·98 | <0·0001 | 1 | 0·264 | 0·006 | 1 | 3·91 | <0·0001 | 1 | 7·7 | 0·57 |
| G × pH | 14 | 0·044 | 0·003 | 14 | 0·004 | 0·065 | 14 | 0·001 | 0·93 | 14 | 9·4 | 0·42 |
| G × N | 7 | 0·021 | 0·323 | 7 | 0·018 | <0·0001 | 7 | 0·033 | <0·0001 | 7 | 22·2 | 0·02 |
| pH × N | 2 | 0·16 | 0·085 | 2 | 0·003 | 0·26 | 2 | 0·009 | 0·082 | 2 | 10·1 | 0·33 |
| G × pH × N | 14 | 0·054 | <0·0001 | 14 | 0·002 | 0·63 | 14 | 0·003 | 0·11 | 14 | 8·44 | 0·53 |
| Error | 240 | 0·018 | 192 | 0·0024 | 192 | 0·002 | 192 | 9·07 | ||||
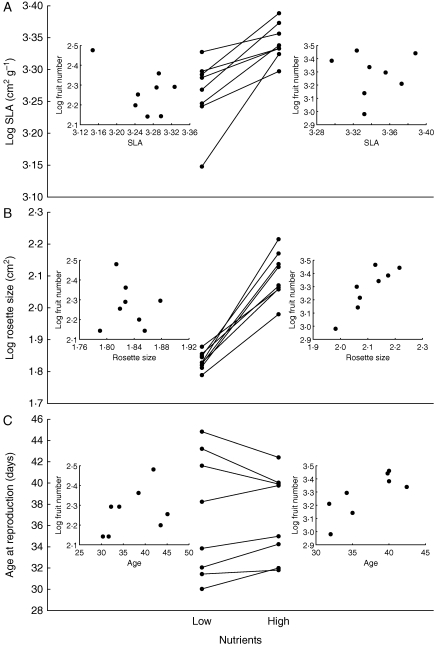
High nutrient addition induced higher SLA and larger rosette size at the age of reproduction (Fig. 2). Variation in SLA was not related to variation in fruit number in any of the nutrient–pH treatment combinations (Table 2, Fig. 2). Whereas SLA was higher in the high nutrient treatment, it was not related to fruit production across genotypes within the high nutrient treatment. Rosette size at reproduction was correlated with high fruit number in the high nutrient treatment at low and neutral pH, but not in the high pH treatment (Table 2, Fig. 2). Rosette size was not correlated with fitness in the low nutrient treatment (at any pH, Table 2). Similarly, age at reproduction was positively correlated with fruit number in the high nutrient treatment at low and neutral pH, but not in the high pH treatment, and not in the low nutrient treatment. Age at reproduction was positively correlated with size at reproduction in the high nutrient treatment at neutral and high pH (Table 2).
Fig. 2.
Mean trait values for genotypes in low and high nutrient treatments at neutral pH for (A) specific leaf area (SLA; cm2 g−1), (B) rosette size at reproduction (cm2) and (C) age at reproduction (days). Lines (reaction norms) connect mean values for a genotype across nutrient treatments. Inset figures show relationships between genotypic mean values for each of the above traits and mean genotype fitness (fruit number) in low nutrient (left inset) and high nutrient (right inset) treatments.
Table 2.
Pairwise Pearson product moment correlation coefficients for fitness functional and life-history traits in each nutrient and pH treatment combination
| Low nutrients |
High nutrients |
||||||
|---|---|---|---|---|---|---|---|
| Fruit number | SLA | Rosette size | Fruit number | SLA | Rosette size | ||
| Low pH | |||||||
| SLA | r | –0.46 | –0·51 | ||||
| P | 0·25 | 0·19 | |||||
| Rosette size | r | 0·03 | 0·53 | 0·76 | –0·47 | ||
| P | 0·95 | 0·18 | 0·03 | 0·24 | |||
| Age at reproduction | r | 0·5 | –0·91 | –0·21 | 0·74 | –0·48 | 0·59 |
| P | 0·21 | 0·002 | 0·62 | 0·03 | 0·23 | 0·12 | |
| Neutral pH | |||||||
| SLA | r | –0·52 | 0·05 | ||||
| P | 0·19 | 0·91 | |||||
| Rosette size | r | –0·08 | 0·32 | 0·88 | 0·09 | ||
| P | 0·85 | 0·43 | 0·004 | 0·83 | |||
| Age at reproduction | r | 0·42 | –0·64 | –0·13 | 0·79 | –0·26 | 0·83 |
| P | 0·31 | 0·09 | 0·76 | 0·02 | 0·54 | 0·01 | |
| High pH | |||||||
| SLA | r | –0·25 | 0·64 | ||||
| P | 0·55 | 0·09 | |||||
| Rosette size | r | –0·01 | 0·17 | –0·45 | –0·82 | ||
| P | 0·98 | 0·69 | 0·26 | 0·01 | |||
| Age at reproduction | r | 0·36 | –0·86 | –0·27 | –0·34 | –0·81 | 0·89 |
| P | 0·38 | 0·007 | 0·53 | 0·41 | 0·02 | 0·003 | |
Some mortality occurred in the experiment, mostly shortly after the beginning of the experiment when the plants were quite young. Mortality was random across genotypes and treatments. For unknown reasons, mortality was higher in the early harvest plants than in the late harvest plants.
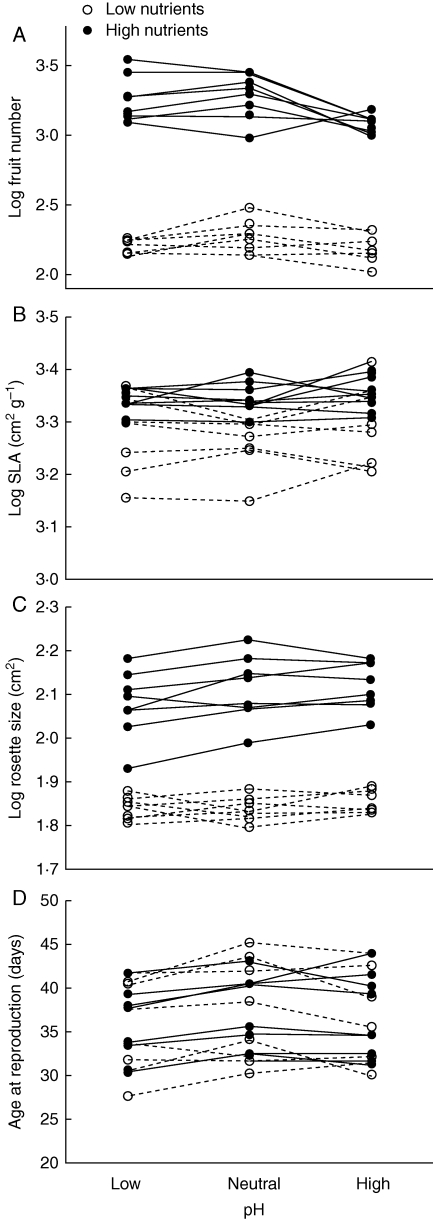
Plasticity across pH treatments
Plants in the high and low pH treatments had significantly lower fruit number than plants in the neutral pH treatment (Table 1, Fig. 3). The significant G × pH interaction (and three-way interaction with nutrients) suggests that some genotypes have adapted to different pH environments. For example, in the high nutrient treatment, the genotype with the lowest fruit number in low and neutral pH treatments had the highest fruit production in the highest pH treatment (Fig. 3). Rosette size also varied significantly across pH treatments. Rosettes were significantly larger at reproduction in the neutral and high pH treatments in the high nutrient treatment but not in the low nutrient treatment (Table 3). Large size at reproduction was also associated with high fruit production in the high nutrient treatment at neutral pH. However, in the high nutrient treatment at high pH, genotypes had large rosettes but relatively low fitness (Table 3, Fig. 3).
Fig. 3.
Mean trait values for genotypes in low, neutral and high pH treatments in low and high nutrient treatments, as indicated, for (A) fruit number, (B) SLA (cm2 g−1), (C) rosette size at reproduction (cm2) and (D) age at reproduction.
Table 3.
Results of paired t-tests comparing the expression of traits for genotypes grown in low (L) and neutral (N) pH treatments, and neutral and high (H) pH treatments for both high and low nutrient treatments
| Trait | Nutrients | Low pH vs neutral pH | t, P | Neutral pH vs high pH | t, P |
|---|---|---|---|---|---|
| Fruit number | High | n.s. | t = 0·6 | N > H | t = –2·78 |
| P = 0·52 | P = 0·028 | ||||
| Low | n.s. | t = 2·25 | N > H | t = 3·03 | |
| P = 0·059 | P = 0·019 | ||||
| Rosette size | High | L < N | t = 3·22 | n.s. | t = 0·56 |
| P = 0·015 | P = 0·59 | ||||
| Low | n.s. | t = 0·43 | n.s. | t = 1·84 | |
| P = 0·68 | P = 0·11 | ||||
| SLA | High | n.s. | t = 0·28 | n.s. | t = 0·26 |
| P = 0·78 | P = 0·80 | ||||
| Low | n.s. | t = 1·13 | n.s. | t = 1·36 | |
| P = 0·29 | P = 0·22 | ||||
| Age at reproduction | High | L < N | t = 8·22 | n.s. | t = 0·38 |
| P < 0·0001 | P = 0·71 | ||||
| Low | L < N | t = 2·37 | n.s. | t = 1·77 | |
| P = 0·05 | P = 0·12 |
Each test reports if a trait is significantly greater in one pH treatment compared with another, or not significantly different (n.s.).
SLA was variable across pH treatments; however, these responses were specific to genotype and nutrient treatment. Similarly, age at reproduction varied across pH treatment (Table 1, Fig. 3). Plants reproduced earlier at low pH than at neutral pH in both the high and the low nutrient treatments. Age at reproduction was not significantly different between neutral and high pH treatments (Table 3, Fig. 3).
Path analysis and causal relationships between leaf form, plant life histories and fitness
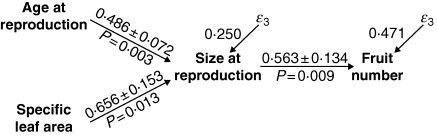
We evaluated six alternative path models describing the cause and effect relationships between SLA, age at reproduction, rosette size at reproduction and the number of fruit produced in two different nutrient environments at neutral pH (Table 4). In the low nutrient treatment we were unable to find any evidence against our six different path models, although both model E and model F appeared to be the best supported (lowest C values). In contrast, in the high nutrient treatment the data clearly supported only model D. All other models were either rejected by the data (models B, E and F) or had only weak support (models A and C). Examination of the best fitting model for the high nutrient treatment (Fig. 4) reveals that rosette size at reproduction significantly predicts the number of fruit produced (r2 = 0·778), with fitness increasing with the size of the plant at reproduction. Moreover, the path model reveals that both age at reproduction and SLA indirectly influence fitness through their impact on size at reproduction, as plants that took longer to flower and had greater SLA were larger at reproduction. In fact, over 90 % of the variation in size at reproduction was explained by the combined variation in age at reproduction and SLA (r2 = 0·937).
Table 4.
The basis sets for the partial independence constraints as implied by each of the six hypothesized models shown in Fig. 1
| Low nutrients, neutral pH |
High nutrients, neutral pH |
|||
|---|---|---|---|---|
| Basis set | Partial r | P (r) | Partial r | P (r) |
| Model A | ||||
| (1,2)|{φ} | –0·640 | 0·088 | –0·088 | 0·851 |
| (1,3)|{φ} | –0·131 | 0·757 | 0·805 | 0·029 |
| (2,3)|{φ} | 0·324 | 0·434 | 0·465 | 0·294 |
| Fisher's C: | χ2 = 7·099, 6 d.f. | χ2 = 9·862, 6 d.f. | ||
| P = 0·312 | P = 0·131 | |||
| Model B | ||||
| (2,3)|{1} | 0·315 | 0·447 | 0·907 | 0·005 |
| (2,4)|{3} | –0·523 | 0·184 | –0·317 | 0·489 |
| (1,4)|{2,3} | 0·117 | 0·783 | –0·149 | 0·751 |
| Fisher's C: | χ2 = 5·490, 6 d.f. | χ2 = 12·663, 6 d.f. | ||
| P = 0·483 | P = 0·049 | |||
| Model C | ||||
| (1,2)|{φ} | –0·640 | 0·088 | –0·088 | 0·851 |
| (1,4)|{3,2} | 0·117 | 0·783 | –0·149 | 0·751 |
| (2,3)|{1} | 0·315 | 0·447 | 0·907 | 0·005 |
| Fisher's C: | χ2 = 6·971, 6 d.f. | χ2 = 11·554, 6 d.f. | ||
| P = 0·324 | P = 0·073 | |||
| Model D | ||||
| (1,2)|{φ} | –0·640 | 0·088 | –0·088 | 0·851 |
| (1,4)|{3} | 0·410 | 0·313 | 0·212 | 0·648 |
| (2,4)|{3} | –0·523 | 0·184 | –0·317 | 0·489 |
| Fisher's C: | χ2 = 10·584, 6 d.f. | χ2 = 2·623, 6 d.f. | ||
| P = 0·102 | P = 0·854 | |||
| Model E | ||||
| (2,4)|{1,3} | –0·373 | 0·363 | –0·281 | 0·542 |
| (1,3)|{2,4} | 0·093 | 0·827 | 0·866 | 0·012 |
| Fisher's C: | χ2 = 2·404, 4 d.f. | χ2 = 10·110, 4 d.f. | ||
| P = 0·662 | P = 0·039 | |||
| Model F | ||||
| (2,4)|{3} | –0·523 | 0·184 | –0·317 | 0·489 |
| (1,3)|{2} | 0·105 | 0·804 | 0·959 | 0·001 |
| (1,4)|{2,3} | 0·117 | 0·783 | –0·149 | 0·751 |
| Fisher's C: | χ2 = 4·314, 6 d.f. | χ2 = 16·748, 6 d.f. | ||
| P = 0·634 | P = 0·010 | |||
The notation (X, Y)|{A, B, … } indicates that variables X and Y are probabilistically independent, conditional on the combined set of variables {A, B, … }, with the symbol φ indicating the null (empty) set. Variables include age at reproduction (1), leaf form (2), size at reproduction (3) and the number of fruit (4). Each conditional independence claim is based on the probability (P(r)) that the partial correlation coefficients (r) are zero. The overall model is tested with Fisher's C statistic, with significance indicating that the model is rejected by the data, and hence lower C values indicating stronger support for the model.
Fig. 4.
The standardized path coefficients for the best fitting path model (model D) in the high nutrient, neutral pH data set. The path coefficients indicate both the direction and the strength of any causal relationships. Error variables, which describe the amount of unexplained variance, are indicated by εi.
DISCUSSION
Variation in nutrient availability induced shifts in the expression of leaf function and life histories. All genotypes initiated reproduction at larger sizes in the high nutrient treatment than in the low nutrient treatment, and large size at reproduction was highly adaptive in the high nutrient treatment. Fecundity in annual or semelparous plants is frequently positively correlated with size at reproduction (Wesselingh et al., 1997; Metcalf et al., 2003). This is generally attributed to the capacity for large plants to allocate more resources (e.g. Andrieu et al., 2007) and meristems (Geber, 1990; Bonser and Aarssen, 2003) to reproduction at final development. Environmental conditions limiting growth or inducing plant mortality (e.g. nutrient limitation) should favour the initiation of reproduction at small sizes. Delaying reproduction could result in reproductive failure if plants are killed before they reproduce or if environmental adversity prevents a plant from achieving the optimal size for reproduction (Bonser and Aarssen, 2009).
Size at reproduction was variable across genotypes, particularly in the high nutrient treatment. Variation in size at reproduction in these favourable environments could be maintained by selection pressures from native environments such as disturbances, environmental variability (e.g. droughts in the growing season) or other factors limiting the potential length of the growing season – conditions not experienced in the current experiment. Alternatively, some genotypes may lack the capacity to grow quickly enough to reach an optimal size for reproduction within a growing season. In the low nutrient treatment, fruit number was not correlated with size or age at reproduction, i.e. neither the smallest nor the largest genotypes had the highest fruit production. Although initiating reproduction at smaller sizes may be an advantage in that it minimizes the chance of reproductive failure, reproducing at a small size is not itself an advantage. Genotypes that reproduced at small sizes but have limited resource acquisition or meristematic capacity to allocate to reproduction did not outperform (or were not outperformed by) plants that reproduced at large sizes but perhaps run out of time or resources to realize their reproductive potential. The results for age at reproduction are generally consistent with these predictions. The largest genotypes take more time to reproduce and the smaller genotypes take less time to reproduce in the low nutrient treatment than in the high nutrient treatment (Fig. 2). Thus, in the low nutrient treatment, large genotypes are more likely to run out of time prior to realizing their reproductive potential (e.g. Bonser and Aarssen, 2009). Developmental decisions to delay reproduction in order to reach a large size may not yield increased fitness when the time to complete reproduction is limited. There are costs and benefits associated with reproducing early and small versus late and large in low nutrient treatments that are not evident in high nutrient environments. Environmental variation could promote the coexistence of multiple life-history strategies within a habitat.
The evolution of leaf function
Leaf functional variability was not directly related to variation in fruit production (fitness) in either nutrient environment. We believe that leaf functional traits may have an integrated role in plant adaptation. For example, large rosette size could be achieved more quickly though producing leaves with high SLA. We found strong evidence through a path analysis that large size at reproduction is achieved through both late age at reproduction and the rapid construction of relatively cheap leaves. This result suggests that the expression of leaf form and function is only an indirect emergent ecological strategy, at least under these experimental conditions in A. thaliana. The evolution of suites of leaf functional traits suggests that these traits define ecological strategies across species (Reich et al., 1997; Westoby et al., 2002; Wright et al., 2004). Patterns of functional trait strategies observed across species must be due to evolutionary change within species, and within-species studies can reveal the conditions promoting the expression of functional traits observed across species.
Why was leaf form variable across genotypes despite the indirect fitness advantage gained by plants with relatively high SLA (at least in the high nutrient treatment)? An integrated view of the expression of leaf traits within the broader context of the individual can also provide a potential explanation for variation in leaf form. Although A. thaliana produces some small leaves on upright green stems after the initiation of reproduction, most leaf biomass throughout the lifetime of the plant is in the rosette. New rosette leaf production ceases at the initiation of reproduction – the terminal meristem is committed to reproduction and no new vegetative production in the rosette is possible (Bonser and Aarssen, 1996). All rosette leaves had senesced at final development but did persist past the initiation of reproduction. Maintaining rosette leaves throughout the adult stage would be advantageous in terms of carbon acquisition and allocation to reproduction. Leaves with high SLA are associated with fast growth rates (e.g. Wright and Westoby, 2000) and fertile habitats (e.g. Poorter and De Jong, 1999). However, in A. thaliana, the rosette leaves produced early in development are probably important photosynthetic organs throughout the plant's life (although the inflorescences can contribute significantly to total carbon gain; see Earley et al., 2009). High carbon allocation to fruit production could be achieved by rapid photosynthesis in a large and highly productive but short-lived rosette. Alternatively, perhaps a second (but less successful in terms of fruit production in our experiment) strategy could exist in which photosynthesis occurs over an extended period in less productive yet long-lived rosette leaves. If SLA is negatively related to leaf life span within A. thaliana, then a range of potentially successful leaf strategies could coexist within a habitat. This hypothesis has yet to be tested.
Adaptive plasticity on a nutrient gradient
As soils become more acidic or basic (relative to neutral pH soils), nutrients generally become less available to the plants (Foth, 1990; Brady and Weil, 2002; Mauseth, 2003). Proton concentration defines soil pH, and protons are also required to disrupt cation (mineral nutrients) attraction to the soil and promote their uptake in the roots (see Maseuth, 2003). A high proton concentration in acidic soils (low pH) mobilizes mineral nutrients in the soil and these nutrients are quickly leached from the soil, reducing soil fertility (Brady and Weil, 2002; Maseuth, 2003; Singh and Agrawal, 2008; Berthrong et al., 2009). Basic soils (high pH) are defined by low concentrations of soil protons. Basic soils can have high nutrient concentrations but the nutrients tend to be unavailable to the plant (Maseuth, 2003). In moderately alkaline soils (pH 8–9), many macronutrients remain available to plants but the availability of phosphorous and many micronutrients (e.g. manganese, zinc and iron) are low enough to limit plant growth significantly (Brady and Weil, 2002). In high nutrient and low pH treatments, rosette size was small, a phenotype consistent with a lower nutrient environment – an environment where the added soil resources were quickly lost and fertility was relatively low. In the high nutrient and high pH treatment, plants expressed rosette traits appropriate for a high nutrient environment despite having relatively low capacity to acquire some necessary nutrients. Mean fruit production for most genotypes was lower in the high pH treatment than in the neutral pH treatment (in both high and low nutrient treatments), suggesting that there is a fitness cost associated with producing a large rosette in an environment where some nutrients are not readily available. We interpret this as adaptive plasticity in rosette size on nutrient gradients; large rosette size at reproduction (where resources are available) yields high fitness, while the same phenotype yields relatively low fitness under limited nutrient availability. The adaptive plasticity hypothesis is a powerful tool in evaluating the adaptive value of plasticity in functional traits (Dudley and Schmitt, 1996; Pigliucci and Schmitt, 1999). To our knowledge, this is the first study to manipulate soil pH to test for adaptive plasticity on nutrient gradients. More research is required to establish whether pH can be used as a tool to effectively assess adaptive plasticity in heterogeneous resource environments. For example, our approach did not allow us to induce a high nutrient phenotype under low nutrient supply. This is not problematic for our study as small rosette size is not itself an adaptive trait in low nutrient treatments (see above). However, the results of this study are promising. This is an important advance because plant adaptation to fertility (productivity) is central to plant strategy theories (e.g. Grime, 1977; Taylor et al., 1990; Bonser and Aarssen, 1996), and our ability to assess adaptive plasticity on fertility gradients has been extremely limited.
Conclusions
Ecologists often assume that many aspects of function and life histories have adaptive value. This is probably a safe assumption as functional and life-history traits are commonly associated with resource acquisition, and strategies of growth and allocation within habitats and across environmental gradients. For example, the importance of leaf functional traits to the physiological ecology of plants has generated a great deal of interest in identifying leaf trait strategies (e.g. Westoby et al., 2002; Wright et al., 2004). We demonstrate that the expression of leaf functional traits is related to nutrient availability. However, interpreting the adaptive value of these functional traits is sometimes only possible when these traits are considered as part of an integrated phenotype. Large size at reproduction is highly adaptive in high nutrient environments and is achieved through a combination of delayed reproduction and high SLA (a leaf functional trait). Thus, leaf function makes an important but indirect contribution to adaptive strategies of A. thaliana genotypes on nutrient gradients. Future research will focus on establishing a more comprehensive understanding of how a range of functional traits contribute to adaptive strategies across different environmental gradients, and in linking the evolution of adaptive strategies within species to the array of strategies observed across species.
ACKNOWLEDGEMENTS
We thank M. VanKluenen, M. Mendez and M. Hanley for helpful comments on earlier versions of this manuscript. This work was supported by the University of New South Wales through a faculty research grant, an early career research grant, and a Vice-Chancellor's research and teaching fellowship to S.P.B.
LITERATURE CITED
- Ackerly DD, Monson RK. Waking the sleeping giant: the evolutionary foundations of plant function. International Journal of Plant Sciences. 2003;164:s1–s6. [Google Scholar]
- Andrieu E, Debussche M, Thompson JD. Size-dependent reproduction and gender modification in the hermaphroditic perennial plant Paeonia officinalis. International Journal of Plant Sciences. 2007;168:435–441. [Google Scholar]
- Arntz MA, Delph LF. Pattern and process: evidence for the evolution of photosynthetic traits in natural populations. Oecologica. 2001;127:455–467. doi: 10.1007/s004420100650. [DOI] [PubMed] [Google Scholar]
- Berthrong ST, Jobbagy EG, Jackson RB. A global meta-analysis of soil exchangeable cations, pH, carbon and nitrogen with afforestation. Ecological Applications. 2009;19:2228–2241. doi: 10.1890/08-1730.1. [DOI] [PubMed] [Google Scholar]
- Bonser SP. Form defining function: interpreting leaf functional variability in integrated plant phenotypes. Oikos. 2006;114:187–190. [Google Scholar]
- Bonser SP, Aarssen LW. Meristem allocation: a new classification theory for adaptive strategies in herbaceous plants. Oikos. 1996;77:347–352. [Google Scholar]
- Bonser SP, Aarssen LW. Allometry and development in herbaceous plants: functional responses of meristem allocation to light and nutrient availability. American Journal of Botany. 2003;90:404–412. doi: 10.3732/ajb.90.3.404. [DOI] [PubMed] [Google Scholar]
- Bonser SP, Aarssen LW. Interpreting reproductive allometry: individual strategies of allocation explain size-dependent reproduction in plant populations. Perspectives in Plant Ecology, Evolution and Systematics. 2009;11:31–40. [Google Scholar]
- Brady NC, Weil RR. Elements of the nature and properties of soils. 2nd edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2002. [Google Scholar]
- Brunt C, Read J, Sanson G. Changes in resource concentration and defence during leaf development in a tough-leaved (Nothofagus moorei) and soft-leaved (Toona ciliata) species. Oecologia. 2006;148:583–592. doi: 10.1007/s00442-006-0369-4. [DOI] [PubMed] [Google Scholar]
- Callahan HS, Pigliucci M. Shade-induced plasticity and its ecological significance in wild populations of Arabidopsis thaliana. Ecology. 2002;83:1965–1980. [Google Scholar]
- Cunningham SA, Summerhayes B, Westoby M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecological Monographs. 1999;69:569–588. [Google Scholar]
- Dudley SA, Schmitt J. Testing the adaptive plasticity hypothesis: density-dependent selection on manipulated stem length in Impatiens capensis. American Naturalist. 1996;147:445–465. [Google Scholar]
- Earley EJ, Ingland B, Winkler J, Tonsor SJ. Inflorescences contribute more than rosettes to lifetime carbon gain in Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 2009;96:786–792. doi: 10.3732/ajb.0800149. [DOI] [PubMed] [Google Scholar]
- Foth HD. Fundamentals in soil science. 8th edn. New York: Wiley; 1990. [Google Scholar]
- Forster MA, Bonser SP. Heteroblastic development and the optimal partitioning of traits among contrasting environments in Acacia implexa. Annals of Botany. 2009;93:95–105. doi: 10.1093/aob/mcn210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geber MA. The cost of meristem limitation in Polygonum arenastrum: negative genetic correlations between fecundity and growth. Evolution. 1990;44:799–819. doi: 10.1111/j.1558-5646.1990.tb03806.x. [DOI] [PubMed] [Google Scholar]
- Geber MA, Dawson TE. Genetic variation in and covariation between leaf gas exchange, morphology, and development in Polygonum arenastrum, an annual plant. Oecologia. 1990;85:153–158. doi: 10.1007/BF00319396. [DOI] [PubMed] [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection on functional traits. International Journal of Plant Sciences. 2003;164:s21–s42. [Google Scholar]
- Grime JP. Evidence for existence of 3 primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Hoffmann WA, Franco AC, Moreira MZ, Haridasan M. Specific leaf area explains differences in leaf trait between congeneric savannah and forest trees. Functional Ecology. 2005;19:932–940. [Google Scholar]
- Kuss P, Rees M, Ægisdóttir HH, Ellner SP, Stöcklin J. Evolutionary demography of long-lived monocarpic perennials: a time-lagged integral projection model. Journal of Ecology. 2008;96:821–832. [Google Scholar]
- Mauseth J. Botany. 3rd edn. Sudbury, MA: Jones and Bartlett; 2003. [Google Scholar]
- Metcalf JC, Rose KE, Rees M. Evolutionary demography of monocarpic perennials. Trends in Ecology and Evolution. 2003;18:471–480. [Google Scholar]
- Pigliucci M, Schmitt J. Genes affecting phenotypic plasticity in Arabidopsis: pleiotropic effects and reproductive fitness of photomorphogenic mutants. Journal of Evolutionary Biology. 1999;12:551–562. [Google Scholar]
- Poorter H, De Jong R. A comparison of specific leaf area, chemical composition and leaf construction costs of field plants from 15 habitats differing in productivity. New Phytologist. 1999;143:163–176. [Google Scholar]
- Read J, Sanson GD, Caldwell E, et al. Correlations between leaf toughness and phenolics among species in contrasting environments of Australia and New Caledonia. Annals of Botany. 2009;103:757–767. doi: 10.1093/aob/mcn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Walters MB, Ellsworth DS. From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences USA. 1997;94:13730–13734. doi: 10.1073/pnas.94.25.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich PB, Wright IJ, Cavender-Bares J, et al. The evolution of plant functional variation: traits, spectra and strategies. International Journal of Plant Sciences. 2003;164:s143–s164. [Google Scholar]
- Shipley B. Cause and correlation in biology: a user's guide to path analysis, structural equations and causal inference. Cambridge: Cambridge University Press; 2000. [Google Scholar]
- Shipley B. Analysing the allometry of multiple interacting traits. Perspectives in Plant Ecology, Evolution and Systematics. 2004;6:235–241. [Google Scholar]
- Singh SA, Agrawal M. Acid rain and its ecological consequences. Journal of Environmental Biology. 2008;29:15–24. [PubMed] [Google Scholar]
- Schmitt J, Dudley SA, Pigliucci M. Manipulative approaches to testing adaptive plasticity: phytochrome-mediated shade-avoidance responses in plants. American Naturalist. 1999;154:s43–s54. doi: 10.1086/303282. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Aarssen LW, Loehle C. On the relationship between r/K selection and environmental carrying capacity: a new habitat templet for plant life history strategies. Oikos. 1990;58:239–250. [Google Scholar]
- Turner IM. Sclerophylly: primarily protective? Functional Ecology. 1994;8:669–675. [Google Scholar]
- Violle C, Navas ML, Vile D, et al. Let the concept of the trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- Vonlanthen CM, Kammer PM, Eugster W, Bühler A, Veit H. Alpine vascular plant species richness: the importance of daily maximum temperature and pH. Plant Ecology. 2006;184:13–25. [Google Scholar]
- Wesselingh RA, Klinkhamer PGL, DeJong TJ, Boorman LA. Threshold size for flowering in different habitats: effects of size-dependent growth and survival. Ecology. 1997;50:2118–2132. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics. 2002;33:125–159. [Google Scholar]
- Wright IJ, Westoby M. Cross-species relationships between seedling relative growth rate, nitrogen productivity and root vs leaf function in 28 Australian woody species. Functional Ecology. 2000;14:97–107. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]