Abstract
Background
The national prevalence and patterns of food allergy (FA) in the United States (US) are not well understood.
Objective
We developed nationally representative estimates of the prevalence of and demographic risk factors for FA, and investigated associations of FA with asthma, hay fever, and eczema.
Methods
8,203 participants in the National Health and Nutrition Examination Survey (NHANES) 2005–2006 had food-specific serum IgE measured to peanut, cow's milk, egg white, and shrimp. Food-specific IgE and age-based criteria were used to define Likely FA (LFA), Possible FA (PFA), and Unlikely FA (UFA), and to develop estimates of clinical FA. Self-reported data were used to evaluate demographic risk factors and associations with asthma and related conditions.
Results
In the US, the estimated prevalence of clinical FA was 2.5% (peanut 1.3%, milk 0.4%, egg 0.2%, shrimp 1.0%, not mutually exclusive). Risk of PFA/LFA was increased in non-Hispanic blacks (odds ratio (OR) 3.06; 95% confidence interval (CI) 2.14-4.36), males (1.87; 1.32-2.66), and children (2.04; 1.42-2.93). Study participants with doctor-diagnosed asthma (vs. no asthma) exhibited increased risk of all measures of food sensitization. Moreover, in those with LFA, the adjusted OR for current asthma (3.8; 1.5-10.7) and an emergency room (ER) visit for asthma in the past year (6.9; 2.4-19.7) were both notably increased.
Conclusion
Population-based serologic data on 4 foods indicate an estimated 2.5% of the US population has FA, and increased risk was found for blacks, males, and children. Additionally, FA could be an under-recognized risk factor for problematic asthma.
Keywords: asthma, eczema, egg, food allergy, food sensitization, food-specific serum IgE, peanut, hay fever, milk, prevalence, risk, shrimp
INTRODUCTION
Food allergy (FA) is a large and growing public health problem in the United States (US). Previous studies have estimated that US FA prevalence may be nearly 4%.1 Targeted studies have also found evidence of higher risk of FA among non-Hispanic blacks and associations between FA and asthma.2, 3, 4 As awareness of the problem continues to grow, child day cares, schools and public institutions are developing protective policies and health programs. Public institutions are also prioritizing funding for FA research.
A comprehensive population-level study on the prevalence of and risks associated with FA in the US would inform these public health efforts. The overall magnitude of the problem has been difficult to estimate, however, due to the lack of a representative nationwide sample of the total population together with an objective, clinical approach to measuring and defining FA. Many reports have focused on specific subpopulations or foods, or depended upon self-reported or clinical manifestations of FA, possibly excluding those with atypical or unrecognized symptoms.5, 6, 7
Knowledge of the clinically-measured, national prevalence of FA and the identification of populations at risk is needed to enable public health policy makers and care providers to prioritize, allocate resources, and develop plans to better recognize and treat FA. Furthermore, evidence suggests that FA is associated with and may exacerbate other immune mediated conditions. This association has not been confirmed in a nationally representative population.
The National Health and Nutrition Examination Survey (NHANES) recently completed the first objective serologic measurement of food sensitivity in a representative sample of the US population. Branum and Lukacs previously reported the prevalence of detectable IgE antibodies in children from these data, while estimating food allergy prevalence among children based upon self-report data from the National Health Interview Survey.2 In this paper, we use serologic data from both children and adults in the NHANES to derive and report the US population-based estimates of clinical FA, identify high-risk populations, and explore associations with other immune-mediated conditions.
METHODS
The NHANES 2005–2006 study cohort
The NHANES is a Centers for Disease Control and Prevention, National Center for Health Statistics program, designed to assess the health and nutritional status of adults and children in the US, that began in the early 1960's. NHANES 2005–2006 was the 7th nationally representative NHANES survey and included 10,348 participants from 30 sites across the continental US.8 The NHANES 2005-2006 protocol was approved by the National Center for Health Statistics, Centers for Disease Control and Prevention, Ethics Review Board.9 Blood was collected from 80.6% of the study participants. Serum was separated and frozen for laboratory testing including food-specific serum IgE panels to four common food allergens. Blood samples were insufficient to perform complete panels in 134 participants (1.3%). The remaining study population consisted of all participants with complete food-specific serum IgE panels (n = 8,203: 79.3%). Participants excluded due to incomplete or missing panels were younger, but otherwise not different with respect to a variety of demographic characteristics.
Participants were asked to self-identify their race and ethnicity, which NHANES combines into one race/ethnicity designation. Instead of actual family income, the Poverty Income Ratio (PIR) was used and represents the ratio of family income to the poverty threshold as defined by the US Census Bureau. PIR values below 1.0 are below the poverty threshold. PIR thresholds of 1.3 and 3.5 were used for classification in adjusted logistic models; these thresholds are used to establish eligibility for US food assistance programs.10
Household education was defined as the highest education level obtained by the household reference person (typically the household head).
Food sensitization and FA risk groups
Food panels consisted of assays to determine serum IgE concentrations specific to peanut, cow's milk, egg white, and shrimp allergens. Clinical studies have demonstrated that higher food-specific IgE levels indicate a greater likelihood of clinical FA.1, 11, 12 Food-specific serum IgE levels to peanut, milk, egg, and shrimp were measured in study participants ages 6+ years old, and to only peanut, milk and egg in 1–5 year old participants; the volume of blood draws and number of assays were limited in general among the youngest NHANES participants. IgE levels were also measured for 15 inhalant allergens.13 Serum IgE assays were performed at a central laboratory (Elmhurst Memorial Hospital, Elmhurst, IL) using standardized methodology (ImmunoCAP 1000; Phadia US; Portage, MI). The lower limit of allergen-specific IgE detection was 0.35 kU/L. Food sensitization was defined as having at least one food-specific serum IgE ≥ 0.35 kU/L and multiple food sensitization was defined as 2 or more food sensitizations out of the panel of four foods.
Among participants above this threshold for food sensitivity, we defined three mutually exclusive FA risk categories; Unlikely FA (UFA), Possible FA (PFA), and Likely FA (LFA). These FA risk groups were based upon prior studies relating food-specific IgE concentrations with diagnosis of food allergy using criteria that include diagnostic oral food challenges. Generally, lower food-specific IgE levels, between 0.35 and 2 kUa/L, have been associated with a relatively low probability (UFA) of reacting (10–20%); moderate IgE levels, between 0.7 and 5 kUa/L, have been associated with ~50% probability of reacting (PFA); and high IgE levels, between 5 and 15 kUa/L, have been associated with a 95% probability of reacting (LFA).1, 11,12 However, the exact 95% probability thresholds vary by age and the specific food type. Therefore, the following 95% predictive levels have been proposed, based on positive predictive values for clinical reactivity: egg, 7 kU/L; milk, 15 kU/L; and peanut, 14 kU/L.1 Clinical studies have also determined that 95% predictive levels differ for young children (i.e., ≤ 2 years old): egg, 2 kU/L14; milk, 5 kU/L.15 There is a lack of data correlating outcomes of allergy for shrimp with IgE levels and thus no well-established IgE cut-point for likely shrimp allergy. Therefore, shrimp was treated in accordance with the typical patterns described above, using a threshold of 5 kU/L. This appears reasonable based upon the limited data that do exist for shrimp.
When considering all four foods together, risk category assignment was based on the highest risk for FA across all food groups. Thus, UFA was defined as having at least one food-specific serum IgE between 0.35 and 2 kU/L, PFA as having at least one food-specific IgE between 2 kU/L and the applicable 95% predictive level, and LFA as having at least one food-specific IgE greater than or equal to the 95% predictive level.
Because participants with IgE sensitization at low levels (i.e., 0.35–2 kU/L) are less likely to have clinical FA, we were particularly interested in those with higher IgE levels associated with PFA or LFA. Clinical FA rates were estimated by summing 50% of participants with PFA plus 95% of participants with LFA, based on the probabilities of reacting in each of those risk categories, as described above. Thus, “clinical FA” may be interpreted to mean that ingestion of that food would reproducibly lead to clinical symptoms consistent with an acute allergic reaction.
Asthma, hay fever and eczema questions
We explored associations between FA and three other immune-mediated conditions: asthma, hay fever, and eczema. These conditions we identified from participant self-report of doctor diagnosis. Specifically, the following NHANES 2005-2006 survey questions were used to define these conditions:
Diagnosed asthma: Has a doctor or other health professional ever told you that you have asthma? (MCQ.010)
Current asthma: Do you still have asthma? (MCQ.035)
>ER visit for asthma in the prior year: During the past 12 months, have you had to visit an emergency room or urgent care center because of asthma? (MCQ.050)
>Diagnosed hay fever: Has a doctor or other health professional ever told you that you have hay fever? (AGQ.010)
Diagnosed eczema: Has a doctor or other health professional ever told you that you have eczema? (AGQ.180)
The 3 asthma questions were asked in the order indicated above. If an answer to an asthma question was not “YES”, then the remaining asthma questions were skipped.
Statistical analyses
NHANES participants are selected using a complex, multistage, probability sampling design and all percentages, means, percentiles, and odds ratios (ORs) were weighted to represent the civilian, non-institutionalized US population. Standard errors, CIs, and p-values were developed in accordance with the complex survey design by using Taylor series linearization methods. Statistical analyses were conducted using SAS statistical software (Version 9.2, Cary, NC) survey procedures. Logistic regression was performed to model participants with PFA or LFA compared to those with no FA with only demographic predictors included in the model (age, gender, race/ethnicity, income), and p-values for main effects reported. Prevalence rates for food sensitization and FA risk groups were calculated for three doctor-diagnosed health outcomes (asthma, hay fever, and eczema) and adjusted ORs were reported based upon logistic regression modeling. Prevalence rates were calculated for food sensitization and FA risk groups for 4 mutually exclusive levels of asthma status (no asthma, doctor-diagnosed asthma but not current, current asthma, and ER visit for asthma in the past 12 months). The Cochran-Armitage trend test was used to test for an increase in the prevalence of FA as asthma status becomes more current and more severe. Logistic regression was performed for the asthma status outcomes and ORs were reported. This logistic regression was performed as an unadjusted model using only FA status as a predictor and also as an adjusted model, which included inhalant sensitization along with the demographic predictors. 95% CIs were developed using t-statistics.
RESULTS
Study population characteristics
The study population was demographically diverse and representative of the US population (Table 1). Allergic sensitization (i.e., serum allergen-specific IgE level ≥ 0.35 kU/L) was common: 16.8% were sensitized to at least one food; 41.3%, to at least one inhalant allergen; and 13.9%, to at least one food and one inhalant allergen. Self-reported doctor-diagnosed asthma (14.6%), hay fever (10.8%), and eczema (9.1%) were also common. Of those with diagnosed asthma, 9.6% reported an emergency room or urgent care (ER) visit for asthma within 12 months.
Table 1.
Demographic and clinical features of the 2005–2006 NHANES FA cohort
| Descriptor | Nd | %e |
|---|---|---|
| Overall | 8203 | |
| Age (years) | ||
| 1-5 | 909 | 5.3 ± 0.4 |
| 6-19 | 2869 | 19.0 ± 0.6 |
| 20-39 | 1672 | 28.0 ± 0.9 |
| 40-59 | 1361 | 30.2 ± 1.1 |
| 60+ | 1392 | 17.6 ± 1.6 |
| Gender | ||
| Male | 3998 | 48.8 ± 0.5 |
| Female | 4205 | 51.2 ± 0.5 |
| Race/Ethnicity | ||
| non-Hispanic white | 3239 | 69.5 ± 2.9 |
| non-Hispanic black | 2124 | 11.8 ± 1.9 |
| Hispanica | 2461 | 13.1 ± 1.4 |
| Other | 379 | 5.6 ± 0.8 |
| Poverty Income Ratio (PIR) | ||
| <= 1.3 | 2669 | 20.0 ± 1.3 |
| 1.3-3.5 | 3003 | 38.5 ± 1.5 |
| > 3.5 | 2171 | 41.5 ± 2.3 |
| Household Education | ||
| High school or less | 4267 | 43.4 ± 2.0 |
| Above high school | 3676 | 56.6 ± 2.0 |
| Disease | ||
| Diagnosed Eczema | 715 | 9.1 ± 0.7 |
| Diagnosed Hay Fever | 586 | 10.8 ± 0.7 |
| Diagnosed Asthma | 1157 | 14.6 ± 0.6 |
| Current Asthma | 730 | 8.8 ± 0.5 |
| ER visitb | 123 | 9.6 ± 1.1 |
| Sensitizationc | ||
| Food | 1729 | 16.8 ± 0.6 |
| Inhalant | 3495 | 41.3 ± 1.1 |
| Both Food and Inhalant | 1350 | 13.9 ± 0.6 |
Defined as “Mexican American” or “Other Hispanic” in NHANES.
Among those with diagnosed asthma.
Sensitization: at least one allergen-specific serum IgE ≥ 0.35 kU/L. Food and inhalant categories are not mutually exclusive.
Unweighted counts.
Weighted % ± Standard Error.
Prevalence of food sensitization and clinical food allergy in the US
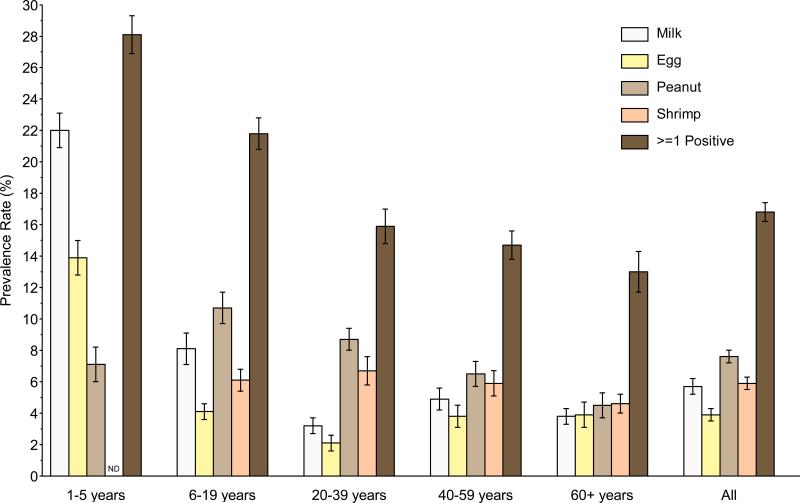
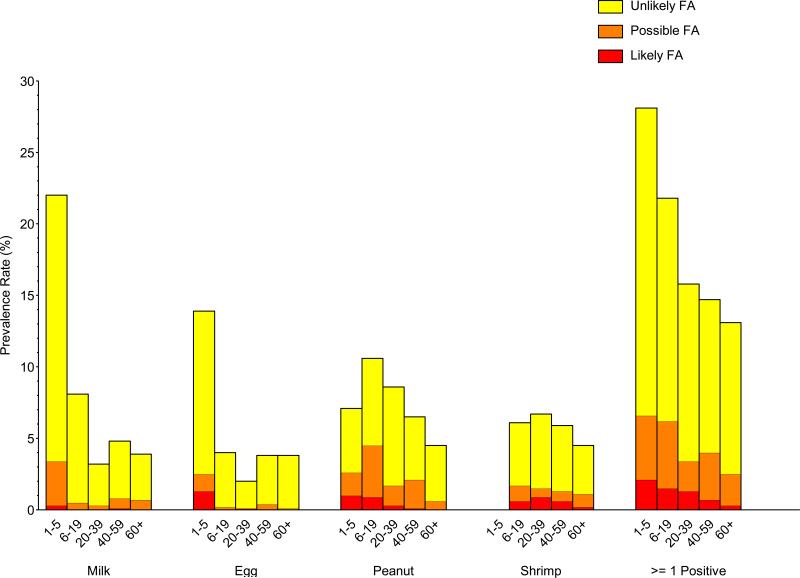
The overall prevalence of food sensitization in the US was 16.8% (Repository Figure E1; Table 2). By age, the highest prevalence was 28.1%, in 1–5 year olds, and declined steadily with age to 13.0%, in adults 60+ years old (Repository Figure E1, Repository Table E1). The prevalence of milk (22.0%) and egg (13.9%) sensitization was highest in 1–5 year old children. Peanut sensitization was most common in older children and young adults (6–19 year olds, 10.7%; 20–39 year olds, 8.7%). Shrimp sensitization, which was not measured in children under age 5, did not vary appreciably by age.
Repository - Unmarked Figure No. E1.
Table 2.
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLf | Likely FA >= PLf | Estimated Clinical FA Rateg | |
|---|---|---|---|---|---|
| Food Sensitizationc | 16.8 ± 0.6 (1729) | 12.7 ± 0.3 (1267) | 3.1 ± 0.4 (320) | 1.0 ± 0.2 (142) | 2.5 ± 0.3 |
| Milk | 5.7 ± 0.5 (650) | 5.0 ± 0.4 (581) | 0.69 ± 0.18 (62) | 0.05 ± 0.04 (7) | 0.40 ± 0.09 |
| Egg | 3.9 ± 0.4 (371) | 3.6 ± 0.4 (327) | 0.24 ± 0.06 (26) | 0.12 ± 0.04 (18) | 0.23 ± 0.05 |
| Peanut | 7.6 ± 0.4 (772) | 5.3 ± 0.3 (509) | 1.9 ± 0.3 (208) | 0.34 ± 0.07 (55) | 1.3 ± 0.1 |
| Shrimpd | 5.9 ± 0.4 (568) | 4.5 ± 0.3 (408) | 0.79 ± 0.13 (84) | 0.62 ± 0.12 (76) | 1.0 ± 0.1 |
| Multiple Food Sensitizatione | 4.7 ± 0.4 (498) | 2.6 ± 0.3 (250) | 1.7 ± 0.2 (169) | 0.53 ± 0.12 (79) | 1.3 ± 0.2 |
Weighted % ± Standard Error (N)
Overall N=8203
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - 95% predictive level: for ages 1-5, 5 kU/L; for age 6+, cow's milk 15kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate: 50% of participants with PFA + 95% of participants with LFA
Repository Table E1.
Food sensitization prevalencea and clinical FA estimates in the United States (2005-2006) by age
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLe | Likely FA >= PLe | Estimated Clinical FA Ratef | |
|---|---|---|---|---|---|
| Food Sensitizationb | |||||
| 1-5 | 28.1 ± 1.2 (291) | 21.5 ± 1.0 (230) | 4.5 ± 0.8 (40) | 2.1 ± 0.4 (21) | 4.3 ± 0.6 |
| 6-19 | 21.8 ± 1.0 (702) | 15.6 ± 0.9 (473) | 4.7 ± 0.6 (150) | 1.5 ± 0.3 (79) | 3.8 ± 0.4 |
| 20-39 | 15.9 ± 1.1 (313) | 12.4 ± 0.7 (236) | 2.1 ± 0.5 (51) | 1.3 ± 0.4 (26) | 2.4 ± 0.5 |
| 40-59 | 14.7 ± 0.9 (217) | 10.7 ± 0.6 (161) | 3.3 ± 0.6 (45) | 0.75 ± 0.30 (11) | 2.3 ± 0.4 |
| 60+ | 13.0 ± 1.3 (206) | 10.6 ± 1.2 (167) | 2.2 ± 0.4 (34) | 0.25 ± 0.15 (5) | 1.3 ± 0.3 |
| Milk | |||||
| 1-5 | 22.0 ± 1.1 (230) | 18.6 ± 0.9 (198) | 3.1 ± 0.7 (27) | 0.28 ± 0.13 (5) | 1.8 ± 0.4 |
| 6-19 | 8.1 ± 1.0 (254) | 7.6 ± 0.9 (240) | 0.50 ± 0.19 (13) | 0.01 ± 0.01 (1) | 0.26 ± 0.10 |
| 20-39 | 3.2 ± 0.5 (50) | 2.9 ± 0.5 (45) | 0.32 ± 0.15 (5) | (0) | 0.16 ± 0.07 |
| 40-59 | 4.9 ± 0.7 (59) | 4.0 ± 0.7 (48) | 0.75 ± 0.34 (10) | 0.12 ± 0.13 (1) | 0.49 ± 0.22 |
| 60+ | 3.8 ± 0.5 (57) | 3.2 ± 0.5 (50) | 0.67 ± 0.31 (7) | (0) | 0.33 ± 0.15 |
| Egg | |||||
| 1-5 | 13.9 ± 1.1 (144) | 11.4 ± 1.1 (117) | 1.2 ± 0.3 (15) | 1.3 ± 0.4 (12) | 1.8 ± 0.4 |
| 6-19 | 4.1 ± 0.5 (99) | 3.8 ± 0.5 (92) | 0.20 ± 0.13 (4) | 0.05 ± 0.03 (3) | 0.14 ± 0.06 |
| 20-39 | 2.1 ± 0.5 (28) | 1.9 ± 0.5 (27) | (0) | 0.13 ± 0.13 (1) | 0.12 ± 0.12 |
| 40-59 | 3.8 ± 0.7 (46) | 3.4 ± 0.6 (40) | 0.41 ± 0.18 (6) | (0) | 0.20 ± 0.09 |
| 60+ | 3.9 ± 0.8 (54) | 3.7 ± 0.8 (51) | 0.11 ± 0.11 (1) | 0.03 ± 0.02 (2) | 0.08 ± 0.06 |
| Peanut | |||||
| 1-5 | 7.1 ± 1.1 (68) | 4.5 ± 0.9 (44) | 1.6 ± 0.7 (14) | 1.0 ± 0.3 (10) | 1.8 ± 0.3 |
| 6-19 | 10.7 ± 1.0 (345) | 6.1 ± 0.8 (192) | 3.6 ± 0.5 (118) | 0.92 ± 0.23 (35) | 2.7 ± 0.3 |
| 20-39 | 8.7 ± 0.7 (178) | 6.9 ± 0.5 (133) | 1.4 ± 0.3 (38) | 0.31 ± 0.16 (7) | 1.0 ± 0.3 |
| 40-59 | 6.5 ± 0.8 (104) | 4.4 ± 0.5 (74) | 2.0 ± 0.5 (27) | 0.09 ± 0.07 (3) | 1.1 ± 0.3 |
| 60+ | 4.5 ± 0.8 (77) | 3.9 ± 0.7 (66) | 0.59 ± 0.11 (11) | (0) | 0.29 ± 0.05 |
| Shrimpc | |||||
| 1-5 | Not Done | Not Done | Not Done | Not Done | Not Done |
| 6-19 | 6.1 ± 0.7 (254) | 4.4 ± 0.6 (167) | 1.1 ± 0.2 (41) | 0.63 ± 0.13 (46) | 1.1 ± 0.2 |
| 20-39 | 6.7 ± 0.9 (141) | 5.2 ± 0.6 (110) | 0.61 ± 0.24 (12) | 0.94 ± 0.26 (19) | 1.2 ± 0.3 |
| 40-59 | 5.9 ± 0.8 (92) | 4.6 ± 0.6 (71) | 0.71 ± 0.26 (13) | 0.56 ± 0.26 (8) | 0.89 ± 0.27 |
| 60+ | 4.6 ± 0.6 (81) | 3.4 ± 0.6 (60) | 0.91 ± 0.32 (18) | 0.22 ± 0.15 (3) | 0.67 ± 0.24 |
| Multiple Food Sensitizationd | |||||
| 1-5 | 11.6 ± 1.4 (117) | 6.1 ± 1.1 (70) | 3.9 ± 0.7 (32) | 1.5 ± 0.5 (15) | 3.4 ± 0.5 |
| 6-19 | 5.9 ± 0.6 (194) | 3.0 ± 0.5 (83) | 2.0 ± 0.4 (67) | 0.89 ± 0.23 (44) | 1.8 ± 0.3 |
| 20-39 | 4.0 ± 0.5 (73) | 2.3 ± 0.4 (37) | 1.0 ± 0.4 (25) | 0.67 ± 0.32 (11) | 1.2 ± 0.3 |
| 40-59 | 4.5 ± 0.8 (61) | 2.3 ± 0.6 (30) | 1.9 ± 0.4 (25) | 0.27 ± 0.15 (6) | 1.2 ± 0.3 |
| 60+ | 3.1 ± 0.6 (53) | 1.9 ± 0.4 (30) | 1.2 ± 0.3 (20) | 0.05 ± 0.03 (3) | 0.65 ± 0.19 |
Weighted % ± Standard Error (N)
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - predictive level: for ages 1-5 years old, 5 kU/L; for age 6+, cow's milk 15 kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate = 50% of participants with PFA + 95% of participants with LFA.
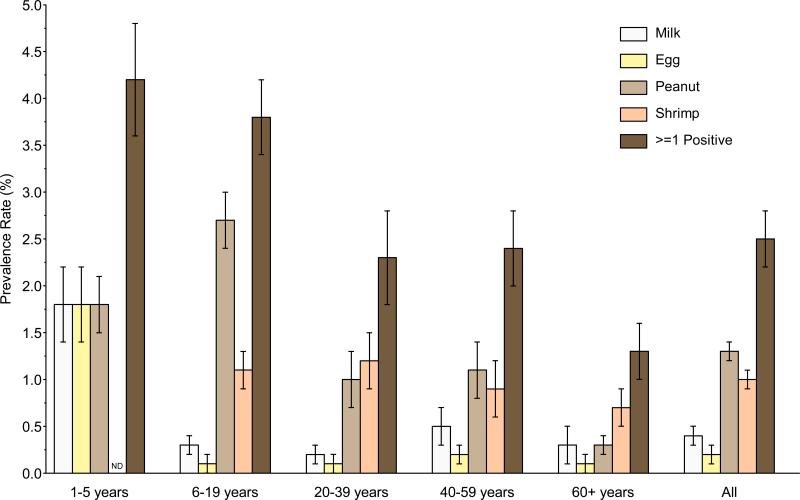
The US population prevalence for PFA and LFA was 3.1% and 1.0%, respectively (Table 2). The estimated US population prevalence of clinical FA was 2.5% (Figure 1, Table 2). These estimates are based upon four common foods and do not account for allergies that are known to occur to other foods; see Discussion section. Overall clinical FA prevalence differed significantly and declined with age, being highest in 1–5 year old children (4.2%) and lowest in adults 60+ years old (1.3%) (Figure 1, Repository Table E1). The clinical FA estimates for individual foods also varied by age. The prevalence of clinical FA to milk, egg, and peanut were each approximately 1.8% in 1–5 year old children; clinical peanut allergy was most prevalent (2.7%) in 6–19 year old children; clinical peanut and shrimp allergy were most prevalent (0.9% to 1.2%) in adults age 20– 59; and shrimp allergy was most prevalent (0.7%) in adults 60+ years old (Figure 1).
Figure 1.
Clinical FA prevalence estimates in the United States (2005-2006) by age
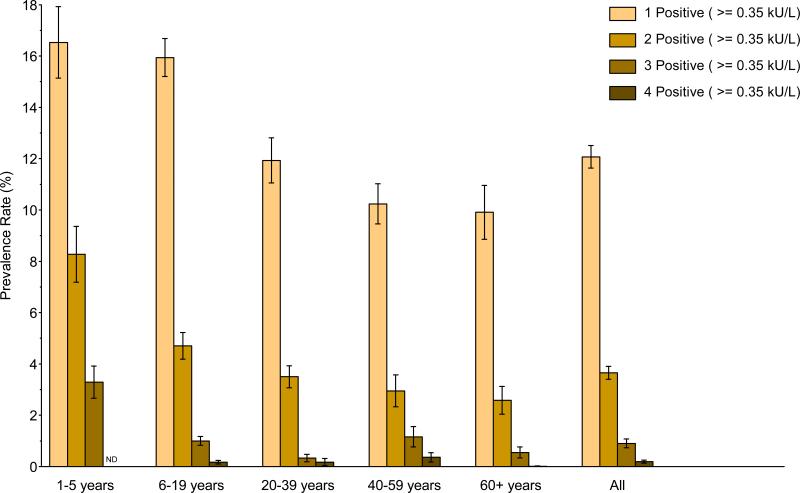
Multiple food sensitizations occurred in 4.7% of the US population (Table 2). By age, the highest prevalence was in the youngest children (1–5 year old, 11.6%) and declined steadily with age to 3.1% in adults 60+ years old (Repository Figure E2; Repository Table E1). Multiple clinical FA was present in 1.3% of the population overall (Table 2). It was more frequent in younger children (1–5 year old, 3.4%) than in older children (1.8%) and adults (1.1 to 1.3%), and was uncommon in adults 60+ years old (0.6%) (Repository Table E1). The most common combinations of PFA/LFA occurred in 1–5 year old children; the prevalence of PFA/LFA to milk, egg, and peanut, to both milk and egg, and to milk and peanut were each 0.4%, in this age group. The next most common PFA/LFA combination was to peanut and shrimp; the prevalence of this combination was 0.3% in 6–19 year olds; 0.1 to 0.2% in 20–59 year olds; and 0.07% in 60+ year olds. Other PFA/LFA combinations were uncommon (< 0.1%).
Repository - Unmarked Figure No. E2.
Demographic risk factors for PFA/LFA
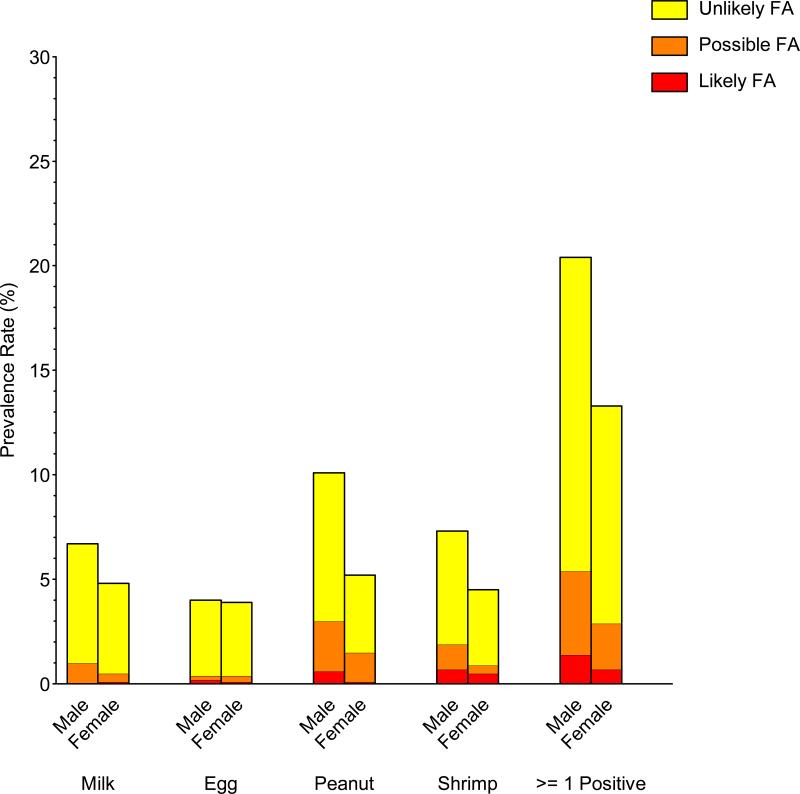
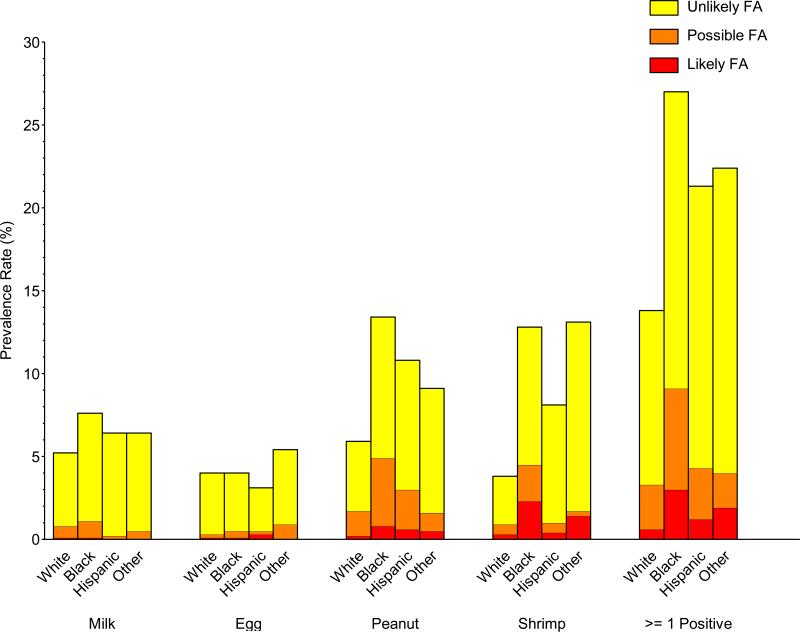
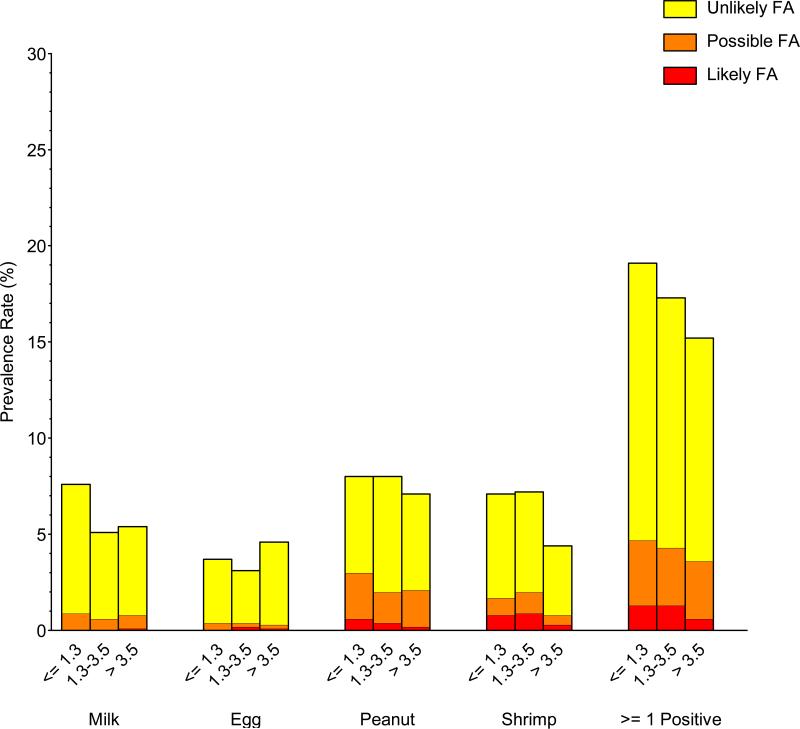
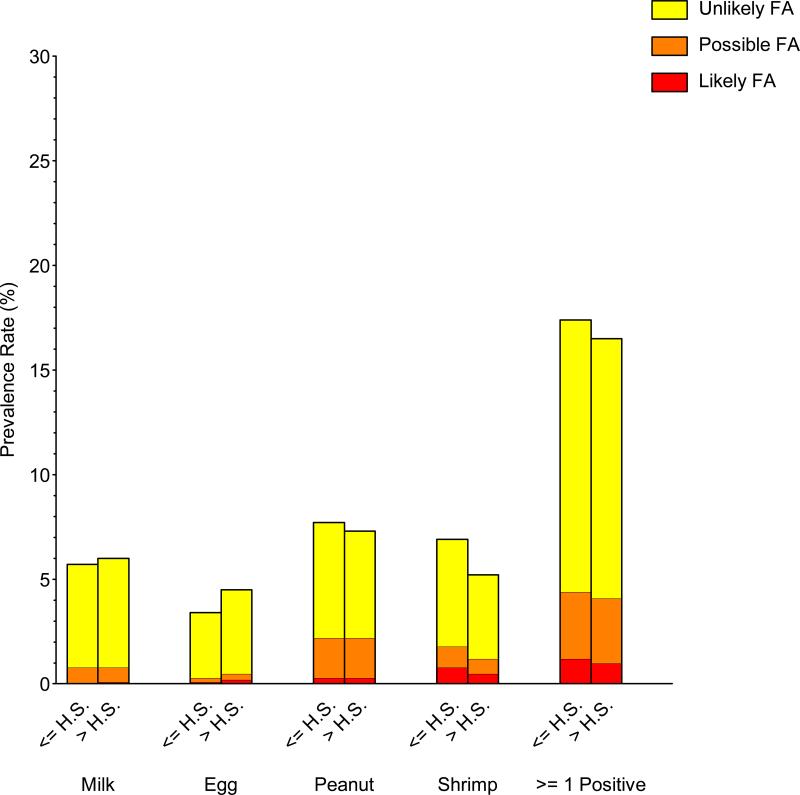
Significant prevalence differences in food sensitization, PFA, and LFA were observed in commonly investigated demographic subgroups (gender, race/ethnicity, and household income). Food sensitization and PFA/LFA were more prevalent in males than females, to peanut, shrimp, and milk, but not egg (Repository Figure E4; Repository Table E2). For example, the prevalence of detectable food sensitization, PFA, and LFA to peanut in males was 10.0%, 2.4%, and 0.6%, respectively, compared with 5.2%, 1.4%, and 0.1% in females. Food sensitization and PFA/LFA were more prevalent in non-Hispanic blacks and least prevalent in non-Hispanic whites (Repository Figure E5; Repository Table E3); these distinctions were greatest for shrimp and peanut. For example, the prevalence of detectable food sensitization, PFA, and LFA to shrimp in non-Hispanic blacks was 12.8%, 2.2%, and 2.3%, respectively, compared with 3.8%, 0.6%, and 0.3% in non-Hispanic whites. Food sensitization and PFA/LFA were generally more prevalent in individuals living in poverty and least prevalent in higher income households (Repository Figure E6; Repository Table E4). For example, the prevalence of detectable food sensitization, PFA, and LFA to at least one food for PIR<=1.3 was 19.0%, 3.4%, and 1.3%, respectively, compared with 15.3%, 3.0%, and 0.6% for PIR>3.5. The prevalence of food sensitization tended to be higher in households with less educated participants, but these differences were not statistically significant (Repository Figure E7; Repository Table E5).
Repository - Unmarked Figure No. E4.
Repository Table E2.
Food sensitization prevalencea and clinical FA estimates in the United States (2005-2006) by gender
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLe | Likely FA >= PLe | Estimated Clinical FA Ratef | |
|---|---|---|---|---|---|
| Food Sensitizationb | |||||
| Male | 20.4 ± 0.9 (967) | 15.0 ± 0.7 (686) | 4.0 ± 0.5 (187) | 1.4 ± 0.2 (94) | 3.3 ± 0.3 |
| Female | 13.4 ± 0.9 (762) | 10.4 ± 0.6 (581) | 2.2 ± 0.4 (133) | 0.75 ± 0.24 (48) | 1.8 ± 0.3 |
| Milk | |||||
| Male | 6.7 ± 0.8 (347) | 5.7 ± 0.6 (312) | 1.0 ± 0.3 (32) | 0.02 ± 0.01 (3) | 0.50 ± 0.15 |
| Female | 4.8 ± 0.5 (303) | 4.3 ± 0.4 (269) | 0.44 ± 0.13 (30) | 0.08 ± 0.08 (4) | 0.30 ± 0.09 |
| Egg | |||||
| Male | 4.0 ± 0.5 (190) | 3.6 ± 0.4 (166) | 0.20 ± 0.09 (12) | 0.17 ± 0.08 (12) | 0.27 ± 0.10 |
| Female | 3.8 ± 0.5 (181) | 3.5 ± 0.5 (161) | 0.28 ± 0.09 (14) | 0.06 ± 0.03 (6) | 0.20 ± 0.06 |
| Peanut | |||||
| Male | 10.0 ± 0.8 (481) | 7.1 ± 0.5 (312) | 2.4 ± 0.4 (130) | 0.56 ± 0.12 (39) | 1.7 ± 0.2 |
| Female | 5.2 ± 0.5 (291) | 3.7 ± 0.4 (197) | 1.4 ± 0.3 (78) | 0.14 ± 0.04 (16) | 0.83 ± 0.17 |
| Shrimpc | |||||
| Male | 7.4 ± 0.5 (329) | 5.4 ± 0.4 (233) | 1.2 ± 0.2 (49) | 0.74 ± 0.17 (47) | 1.3 ± 0.2 |
| Female | 4.6 ± 0.5 (239) | 3.6 ± 0.4 (175) | 0.42 ± 0.12 (35) | 0.52 ± 0.18 (29) | 0.70 ± 0.19 |
| Multiple Food Sensitizationd | |||||
| Male | 5.7 ± 0.5 (290) | 3.0 ± 0.3 (134) | 1.9 ± 0.3 (100) | 0.83 ± 0.21 (56) | 1.7 ± 0.3 |
| Female | 3.9 ± 0.5 (208) | 2.2 ± 0.4 (116) | 1.5 ± 0.3 (69) | 0.24 ± 0.09 (23) | 1.0 ± 0.2 |
Weighted % ± Standard Error (N)
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - predictive level: for ages 1-5 years old, 5 kU/L; for age 6+, cow's milk 15 kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate = 50% of participants with PFA + 95% of participants with LFA.
Repository - Unmarked Figure No. E5.
Repository Table E3.
Food sensitization prevalencea and clinical FA estimates in the United States (2005-2006) by race/ethnicity
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLe | Likely FA >= PLe | Estimated Clinical FA Ratef | |
|---|---|---|---|---|---|
| Food Sensitizationb | |||||
| Non-Hispanic White | 13.8 ± 0.7 (478) | 10.5 ± 0.6 (373) | 2.7 ± 0.4 (87) | 0.63 ± 0.16 (18) | 1.9 ± 0.3 |
| Non-Hispanic Black | 27.0 ± 1.2 (617) | 17.9 ± 1.0 (403) | 6.1 ± 0.7 (135) | 3.0 ± 0.5 (79) | 5.9 ± 0.6 |
| Hispanic | 21.2 ± 1.4 (545) | 17.0 ± 1.2 (419) | 3.1 ± 0.4 (87) | 1.2 ± 0.3 (39) | 2.7 ± 0.3 |
| Other | 22.3 ± 4.0 (89) | 18.4 ± 3.6 (72) | 2.1 ± 0.6 (11) | 1.9 ± 0.9 (6) | 2.8 ± 1.0 |
| Milk | |||||
| Non-Hispanic White | 5.2 ± 0.6 (193) | 4.4 ± 0.5 (167) | 0.75 ± 0.24 (25) | 0.05 ± 0.06 (1) | 0.43 ± 0.13 |
| Non-Hispanic Black | 7.6 ± 0.9 (209) | 6.5 ± 0.8 (185) | 1.0 ± 0.3 (20) | 0.10 ± 0.05 (4) | 0.58 ± 0.14 |
| Hispanic | 6.5 ± 0.4 (213) | 6.2 ± 0.4 (198) | 0.24 ± 0.05 (13) | 0.04 ± 0.03 (2) | 0.16 ± 0.04 |
| Other | 6.4 ± 2.1 (35) | 5.9 ± 1.9 (31) | 0.47 ± 0.26 (4) | (0) | 0.23 ± 0.13 |
| Egg | |||||
| Non-Hispanic White | 3.9 ± 0.4 (136) | 3.7 ± 0.4 (127) | 0.16 ± 0.08 (5) | 0.09 ± 0.06 (4) | 0.17 ± 0.07 |
| Non-Hispanic Black | 4.0 ± 0.5 (105) | 3.5 ± 0.5 (90) | 0.44 ± 0.17 (11) | 0.09 ± 0.07 (4) | 0.31 ± 0.09 |
| Hispanic | 3.1 ± 0.3 (107) | 2.6 ± 0.3 (91) | 0.19 ± 0.11 (6) | 0.32 ± 0.14 (10) | 0.39 ± 0.15 |
| Other | 5.4 ± 1.4 (23) | 4.5 ± 1.4 (19) | 0.94 ± 0.57 (4) | (0) | 0.47 ± 0.28 |
| Peanut | |||||
| Non-Hispanic White | 5.9 ± 0.5 (191) | 4.2 ± 0.4 (134) | 1.5 ± 0.3 (49) | 0.19 ± 0.08 (8) | 0.93 ± 0.15 |
| Non-Hispanic Black | 13.4 ± 0.9 (300) | 8.5 ± 0.6 (177) | 4.1 ± 0.6 (96) | 0.85 ± 0.24 (27) | 2.8 ± 0.4 |
| Hispanic | 10.8 ± 1.0 (250) | 7.8 ± 0.9 (174) | 2.4 ± 0.4 (59) | 0.60 ± 0.21 (17) | 1.7 ± 0.3 |
| Other | 9.1 ± 1.7 (31) | 7.5 ± 1.6 (24) | 1.1 ± 0.3 (4) | 0.53 ± 0.35 (3) | 1.0 ± 0.4 |
| Shrimpc | |||||
| Non-Hispanic White | 3.8 ± 0.3 (117) | 2.9 ± 0.3 (92) | 0.63 ± 0.15 (19) | 0.33 ± 0.13 (6) | 0.63 ± 0.14 |
| Non-Hispanic Black | 12.8 ± 1.2 (247) | 8.3 ± 0.8 (152) | 2.2 ± 0.4 (42) | 2.3 ± 0.4 (53) | 3.3 ± 0.4 |
| Hispanic | 8.0 ± 0.8 (171) | 7.1 ± 0.8 (137) | 0.57 ± 0.11 (20) | 0.35 ± 0.13 (14) | 0.62 ± 0.12 |
| Other | 13.2 ± 4.0 (33) | 11.4 ± 3.9 (27) | 0.34 ± 0.23 (3) | 1.4 ± 1.0 (3) | 1.5 ± 0.9 |
| Multiple Food Sensitizationd | |||||
| Non-Hispanic White | 3.9 ± 0.4 (128) | 2.1 ± 0.3 (69) | 1.5 ± 0.3 (50) | 0.26 ± 0.11 (9) | 1.0 ± 0.2 |
| Non-Hispanic Black | 7.9 ± 0.8 (186) | 3.3 ± 0.5 (77) | 3.1 ± 0.4 (67) | 1.5 ± 0.4 (42) | 3.0 ± 0.5 |
| Hispanic | 5.5 ± 0.5 (160) | 3.0 ± 0.4 (87) | 1.6 ± 0.4 (48) | 0.82 ± 0.23 (25) | 1.6 ± 0.2 |
| Other | 7.3 ± 2.5 (24) | 5.8 ± 1.9 (17) | 0.36 ± 0.20 (4) | 1.1 ± 0.7 (3) | 1.2 ± 0.7 |
Weighted % ± Standard Error (N)
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - predictive level: for ages 1-5 years old, 5 kU/L; for age 6+, cow's milk 15 kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate = 50% of participants with PFA + 95% of participants with LFA.
Repository - Unmarked Figure No. E6.
Repository Table E4.
Food sensitization prevalencea and clinical FA estimates in the United States (2005-2006) by poverty income ratio
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLe | Likely FA >= PLe | Estimated Clinical FA Ratef | |
|---|---|---|---|---|---|
| Food Sensitizationb | |||||
| <= 1.3 | 19.0 ± 1.1 (593) | 14.4 ± 0.9 (443) | 3.4 ± 0.3 (96) | 1.3 ± 0.2 (54) | 2.9 ± 0.3 |
| 1.3-3.5 | 17.3 ± 1.0 (653) | 13.0 ± 0.7 (480) | 3.0 ± 0.4 (114) | 1.3 ± 0.4 (59) | 2.8 ± 0.4 |
| > 3.5 | 15.3 ± 1.0 (401) | 11.6 ± 0.8 (288) | 3.0 ± 0.5 (90) | 0.64 ± 0.19 (23) | 2.1 ± 0.3 |
| Milk | |||||
| <= 1.3 | 7.6 ± 0.8 (258) | 6.7 ± 0.7 (233) | 0.89 ± 0.26 (23) | 0.04 ± 0.03 (2) | 0.48 ± 0.12 |
| 1.3-3.5 | 5.1 ± 0.7 (225) | 4.5 ± 0.6 (202) | 0.60 ± 0.19 (20) | 0.02 ± 0.01 (3) | 0.32 ± 0.10 |
| > 3.5 | 5.4 ± 0.7 (135) | 4.6 ± 0.7 (117) | 0.69 ± 0.24 (16) | 0.10 ± 0.09 (2) | 0.44 ± 0.15 |
| Egg | |||||
| <= 1.3 | 3.7 ± 0.5 (119) | 3.3 ± 0.4 (108) | 0.35 ± 0.18 (9) | 0.03 ± 0.02 (2) | 0.20 ± 0.11 |
| 1.3-3.5 | 3.1 ± 0.4 (122) | 2.7 ± 0.4 (105) | 0.16 ± 0.06 (7) | 0.20 ± 0.11 (10) | 0.27 ± 0.11 |
| > 3.5 | 4.5 ± 0.6 (100) | 4.3 ± 0.7 (89) | 0.21 ± 0.11 (6) | 0.05 ± 0.03 (5) | 0.16 ± 0.06 |
| Peanut | |||||
| <= 1.3 | 7.9 ± 0.6 (224) | 5.0 ± 0.5 (142) | 2.4 ± 0.4 (60) | 0.58 ± 0.16 (22) | 1.7 ± 0.2 |
| 1.3-3.5 | 7.9 ± 0.5 (299) | 6.0 ± 0.3 (211) | 1.6 ± 0.3 (68) | 0.38 ± 0.16 (20) | 1.1 ± 0.2 |
| > 3.5 | 7.1 ± 0.8 (208) | 5.0 ± 0.6 (129) | 1.9 ± 0.5 (68) | 0.21 ± 0.08 (11) | 1.2 ± 0.2 |
| Shrimpc | |||||
| <= 1.3 | 7.1 ± 0.6 (202) | 5.4 ± 0.5 (143) | 0.92 ± 0.29 (28) | 0.80 ± 0.24 (31) | 1.2 ± 0.3 |
| 1.3-3.5 | 7.1 ± 0.8 (226) | 5.2 ± 0.5 (156) | 1.1 ± 0.3 (37) | 0.86 ± 0.24 (33) | 1.4 ± 0.3 |
| > 3.5 | 4.4 ± 0.5 (115) | 3.6 ± 0.4 (91) | 0.47 ± 0.16 (15) | 0.34 ± 0.18 (9) | 0.56 ± 0.17 |
| Multiple Food Sensitizationd | |||||
| <= 1.3 | 5.3 ± 0.3 (171) | 2.5 ± 0.2 (81) | 2.2 ± 0.2 (64) | 0.60 ± 0.13 (26) | 1.7 ± 0.2 |
| 1.3-3.5 | 4.4 ± 0.5 (169) | 2.3 ± 0.3 (87) | 1.3 ± 0.3 (48) | 0.72 ± 0.29 (34) | 1.3 ± 0.3 |
| > 3.5 | 4.6 ± 0.7 (121) | 2.7 ± 0.5 (62) | 1.7 ± 0.4 (45) | 0.26 ± 0.08 (14) | 1.1 ± 0.2 |
Weighted % ± Standard Error (N)
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - predictive level: for ages 1-5 years old, 5 kU/L; for age 6+, cow's milk 15 kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate = 50% of participants with PFA + 95% of participants with LFA.
Repository - Unmarked Figure No. E7.
Repository Table E5.
Food sensitization prevalencea and clinical FA estimates in the United States (2005-2006) by household education
| Sensitization >=0.35 kU/L | Unlikely FA 0.35-2.0 kU/L | Possible FA 2.0 kU/L-PLe | Likely FA >= PLe | Estimated Clinical FA Ratef | |
|---|---|---|---|---|---|
| Food Sensitizationb | |||||
| High school or less | 17.3 ± 0.6 (928) | 13.0 ± 0.4 (687) | 3.2 ± 0.2 (163) | 1.2 ± 0.2 (78) | 2.7 ± 0.3 |
| Above high school | 16.5 ± 0.9 (744) | 12.4 ± 0.7 (530) | 3.1 ± 0.6 (153) | 1.0 ± 0.2 (61) | 2.5 ± 0.4 |
| Milk | |||||
| High school or less | 5.7 ± 0.5 (367) | 4.9 ± 0.3 (332) | 0.77 ± 0.28 (32) | 0.02 ± 0.01 (3) | 0.41 ± 0.13 |
| Above high school | 5.9 ± 0.7 (272) | 5.2 ± 0.6 (238) | 0.66 ± 0.19 (30) | 0.08 ± 0.07 (4) | 0.41 ± 0.13 |
| Egg | |||||
| High school or less | 3.4 ± 0.5 (186) | 3.1 ± 0.5 (169) | 0.22 ± 0.10 (11) | 0.06 ± 0.03 (6) | 0.17 ± 0.06 |
| Above high school | 4.4 ± 0.5 (183) | 4.0 ± 0.5 (156) | 0.26 ± 0.09 (15) | 0.17 ± 0.08 (12) | 0.29 ± 0.08 |
| Peanut | |||||
| High school or less | 7.7 ± 0.5 (381) | 5.5 ± 0.4 (257) | 1.9 ± 0.2 (99) | 0.33 ± 0.09 (25) | 1.3 ± 0.1 |
| Above high school | 7.4 ± 0.7 (359) | 5.1 ± 0.5 (226) | 1.9 ± 0.4 (105) | 0.32 ± 0.08 (28) | 1.3 ± 0.2 |
| Shrimpc | |||||
| High school or less | 6.9 ± 0.5 (334) | 5.1 ± 0.4 (236) | 1.0 ± 0.2 (50) | 0.84 ± 0.20 (48) | 1.3 ± 0.2 |
| Above high school | 5.2 ± 0.5 (213) | 4.0 ± 0.4 (152) | 0.70 ± 0.15 (34) | 0.48 ± 0.17 (27) | 0.81 ± 0.16 |
| Multiple Food Sensitizationd | |||||
| High school or less | 4.8 ± 0.4 (272) | 2.5 ± 0.3 (138) | 1.8 ± 0.2 (93) | 0.49 ± 0.11 (41) | 1.4 ± 0.2 |
| Above high school | 4.8 ± 0.6 (217) | 2.6 ± 0.4 (106) | 1.6 ± 0.4 (75) | 0.54 ± 0.18 (36) | 1.3 ± 0.3 |
Weighted % ± Standard Error (N)
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
PL - predictive level: for ages 1-5 years old, 5 kU/L; for age 6+, cow's milk 15 kU/L, egg white 7 kU/L, peanut 14 kU/L, shrimp 5 kU/L
Estimated Clinical FA Rate = 50% of participants with PFA + 95% of participants with LFA.
We also performed logistic regression modeling to determine the relative significance of these demographic factors in PFA/LFA risk. There were significantly increased odds of PFA/LFA in children, non-Hispanic blacks, and males (Table 3). Further subpopulation analyses revealed that the unadjusted OR (95% CI) of PFA/LFA in black male children compared to the remainder of the population was 4.4 (2.8–6.7). Household income and education level were not associated with PFA/LFA in this model.
Table 3.
Demographic predictors of Possible FA or Likely FAa
| Odds Ratio (95% CI)b | Effect P-Valuec | |
|---|---|---|
| Age (years) | ||
| 1-5 | 2.04 (1.42, 2.93) | < 0.001 |
| 6-19 | 1.80 (1.46, 2.23) | |
| 20+ | 1.00 | |
| Race/Ethnicity | ||
| non-Hispanic black | 3.06 (2.14, 4.36) | < 0.001 |
| Hispanic | 1.14 (0.71, 1.81) | |
| Other | 1.09 (0.56, 2.14) | |
| non-Hispanic white | 1.00 | |
| Gender | ||
| Male | 1.87 (1.32, 2.66) | < 0.001 |
| Female | 1.00 | |
| Poverty Income Ratio (PIR) | ||
| <= 1.3 | 0.94 (0.65, 1.34) | 0.46 |
| 1.3-3.5 | 1.06 (0.79, 1.43) | |
| > 3.5 | 1.00 | |
| Household Education | ||
| High school or less | 1.03 (0.79, 1.34) | 0.83 |
| Above high school | 1.00 |
Overall N=6557
Adjusted Model: PFA/LFA vs. no FA = Age + Race/Ethnicity + Gender + PIR + Household Education
Wald chi-square test for significance of each factor
Relationship between food sensitization, food allergy, and asthma
In weighted, bivariate analyses, the prevalences of all food sensitizations and FA risk groups were higher in participants with doctor-diagnosed asthma compared to those with no asthma. The odds of food sensitization (2.3), sensitization to milk (2.0), egg (2.9), peanut (2.3) shrimp (1.9), and multiple foods (2.4), as well as UFA (1.9), PFA (2.8), and LFA (2.0) were all significantly increased (Table 4). As asthma increased in persistence and severity, the prevalences of food sensitization and FA significantly increased. For example, the prevalences of food sensitization in those with no asthma, diagnosed but not current asthma, current asthma without recent ER visits, and current asthma with recent ER visits were 14.9%, 20.6%, 31.5%, and 35.0%, respectively (p<0.001, Cochran-Armitage test for trend; Table 5). Notably, the prevalence of LFA in those with an ER visit for asthma in the prior year (8.5%) was remarkably higher than in those with current asthma but without a recent ER visit (1.3%), diagnosed but not current asthma (1.1%), and no asthma (0.9%) (p<0.001 for trend).
Table 4.
Prevalence and risk of food sensitization and FA in diagnosed asthma
| % Asthmae | % No Asthma | Odds Ratiof (95% CI) | |
|---|---|---|---|
| Countsa | n=1157 | n=7037 | |
| Food Sensitizationb | 27.5 | 14.9 | 2.3 (1.9-2.7) |
| Milk | 9.1 | 5.2 | 2.0 (1.5-2.5) |
| Egg | 8.2 | 3.2 | 2.9 (2.4-3.5) |
| Peanut | 13.3 | 6.6 | 2.3 (1.9-2.8) |
| Shrimpc | 9.1 | 5.3 | 1.9 (1.2-2.9) |
| Multiple Food Sensitizationd | 8.8 | 4.0 | 2.4 (1.9-3.1) |
| Unlikely FA | 19.0 | 11.5 | 1.9 (1.7-2.1) |
| Possible FA | 6.6 | 2.5 | 2.8 (1.7-4.5) |
| Likely FA | 1.9 | 0.9 | 2.0 (1.1-3.7) |
Unweighted counts
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
Self-reported diagnosis by doctor or other health professional
Adjusted Model: Food Sensitization / Allergy = Diagnosed Asthma + Age + Gender + Race + Poverty Income Ratio
Table 5.
Prevalencea of food sensitization and FA by asthma status
| % No Asthmaf | % Diagnosed Asthma (not current) | % Current Asthma but no ER visit for asthma | % Current Asthma with ER visit for asthma in past year | P-Valueg,h | |
|---|---|---|---|---|---|
| Countsb | n=7037 | n=427 | n=607 | n=123 | N/A |
| Food Sensitizationc | 14.9 ± 0.7 | 20.6 ± 2.8 | 31.5 ± 2.4 | 35.0 ± 5.7 | <.001 |
| Milk | 5.2 ± 0.5 | 8.2 ± 1.5 | 9.6 ± 1.6 | 10.9 ± 3.8 | <.001 |
| Egg | 3.2 ± 0.3 | 8.2 ± 1.2 | 8.0 ± 1.6 | 9.6 ± 3.0 | <.001 |
| Peanut | 6.6 ± 0.5 | 6.8 ± 1.4 | 17.5 ± 2.3 | 17.5 ± 2.6 | <.001 |
| Shrimpd | 5.3 ± 0.5 | 6.9 ± 1.6 | 9.6 ± 1.9 | 16.0 ± 5.2 | 0.002 |
| Multiple Food Sensitizatione | 4.0 ± 0.3 | 7.3 ± 0.9 | 9.1 ± 2.0 | 13.8 ± 4.3 | <.001 |
| Unlikely FA | 11.5 ± 0.4 | 16.2 ± 2.5 | 21.2 ± 2.4 | 19.2 ± 4.7 | <.001 |
| Possible FA | 2.5 ± 0.4 | 3.4 ± 0.8 | 9.0 ± 1.9 | 7.3 ± 1.8 | <.001 |
| Likely FA | 0.9 ± 0.2 | 1.1 ± 0.4 | 1.3 ± 0.3 | 8.5 ± 3.3 | <.001 |
Weighted % ± Standard Error (N)
Unweighted counts
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
Self-reported diagnosis by doctor or other health professional.
P-value result of Cochran-Armitage test for linear trend.
Adjusted Model: Food Sensitization / Allergy = Asthma Severity + Age + Gender + Race + Poverty Income Ratio
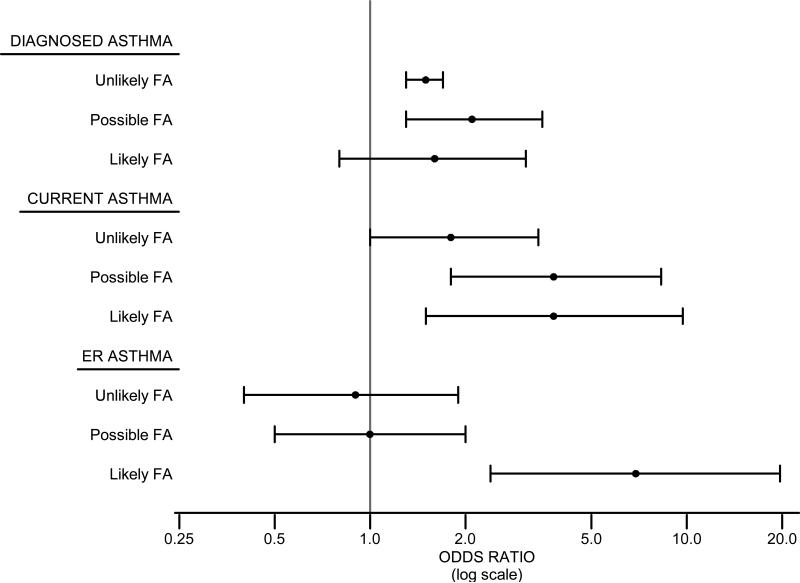
To better understand the relationship between asthma, especially asthma persistence and recent severe asthma exacerbations, and FA risk groups, we developed logistic regression models, after adjusting for inhalant sensitization and demographic variables (age, gender, race/ethnicity, household income) (Figure 2; Repository Table E6). The adjusted odds of diagnosed asthma (vs. no asthma) were significantly increased in those with UFA and PFA, and tended to be increased in LFA. Similarly, the adjusted odds of current asthma (vs. diagnosed asthma, not current) were significantly increased in those with UFA, PFA, and LFA. Importantly, the adjusted odds of an ER visit for asthma in the prior year (vs. diagnosed asthma, no ER visit) were highest in those with LFA (OR 6.9, 95% CI 2.4–19.7), but not increased in UFA or PFA.
Figure 2.
FA and asthma risk in United States (2005–2006). Adjusteda odds ratios for diagnosed asthmab, current asthmac, and ER visitd for asthma in the prior year in those with UFA, PFA and LFA.
a: Adjusted model: Asthma outcome = Food Sensitization + Inhalant Sensitization + Age + Gender + Race + Poverty Income Ratio
b: Diagnosed Asthma modeled against all participants without asthma.
c: Current Asthma modeled against all participants diagnosed with asthma but not currently having asthma.
d: ER Asthma modeled against all participants diagnosed with asthma or currently having asthma but not having an ER visit related to asthma.
Repository Table E6.
Adjusted Odds Ratiosa for Asthma Risk
| Diagnosed Asthmab | Current Asthmac | ER Visit for Asthmad | |
|---|---|---|---|
| Unlikely FA | 1.5 (1.3-1.7) | 1.8 (1.0-3.4) | 0.9 (0.4-1.9) |
| Possible FA | 2.1 (1.3-3.5) | 3.8 (1.8-8.3) | 1.0 (0.5-2.0) |
| Likely FA | 1.6 (0.8-3.1) | 3.8 (1.5-9.7) | 6.9 (2.4-19.7) |
| No Sensitization | 1.0 | 1.0 | 1.0 |
Adjusted Model: Asthma outcome = Food Sensitization + Inhalant Sensitization + Age + Gender + Race + Poverty Income Ratio
Diagnosed Asthma modeled against all participants without asthma.
Current Asthma modeled against all participants diagnosed with asthma but not currently having asthma.
ER Asthma modeled against all participants diagnosed with asthma or currently having asthma but not having an ER visit related to asthma.
Relationship between food sensitization, food allergy, and hay fever and eczema
In participants with hay fever, the prevalence and odds of food sensitization and FA risk groups were significantly increased, although not for LFA (Table 6). In participants with eczema, only the odds of LFA were significantly increased. In a fully adjusted model (including inhalant sensitization and demographic variables), the odds of diagnosed hay fever were significantly increased only in those with PFA (OR 2.0; 95% CI 1.4-2.8) (Repository Table E7). The odds of diagnosed eczema were not significantly increased for any FA risk group (Repository Table E7), except that in an unadjusted model, the odds of eczema were increased only in those with LFA (OR 2.1; 95% CI 1.2-.3.7).
Table 6.
Prevalence and risk of food sensitization and FA in diagnosed hay fever and eczema
| Hay Fevere | Eczemae | |||||
|---|---|---|---|---|---|---|
| Yes | No | Odds Ratiof (95% CI) | Yes | No | Odds Ratiof (95% CI) | |
| Countsa | n=586 | n=7599 | n=715 | n=7470 | ||
| Food Sensitizationb | 24.9 | 15.7 | 2.4 (1.8-3.2) | 17.8 | 16.7 | 1.1 (0.8-1.5) |
| Milk | 6.8 | 5.5 | 1.9 (1.2-2.9) | 7.0 | 5.6 | 1.0 (0.6-1.7) |
| Egg | 6.9 | 3.6 | 2.6 (1.5-4.3) | 6.4 | 3.7 | 1.5 (0.9-2.6) |
| Peanut | 15.0 | 6.6 | 3.4 (2.7-4.5) | 7.6 | 7.6 | 1.0 (0.8-1.4) |
| Shrimpc | 7.3 | 5.8 | 1.7 (1.1-2.5) | 5.8 | 6.0 | 1.1 (0.8-1.6) |
| Multiple Food Sensitizationd | 8.0 | 4.3 | 2.6 (1.8-3.9) | 6.1 | 4.6 | 1.3 (0.8-2.0) |
| Unlikely FA | 17.7 | 12.0 | 2.0 (1.5-2.8) | 12.7 | 12.6 | 1.0 (0.7-1.5) |
| Possible FA | 6.4 | 2.7 | 3.3 (2.4-4.7) | 3.2 | 3.1 | 1.0 (0.6-1.5) |
| Likely FA | 0.9 | 1.0 | 1.2 (0.4-4.1) | 1.9 | 1.0 | 2.0 (1.1-3.8) |
Unweighted counts
Food Sensitization: at least one food ≥ 0.35 kU/L
Information only available for ≥ 6 year olds
Multiple Food Sensitization: two or more foods ≥ 0.35 kU/L
Self-reported diagnosis by doctor or other health professional
Adjusted Model: Food Sensitization / Allergy = Hay Fever/Eczema + Age + Gender + Race + Poverty Income Ratio
Repository Table E7.
Adjusted Odds Ratiosa for Eczema and Hay Fever
| Diagnosed Eczemab | Diagnosed Hay Feverc | |
|---|---|---|
| Unlikely FA | 0.9 (0.6-1.3) | 1.3 (0.9-1.7) |
| Possible FA | 0.8 (0.5-1.3) | 2.0 (1.4-2.8) |
| Likely FA | 1.7 (0.9-3.3) | 0.7 (0.2-2.6) |
| No Sensitization | 1.0 | 1.0 |
Adjusted Model: Outcome = Food Sensitization + Inhalant Sensitization + Age + Gender + Race + Poverty Income Ratio
Diagnosed Eczema modeled against all participants without eczema.
Diagnosed Hay Fever modeled against all participants without hay fever.
DISCUSSION
This study found that the prevalence estimate of clinical allergy to peanut, milk, egg, and/or shrimp was 2.5% and was associated with childhood, male gender, and non-Hispanic black race/ethnicity. We also found an association of Likely FA with current asthma and emergency room (ER) visits for asthma in the prior year.
For the first time in a US nationally representative sample, specific serum IgE levels were used to quantify allergic sensitization to common foods. This is important because clinical studies have demonstrated that higher food-specific IgE levels indicate a greater likelihood of clinical FA. While age-specific distinctions in food sensitization and FA have long been suspected, this was also the first time in a US national study that food sensitization was assessed in younger children (1–5 years old) together with older adults (at least 60 years old). These cohort strengths allowed for a comprehensive, contemporary US public health perspective on the prevalence of and risks associated with higher food-specific serum IgE levels, indicative of PFA or LFA.
This investigation estimated clinical FA rates of 2.5% in the US population. They are highest in children (4.2% in 1–5 year olds, 3.8% in 6–19 year olds), and lowest in older adults (1.3% in 60+ year olds). These overall estimates are lower than some other studies. Sampson estimated the prevalence of FA in the US to be 3.5 to 4.0%.1 Bock et al. reported a 6% prevalence of food challenge-confirmed FA in infants less than 3 years of age.7 Our estimates were remarkably similar to those reported by Branum and colleagues from the 2007 National Health Interview Survey, in which the prevalence of self-reported food or digestive allergy in children was 3.9%.2 Our US prevalence of peanut sensitization of 7.6% is consistent with that observed by Arbes and colleagues in NHANES III (1988–1994). These investigators reported an 8.6% prevalence of peanut sensitization in 6–59 year olds by allergy prick skin testing. Importantly, NHANES III did not include younger children or older adults, whereas our data indicate that peanut sensitization is less prevalent in these age groups5 and excluding them to achieve a comparable age population to NHANES III increases the estimated peanut sensitization prevalence to 8.3%. Our clinical peanut allergy estimate of 1.3% is higher than in a US telephone survey in 2002 by Sicherer and colleagues that determined self-reported peanut allergy to be present in 0.6% of the population.6 While there were limited shrimp-specific data upon which to base our threshold partitioning PFA and LFA, our clinical shrimp allergy estimate of 1.0% appears consistent with another US telephone survey conducted by Sicherer and colleagues, which found 2.0% prevalence of physician-diagnosed or convincing reactions to any type of shellfish.3 Furthermore, a sensitivity analysis showed that the estimated prevalence of clinical food allergy reported in this paper is very robust to the particular choice of threshold used to differentiate PFA and LFA to shrimp.
Prevalence differences between studies are attributable to differences in cohort enrollment, how food sensitization or FA was determined, and the panel of foods tested. Our study was strengthened by the nationally representative nature of the cohort, the consistent methodologies employed by the NHANES, and by using standardized food-specific serum IgE, increasing levels of which predict an increasing probability of clinical food reactivity.1, 11, 12 Those with low-level sensitization (0.35–2 kU/L) were excluded from clinical FA estimates in order to minimize the impact of low-level sensitization, which is thought to be associated with a relatively low probability of clinical allergic reactivity, on the results. This study was limited by the fact that the NHANES relied solely on IgE levels for determination of clinical FA estimates, without detailed clinical or questionnaire-based information to corroborate the relevance of these findings. Moreover, there are no predictive values available from studies of adults. Therefore, this study might overestimate FA prevalence since it is possible that higher IgE levels in adults do not correlate as highly with allergy, or it might underestimate FA prevalence by measuring specific IgE to only 4 common foods. Including other common allergenic foods (e.g., tree nuts, sesame, fish, wheat, soy) would likely have increased overall prevalence estimates. Nevertheless, this study provides a consistent, contemporary picture of FA prevalence in the US.
Childhood (1–19 years old), male gender, and non-Hispanic black race/ethnicity were identified as significant risk factors for FA. In fact, the odds of black male children having FA were 4.4 times higher than others in the general population. Childhood is well recognized as a time when food allergies, especially to milk and egg, are more prevalent. Our study's observation of the high peanut allergy prevalence through childhood (1.8% in 1–5 year olds, 2.7% in 6–19 year olds), and the low peanut allergy prevalence in older adults (0.3% in 60+ year olds) has not been previously observed.6 The overall decreasing trend in FA with increasing age may reflect a combination of two factors: (1) the general loss of sensitization with age, noting that allergy to foods such as milk and egg typically resolve over time whereas peanut and shellfish more typically persist, together with (2) a possible cohort effect associated with the increasing prevalence of allergy in children in recent years.16
Male gender and black race/ethnicity were significant risk factors for inhalant allergen sensitization in NHANES II17 and NHANES III.5 With specific regard to FA, Sicherer et al3 found that self-reported shellfish allergy was more prevalent among blacks than whites, while Branum and Lukacs2 found higher rates of sensitization to shrimp, milk, and peanuts among non-Hispanic black children than other children. Our results are consistent with these but are supported by, to our knowledge, the first population-based study among all ages using objective clinical data to identify male gender and black race/ethnicity as risk factors for FA. FA may be under-recognized in blacks, males, and children, because it was self-reported in the other US-wide studies instead of being determined by food-specific serum IgE levels.
The higher prevalence of food sensitization and FA risk categories in participants with asthma, hay fever and eczema is consistent with other national studies.18, 19 In our study, the link between FA and asthma appeared especially strong. There was increased prevalence of all food sensitization and FA risk categories in those with diagnosed asthma, as well as, increased prevalence and likelihood of FA in those with current asthma (indicative of asthma persistence) and ER visits for asthma in the prior year (indicative of severe asthma exacerbations). Indeed, the odds of asthmatics with FA experiencing a recent severe asthma exacerbation were 6.9 times higher than those without FA.
This relationship between FA and asthma severity may or may not be causal in nature. For example, severe asthma may be associated with greater atopy in general.20, 21 In this context, FA might be a marker for a generalized atopic phenotype of severe asthma, but ingesting the allergenic food might not be directly responsible for the asthma severity and severe asthma attacks.
Alternatively, the relationship between FA and asthma severity could be causal, noting that asthma symptoms are induced by foods in food-allergic people up to 30% of the time, although usually with other allergic symptoms.4, 22 However, bronchial hyperreactivity and asthma worsening can be induced, in the absence of immediate bronchospasm, by the ingestion of small amounts of food allergens in sensitized individuals.23, 24 FA has been recently found to be a major risk factor for severe asthma and life-threatening asthma episodes. Several smaller studies have identified food sensitization as a significant risk factor for severe asthma.25, 26 Roberts and colleagues reported 54% of children with asthma requiring intubation for a severe asthma exacerbation had food sensitization, compared with 10% of hospitalized children with asthma at the same hospital.27 Asthma is coincident in nearly all people who suffer fatal anaphylactic reactions and severe asthma is a common manifestation.28, 29
Thus, while the relationship may or may not be causal, this study provides further credence and a national perspective to the concern that FA may be an under recognized trigger of severe asthma exacerbations. Offending foods are notoriously occult and are often hidden in other foods, leading to accidental ingestion. Food-allergic reactions might only be triggered when combined with exercise. In these circumstances, the onset of food-induced symptoms can often be delayed by 2–4 hours after ingestion and only manifest after vigorous physical exertion.30, 31 With these challenges to clinical detection of food-triggered asthma, heightened suspicion is necessary to identify food-triggered asthma episodes. Recognition of FA-triggered asthma exacerbations might improve preventive and therapeutic management and result in better clinical outcomes.
In summary, this NHANES investigation provides a comprehensive, contemporary US public health perspective on the prevalence and risks associated with higher food-specific serum IgE levels indicative of PFA or LFA. Black race/ethnicity and male gender were identified as important risk factors for FA. Along with childhood, these risk factors might identify at-risk populations in which FA is under recognized. A US population-level association between FA and severe asthma exacerbations was also identified. Because the cross-sectional design of this study does not substantiate inferences of causation, prospective clinical investigation of this association is needed. Nevertheless, these findings raise awareness that FA may be contributing to severe asthma episodes.
Repository - Unmarked Figure No. E3.
Acknowledgments
Grant support:
Supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences
Abbreviations
- CI
Confidence Interval
- ER
Emergency Room
- FA
Food Allergy
- LFA
Likely Food Allergy
- NHANES
National Health and Nutrition Examination Survey
- OR
Odds Ratio
- PFA
Possible Food Allergy
- PIR
Poverty Income Ratio
- PL
Predictive Level
- UFA
Unlikely Food Allergy
Footnotes
Clinical Implications
Blacks, males, and children represent at-risk populations for whom clinical food allergy may be under-recognized. Furthermore, population-level associations suggest that food allergy may contribute to severe asthma episodes.
Capsule summary.
The first nationally representative serologic measurement of food sensitivity in the US population supports estimation of the prevalence of clinical food allergy, identification of high-risk groups, and detection of associations with severe asthma episodes.
Contributor Information
Andrew H. Liu, National Jewish Health and University of Colorado Denver School of Medicine, Denver, CO.
Renee Jaramillo, SRA International, Inc., Durham, NC.
Scott H. Sicherer, Jaffe Food Allergy Institute, Mount Sinai School of Medicine New York, NY.
Robert A. Wood, Johns Hopkins University School of Medicine, Baltimore, MD.
S. Allan Bock, National Jewish Health and University of Colorado School of Medicine, Denver, CO.
A. Wesley Burks, Duke University Medical Center, Durham, NC.
Mark Massing, SRA International, Inc., Durham, NC.
Richard D. Cohn, SRA International, Inc., Durham, NC.
Darryl C. Zeldin, NIH/National Institute of Environmental Health Sciences, Research Triangle Park, NC.
References
- 1.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004 May;113(5):805–19. doi: 10.1016/j.jaci.2004.03.014. quiz 820. Review. PubMed PMID: 15131561. [DOI] [PubMed] [Google Scholar]
- 2.Branum AM, Lukacs SL. Food allergy among children in the United States. Pediatrics. 2009 Dec;124(6):1549–55. doi: 10.1542/peds.2009-1210. Epub 2009 Nov 16. PubMed PMID: 19917585. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004 Jul;114(1):159–65. doi: 10.1016/j.jaci.2004.04.018. PubMed PMID: 15241360. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA. Respiratory reactions induced by food challenges in children with pulmonary disease. Ped Allergy Immunol. 1992 Dec;3(4):188–194. [Google Scholar]
- 5.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005 Aug;116(2):377–83. doi: 10.1016/j.jaci.2005.05.017. PubMed PMID: 16083793. [DOI] [PubMed] [Google Scholar]
- 6.Sicherer SH, Muñoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003 Dec;112(6):1203–7. doi: 10.1016/s0091-6749(03)02026-8. PubMed PMID: 14657884. [DOI] [PubMed] [Google Scholar]
- 7.Bock SA. Prospective appraisal of complaints of adverse reactions to foods in children during the first 3 years of life. Pediatrics. 1987 May;79(5):683–8. PubMed PMID: 3575022. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2006. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/current_nhanes_05_06.htm. [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: 2006. Available at: http://www.cdc.gov/nchs/nhanes/irba98.htm. [Google Scholar]
- 10. [January 6, 2010];ANALYTIC AND REPORTING GUIDELINES: The Third National Health and Nutrition Examination Survey, NHANES III (1988-94) 1996 October; Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 11.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997 Oct;100(4):444–51. doi: 10.1016/s0091-6749(97)70133-7. PubMed PMID: 9338535. [DOI] [PubMed] [Google Scholar]
- 12.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001 May;107(5):891–6. doi: 10.1067/mai.2001.114708. PubMed PMID: 11344358. [DOI] [PubMed] [Google Scholar]
- 13.Gergen PJ, Arbes SJ, Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2009 Sep;124(3):447–53. doi: 10.1016/j.jaci.2009.06.011. Epub 2009 Aug 3. PMID: 19647861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyano Martínez T, García-Ara C, Díaz-Pena JM, Muñoz FM, García Sánchez G, Esteban MM. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy. 2001 Sep;31(9):1464–9. doi: 10.1046/j.1365-2222.2001.01175.x. PubMed PMID: 11591198. [DOI] [PubMed] [Google Scholar]
- 15.García-Ara C, Boyano-Martínez T, Díaz-Pena JM, Martín-Muñoz F, Reche-Frutos M, Martín-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cows’ milk protein in the infant. J Allergy Clin Immunol. 2001 Jan;107(1):185–90. doi: 10.1067/mai.2001.111592. PubMed PMID: 11150010. [DOI] [PubMed] [Google Scholar]
- 16.Ariana C, Yang AC, Arruda LK, Santos ABR, Barbosa MCR, Chapman MD, Galvao CES, Kalil J, Morato-Castro FF. Measurement of IgE antibodies to shrimp tropomyosin is superior to skin prick testing with commercial extract and measurement of IgE to shrimp for predicting clinically relevant allergic reactions after shrimp ingestion. J Allergy Clin Immunol. 2010 Apr;125(4):872–8. doi: 10.1016/j.jaci.2009.11.043. PubMed PMID: 20226506. [DOI] [PubMed] [Google Scholar]
- 17.Gergen PJ, Turkeltaub PC, Kovar MG. The prevalence of allergic skin test reactivity to eight common aeroallergens in the U.S. population: results from the second National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 1987 Nov;80(5):669–79. doi: 10.1016/0091-6749(87)90286-7. PubMed PMID: 3680811. [DOI] [PubMed] [Google Scholar]
- 18.Branum AM, Lukacs SL. Food allergy among U.S. children: trends in prevalence and hospitalizations. NCHS Data Brief. 2008 Oct;(10):1–8. PubMed PMID: 19389315. [PubMed] [Google Scholar]
- 19.Wickman M, Lilja G, Söderström L, van Hage-Hamsten M, Ahlstedt S. Quantitative analysis of IgE antibodies to food and inhalant allergens in 4-year-old children reflects their likelihood of allergic disease. Allergy. 2005 May;60(5):650–7. doi: 10.1111/j.1398-9995.2004.00764.x. Erratum in: Allergy. 2005 Nov;60(11):1458. van HageHamsten, M [corrected to van Hage-Hamsten, M]. PubMed PMID: 15813811.
- 20.Moore WC, Bleecker ER, Curran-Everett D, Erzurum SC, Ameredes BT, Bacharier L, et al. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007 Feb;119(2):405–13. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fitzpatrick AM, Gaston BM, Erzurum SC, Teague WG, National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program Features of severe asthma in school-age children: Atopy and increased exhaled nitric oxide. J Allergy Clin Immunol. 2006 Dec;118(6):1218–25. doi: 10.1016/j.jaci.2006.08.019. Epub 2006 Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James JM, Bernhisel-Broadbent J, Sampson HA. Respiratory reactions provoked by double-blind food challenges in children. Am J Respir Crit Care Med. 1994 Jan;149(1):59–64. doi: 10.1164/ajrccm.149.1.8111598. PubMed PMID: 8111598. [DOI] [PubMed] [Google Scholar]
- 23.Zwetchkenbaum JF, Skufca R, Nelson HS. An examination of food hypersensitivity as a cause of increased bronchial responsiveness to inhaled methacholine. J Allergy Clin Immunol. 1991 Sep;88(3 Pt 1):360–4. doi: 10.1016/0091-6749(91)90098-9. PubMed PMID: 1890264. [DOI] [PubMed] [Google Scholar]
- 24.James JM, Eigenmann PA, Eggleston PA, Sampson HA. Airway reactivity changes in asthmatic patients undergoing blinded food challenges. Am J Respir Crit Care Med. 1996 Feb;153(2):597–603. doi: 10.1164/ajrccm.153.2.8564104. PubMed PMID: 8564104. [DOI] [PubMed] [Google Scholar]
- 25.Berns SH, Halm EA, Sampson HA, Sicherer SH, Busse PJ, Wisnivesky JP. Food allergy as a risk factor for asthma morbidity in adults. J Asthma. 2007 Jun;44(5):377–81. doi: 10.1080/02770900701364031. PubMed PMID: 17613633. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005 May;115(5):1076–80. doi: 10.1016/j.jaci.2005.02.014. PubMed PMID: 15867869. [DOI] [PubMed] [Google Scholar]
- 27.Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life-threatening asthma in childhood: a case-controlled study. J Allergy Clin Immunol. 2003 Jul;112(1):168–74. doi: 10.1067/mai.2003.1569. PubMed PMID: 12847494. [DOI] [PubMed] [Google Scholar]
- 28.Sampson HA. Clinical practice. Peanut allergy. N Engl J Med. 2002 Apr 25;346(17):1294–9. doi: 10.1056/NEJMcp012667. PubMed PMID: 11973367. [DOI] [PubMed] [Google Scholar]
- 29.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001 Jan;107(1):191–3. doi: 10.1067/mai.2001.112031. PubMed PMID: 11150011. [DOI] [PubMed] [Google Scholar]
- 30.Varjonen E, Vainio E, Kalimo K. Life-threatening, recurrent anaphylaxis caused by allergy to gliadin and exercise. Clin Exp Allergy. 1997 Feb;27(2):162–6. PubMed PMID: 9061215. [PubMed] [Google Scholar]
- 31.Castells MC, Horan RF, Sheffer AL. Exercise-induced anaphylaxis (EIA). Clin Rev Allergy Immunol. 1999;17(4):413–24. doi: 10.1007/BF02737646. Winter. Review. PubMed PMID: 10829811. [DOI] [PubMed] [Google Scholar]