Abstract
Background and Aims
Trithuria, the sole genus in the family Hydatellaceae, is an important group for understanding early angiosperm evolution because of its sister relationship to the ancient lineage, Nymphaeales (water lilies). Although also aquatic, Trithuria differs from water lilies in that all species are extremely small, and most have an annual life form and grow in seasonal wetlands. Very little is known about their reproductive ecology. This paper reports on reproductive timing, mode of pollination and characteristics of the breeding system of Trithuria submersa in Western Australia.
Methods
Mass collections of open-pollinated plants from different ecological settings were used to characterize the reproductive developmental sequence and natural pollen reception. Hand-pollination, caging and emasculation experiments were used to measure outcross + geitonogamous pollen reception versus autonomous self-pollination in two populations over two field seasons.
Key Results
Natural outcross or geitonogamous pollination was by wind, not by water or insects, but pollen reception was extremely low. Pollen production was very low and pollen release was non-synchronous within populations. The pollen to ovule (P/O) ratio was 23·9, compared with 1569·1 in dioecious Trithuria austinensis. Stigmas became receptive before male phase and remained so until anthers dehisced and autonomous self-pollination occurred. Natural pollen loads are composed primarily of self pollen. Self- and open-pollinated plants had equivalent seed set (both >70 %). Self-pollinated plants produced seed within 17 d.
Conclusions
Autonomous self-pollination and self-fertilization are predominant in T. submersa. The low P/O ratio is not an artefact of small plant size and is inconsistent with long-term pollination by wind. It indicates that T. submersa has evolved a primarily autogamous breeding system. Selfing, along with the effect of small plant size on the speed of reproduction, has enabled T. submersa to colonize marginal ephemeral wetlands in the face of unpredictable pollination.
Keywords: Autogamy, basal angiosperm, delayed self-pollination, Hydatellaceae, Nymphaeales, wind pollination, reproductive assurance, reproductive timing, stigma receptivity, Trithuria submersa
INTRODUCTION
Nymphaeales is an ancient lineage that diverges from the basal-most or next most basal node of the flowering plant phylogenetic tree (Qiu et al., 1999; APG III, 2009) and is represented among the oldest known angiosperm macrofossils (Friis et al., 2001, 2009; Wang and Dilcher, 2006; Mohr et al., 2008; Taylor et al., 2008). Trithuria, the sole genus within the family Hydatellaceae, has recently been placed as sister to the water lilies, Nymphaeales sensu stricto (s.s.) (Nymphaeaceae + Cabombaceae; Saarela et al., 2007; Borsch et al., 2008). A number of morphological and anatomical features unite these two ancient groups (e.g. Rudall et al., 2007; Friedman, 2008; Endress and Doyle, 2009). However, the reproductive ecology and life history of Trithuria species are likely to be quite different from water lilies and have not yet been studied in detail in the wild.
Water lilies are herbaceous perennials that typically inhabit stable, permanently inundated habitats. Ondinea purpurea (= Nymphaea ondinea, Löhne et al., 2009) and Barclaya rotundifolia (both in Nymphaeaceae) are known to occupy habitats that experience seasonal dry-down. Both are perennials that survive seasonal dry periods as persistent rhizomes and tubers (Schneider and Carlquist, 1995; Williamson and Moseley, 1989). In contrast, ten of 12 species of Trithuria occupy ephemeral aquatic habitats. All ten are reported to be annuals that survive the dry period as seeds (Hamann, 1998; Gaikwad and Yadav, 2003; Sokoloff et al., 2008a). Annuals are extremely rare among extant basal angiosperms and are found only in Trithuria, and possibly in two species of Nymphaeaceae, Euryale ferox (Kadono and Schneider, 1987) and Victoria cruziana (C. Magdalena, Royal Botanic Gardens, Kew, UK, pers. comm.).
Water lilies are large plants, with large floating leaves, extensive rhizomes and flower sizes that range from 1–2 cm in diameter in Cabombaceae (Williamson and Schneider, 1993) to 30–50 cm wide in Victoria (Schneider and Williamson, 1993). In contrast, whole plants of Trithuria are often less than 1 cm in diameter and bear tiny ‘flowers’ (Fig. 1A, B). These small reproductive structures have characteristics of both flowers and inflorescences and they may represent a transitional, pre-floral stage in the evolution of the flower (Endress and Doyle, 2009; Rudall et al., 2009). Hereafter, we refer to them as ‘reproductive units,’ occasionally abbreviated as ‘RU’ (Rudall et al., 2007, 2009).
Fig. 1.
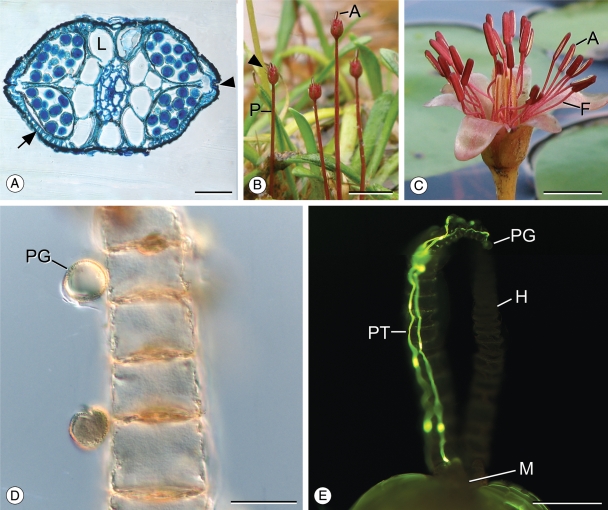
Morphology and development of Trithuria submersa. (A) Habit of T. submersa at Kulunilup Swamp, Kulunilup Nature Reserve. T. submersa plants are red (bright green leaves are immature Goodenia claytoniacea). (B) Reproductive units at various stages of emergence; completely submerged (stage 1; arrow), at the water level creating a depression (stage 2; arrowhead) and fully emergent (stage 3; asterisk). Scale bar = 5 mm. (C) One reproductive unit showing four bracts surrounding carpels with elongating stigmatic hairs and a single central stamen comprising a partially elongated filament and non-dehiscent anther (developmental stage 2). Note that one stigmatic hair is typically longer than the others. Scale bar = 500 µm. (D) One reproductive unit with two dehiscent anthers (developmental stage 4). Some stigmatic hairs have received pollen and cells have collapsed, causing stigmatic hairs to bend (arrowhead). Scale bar = 500 µm. (E) A single carpel from a stage 1 reproductive unit. Stigmatic hairs are short and cell length is much greater along the axis perpendicular to the stigmatic hair. Scale bar = 100 µm. (F) A composite image (two focal planes) showing a single carpel from a mature reproductive unit (stage 4) with two elongated and one short stigmatic hair. Stigmatic cells have elongated and some have collapsed (arrowheads). Grey line indicates the border between images. Scale bar = 100 µm. Abbreviations: A, anther; B, bract; C, carpel; F, filament; H, stigmatic hair; M, carpel mouth; O, ovule.
Given its phylogenetic position as sister to the rest of water lilies, Trithuria may offer important clues to the evolution of reproductive function among early angiosperms and Nymphaeales sensu lato (s.l.) in particular. To date, studies of Trithuria have concentrated on characterizing vegetative morphology (Edgar, 1966; Gaikwad and Yadav, 2003), pollen morphology (Bortenschlager et al., 1966; Remizowa et al., 2008), and developmental aspects of the reproductive unit (Rudall et al., 2007, 2009), shoot (Sokoloff et al., 2009), female gametophyte (Friedman, 2008; Rudall et al., 2008), seeds (Tuckett et al., 2010a, b) and seedlings (Cooke, 1983; Tillich et al., 2007; Sokoloff, et al., 2008b).
All Trithuria species are thought to be abiotically pollinated. Wind pollination has been hypothesized for Trithuria konkanensis and other species with emergent reproductive units, on the basis of floral morphology (Hamann, 1998; Gaikwad and Yadav, 2003). Water pollination has also been hypothesized as a possibility, particularly in the two permanently submerged species (Rudall et al., 2007). Nothing is known of breeding systems, apart from the fact that four species are dioecious, and hence obligately outcrossing (Yadav and Janarthanam, 1995; Sokoloff et al., 2008a). There are no data on the relative timing and duration of male and female function, a potentially important aspect of the breeding system of the other species that are monoecious or have bisexual reproductive units.
The objective of this study was to understand the reproductive ecology of Trithuria submersa, a species found in seasonal, rain-fed wetlands of south-western Western Australia, as well as parts of southern New South Wales, South Australia, Victoria and Tasmania (Sokoloff et al., 2008a). T. submersa was chosen because it is a widespread species with emergent, bisexual reproductive units. Our aims were to: (1) determine the primary pollen vector, (2) describe the relative timing and duration of anther dehiscence and stigma receptivity and (3) determine if self-pollination and self seed-set occur. We also report the pollen/ovule ratio (P/O) of dioecious Trithuria austinensis and pollen load size in Brasenia schreberi to enable a discussion of alternative life-history strategies among wind-pollinated Nymphaeales.
METHODS
Reproductive biology of T. submersa
Fieldwork on Trithuria submersa Hook.f. was undertaken in November/December 2008 at Kulunilup Swamp (Fig. 1A), Kulunilup Nature Reserve, Western Australia (34°19′S, 116°46′E). A second field season was undertaken in November/December 2009 at Kulunilup Swamp and nearby Frying Pan Swamp (34°16′S, 116°42′E). Laboratory work was conducted at the Department of Environment and Conservation Science Division facility in Manjimup, Western Australia, and at the University of Tennessee, Knoxville. Voucher specimens have been deposited in the University of Tennessee herbarium (TENN).
South-western Western Australia experiences cool, wet winters and hot, dry summers and exhibits a vast network of wetlands that undergo a strong seasonal hydrological cycle of winter flooding and summer drawdown (Hill et al., 1996). ‘Swamps’, or ‘sumplands’, are characterized by shallow standing water in late winter and spring, followed by complete evaporation of water over the course of a few days to a few weeks (Hill et al., 1996). These swamps can also be considered vernal pools (Holland and Jain, 1988; Rheinhardt and Hollands, 2008). Seeds of T. submersa germinate in swamps and plants mature while entirely submerged. Reproductive units become gradually exposed as the water level falls and flowering and fruit-set must be completed before the swamp dries out. T. austinensis, Trithuria australis and Trithuria bibracteata are also found in this region, with T. australis and T. bibracteata sometimes growing alongside T. submersa.
Reproductive development
Because of the small size of Trithuria plants, reproductive development was characterized by relative stages rather than by absolute time. A developmental sequence was reconstructed from a mass collection of open-pollinated T. submersa reproductive units (made without knowledge of developmental stage). To understand the effect of environment on reproductive development, the mass collection comprised equal numbers of haphazardly collected RUs in four distinct ecological settings: (1) reproductive units entirely submerged, (2) reproductive units newly emergent with between 50 and 75 % of the reproductive unit above water level, (3) reproductive units fully emergent but plants still partially submerged and (4) plants completely emergent (hereafter termed long emergent; see Fig. 1A, B).
Reproductive units were fixed in FAA (2 : 1 : 10 40 % formaldehyde, glacial acetic acid, 95 % ethanol) or 3 : 1 (95 % ethanol : glacial acetic acid) for 24 h and then stored in 70 % ethanol. Carpels were removed, stained with aniline blue for 4–8 h and viewed under UV light to visualize pollen grains (methods as described by Taylor and Williams, 2009). Onset and duration of pollen reception and stigma receptivity were assessed by recording the number of germinated pollen grains on each stigmatic hair at each developmental/ecological stage and the proportion of carpels exhibiting pollen germination and pollen loads were compared. As ungerminated pollen grains can wash off stigmatic hairs during fixation, only germinated grains were compared. If data were non-normally distributed, a non-parametric Wilcoxon rank sums test was performed. If variances were not equal, an unequal variance t-test was used (Ruxton, 2006). Analyses were performed via JMP v.7.0.2 statistical software (SAS Institute, Cary, NC, USA). When comparing three or more means after a significant one-way ANOVA, a Games–Howell post-hoc test was performed with SPSS 16·0 (SPSS Inc., Chicago, IL, USA). Individual plants were the experimental unit. All measures of variation in the text are standard deviations.
A second experiment used hand-pollinations of stage two and three reproductive units to determine if stigmas were receptive before anther dehiscence. Foreign pollen was excluded from haphazardly selected, fully submerged plants by covering them with clear plastic cups staked into the ground with wire. All reproductive units except the focal unit were first removed to prevent geitonogamous pollination within the ‘cage’. After the reproductive unit emerged within the cage, but before anthers dehisced, carpels were pollinated by gently brushing a dehiscent anther across stigmatic hairs. The pollen donor plant was 1–5 m distant from the caged plant and there was no evidence for rhizome connections between plants. Hand-pollinated reproductive units were collected within 6 h after pollination and fixed in 3 : 1. Carpels were scored for number of germinated pollen grains.
Pollination syndrome
To determine if entomophily occurred, insect behaviour in the population was assessed by direct observation (approx. 75 h). Twenty plants were marked with coloured thread and their reproductive units were observed periodically from emergence until anther opening and then continually until anthers were judged to be empty. The number of events in which insects contacted reproductive units and the activity of the insect (resting or foraging) was recorded.
To test for anemophily, 11 glass slides were thinly coated with petroleum jelly and placed at 1-m intervals in a transect through the Kulunilup Swamp population to trap wind-borne pollen. As anther dehiscence was common in late morning and early afternoon, slides were set at 1000 h and collected after 6 h to prevent exposure to afternoon rain. Pollen grains were counted and typed (no other Trithuria species in the population were reproductive at the time).
To determine the potential for hydrophily, 20 plants with developing reproductive units were kept submerged in the laboratory for 15 d. Anthers were monitored periodically, and RUs were collected well after bracts reflexed and were scored for pollen reception and anther opening.
Breeding system
A caging experiment tested for autonomous self-pollination and self-fertilization. Thirty plants with a single reproductive unit at Kulunilup Swamp were covered with cups as above to prevent cross-pollination and geitonogamy (cups also excluded wind as a pollen vector). Twelve plants were collected 3–5 d after emergence, fixed in 3 : 1 and the germinated pollen load was determined. The remaining 18 plants were collected 3 weeks later, after seeds were mature. Seed-set was calculated as the number of developed seeds divided by the total number of ovules and seeds (ovules could be easily seen through the carpel wall with a stereomicroscope). A developed seed was conspicuously larger than a mature ovule in an unpollinated reproductive unit and had a hard seed coat.
An emasculation experiment was designed to determine pollen load sizes with and without self-pollination. Immature anthers were removed from 23 reproductive units, which were then allowed to receive pollen naturally, including geitonogamous pollen. The 23 emasculated reproductive units and 23 untreated reproductive units were collected 15 d after emasculation or anther abscission to ensure maximum pollen reception. Carpels were fixed and stained with aniline blue and pollen was counted.
For analysis of pollen production, anthers of T. submersa were each macerated in 200 µl of 1 % polyethylene glycol (PEG) in 95 % ethanol and gently vortexed for 30 s. One tenth (20 µl) of the pollen mixture was placed on a glass slide and the entire cover slip was scanned. The number of observed pollen grains was multiplied by 10 to estimate the total number of grains per anther and this was multiplied by the number of anthers in the reproductive unit to estimate the total number per unit. The number of pollen grains per reproductive unit was divided by the number of carpels in the unit (one ovule per carpel) to obtain the P/O ratio.
For comparison, pollen production and P/O ratio in dioecious T. austinensis D. D. Sokoloff, Remizowa, T. D. Macfarl. & Rudall were calculated using 11 male and 11 female mature reproductive units collected at Branchinella Lake, shire of Manjimup, Western Australia (34°21′S, 116°43′E). One anther from each male reproductive unit was macerated in PEG and pollen production per anther and reproductive unit was determined as above. As T. austinensis is dioecious, average pollen production per male reproductive unit was divided by the average number of carpels (one ovule per carpel) in female reproductive units to obtain the P/O ratio. Also for comparison, stigmatic pollen loads were determined for open-pollinated flowers of B. schreberi J.F. Gmel (n = 16 plants; methods and location given by Taylor and Williams, 2009).
RESULTS
Reproductive development
Individual plants produced from one to 18 reproductive units (mean = 4·8 ± 3·7 in Kulunilup Swamp, 5·4 ± 4·4 in Frying Pan Swamp) over the course of the season. Reproductive units were borne singly on peduncles of different heights and emerged at different times (Fig. 1B). Most reproductive units possessed a single stamen (Fig. 1C), but 3 % from Kulunilup Swamp and 24 % from Frying Pan Swamp had two (Fig. 1D; n = 180 and 67, respectively). Reproductive units contained an average of 19·3 ± 6·4 carpels (n = 280, range 3–37) each with a single ovule and three uniseriate stigmatic hairs.
Buds enlarged under water and mature bracts partially reflexed (Fig. 1B–D), whether or not the reproductive unit had emerged. Once they reflexed, the bracts did not close again. The stamens and carpels continued to develop whether or not they were under water, but anther dehiscence did not occur until the reproductive unit had emerged.
Within open reproductive units, five distinct developmental stages could be characterized with respect to stamen development (Table 1). In stage 1, anthers and carpels were positioned at similar heights. Ovaries and their ovules had already attained their mature size (cf. Fig. 1E, F). Each of the three uniseriate stigmatic hairs was fully formed, but the cells had not expanded (mean height to width ratio of fifth cell = 0·31, n = 10; Fig. 1E).
Table 1.
Developmental sequences of reproductive units in Trithuria submersa
| Developmental stage | Stamen | Carpel |
|---|---|---|
| 1 | Filament not elongated, anther not above carpels | Stigmatic hairs short (mean of longest hair = 253 ± 58 µm; n = 7) |
| 2 | Filament partially elongated, anther beginning to emerge above carpel body, but not above stigmatic hairs | At least one stigmatic hair per carpel has begun elongating |
| 3 | Filament fully elongated, anther above carpel tops and many of the stigmatic hairs. Anther not dehiscent | Typically two stigmatic hairs per carpel have elongated (mean = 463 ± 172 µm; n = 15) |
| 4 | Filament fully elongated as in stage 3. Anther dehiscent | Stigmatic hairs as in stage 3, some hairs bent with collapsed cells |
| 5 | Anther abscised | Stigmatic hair length as in stage 3 (mean = 480 ± 239 µm; n = 10) |
In stage 2, anther filaments had elongated but had not reached their full length, and the anthers protruded above carpels, but not the stigmatic hairs (Fig. 1C). The stigmatic hairs had partially elongated via cell expansion, with one hair typically longer than the others. Cells near the base of the stigmatic hair elongated first, whereas cells at the tip rarely expanded (Fig. 1C, F).
In stage 3, the anthers were positioned above most or all of the stigmatic hairs. The longest stigmatic hairs had more than doubled in length to their mature size (mean height to width ratio = 0·76, n = 15; Fig. 1F). The second stigmatic hair often remained slightly shorter than the first whereas the third stigmatic hair did not elongate in any of the carpels observed (Fig. 1F).
Stage 4 was characterized by anther dehiscence (Fig. 1D). Anthers opened along two longitudinal lines of dehiscence that extended the length of the anther (Fig. 2A, B). Dehiscence was observed at all times of day, and anthers generally emptied within a few minutes after opening. At this stage stigmatic hairs often exhibited one or more collapsed cells, causing the stigmatic hair to bend or curl. Cells in the top half of the hair were more prone to collapse than those near the base (Fig. 1D, F). Occasionally, filaments were also observed to bend, lowering the dehiscent anther toward the stigmatic hairs (Fig. 1D). After dehiscence, anthers abscised, leaving the filament – this indicated the onset of stage 5 (Table 1).
Fig. 2.
Pollination and pollen reception in Trithuria submersa. (A) Cross-section of a dehiscent anther showing numerous endothecial bands (arrow), aerenychma ground tissue and the two stomia just beginning to open (one indicated by an arrowhead). Scale bar = 50 µm. (B) Mature reproductive units of T. submersa with fully elongated filaments supporting dehiscing anthers (developmental stage 4). The stigmatic hairs are visible above and between the reflexed bracts (arrowhead). Scale bar = 5 mm. (C) Staminate flower of wind-pollinated Brasenia schreberi with dehiscing anthers supported by long, slender filaments. Compare to B. Scale bar = 5 mm. (D) Receptive stigmatic hair (from a developmental stage 3 reproductive unit) with germinating pollen grains (two in plane of focus). Scale bar = 25 µm. (E) Carpel in which cross-pollination was prohibited, with self-pollen tubes successfully reaching the carpel mouth. Scale bar = 150 µm. Abbreviations: A, anther; F, filament; L, aerenchyma; M, carpel mouth; H, stigmatic hair; P, peduncle; PG, pollen grain; PT, pollen tube.
In contrast to other water lilies, in which the entire perianth closes after anthesis, bracts of T. submersa remained reflexed throughout fruit development. Fruits appeared mature and were falling out of reproductive units within 17 d of dehiscence. Seeds from reproductive units that were naturally pollinated on 10–16 November 2008 were collected on 3 December, stored dry at room temperature (approx. 21 °C) and planted in saturated soil (18 °C), on 15 March 2009. These germinated while submerged and began flowering on 6 October 2009. Seeds collected on 1 December 2009 were stored as above and planted in chilled (10 °C), saturated soil on 1 July 2010. These first germinated 30 d later.
Pollination syndrome
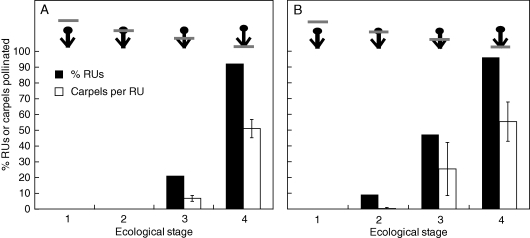
Two indications that T. submersa might experience pollination by water were (1) buds opened, stigmatic hairs and stamen filaments elongated, and pollen matured under water, and (2) aerenchyma was present in the connective tissue of the anther (Fig. 2A). However, anthers were never dehiscent in either naturally or experimentally submerged reproductive units. Furthermore, submerged reproductive units (ecological stage 1) never received pollen (Fig. 3A, B), despite having fully elongated stigmatic hairs. Even among newly emergent reproductive units (ecological stage 2) only a single stigmatic hair received pollen (Fig. 3B). Only emergent reproductive units received pollen (Fig. 3).
Fig. 3.
Natural pollen reception by ecological stage of Trithuria submersa. Pollen reception occurs only in partially or fully emergent reproductive units. The percentage of reproductive units (black bars) or carpels per reproductive unit (white bars) that were naturally pollinated at each of the four ecological stages (1–4) is shown for each population. (A) Kulunilup Swamp (2008; n = 25, 25, 82, 35). (B) Frying Pan Swamp (2009; n = 15, 11, 17, 13). The position of the water level (grey bar) is represented relative to the reproductive unit (black circles). Error bars = 95 % confidence intervals.
Nine insect visits to Trithuria plants were recorded over the course of two years of field observations. In all nine cases insects only rested on bracts. No foraging behaviour within reproductive units was ever observed, although two of 264 anthers in the fixed material appeared to have been partially eaten.
Sixty-four of 427 pollen grains on glass slides were from T. submersa. Anthers were also observed shedding pollen in the wind. Pollen was not sticky and exhibited a smooth exine (Fig. 2D). On stigmas, pollen grains measured 18·5 ±1·7 µm by 15·8 ±1·8 µm. A dehiscent anther(s) is held above the reproductive unit, which is in turn held above the vegetative body by a long slender peduncle (0·5–3·0 cm long and 0·4 mm wide; Figs 1A, B and 2B). Anther morphology of T. submersa was nearly identical to that of wind-pollinated water lily, B. schreberi (Cabombaceae; Fig. 2B, C), including the presence of robust endothecial bands (Fig. 2A; see also Taylor and Osborn, 2006).
Breeding system
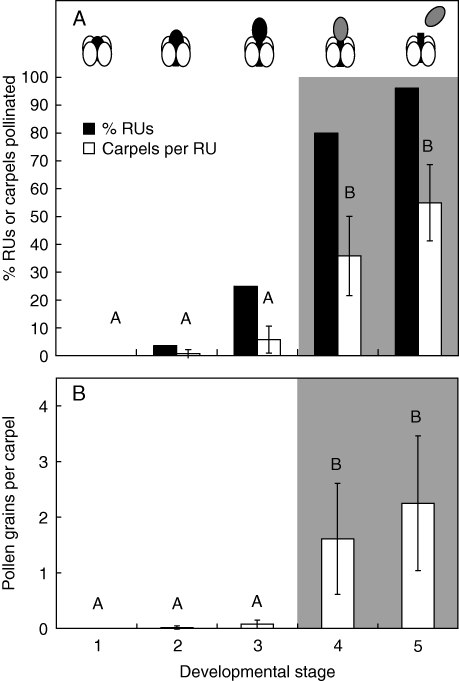
In the mass collection experiment, only one emergent reproductive unit received pollen during developmental stages 1 and 2 and no pollen germination was observed (Fig. 4A, B). In stage 3, 25 % of the reproductive units and 6 % of carpels per reproductive unit exhibited stigmas with germinated pollen (Fig. 4A). However, the average germinated pollen load in stage 3 was small (0·08 grains per carpel; Fig. 4B). Within reproductive units that received pollen, 23 % of carpels received pollen and the average pollen load per carpel was 1·2 ± 0·5. The maximum pollen load was 3. In the hand-pollination experiment, nine of 21 reproductive units, all in stage 3, had growing pollen tubes. As anthers were indehiscent when stigmatic hairs became receptive in stage 3, the onset of female function occurs before the onset of male function in the bisexual reproductive unit in T. submersa.
Fig. 4.
Natural pollen reception by stamen developmental stage of Trithuria submersa at Kulunilup Swamp. Outcross pollen reception can occur during developmental stages 1–3, whereas potential for self-pollination occurs during stages 4 and 5 (grey background). Developmental stages are categorized by the position of non-dehiscent anthers (black ovals) or dehiscent anthers (grey ovals) relative to that of the carpels (white ovals). (A) Percentage reproductive units (black bars) and carpels per reproductive unit (white bars) that received pollen at each developmental stage (1–5). The percentage of carpels pollinated is significantly higher in stages 4 and 5 than in stages 1–3 (ANOVA/Games–Howell: F = 32·32, d.f. = 4, P < 0·0001). (B) Pollen load per carpel in emergent reproductive units at each developmental stage. Pollen loads are significantly higher in stages 4 and 5 than in stages 1–3 (ANOVA/Games–Howell test: F = 11·58, d.f. = 4, P < 0·0001). Error bars = 95 % confidence interval. n = 15, 28, 28, 18, 22, respectively. Submerged and water level reproductive units (ecological stages 1 and 2) were excluded from analyses of pollen reception by developmental stage.
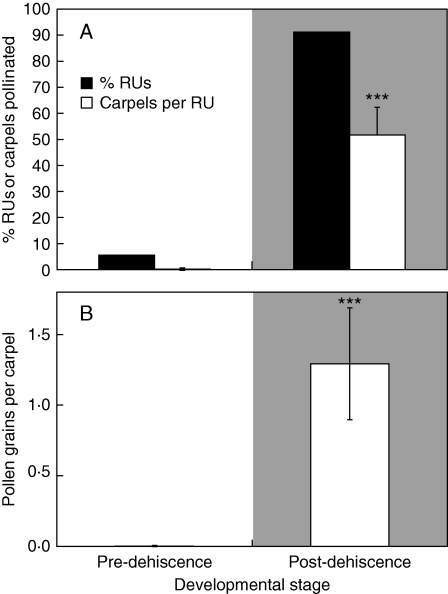
Reproductive units with dehiscent anthers (stages 4 and 5) received much more pollen than those prior to dehiscence (stages 1–3). In 2008, almost all post-dehiscent reproductive units had received pollen; the percentage of pollinated carpels per RU was significantly higher than that of pre-dehiscent RUs (Fig. 4A), and pollen loads were also higher (Fig. 4B). In 2009, 6 % of reproductive units received pollen prior to anther dehiscence, whereas 91 % received pollen after dehiscence (Fig. 5A), and both the percentage of carpels pollinated and pollen loads were significantly higher (Fig. 5A, B).
Fig. 5.
Natural pollen reception by stamen developmental stage in Trithuria submersa at Frying Pan Swamp. Reproductive units with dehisced anthers could have received autonomous self pollen, as well as geitonogamous self and outcross pollen (grey background), whereas those without dehiscent anthers could only have received outcross or geitonogamous self pollen (white background). (A) Percentage reproductive units (black bars) or carpels per reproductive unit (white bars) that received pollen (Wilcoxon rank sums: χ2 = 30·21, d.f. = 1, ***P < 0·0001). (B) Pollen load per carpel in emergent reproductive units before or after anther dehiscence (Wilcoxon rank sums: χ2 = 30·20, d.f. = 1, ***P < 0·0001). Error bars = 95 % confidence interval. n = 18 and 23, respectively. Submerged reproductive units (ecological stage 1) were excluded from this analysis.
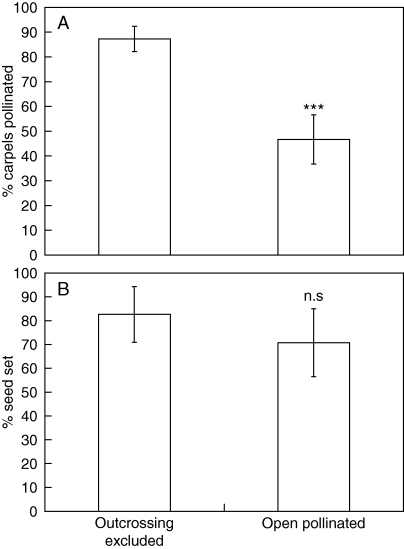
In the caging experiment, outcross or geitonogamous self-pollination by wind was prevented so that only autonomous self-pollination by gravity could occur. In caged plants, 100 % of reproductive units and a mean of 87 % of carpels per RU received germinable pollen, a significantly higher percentage than that of open-pollinated plants (Fig. 6A). The average stigmatic pollen load was 5·8 ± 1·8 on caged plants versus 2·3 ± 2·7 on stage 5 open-pollinated plants. Mean seed-set of the 18 caged plants was not significantly different from the open-pollinated control (Fig. 6B).
Fig. 6.
Self-pollen reception by caged and uncaged plants. (A) The percentage of carpels per reproductive unit that received pollen when cross-pollination was either experimentally excluded to allow only autonomous self-pollination (n = 12) or allowed to occur naturally (n = 46). Treatments were significantly different (Wilcoxon rank sums: χ2 = 14·82, d.f. = 1, ***P < 0·0001). (B) Seed set in reproductive units in which cross-pollination was experimentally excluded (n = 18) or not (n = 14). Treatments were not significantly different (Wilcoxon rank sums: χ2 = 3·40, d.f. = 1, P = 0·065). Error bars = 95 % confidence interval.
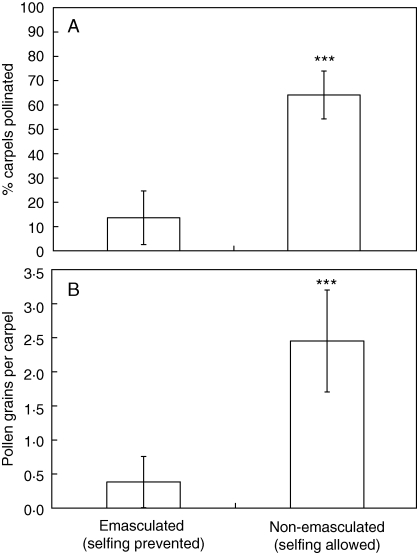
The emasculation experiment showed that reproductive units received outcross or geitonogamous pollen. These plants received significantly fewer pollen grains (14 % versus 64 % of carpels per RU; Fig. 7A) and had smaller pollen loads than the open-pollinated control (0·38 ± 0·9 versus 2·5 ± 1·7 pollen grains per carpel; Fig. 7B). For comparison, the mean pollen load per carpel of B. schreberi, which receives only outcross or geitonogamous pollen from a separate inflorescence, was 3·0 ± 3·1.
Fig. 7.
Outcross pollination in emasculated reproductive units. (A) The percentage of carpels per reproductive unit that received pollen when self-pollination was prevented by emasculation (n = 23) compared with untreated reproductive units in which self-pollination could occur (n = 23). Treatments were significantly different (t = 7·07, d.f. = 44, ***P < 0·0001). (B) Pollen load per carpel in emasculated reproductive units (n = 23) compared with open-pollinated reproductive units (n = 23). Treatments were significantly different (unequal variance t-test: t′ = 5·12, d.f. = 32·42, ***P < 0·0001). Error bars = 95 % confidence interval.
Mature anthers in T. submersa contained 426·0 ± 149·4 pollen grains and the average P/O ratio was 23·9 (n = 27). Reproductive units of T. austinensis had similar numbers of ovules as T. submersa (17·1 vs. 19·3), but more anthers (7·9 vs. 1·1) and much greater pollen production of 3526 grains per anther and 27 650 grains per RU. Its P/O ratio was 1569·1 (n = 11). Anthers in T. austinensis measured 1·96 mm long × 0·52 mm wide × 0·29 deep, compared with 0·71 × 0·32 × 0·22 mm in T. submersa (n = 5 each).
DISCUSSION
Trithuria species are similar to other Nymphaeales in that they begin development while totally submerged. However, unlike most water lilies that occur in more or less permanent aquatic environments, nearly all Trithuria species are found in ephemeral wetlands. Vernal pool plants are typically small and exhibit fast vegetative and reproductive development (Zedler, 1990). Plants of T. submersa are quite small (<1 cm in diameter) and their reproductive function, triggered by water drawdown, was short and locally unpredictable. The period between fertilization and seed dispersal was also short, only 17 d in 2008. Small plant size and the brief window for reproduction underlie many aspects of the reproductive biology of T. submersa. Below we discuss the reproductive development of T. submersa in light of its probable aquatic, perennial ancestry and the evolution of its ephemeral wetland ecology.
Pollination syndrome
Hydrophily in T. submersa was a distinct possibility because reproductive units often opened under water, and both stamens and carpels reached mature sizes while submerged. At least two other Trithuria species may carry out their entire life history under water (Edgar, 1966; Pledge, 1974; Rudall et al., 2007). However, two observations indicate that desiccation was necessary for anther dehiscence. First, only emergent anthers dehisced, whether in collected material or in plants that were kept artificially submerged for up to 15 d. Second, anthers possessed endothecial bands, which are known to facilitate anther opening upon desiccation and are not present in submerged anthers of water-pollinated plants (D'Arcy, 1996; Endress, 1996). Our data also show that emergence was necessary for pollen reception. Stigmatic hairs from mass-collected submerged reproductive units received no pollen and artificially submerged plants set no seed.
Emerging reproductive units created an indentation in the water surface that might serve to draw floating pollen or abscised anthers toward the stigmatic hairs (Fig. 1B). Many aquatic taxa, including several seagrasses (Cymodoceaceae, Hydrocharitaceae, Zosteraceae), achieve pollination via floating pollen or anthers that physically contact receptive stigmas (Cox, 1988; Cox and Humphries, 1993). Among basal angiosperms, both Ceratophyllum (Ceratophyllaceae) and Lepilaena cylindrocarpa (Potamogetonaceae) have anthers that abscise under water and float to the surface to release pollen (Cox, 1988). Anthers of T. submersa also abscise and the large lacunae in the connective tissue suggest anthers could float on the water surface. However, in T. submersa pollen was dry, anthers emptied before they abscised and partially emergent reproductive units received very little pollen. We conclude that T. submersa is not water-pollinated.
We also ruled out insect pollination. Extensive observation over the course of two field seasons in several populations indicated that insects did not interact with Trithuria reproductive units, and there was very little evidence of pollen scavenging. Pollinivory is quite common in many basal angiosperms (Thien et al., 2009), but in T. submersa the reward would be quite small, given how little pollen was present in an anther. Under insect pollination, large pollen loads might be expected within at least some RUs, but on emasculated RUs, no stigma received more than three pollen grains and most received no pollen at all.
Experimental, observational and anatomical evidence indicates that T. submersa at least occasionally exhibits wind-pollination. Pollen traps captured wind-borne pollen and small numbers of viable pollen were received on stigmas of emasculated and female phase reproductive units. Pollen in T. submersa is not sticky, lacks ornamentation and is at the small end of the size range of wind-dispersed pollen (Friedman and Barrett, 2009). Reproductive units lack a perianth or showy bracts, dehiscent anthers are elevated on elongated filaments, and receptive stigmatic hairs often extended beyond the bracts. Organ placement that reduces interference is common in wind-pollinated plants (Whitehead, 1969; Friedman and Barrett, 2009).
Pollination and the breeding system
T. submersa can be characterized as self-pollinated, self-compatible and primarily autogamous. Stigmas are receptive before and during anther dehiscence. Thus, stigmas can receive outcross or geitonogamous (self) pollen prior to autonomous self-pollination by the overarching anther. Outcross pollination was exceptionally rare – when reproductive units were emasculated more than 86 % of carpels received no pollen at all. Yet in unemasculated plants that could outcross and self, over 60 % of carpels received pollen. Pollen reception was even greater on caged plants in which only autonomous self-pollination could occur. These results indicate autonomous self-pollination compensates for the lack of outcross pollen reaching stigmas of T. submersa.
Open-pollinated plants had 71 % seed-set, a result inconsistent with the extremely low levels of cross-pollination, unless self-pollination is ubiquitous and plants are self-compatible. Self-compatibility was confirmed by the 83 % seed-set of self-pollinated plants in the caging experiment. Germination of open-pollinated seeds in the greenhouse indicates seeds have high viability, as also found by Tuckett et al. (2010b) for the same species. Our data show that such seeds were probably self-fertilized, and therefore have low levels of inbreeding depression. Another indication of long-term inbreeding is the low P/O ratio of 24. Such a low P/O ratio is consistent with obligate autogamy (Cruden, 1977).
It could be argued that the exceptionally low P/O ratio of T. submersa evolved in large part because extreme reduction in plant size caused reduced pollen production, leading to the subsequent evolution of autogamy. Its reproductive units are only 2 mm wide and have a mean of 1·1 anthers with 426 pollen grains per anther. However, T. austinensis, a close relative that is of similarly small size, produces about eight times more anthers and eight times more pollen per anther, even though it has slightly larger pollen than T. submersa. Thus, the extremely low pollen production of T. submersa, the proximate cause of its low P/O ratio, cannot be due to small plant size alone. To underline the point, the P/O ratio of 1569 of T. austinensis is within the low end of the range of those of other dioecious and wind-pollinated species (Cruden, 2000; Michalski and Durka, 2010). Thus, we interpret the low P/O ratio of T. submersa as being causally linked to the evolution of an obligately autogamous breeding system.
Given our conclusions, traits typically associated with cross-pollination, such as the onset of stigma receptivity prior to anther dehiscence, pollination by wind and a somewhat sequential maturation of RUs, now seem to have little function. Perhaps one of the best-supported conclusions of recent comparative studies of basal angiosperms is that bisexual flowers were ancestrally protogynous (Endress, 2010). As such, the prior onset of stigma receptivity in T. submersa is probably an historical effect and, if so, would indicate a similar level of developmental integration within a bisexual RU (Trithuria) as within a bisexual flower (other basal angiosperms). Its retention in present-day T. submersa may be less related to facilitating cross-pollination than to its function in ensuring self-pollination. Early onset of a long period of stigmatic receptivity is currently maintained because of the uncertainty in timing of anther dehiscence and the predominant mode of pollen reception – autonomous self-pollination.
Reproductive strategies of wind-pollinated Nymphaeales
Many of the structural features associated with wind pollination in T. submersa (pollen, anthers and reproductive units) are also present in other Trithuria species. It may be that all but the two perennial species with submerged reproductive units have some degree of wind-pollination, as hypothesized by Hamann (1998). Within the sister lineage to Hydatellaceae, Nymphaeales s.s., insect pollination is generally thought to be plesiomorphic (e.g. Friis et al., 2001; Borsch et al., 2008) and the only documentation of wind-pollination in the group is in B. schreberi (Osborn and Schneider, 1988). Here we compare ecological and historical aspects of the evolution of anemophily in Trithuria and Brasenia, as well as the subsequent shift from a primarily wind-pollinated to a primarily autonomously self-pollinated reproductive system in present-day T. submersa.
There are strong indications that the common ancestor of Nymphaeales s.l. was an aquatic perennial, that was homoecious (monoecious or with bisexual reproductive units) with female organs maturing before male organs (Friis et al., 2001; Crepet et al., 2004; Wang and Dilcher, 2006; Mohr et al., 2008; Taylor et al., 2008; Endress and Doyle, 2009; Endress, 2010). The major ecological difference between Nymphaeales s.s. (Cabombaceae + Nymphaeaceae) and Hydatellaceae involves their aquatic environment – the former are large perennials that typically occupy permanently inundated habitats (Williamson and Schneider, 1993), whereas the latter are extremely small and most are annuals that live in a seasonal aquatic environment (only two species of Trithuria occur in permanently inundated habitat and both retain the ancestral perennial habit) (Edgar, 1966; Pledge, 1974; Sokoloff et al., 2008a).
Wind pollination is favoured in dry, open environments (Whitehead, 1969; Culley et al., 2002; Friedman and Barrett, 2009), yet wind can also be a reliable pollen vector in the large, open habitats of many aquatic plants that flower above water. Long-term persistence in predictable environments with seasonal cues can also enable a high degree of synchronization of flowering within a population to evolve (Whitehead, 1969). Synchrony of flowering is much stronger in B. schreberi (closely coincident onset timing and of much shorter duration) than in other insect-pollinated Nymphaeales (Osborn and Schneider, 1988; Taylor and Williams, 2009). It also occurs in the context of the strict dichogamy typical of Nymphaeales – female phase occurs on the first day of anthesis and male phase begins on the second day, a pattern present in most basal angiosperms (Endress, 2010). Floral buds also open sequentially on B. schreberi shoots, minimizing the potential for geitonogamy. We found that outcross pollen transfer by wind was successful in B. schreberi and we have observed high seed set in several populations, both in the south-eastern USA and in Australia (M. L. Taylor and J. H. Williams, 2009, pers. observ.). The species has a cosmopolitan distribution (Williamson and Schneider, 1993).
The evolution of wind pollination in Trithuria has a quite different history. Extreme reduction in plant size occurred before the radiation of extant species. Subsequently, the annual species have shifted from their ancestral perennial habit in permanent wetlands to seasonal wetlands. T. austinensis reflects one outcome of such a shift. It typically grows in shallow wetlands in a very dense carpet, forming large populations that are fairly constant in size from year to year. Gradual water drawdown causes many neighbouring plants along the receding wetland margins to emerge and to flower simultaneously in an open environment. Pollen production and P/O ratios were relatively high, consistent with wind-pollination and outcrossing. Large population size, dioecy and predictable environmental cues all favour the origin and maintenance of outcrossing by wind-pollination (Friedman and Barrett, 2008, 2009).
An alternative, and perhaps more derived strategy is represented by T. submersa, which has bisexual reproductive units, but in which dichogamy has been lost. In T. submersa cross-pollination via wind can occur but was rare in both study years. T. submersa is found in disturbed, early-successional wetland habitats, such as in roadside ditches or in shallow depressions in recently burned areas. Relative to T. austinensis, its populations are small and ephemeral, and individuals are not as densely distributed. Furthermore, the topography and surrounding vegetation in their small patchy habitats forms a more closed environment for these small plants, greatly reducing exposure to wind. Although plants matured fairly synchronously in both years, their reproductive units did not. Small differences in plant height or relative ground level often caused neighbouring reproductive units to emerge, and anthers to dehisce, hours or even days apart. Thus, low population density, non-synchrony of pollen dispersal and the often closed habitat are all causes of ineffective outcross pollen transfer by wind. The shift to an effective system of autonomous self-pollination and primarily autogamous reproduction has exacerbated the effect by allowing lower pollen production.
Self-fertilization has long been associated with the ability to colonize and succeed in pioneer habitats (Stebbins, 1970) and one indicator of success is that T. submersa is one of the most widespread Trithuria species. Pioneer habitats are not typically colonized by obligately outcrossing T. austinensis. On the other hand, T. bibracteata is considered to be a pioneer species, and although its breeding system is unknown, it has bisexual reproductive units like T. submersa. Understanding the significance of the great reproductive diversity in Trithuria in the context of early angiosperm evolution will certainly require a greater appreciation of the intimate connection between their reproductive biology and their natural ecological settings.
Conclusions
Nymphaeales is thought to be ancestrally aquatic, perennial and probably insect-pollinated. In contrast, most species of Trithuria are annuals and inhabit seasonal wetlands. Among these, T. submersa has evolved to colonize disturbed, early-successional habitats. Protogyny and prolonged stigma receptivity may have initially promoted outcrossing with reproductive assurance via delayed selfing. The overlapping male and female phases now seem to function primarily to ensure autonomous self-pollination and high seed set in an unpredictable and heterogeneous pollination environment.
ACKNOWLEDGEMENTS
We thank R. Robinson and R. Bowles at the Department of Environment and Conservation Science Division, Manjimup, WA, for providing logistical support and use of facilities, as well as R. Hearn for invaluable assistance and discussion. We also thank M. T. Lettre for assistance with pollen counts, A. K. Becker for field assistance, and J. M. Abercrombie, N. E. Buckley, T. S. Feild, E. E Schilling and especially four anonymous reviewers for comments on the manuscript. This work was supported by a National Science Foundation (NSF) Doctoral Dissertation Improvement Grant to M.L.T. (DEB 0910171) and by an NSF award (DEB 0640792) to J.H.W.
LITERATURE CITED
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnaean Society. 2009;161:105–121. [Google Scholar]
- Borsch T, Löhne C, Wiersema J. Phylogeny and evolutionary patterns in Nymphaeales: integrating genes, genomes and morphology. Taxon. 2008;57:1052–1081. [Google Scholar]
- Bortenschlager S, Erdtman G, Praglowski J. Pollenmorphologische Notizen über einige Blütenpflanzen incertae sedis. Botaniska Notiser. 1966;119:160–168. [Google Scholar]
- Cooke DA. The seedlings of Trithuria (Hydatellaceae) Victorian Naturalist. 1983;100:68–69. [Google Scholar]
- Cox PA. Hydrophilous pollination. Annual Review of Ecology and Systematics. 1988;19:261–280. [Google Scholar]
- Cox PA, Humphries CJ. Hydrophilous pollination and breeding system evolution in seagrasses: a phylogenetic approach to the evolutionary ecology of the Cymodoceaceae. Botanical Journal of the Linnean Society. 1993;113:217–226. [Google Scholar]
- Crepet WL, Nixon KC, Gandolfo MA. Fossil evidence and phylogeny: the age of major angiosperm clades based on mesofossil and macrofossil evidence from Cretaceous deposits. American Journal of Botany. 2004;91:1666–1682. doi: 10.3732/ajb.91.10.1666. [DOI] [PubMed] [Google Scholar]
- Cruden RW. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution. 1977;31:32–46. doi: 10.1111/j.1558-5646.1977.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Cruden RW. Pollen grains: why so many? Plant Systematics and Evolution. 2000;222:143–165. [Google Scholar]
- Culley TM, Weller SG, Sakai AK. The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution. 2002;17:361–369. [Google Scholar]
- D'Arcy WG. Anthers and stamens and what they do. In: D'Arcy WG, Keating RC, editors. The anther: form, function, and phylogeny. New York: Cambridge University Press; 1996. pp. 1–24. [Google Scholar]
- Edgar E. The male flowers of Hydatella inconspicua (Cheesem.) Cheesem. (Centrolepidaceae) New Zealand Journal of Botany. 1966;4:153–158. [Google Scholar]
- Endress PK. Diversity and evolutionary trends in angiosperm anthers. In: D'Arcy WG, Keating RC, editors. The anther: form, function, and phylogeny. Cambridge: Cambridge University Press; 1996. pp. 92–110. [Google Scholar]
- Endress PK. The evolution of floral biology in basal angiosperms. Philosophical Transactions of the Royal Society B. 2010;365:411–421. doi: 10.1098/rstb.2009.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK, Doyle JA. Reconstructing the ancestral angiosperm flower and its initial specializations. American Journal of Botany. 2009;96:22–66. doi: 10.3732/ajb.0800047. [DOI] [PubMed] [Google Scholar]
- Friedman J, Barrett SCH. A phylogenetic analysis of the evolution of wind pollination in the angiosperms. International Journal of Plant Sciences. 2008;169:49–58. [Google Scholar]
- Friedman J, Barrett SCH. Wind of change: new insights on the ecology and evolution of pollination and mating in wind-pollinated plants. Annals of Botany. 2009;103:1515–1527. doi: 10.1093/aob/mcp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman WE. Hydatellaceae are water lilies with gymnospermous tendencies. Nature. 2008;453:94–97. doi: 10.1038/nature06733. [DOI] [PubMed] [Google Scholar]
- Friis EM, Pedersen KR, Crane PR. Fossil evidence of water lilies (Nymphaeales) in the Early Cretaceous. Nature. 2001;410:357–360. doi: 10.1038/35066557. [DOI] [PubMed] [Google Scholar]
- Friis EM, Pedersen KR, von Balthazar M, Grimm GW, Crane PR. Monetianthus mirus gen. et sp nov., A Nymphaealean flower from the Early Cretaceous of Portugal. International Journal of Plant Sciences. 2009;170:1086–1101. [Google Scholar]
- Gaikwad SP, Yadav SR. Further morphotaxonomical contribution to the understanding of family Hydatellaceae. The Journal of the Swamy Botanical Club. 2003;20:1–10. [Google Scholar]
- Hamann U. Hydatellaceae. In: Kubitzki K, editor. Flowering plants, monocotyledons: Alismatanae and Commelinanae (except Gramineae). Berlin: Springer-Verlag; 1998. pp. 231–234. [Google Scholar]
- Hill AL, Semeniuk CA, Semeniuk V, Del Marco A. Wetlands of the Swan Coastal Plain vol. 2a. Wetland mapping, classification and evaluation. 1996 Report for Water and Rivers Commission and Department of Environmental Protection; Perth, Western Australia. [Google Scholar]
- Holland RF, Jain SK. Barbour MG, Major J, editors. Vernal pools. Terrestrial vegetation of California. California Native Plant Society Special Publication. 1988;9:515–531. [Google Scholar]
- Kadono Y, Schneider EL. The life history of Euryale ferox Salisb. in southwestern Japan with special reference to reproductive ecology. Plant Species Biology. 1987;2:109–115. [Google Scholar]
- Löhne C, Wiersema JH, Borsch T. The unusual Ondinea, actually just another Australian water-lily of Nymphaea subg. Anecphya (Nymphaeaceae) Willdenowia. 2009;39:55–58. [Google Scholar]
- Michalski SG, Durka W. Pollen and ovule production in wind-pollinated species with special reference to Juncus. Plant Systematics and Evolution. 2010;286:191–197. [Google Scholar]
- Mohr BAR, Bernardes-de-Oliveira MEC, Taylor DW. Pluricarpellatia, a nymphaealean angiosperm from the Lower Cretaceous of northern Gondwana (Crato Formation, Brazil) Taxon. 2008;57:1147–1158. [Google Scholar]
- Osborn JM, Schneider EL. Morphological studies of the Nymphaeaceae sensu lato. XVI. The floral biology of Brasenia schreberi. Annals of the Missouri Botanical Garden. 1988;75:778–794. [Google Scholar]
- Pledge DH. Some observations on Hydatella inconspicua (Cheesem.) Cheesem. (Centrolepidaceae) New Zealand Journal of Botany. 1974;12:559–561. [Google Scholar]
- Qiu Y-L, Lee J, Bernasconi-Quadroni F, et al. The earliest angiosperms: evidence from mitochondrial, plastid, and nuclear genomes. Nature. 1999;402:404–407. doi: 10.1038/46536. [DOI] [PubMed] [Google Scholar]
- Remizowa MV, Sokoloff DD, Macfarlane TD, Yadav SR, Prychid CJ, Rudall PJ. Comparative pollen morphology in the early-divergent angiosperm family Hydatellaceae reveals variation at the infraspecific level. Grana. 2008;47:81–100. [Google Scholar]
- Rheinhardt RD, Hollands GG. Classification of vernal pools: geomorphic setting and distribution. In: Calhoun AJK, deMaynadier PG, editors. Science and conservation of vernal pools in northeastern North America. Boca Raton, FL: CRC Press; 2008. pp. 11–29. [Google Scholar]
- Rudall PJ, Sokoloff DD, Remizowa MV, et al. Morphology of Hydatellaceae, an anomalous aquatic family recently recognized as an early-divergent angiosperm lineage. American Journal of Botany. 2007;94:1073–1092. doi: 10.3732/ajb.94.7.1073. [DOI] [PubMed] [Google Scholar]
- Rudall PJ, Remizowa MV, Beer AS, et al. Comparative ovule and megagametophyte development in Hydatellaceae and water lilies reveal a mosaic of features among earliest angiosperms. Annals of Botany. 2008;101:941–956. doi: 10.1093/aob/mcn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudall PJ, Remizowa MV, Prenner G, Prychid CJ, Tuckett RE, Sokoloff DD. Nonflowers near the base of extant angiosperms? Spatiotemporal arrangement of organs in reproductive units of Hydatellaceae and its bearing on the origin of the flower. American Journal of Botany. 2009;96:67–82. doi: 10.3732/ajb.0800027. [DOI] [PubMed] [Google Scholar]
- Ruxton GD. The unequal variance t-test is an underused alternative to Student's t-test and the Mann–Whitney U test. Behavioral Ecology. 2006;17:688–690. [Google Scholar]
- Saarela JM, Rai HS, Doyle JA, et al. Hydatellaceae identified as a new branch near the base of the angiosperm phylogenetic tree. Nature. 2007;446:312–315. doi: 10.1038/nature05612. [DOI] [PubMed] [Google Scholar]
- Schneider EL, Carlquist S. Vessels in the roots of Barclaya rotundifolia (Nymphaeaceae) American Journal of Botany. 1995;82:1343–1349. [Google Scholar]
- Schneider EL, Williamson PS. Nymphaeaceae. In: Kubitzki K, Rohwer JG, Bitttrich V, editors. The families and genera of vascular plants, vol. 2. Flowering plants: dicotyledons. magnoliid, hamamelid and caryophyllid families. Berlin: Springer-Verlag; 1993. pp. 486–493. [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, Rudall PJ. Classification of the early-divergent angiosperm family Hydatellaceae: one genus instead of two, four new species and sexual dimorphism in dioecious taxa. Taxon. 2008a;57:179–200. [Google Scholar]
- Sokoloff DD, Remizowa MV, Macfarlane TD, et al. Seedling diversity in Hydatellaceae: implications for the evolution of angiosperm cotyledons. Annals of Botany. 2008b;101:153–164. doi: 10.1093/aob/mcm274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff DD, Remizowa MV, Briggs BG, Rudall PJ. Shoot architecture and branching pattern in perennial Hydatellaceae (Nymphaeales) International Journal of Plant Sciences. 2009;170:869–884. [Google Scholar]
- Stebbins GL. Adaptive radiation of reproductive characteristics in Angiosperms, I. Pollination mechanisms. Annual Review of Ecology and Systematics. 1970;1:307–326. [Google Scholar]
- Taylor DW, Brenner GJ, Basha SH. Scutifolium jordanicum gen. et sp. nov. (Cabombaceae), an aquatic fossil plant from the Lower Cretaceous of Jordan, and the relationships of related leaf fossils to living genera. American Journal of Botany. 2008;95:340–352. doi: 10.3732/ajb.95.3.340. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Osborn JM. Pollen ontogeny in Brasenia (Cabombaceae; Nymphaeales) American Journal of Botany. 2006;93:344–356. doi: 10.3732/ajb.93.3.344. [DOI] [PubMed] [Google Scholar]
- Taylor ML, Williams JH. Consequences of pollination syndrome evolution for post-pollination biology in an ancient angiosperm family. International Journal of Plant Sciences. 2009;170:584–598. [Google Scholar]
- Thien LB, Bernhardt P, Devall MS, et al. Pollination biology of basal angiosperms (ANITA grade) American Journal of Botany. 2009;96:166–182. doi: 10.3732/ajb.0800016. [DOI] [PubMed] [Google Scholar]
- Tillich H-J, Tuckett R, Facher E. Do Hydatellaceae belong to the monocotyledons or basal angiosperms? Evidence from seedling morphology. Willdenowia. 2007;37:399–406. [Google Scholar]
- Tuckett RE, Merritt DJ, Hay FR, Hopper SD, Dixon KW. Comparative longevity and low-temperature storage of seeds of Hydatellaceae and temporary pool species of south-west Australia. Australian Journal of Botany. 2010a;58:327–334. [Google Scholar]
- Tuckett RE, Merritt DJ, Rudall PJ, et al. A new type of specialized morphophysiological dormancy and seed storage behaviour in Hydatellaceae, an early-divergent angiosperm family. Annals of Botany. 2010b;105:1053–1061. doi: 10.1093/aob/mcq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Dilcher DL. Aquatic angiosperms from the Dakota Formation (Albian, Lower Cretaceous), Hoisington III locality, Kansas, USA. International Journal of Plant Sciences. 2006;167:385–401. [Google Scholar]
- Whitehead DR. Wind pollination in the angiosperms: evolutionary and environmental considerations. Evolution. 1969;23:28–35. doi: 10.1111/j.1558-5646.1969.tb03490.x. [DOI] [PubMed] [Google Scholar]
- Williamson PS, Moseley MF. Morphological studies of the Nymphaeaceae sensu lato. XVII. Floral anatomy of Ondinea purpurea subspecies purpurea (Nymphaeaceae) American Journal of Botany. 1989;76:1779–1794. [Google Scholar]
- Williamson PS, Schneider EL. Cabombaceae. In: Kubitzki K, Rohwer JG, Bitttrich V, editors. The families and genera of vascular plants, vol. 2. Flowering plants: dicotyledons. magnoliid, hamamelid and caryophyllid families. Berlin: Springer-Verlag; 1993. pp. 157–161. [Google Scholar]
- Yadav SR, Janarthanam MK. Trithuria konkanénsis (Hydatellaceae), eine neue Art aus Indien. Aqua-planta. 1995;3:91–97. [Google Scholar]
- Zedler PH. Vernal pool plants: their habitat and biology. Chico: California State University; 1990. Life histories of vernal pool vascular plants; pp. 123–146. Studies from the Herbarium No. 8. [Google Scholar]