SUMMARY
Background
Non-alcoholic steatohepatitis (NASH) is a form of progressive fatty liver disease that is strongly associated with insulin resistance, which suggests that insulin sensitizing agents such as metformin may be beneficial for NASH.
Aim
To assess the effects of metformin on insulin sensitivity, body composition, serum alanine aminotransferase (ALT) levels and liver histology in patients with NASH.
Methods
Patients underwent liver biopsy, metabolic profiling and imaging studies before and at the end 48 weeks of metformin (2000 mg/day) therapy. The primary endpoint was a three-point improvement in the histological NASH activity index.
Results
Of 28 patients enrolled, 26 (13 females; average age 44 years) completed 48 weeks of treatment and underwent repeat metabolic studies, imaging and liver biopsy. Thirty per cent achieved a histological response. Most patients lost weight, the average being 6 kg. There was a marked association between weight loss and improvements in NASH activity index and ALT levels (both, P < 0.01). Insulin sensitivity also improved, but the degree of change did not correlate with histological improvement.
Conclusion
Metformin leads to improvements in liver histology and ALT levels in 30% of patients with NASH, probably by its effects in causing weight loss.
INTRODUCTION
Non-alcoholic steatohepatitis (NASH) is a clinicopathological entity characterized by histological features resembling alcoholic liver disease that occurs in persons who consume little or no alcohol.1 NASH is a part of the spectrum of non-alcoholic fatty liver disease (NAFLD) and is defined by of the presence of ballooning (zone 3) hepatocellular injury and inflammation in addition to steatosis.2 NASH is a progressive liver disease that can result in fibrosis, cirrhosis and, in some cases, liver cancer.3 While some degree of fatty liver is present in up to 30% of adult Americans, frank NASH occurs in <5%.4 NASH is typically associated with obesity, type II diabetes, dyslipidaemia and the metabolic syndrome.4 Because of the steady rise in obesity in the US, the prevalence of liver disease caused by NASH is likely to increase in the next two decades. The close association of obesity and diabetes with NASH suggests that insulin resistance may play a role in its pathogenesis.5
How insulin resistance leads to fatty liver injury is not clear. The ‘two-hit’ hypothesis of the pathogenesis of NASH proposes that a first hit (obesity, diet) induces fatty liver and a second leads to the inflammation and cell injury.5 Factors that have been suggested to cause the second hit include insulin resistance, cytokines, free fatty acids or intracellular oxidative stress. In vitro and in vivo models of fatty liver disease suggest that cytokines produced by adipocytes (‘adipokines’) might be important in NASH leading to dysfunction in fatty acid oxidation in the liver and preventing export of free fatty acids and triglycerides. 6, 7 These adipokines may also activate stellate cells and lead to deposition of collagen and fibrosis.8 Improving insulin sensitivity by weight loss or by pharmacological agents therefore may reverse some of these processes either directly or by decreasing oxidative stress and lowering proinflammatory cytokine secretion.
Several pilot studies in patients with NASH have shown that treatment with the insulin-sensitizing thiazolidinediones (TZDs) can lead to improvements in biochemical and histological features of NASH.9–13 The degree of improvement in liver disease correlates to some degree with the degree of improvement in insulin sensitivity, but appears to be more directly associated with changes in fatty acid metabolism and fat distribution. Importantly, use of TZDs is often associated with weight gain, which may ultimately limit the efficacy of these agents and is distressing to patients, many of whom are already overweight or obese. Metformin is another anti-diabetic medication that improves insulin sensitivity but is associated with weight loss rather than weight gain. Metformin is also relatively inexpensive and has an excellent long-term track record for safety and tolerance. Metformin has been shown to cause reversal of fatty liver in leptin-deficient mice, an animal model for NASH.14 In patients with NAFLD, several groups have now shown that metformin leads to improvement in serum aminotransferase levels and liver histology;15–17 but, detailed changes in histological, anthropometric and metabolic parameters and measures of insulin sensitivity and their correlations have not been described.
We have conducted a small, open-label study of a 1-year course of metformin in 28 patients with well-characterized NASH and report results on clinical, biochemical, metabolic, radiographic and histological features.
METHODS
Patient population
Enrolment criteria for this open-label study were (i) age above 18 years, (ii) elevations in alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels on at least two occasions or fatty liver on ultrasound during the previous 6 months, (iii) alcohol intake of <1 drink/day (10–12 g of alcohol) and (iv) liver biopsy findings diagnostic for NASH (see below). Exclusion criteria included previous use of metformin or other anti-diabetic medications, other known forms of liver disease, decompensated liver disease, bariatric surgery, recent exposure to hepatotoxic medications, active substance abuse, pregnancy or inability to practice birth control and presence of any other medical (symptomatic coronary artery disease, congestive heart failure, renal failure, severe lung disease or malignancy) or psychiatric condition that might interfere with the treatment and assessment of response.
Baseline evaluation
Patients were initially screened during an out-patient clinic visit with brief medical history, review of outside medical records and routine blood tests. Patients suspected of having NASH were given instructions to lose weight and follow a healthy diet and were encouraged to stop all herbal, vitamins and over-the-counter medications or supplements. None of the patients took any herbal product or medication that is known to cause weight loss or affect hepatic steatosis during the trial. All patients received standard nutritional counselling by a trained nutritionist emphasizing the need to maintain a healthy life style and gradual weight loss (~0.45 kg/week).
Patients found to be eligible during screening were monitored with clinic visits for routine blood tests at 4-week intervals during which they also underwent a 2-h oral glucose tolerance test (OGTT), a frequently sampled intravenous glucose tolerance test (FSIGT), careful measurements of weight and waist hip ratio and abdominal ultrasound. Body fat composition was measured by dual-energy X-ray absorptiometry (DEXA). Hepatic fat content and liver volume were determined by magnetic resonance imaging (MRI) of the liver. Visceral and subcutaneous abdominal fat were measured using computed tomography (CT) of the abdomen. Details of the conduct and interpretation of imaging results and glucose tolerance testing have been previously described8 and are described in the supplementary materials. Careful determination of waist and hip circumference and body mass index [BMI = weight in kg/(height in m)2] was made at baseline and at 48 weeks.
Patients who did not have a liver biopsy that was available for interpretation within the previous 12 months underwent percutaneous liver biopsy. Biopsy specimens were read under code and scored using defined criteria for degree of steatosis (0–4), cellular injury or ballooning degeneration (0–4), parenchymal inflammation (0–4) and perisinusoidal fibrosis (0–4).9 The presence of cirrhosis and Mallory bodies was also recorded. The criteria for the diagnosis of NASH for this study were the presence of steatosis and a total score of 4 or more of the NASH activity index which was defined as the sum of the individual scores for steatosis, cellular injury and inflammation, thus ranging from 0 to 12.
Insulin resistance assessment
Insulin resistance was assessed by two different techniques. Using fasting glucose and insulin values on the morning of the OGTT, insulin resistance was determined using the homeostasis model assessment (HOMA).18 HOMA was calculated from fasting glucose and insulin levels in all 26 subjects who completed the study. Twenty-one subjects also had insulin resistance determined from the insulin sensitivity index using data from the FSIGT.19
Therapy and monitoring
After complete medical evaluation and liver biopsy, patients who qualified for therapy were started on metformin in an initial dose of 500 mg once daily. After 2 weeks, the dose was increased to 500 mg twice daily and after 4 weeks to the full dose of 1000 mg twice daily. Subsequent dose reductions were carried out based on tolerance, with particular attention to gastrointestinal upset and abdominal bloating. Patients were seen in the out-patient clinic, had a brief medical history and examination and routine blood tests at 2 and 4 weeks after enrolment and every 4 weeks thereafter. The oral and intravenous glucose tolerance tests were repeated after 40 and 44 weeks respectively and liver biopsy and imaging tests at 48 weeks. Metformin was discontinued after 48 weeks in patients without diabetes on the pre-treatment evaluation.
The primary outcome measure was improvement in liver histology, which was defined as a three-point decrease in the NASH activity index with a decrease in at least two of the component scores and no worsening of fibrosis or increase in Mallory bodies. Secondary endpoints included improvements in serum aminotransferase levels and insulin sensitivity. All components of this study were approved by the Institutional Review Board of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and patients gave written informed consent. An investigation of new drug application (no. 67,551) for use of metformin in patients with NASH was held by the senior investigator (JHH). This study was registered in Clinicaltrials.gov (NCT00063232).
Statistical analyses
Statistical analysis plan was defined a priori. Paired t-tests were performed to test the statistical significance of the primary endpoint and all the before and after treatment measures. Two sample t-tests were used to compare the means for continuous variables and Mann–Whitney U-tests were used for nonparametric variables. Categorical variables were tested using Fisher’s exact test. Spearman rank correlation coefficient was used to examine the association between selected variables. Statistical analysis was performed using GraphPad Prism 4 Software (Graph Pad Software Inc., San Diego, CA, USA). A two-tailed P-value of 0.05 was considered statistically significant.
RESULTS
Patients enrolled
Twenty-eight adult patients with biopsy-proven NASH were enrolled in the study and 26 completed the 48 weeks of therapy and underwent follow-up metabolic testing, imaging and repeat liver biopsy. The two drop-outs included a 26-year-old woman who stopped therapy after 12 weeks because she had moved and her employment did not allow time for travel for outpatient visits and a 53-year-old woman who stopped therapy after 24 weeks because of desire to pursue other treatments. These patients were not included in further analyses.
Baseline features
The 26 patients included 13 women and the average age was 44 years. All patients were either overweight (31%) or obese (69%) (Table 1). Although no patient was being treated for diabetes at the time of enrolment, seven patients (27%) fulfilled the criteria for having diabetes and another eight (31%) had impaired glucose tolerance. Average serum ALT levels were 70 U/L (normal = 6–41 U/L) and AST 47 U/L (normal 9–34 U/L). On the day metformin was started, five patients had normal ALT and seven had normal AST levels. All patients had NASH on liver biopsy and NASH activity index scores ranged from 4 to 10 (median = 8). All but 4 patients had some degree of fibrosis and one had cirrhosis on pre-treatment liver biopsy.
Table 1.
Baseline demographics
| Characteristics | n (%) |
|---|---|
| Number of patients | 26* |
| Mean age (s.d.) in years | 44 (13.7) |
| Female gender | 13 (50) |
| Race/ethnicity | |
| Non-Hispanic White | 17 (65) |
| Hispanic White | 4 (15) |
| Asian | 5 (19) |
| African-American | 0 (0) |
| Overweight (BMI ≥25 and <30 kg/m2) | 8 (31) |
| Obese (BMI ≥30 and <40 kg/m2) | 13 (50) |
| Severely obese (BMI ≥40 kg/m2) | 5 (19) |
| Impaired glucose tolerance† | 8 (31) |
| Diabetes† | 7 (29) |
BMI, body mass index; s.d., standard deviation.
Two patients dropped out (weeks 12 and 24) and are not included in the analysis.
Impaired glucose tolerance is defined by fasting glucose 110–125 or ≥160 mg/dL after 2-h glucose load, while diabetes is defined by a fasting glucose ≥125 or ≥200 mg/dL after a 2-h glucose load.
Metformin therapy
Metformin treatment was well tolerated. No serious adverse events occurred and no patient stopped therapy because of side effects. The most common side effect was mild and intermittent diarrhoea with variable amounts of abdominal bloating and discomfort that affected most patients to some degree during the first few weeks of treatment. However, diarrhoea and intestinal upset resolved despite continuation of full doses of therapy in all except two patients who underwent dose reduction at 12 and 16 weeks; one being maintained on 1500 mg and one on 1000 mg daily. No patient developed hypoglycaemia, evidence of lactic acidosis, congestive heart failure or renal dysfunction.
Biochemical results
Overall, average serum ALT and AST levels improved minimally during the study and the differences between mean baseline and end-of-treatment aminotransferase values were not statistically significant (Table 2). Changes in aminotransferase levels appeared to be dichotomous, in that ALT levels fell into the normal range in nine patients and AST levels in six patients by the end of treatment, but changed minimally overall. ALT levels remained in the normal range in four of the five patients who had normal values at the start of therapy so that the proportion of patients with normal ALT levels was significantly higher at the end of treatment (50% vs. 19%; Fisher’s exact test: P = 0.04). Other biochemical test results did not change appreciably including serum bilirubin, albumin, prothrombin time and cholesterol or triglyceride levels. There were mild increases in mean HDL cholesterol levels. Blood counts, including haematocrit, total white blood cell count and platelet count did not change (data not shown).
Table 2.
Biochemical data at baseline and after 48 weeks of metformin
| Parameter | Pre | Week 48 | P-value* |
|---|---|---|---|
| ALT (U/L)† | 70 (59) | 63 (51) | 0.63 |
| Normal ALT (<42 U/L) | 5 (19%) | 13 (50%) | 0.04 |
| AST (U/L)† | 47 (28) | 43 (23) | 0.64 |
| Normal AST (<35 U/L) | 7 (27%) | 12 (46%) | 0.14 |
| Total bilirubin (mg/dL)† | 0.8 (0.4) | 0.9 (0.3) | 0.91 |
| Albumin (gm/dL)† | 4.0 (0.3) | 4.1 (0.3) | 0.34 |
| Total Cholesterol (mg/dL)† | 184 (32) | 184 (33) | 0.98 |
| Triglycerides (mg/dL)† | 165 (62) | 172 (88) | 0.76 |
| LDL Cholesterol (mg/dL)† | 122 (29) | 116 (32) | 0.44 |
| HDL Cholesterol (mg/dL)† | 42 (8) | 48 (16) | 0.09 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
For continuous data, P-value based on two-sided paired t-test; for dichotomous data, P-value based on Fisher’s exact test.
Values are given as mean (s.d.).
Liver histology
Twenty-six patients underwent follow-up liver biopsy after 48 weeks of metformin treatment (Table 3). All features of the NASH activity index improved somewhat, the changes being the greatest for cellular injury and less for parenchymal inflammation and steatosis. Mallory bodies were less evident in follow-up liver biopsies. The average NASH activity index improved significantly, falling from 8.2 to 5.9 (P < 0.001). The primary outcome (a three-point improvement in NASH activity index with improvements in at least two components of the score and no worsening of fibrosis or increase in Mallory bodies) was achieved by eight patients (31%), all of whom also had improvements in serum aminotransferase elevations. Among the 26 patients, 20 showed improvement (nine had a threepoint or greater, 11 a one- to two-point improvement), five had no change and one had a two-point increase in the NASH activity index.
Table 3.
Histological data at baseline and after 48 weeks of metformin
| Parameter (0–4) | Pre (n = 26) | Week 48 (n = 26) | P-value* |
|---|---|---|---|
| Presence of NASH | 26 (100%) | 18 (69%) | <0.01 |
| Portal inflammation† | 1.9 (1.1) | 1.6 (1.0) | 0.23 |
| Parenchymal inflammation† | 3.5 (0.8) | 2.9 (1.1) | 0.03 |
| Cellular injury† | 2.1 (0.6) | 1.0 (0.6) | <0.001 |
| Mallory’s hyalin† | 2.3 (1.6) | 1.6 (1.4) | 0.07 |
| Steatosis† | 2.6 (1.1) | 2.0 (1.2) | 0.06 |
| Fibrosis† | 1.7 (1.1) | 1.5 (1.0) | 0.43 |
| NASH activity index (0–12) †,‡ | 8.2 (1.5) | 5.9 (2.2) | <0.001 |
NASH, non-alcoholic steatohepatitis.
For continuous data, P-values are calculated by two-sided paired t-tests; for dichotomous data, P-values are based on Fisher’s exact test.
Mean (s.d.).
NASH activity index represents the sum of scores for parenchymal inflammation (0–4), cellular injury (0–4) and steatosis (0–4).
In contrast to activity scores, liver fibrosis scores decreased minimally and not significantly after 48 weeks of therapy (mean NASH fibrosis scores 1.7 initially vs. 1.5 at end of treatment). Overall, eight patients had a one or more point improvement in fibrosis scores, 14 had no change and four had worsening of one or two points.
Anthropometric changes
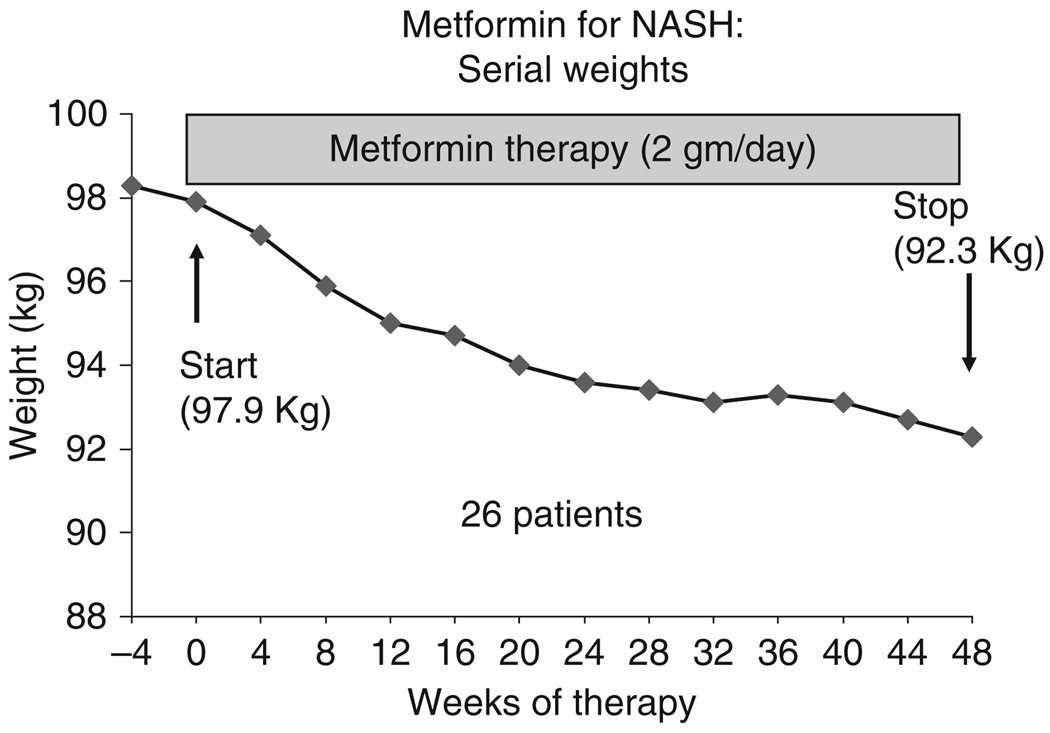
Patients on metformin often lost weight and the average weight loss during the 48 weeks of treatment was 6 kg (change in weight range: +1.3 to −18.9 kg; Table 4). The weight loss was maximal during the first 6 months, but continued throughout the treatment period (Figure 1). Weight loss, similar to the histological and biochemical improvements, appeared to be dichotomous: 18 patients (69%) lost at least 2 kg and five (19%) lost more than 10 kg of weight. In the remaining eight patients, average weight did not change. There was a strong positive correlation between weight change and changes in serum aminotransferase levels (for ALT: r = 0.52, P = 0.005; for AST, r = 0.50, P = 0.009) and between weight loss and decreases in NASH activity index scores (r = 0.76, P < .0001).
Table 4.
Anthropometric data at baseline and after 48 weeks of metformin
| Parameter | Pre | Week 48 | P-value* |
|---|---|---|---|
| Weight (kg) | 98.1 (24.6) | 92.2 (25.5) | 0.40 |
| BMI (kg/m2) | 33.9 (6.8) | 31.8 (7.3) | 0.28 |
| Waist circumference (cm) | 111.0 (16.6) | 107.6 (16.5) | 0.47 |
| Hip circumference (cm) | 113.2 (13.6) | 110.2 (13.7) | 0.17 |
| Waist/hip ratio | 0.98 (0.06) | 0.98 (0.05) | 0.86 |
| Total body fat (%) | 34.3 (9) | 33.2 (7.5) | 0.53 |
| Fat mass (kg) | 32.1 (10.0) | 29.5 (9.7) | 0.22 |
| Lean mass (kg) | 59.4 (15.3) | 57.3 (16.1) | 0.61 |
| Liver fat by MRI† (%) | 14.9 (9.4) | 13.0 (8.4) | 0.50 |
| Liver volume by MRI† (cc) | 2213 (547) | 2156 (580) | 0.74 |
| Total fat by CT (cm2) | 453 (210) | 460 (223) | 0.66 |
| Visceral fat by CT (cm2) | 200 (76) | 170 (66) | 0.001 |
| Subcutaneous fat by CT (cm2) | 254 (161) | 290 (185) | 0.008 |
Values are given as mean (s.d.).
BMI, body mass index; MRI, magnetic resonance imaging; CT, computerized tomography.
P-value based on two-sided paired t-test.
n = 16 patients.
Figure 1.
Changes in body weight in 26 patients treated with metformin for 48 weeks.
There was a slight average decrease in waist circumference, but a similar decrease in hip circumference, so that the average waist/hip ratio did not change (Table 4). Whole body composition by DEXA showed that the weight loss was because of decreases in both lean body and fat mass so that the per cent body fat decreased minimally (34.3–33.2%). The liver fat as assessed by MRI also decreased minimally from 14.9% to 13.0% (P = 0.50) without a significant change in total liver volume. Total abdominal fat did not change; however, visceral fat decreased (200–170 cm2), while subcutaneous fat increased (254–290 cm2) as estimated by cross sectional CT.
Changes in measures of insulin sensitivity
Improvements in fasting blood glucose, insulin, C-peptide and free fatty acid levels occurred during metformin treatment, but most of the changes did not reach statistical significance (Table 5). Impaired glucose tolerance was present in eight and diabetes in seven patients before therapy, this distribution shifting to 13 with impaired glucose tolerance and three with diabetes at the end of therapy. Fasting blood glucose and insulin results showed significant improvements in HOMA by 44% (P = 0.04). HOMA values improved in all except four patients and improved more in patients with a histological response. FSIGT results (in 21 patients who were tested before and after treatment) showed no significant change in acute insulin response to glucose (AIRG), insulin sensitivity index (SI), glucose effectiveness (SG) or glucose disposition index (DI).
Table 5.
Insulin sensitivity at baseline and after 40 weeks of metformin
| Parameter | Pre | Week 40–48 | P-value* |
|---|---|---|---|
| Normal glucose tolerance (n) | 11 | 10 | 0.29 |
| Impaired glucose tolerance (n) | 8 | 13 | |
| Diabetes (n) | 7 | 3 | |
| Fasting glucose (mg/dL)† | 106 (45) | 99 (15) | 0.43 |
| Fasting insulin (µU/mL)† | 32 (36) | 22 (15) | 0.19 |
| Fasting C-peptide (ng/mL)† | 4.7 (2.1) | 3.9 (1.4) | 0.12 |
| Fasting free fatty acids (µEq/L)† | 587 (256) | 546 (224) | 0.53 |
| Oral glucose tolerance test HOMA-IR† | 7.8 (7.5) | 4.4 (3.6) | 0.04 |
| FSIGT (21 pts with paired testing) SI (×10−4)‡ | 2.92 | 2.41 | 0.46 |
| SG‡ | 0.014 | 0.015 | 0.82 |
| AIRG‡ | 541.4 | 633.5 | 0.42 |
| DI‡ | 1067.3 | 1114.4 | 0.87 |
HOMA-IR, homeostatic model assessment of insulin resistance; FSIGT, frequently sampled intravenous glucose tolerance; SI, insulin sensitivity index; SG, glucose effectiveness; AIRG, acute insulin response to glucose; DI, disposition index.
P-value based on two-sided paired t-test.
Values are given as mean (s.d.).
n = 21 patients.
Differences between responders and nonresponders
Eight patients (31%) met the histological criteria of response and the remaining 18 were classified as nonresponders, although many of them had some degree of histological improvement (Table 6). At baseline, responders had a lower average BMI than nonresponders, but this difference was not statistically significant. None of the patients with baseline BMI of 40 or more achieved a histological response on treatment. There was no significant difference between responders and nonresponders at baseline with regard to other features such as average age, ALT, AST, HOMA, NASH activity index and fibrosis scores.
Table 6.
Comparison of histological responders and nonresponders to metformin therapy
| Parameter | Responders (n = 8) | Nonresponders (n = 18) | P-value* |
|---|---|---|---|
| Age (years)† | 47.8 (13.0) | 42.8 (14.1) | 0.38 |
| Baseline weight (kg)† | 89.5 (20.5) | 102.1 (25.7) | 0.20 |
| Baseline BMI 25–30 kg/m2 | 3 (38%) | 5 (28%) | 0.28 |
| Baseline BMI 30–40 kg/m2 | 5 (63%) | 8 (44%) | |
| Baseline BMI >40 kg/m2 | 0 | 5 (28%) | |
| Initial ALT (U/L)† | 60 (27) | 75 (69) | 0.43 |
| Initial AST (U/L)† | 40 (13) | 50 (29) | 0.26 |
| Initial HOMA† | 8.5 (7.6) | 7.5 (7.6) | 0.77 |
| Initial NASH activity index† | 8.8 (0.7) | 7.9 (1.7) | 0.09 |
| Initial fibrosis score† | 2.1 (0.6) | 1.6 (1.2) | 0.13 |
| Change in weight (kg)† | −10.5 (5.7) | −4.0 (4.6) | 0.02 |
| Change in BMI (kg/m2)† | −3.8 (2.2) | −1.4 (1.7) | 0.02 |
| Change in ALT (U/L)† | −29 (30.5) | +2.1 (53) | 0.08 |
| Change in AST (U/L)† | −13 (15.1) | +1.2 (30) | 0.13 |
| Change in HOMA-IR† | −6.5 (7.8) | −2.0 (6.7) | 0.19 |
| Change in NASH fibrosis† | −0.6 (1.1) | −0.1 (0.9) | 0.21 |
| Change in hepatic fat (% by MRI) | −0.8% (2.5) | −0.2% (4.5) | 0.67 |
| Change in hepatic volume (cc) | −107 (205) | −66 (321) | 0.72 |
| Change in total abdominal fat (cc) | −10 (72) | +14 (71) | 0.45 |
| Change in subcutaneous fat (cc) | +30 (69) | +39 (64) | 0.38 |
| Change in visceral fat (cc) | −41 (36) | −25 (46) | 0.37 |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA, homeostatic model assessment; NASH, non-alcoholic steatohepatitis; MRI, magnetic resonance imaging.
P-value <0.05 was considered statistically significant.
Categorical variables are presented as proportion and continuous variables are presented as (†)mean (s.d.). A two-sided Mann–Whitney U-test and Fisher’s exact test for categorical variables were conducted to assess statistical significance.
On therapy, responders were more likely to lose weight. Furthermore, there was a trend for decreases in ALT and AST being greater among the responders than nonresponders (Table 6). However, the histological responders and nonresponders did not different with regard to improvements in HOMA, decreases in hepatic fat or hepatic volume (by MRI) or changes in total, visceral and subcutaneous fat.
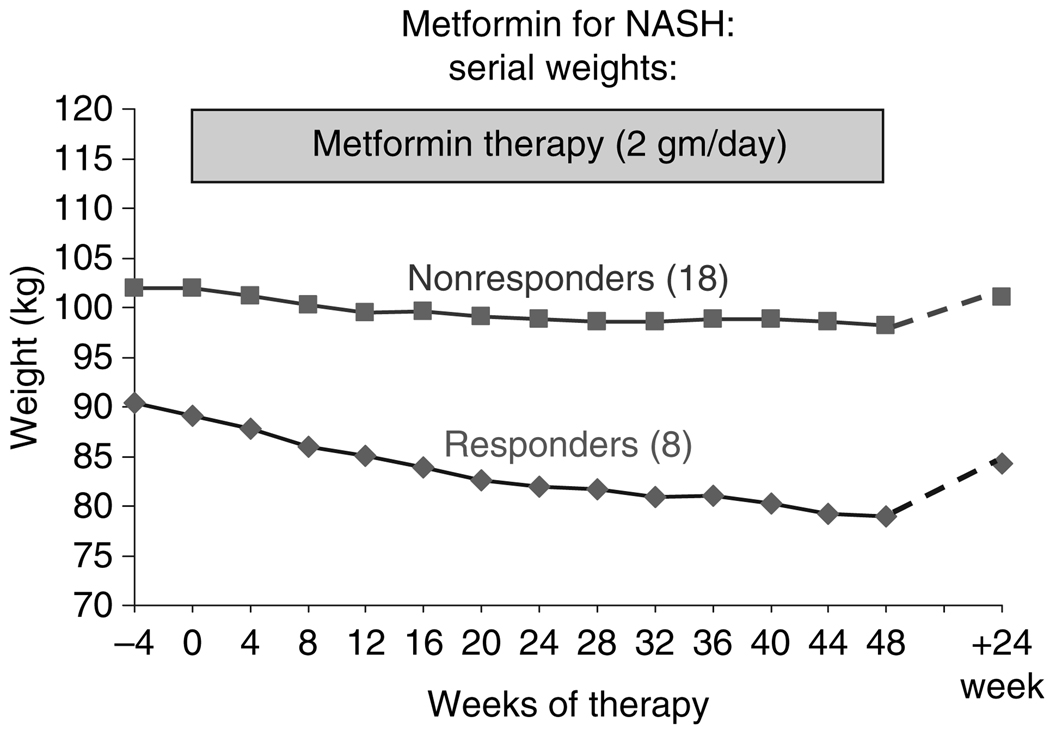
Changes in weight over the 48 weeks of therapy in relationship to response are shown in Figure 2. After 48 weeks of metformin treatment, the average weight change was −10.5 kg in responders vs. −4.0 kg in nonresponders (P = 0.02). All histological responders lost more than 5 kg during therapy (range 5.7–18.7 kg) and three responders were no longer overweight (BMI <25) at the end of 48 weeks of treatment.
Figure 2.
Changes in average weight in eight responders and 18 nonresponders to a 48-week course of metformin. The diamonds represent the mean weights of the eight responders and the squares, the mean weights of the 18 nonresponders during therapy and then again when measured 6 months later (in eight responders and 16 nonresponders who were seen between 20 and 32 weeks after stopping therapy).
Figure 2 also shows the changes in weight during follow-up to at least 24 weeks after treatment which was available on 24 patients, five of whom (one responder and four nonresponders) had diabetes and asked to remain on metformin. During follow-up, the average body weight increased in both responders and nonresponders. Indeed, all except two patients gained at least one kilogram in weight, the average weight gain being 4.1 kg. The two exceptions to the weight gain comprised one patient with diabetes on metformin (−0.1 kg weight change) and another patient who attributed her weight loss (−5.7 kg) to severe situational depression.
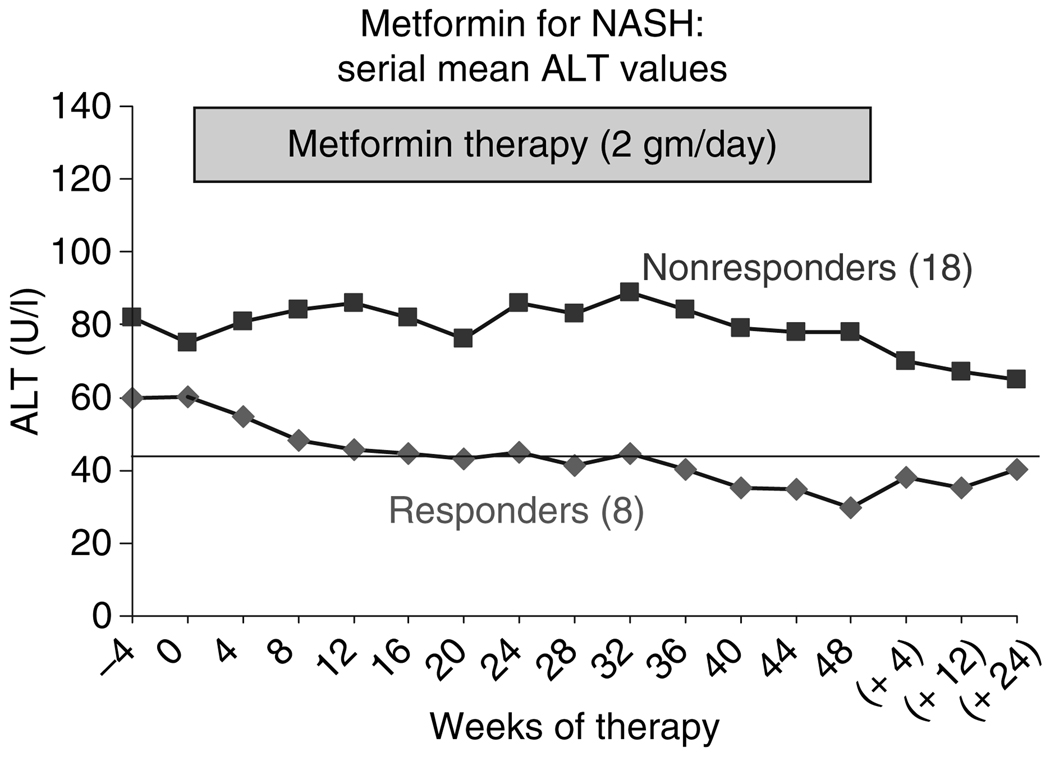
Responders were also more likely to have improvements in serum ALT levels during therapy than nonresponders; the average levels in responders decreasing to within the normal range by the end of therapy (Figure 3). At the end of treatment, ALT levels were normal in all except one responder (whose ALT level was 59 U/L). When metformin was stopped, however, serum ALT levels tended to rise towards baseline.
Figure 3.
Changes in mean serum alanine aminotransferase (ALT) in eight responders and 18 nonresponders during a 48-week course of metformin. Squares represent the mean values of the 18 nonresponders, the diamonds, the mean values for the eight responders. While ALT levels did not change overall, there was a gradual fall into the normal range in the responders and by the end of therapy, the mean level was 30 U/L and levels were normal in seven of the eight patients. After stopping therapy, ALT levels rose in the eight nonresponders, but remained largely unchanged in the 16 nonresponders who were followed up (including four nonresponders who remained on metformin).
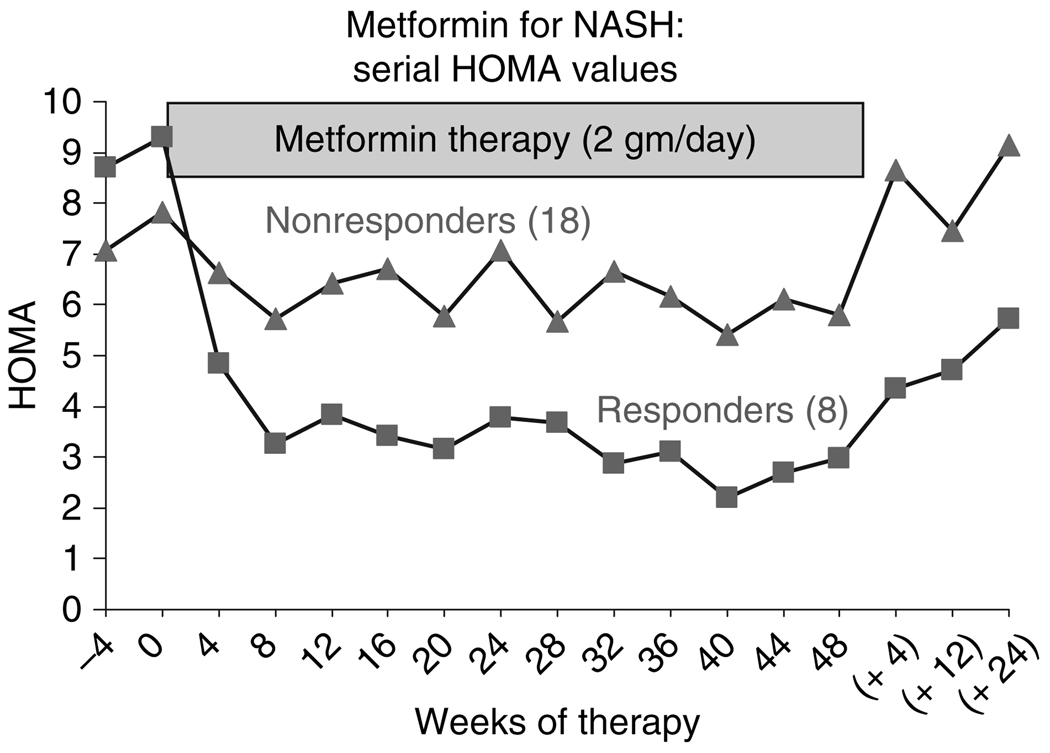
Finally, HOMA values improved markedly during the first 8 weeks of starting metformin, improving more among responders than among nonresponders; thereafter, HOMA values remained constant, although decreased slightly among responders (Figure 4). By 48 weeks, HOMA values in responders ranged from 1.2 to 3.1 (mean = 1.9) and were normal (<2.5) in six of the eight; in contrast, in nonresponders, HOMA values ranged between 1.1 and 17.4 (mean = 5.5) and were normal in only four of 18. With stopping therapy, HOMA values began to rise towards baseline levels among both responders and nonresponders.
Figure 4.
Changes in average homeostasis model assessment (HOMA) values in eight responders and 18 nonresponders to a 48-week course of metformin. The diamonds represent the mean HOMA values of the eight responders and the squares, those of the 18 nonresponders during therapy and then again when measured 4, 12 and 24 weeks later (in eight responders, only one remaining on metformin and 16 nonresponders, four remaining on metformin).
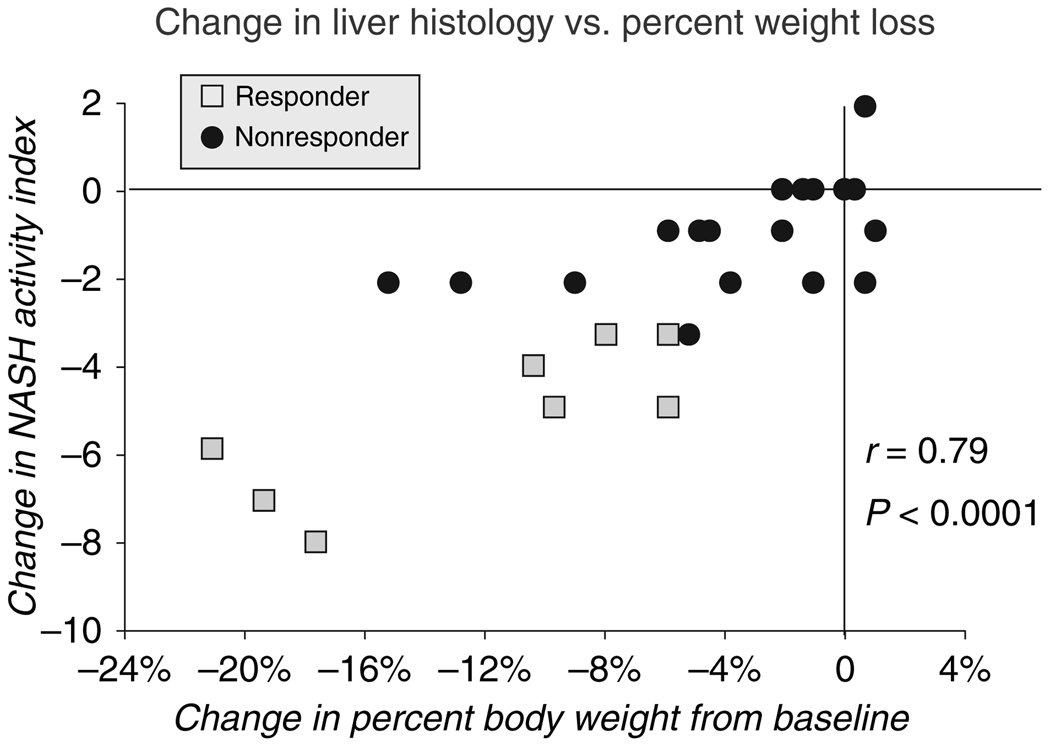
Analysis by degree of improvement in NASH activity index showed a close association of improved histology with weight loss during treatment, which was the strongest when expressed as per cent weight loss (r = 0.79, P < 0.00001) (Figure 5). Improvements in NASH activity index scores also correlated with the degree of improvement in serum ALT levels (r = 0.6, P < 0.01), but not with improvement in HOMA values expressed as either absolute or per cent change (r = 0.26, P = 0.18).
Figure 5.
Correlation between per cent change in body weight and change in NASH activity index after 48 weeks of metformin. The eight squares represent responders and the 18 circles represent nonresponders. There was a positive significant correlation between decrease in NASH activity index score and decrease in body weight in kilograms (r = 0.78: P < 0.0001).
DISCUSSION
In this pilot study, a 48-week course of metformin in doses of 2 gm daily was associated with improvements in liver histology and insulin sensitivity in over half of patients with NASH. Approximately 30% of patients met strict predefined histological criteria for response, and in these patients, there was also an improvement in serum aminotransferase levels. Histological improvements were found predominantly in hepatocellular injury and parenchymal inflammation, with fewer effects on steatosis and little measurable effects on portal inflammation or fibrosis. Importantly, metformin also caused weight loss. Twenty one of the 26 patients (81%) lost weight during the 48 weeks of treatment and in five (19%), the weight loss was marked (>10 kg). Strikingly, the patients with the most weight loss tended to have the greatest improvement in liver histology and those with no weight loss tended to have no improvement in histology. Statistical analyses showed that improvements in hepatic histology correlated most closely with weight loss rather than improvements in insulin sensitivity.
Many studies have demonstrated that metformin therapy can lead to weight loss;15, 16, 20–22 and for this reason, it is considered the first choice of anti-diabetic medications for treatment of type 2 diabetes in obese patients. The mechanism by which metformin causes weight loss remains unknown. When questioned, patients in this study with weight loss attributed it to the drug, which they felt allowed them to control their appetite better. The metabolic effects of metformin are thought to be mediated through the activation of adenosine monophosphate (AMP) activated protein kinase in hepatic and peripheral tissues either directly or indirectly through another serine–threonine kinase known as LKB1.23 The activation of AMP kinase leads to transactivation of genes that inhibit gluconeogenesis and lipogenesis and promote fatty acid and glucose uptake in liver and muscle. These effects appear to be responsible for the insulin sensitizing activity of metformin. AMP kinase is also present in the hypothalamus where it may play a role in the regulation of food intake. Thus, AMP kinase plays a central and critical role in the regulation of energy balance and fatty acid and glucose metabolism as well as appetite and satiety.
There are no currently proven and approved therapies for NAFLD or NASH, although several agents have appeared promising in pilot studies. In particular, the TZDs have been found to induce biochemical and histological remission in disease in a high proportion of patients. In three open-label studies and in a small randomized, placebo controlled trial, 6- or 12-month courses of pioglitazone or rosiglitazone were associated with improvements in serum aminotransferase levels and decreases in hepatic steatosis and cell injury in up to 70% of patients.9, 10, 13 Unlike with metformin, the improvements in NASH associated with TZD therapy were accompanied by weight gain rather than loss. Furthermore, the weight gain on TZDs was primarily in fat mass and was associated with a redistribution of fat, away from the liver and central sites and more to the periphery and subcutaneous areas of the body.9 This redistribution of fat was also associated with improvements in insulin signalling and increases in adiponectin levels. Thus, the beneficial effects of the TZDs in NASH may have a cause different from that associated with metformin. With metformin therapy, there was little evidence of redistribution of fat and weight loss that occurred was associated with decreases in both lean body and fat mass. These findings suggest that the beneficial effects of metformin therapy on NASH are mediated, at least in part, by weight loss. It is unclear whether the benefits of metformin are greater than might be achieved with weight loss from diet and exercise alone or with a weight loss medication that does not directly affect insulin sensitivity.15
This study enrolled patients with well-characterized NASH and used strict histological criteria for diagnosis as well as assessment of benefit. The strengths of this study were the use of maximum doses of metformin and use of multiple measures of body composition, insulin sensitivity and liver disease status in a carefully followed up cohort with excellent compliance and follow-up. The major limitation of this study was the lack of a control group. Previous controlled trials of therapy of NASH have shown that spontaneous improvements in serum aminotransferase levels and hepatic steatosis can occur even in placebo-treated patients.24 However, the same studies showed little spontaneous improvement in hepatocellular injury or inflammation, the major effects that were seen with TZD and metformin therapy.9, 24 In a recent pooled analysis of placebo-treated patients from five randomized controlled studies in NASH, a two-point improvement in steatosis score or any improvement in hepatocellular injury was rarely seen with placebo treatment.25 Furthermore, significant weight loss was uncommon in placebo-treated patients.
Another limitation of this study was that patients were treated for 48 weeks only. Weight loss and improvements in ALT levels continued for the duration of therapy, but stopping treatment was followed promptly with weight gain followed by increases in serum aminotransferase levels. Therapies for NASH are likely to require long-term treatment, and it is not clear whether the apparent beneficial effects of metformin on weight and liver histology would be sustained with longer treatment. On the other hand, long-term therapy might be required to demonstrate decreases in hepatic fibrosis. Future studies are needed to assess metformin given over a longer period of time and in comparison with a control group on placebo therapy or in a behavioural weight loss programme.
Thus, in a small, open label trial in well-characterized patients with NASH, metformin therapy was associated with improvements in insulin sensitivity in most patients and with weight loss, decreases in serum aminotransferase levels and improvements in liver histology in approximately 30% of patients. These results provide evidence that metformin is effective in a subset of NASH patients and that the beneficial effects may be mediated largely through weight loss.
Supplementary Material
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Protocol used to evaluate insulin sensitivity and imaging and anthropometric measures.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
ACKNOWLEDGEMENTS
Declaration of personal interests: None. All authors had access to the data. Analysis was conducted by the first (RL) and the senior author (JHH). The authors thank Drs Edward Doo, Marc Ghany, Theo Heller and Yaron Rotman, and Mrs Yoon Park of the Liver Diseases Branch, NIDDK for the help in managing patients; Ms Francine Thomas, Radiology Department, Clinical Center, NIH for providing help in MRI and CT measurements; Dr Barbara Frempong, Clinical Endocrinology Branch, NIDDK, NIH for conducting FSIGT; Dr Gloria Lena Vega, Clinical Nutrition Center, University of Texas Medical Center, Dallas, Texas, for providing measurements of fasting free fatty acid levels; Ms Blakeley Denkinger and Nancy Sebring, Nutrition Service, Clinical Center, NIH for providing nutritional counseling to the patients. Declaration of funding interests: This study was funded in full by the intramural research program of the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–1219. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 5.James OF, Day CP. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 6.Finck BN, Gropler MC, Chen Z, et al. Lipin 1 is an inducible amplifier of the hepatic PGC-1 alpha/PPAR alpha regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 8.Ding X, Saxena NK, Lin S, Xu A, Srinivasan S, Anania FA. The roles of leptin and adiponectin: a novel paradigm in adipocytokine regulation of liver fibrosis and stellate cell biology. Am J Pathol. 2005;166:1655–1669. doi: 10.1016/S0002-9440(10)62476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 10.Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008–1017. doi: 10.1053/jhep.2003.50420. [DOI] [PubMed] [Google Scholar]
- 11.Caldwell SH, Hespenheide EE, Redick JA, Iezzoni JC, Battle EH, Sheppard BL. A pilot study of a thiazolidinedione, troglitazone, in nonalcoholic steatohepatitis. Am J Gastroenterol. 2001;96:519–525. doi: 10.1111/j.1572-0241.2001.03553.x. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal AJ, Mofrad PS, Contos MJ, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–1115. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 13.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 14.Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000;6:998–1003. doi: 10.1038/79697. [DOI] [PubMed] [Google Scholar]
- 15.Bugianesi E, Gentilcore E, Manini R, et al. A randomized controlled trial of metformin versus vitamin E or prescriptive diet in nonalcoholic fatty liver disease. Am J Gastroenterol. 2005;100:1082–1090. doi: 10.1111/j.1572-0241.2005.41583.x. [DOI] [PubMed] [Google Scholar]
- 16.Nair S, Diehl AM, Wiseman M, Farr GH, Jr, Perrillo RP. Metformin in the treatment of non-alcoholic steatohepatitis: a pilot open label trial. Aliment Pharmacol Ther. 2004;20:23–28. doi: 10.1111/j.1365-2036.2004.02025.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Middleton MS, Deutsch R, Lavine JE. A phase 2 clinical trial of metformin as a treatment for non-diabetic paediatric non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2005;21:871–879. doi: 10.1111/j.1365-2036.2005.02420.x. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5:1003–1015. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 20.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquali R, Gambineri A, Biscotti D, et al. Effect of long-term treatment with metformin added to hypocaloric diet on body composition, fat distribution, and androgen and insulin levels in abdominally obese women with and without the polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:2767–2774. doi: 10.1210/jcem.85.8.6738. [DOI] [PubMed] [Google Scholar]
- 22.Kay JP, Alemzadeh R, Langley G, D’Angelo L, Smith P, Holshouser S. Beneficial effects of metformin in normoglycemic morbidly obese adolescents. Metabolism. 2001;50:1457–1461. doi: 10.1053/meta.2001.28078. [DOI] [PubMed] [Google Scholar]
- 23.Watt MJ, Dzamko N, Thomas WG, et al. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nat Med. 2006;12:541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 24.Lindor KD, Kowdley KV, Heathcote EJ, et al. Ursodeoxycholic acid for treatment of nonalcoholic steatohepatitis: results of a randomized trial. Hepatology. 2004;39:770–778. doi: 10.1002/hep.20092. [DOI] [PubMed] [Google Scholar]
- 25.Loomba R, Wesley R, Pucino F, Liang TJ, Kleiner DE, Lavine JE. Placebo in nonalcoholic steatohepatitis: insights into natural history and implications for future clinical trials. Clin Gastroenterol Hepatol. 2008;6:1243–1248. doi: 10.1016/j.cgh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Protocol used to evaluate insulin sensitivity and imaging and anthropometric measures.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.