Abstract
Objective
Reduced capillarization in hemiparetic skeletal muscle of chronic stroke patients can limit insulin, glucose, and oxygen supply to muscle, thereby contributing to impaired glucose metabolism and cardiovascular deconditioning. We hypothesized that compared to sedentary controls, stroke subjects have reduced skeletal muscle capillarization that is associated with glucose intolerance and reduced peak oxygen consumption (Vo2peak).
Methods
Twelve chronic stroke subjects (ages, 62.1±2.8 years), and matched sedentary controls with impaired (n = 12) or normal (n = 12) glucose tolerance underwent oral glucose tolerance tests, exercise tests, and vastus lateralis biopsies.
Results
Stroke subjects had lower capillarization in hemiparetic muscle than in nonparetic muscle and normal glucose tolerant controls (∼22 and ∼28%, respectively; P <0.05) and had similar bilateral capillarization, compared to controls with impaired glucose tolerance. Capillary density in hemiparetic muscle inversely correlated with 120-minute glucose (r = −0.70, P <0.01) and glucose area under the curve (r = −0.78, P <0.01). Vo2peak was ∼40% lower in stroke subjects, compared to controls (P <0.001), but did not correlate with capillarization (P = n.s.).
Conclusions
Hemiparetic muscle capillarization is reduced after stroke, and reduced capillarization is associated with glucose intolerance in stroke and control subjects. Interventions to increase skeletal muscle capillarization may prove beneficial for improving glucose metabolism in chronic stroke patients.
Keywords: diabetes, insulin resistance, capillaries, hemiparesis, skeletal muscle
Reduced capillarization in skeletal muscle may affect a number of physiological processes, because transcapillary delivery of metabolic substrates and hormones can be limited by the number of perfused capillaries [10]. Transcapillary transport of insulin is an important determinant of glucose uptake in skeletal muscle [1]; therefore, a reduction in capillarization could contribute to impairments in glucose metabolism. In experimental rodent models, occlusion of capillaries reduces insulin delivery to skeletal muscle [38] and insulin-mediated glucose uptake [30], independent of changes in blood flow.
In healthy individuals, there is a direct correlation between skeletal muscle capillarization and insulin-stimulated glucose uptake [14,21]. Likewise, reduced skeletal muscle capillarization can negatively impact peak oxygen consumption (Vo2peak) and may contribute to poor fitness levels [31].
The prevalences of impaired glucose metabolism and low cardiorespiratory fitness are both increased after stroke. More than 75% of individuals with chronic hemiparetic stroke have impaired glucose tolerance or type 2 diabetes [18,19], and Vo2peak is 40–50% lower in chronic stroke subjects than in healthy subjects of similar age [25,29,32]. Many impairments after stroke are largely attributed to upper motor neuron defects; however, changes in skeletal muscle morphology secondary to physiological changes and reduced ambulatory physical activity levels after stroke [11,25] may further contribute to physical disability, impaired metabolism, and increased risk for cardiovascular disease [7,12,20,33].
To our knowledge, only one study has investigated skeletal muscle capillarization in stroke subjects, finding approximately 10% lower capillarization in the hemiparetic vs. nonparetic vastus lateralis muscle [36] without a comparison to control subjects. While there are direct relationships between skeletal muscle capillarization, the proportion of type I muscle fibers, and glucose metabolism [14,21], as well as between capillarization and maximal oxygen consumption [3,6,24] in healthy individuals, these have not been investigated in stroke subjects. We hypothesized that hemiparetic and nonparetic muscle from stroke subjects would have lower capillarization in overall muscle and in type I fibers than that from sedentary control subjects with normal glucose tolerance, and that reduced capillarization would be associated with reduced Vo2peak and impaired glucose metabolism in stroke subjects.
Materials and Methods
Subjects
Men and women (n = 6 and n = 6, respectively), aged 47–77 years with chronic hemiparetic gait deficits from an ischemic stroke, were recruited by advertisement in local media (newspaper and television advertisements) and local area outpatient centers. Subjects were studied at least six months after stroke onset to avoid potential confounding by early neurological recovery or ongoing rehabilitation therapy on skeletal muscle morphology. Participants had completed all conventional rehabilitation therapy more than 12 weeks prior to the study and had residual mild to moderate gait deficits. Participants had preserved capacity for ambulation with or without assistive devices. Exclusion criteria for the study included known history of diabetes mellitus, peripheral vascular disease, active infection or inflammation, known muscle disease, anti-inflammatory or anticoagulant medications, dementia, untreated major depression, and aphasia (operationally defined as incapacity to follow two-point commands).
Subjects with normal glucose tolerance (NGT) and impaired glucose tolerance (IGT) (n = 12 per group) were selected as control subjects from ongoing studies of sedentary (self-reported exercise less than 20 minutes on two or fewer days per week), older (50–77 years) individuals in the University of Maryland School of Medicine, Division of Gerontology and Baltimore Veterans Affairs Medical Center (Baltimore, Maryland, USA). Control subjects were matched to stroke subjects based on sex, body mass index (BMI) (± 2 kg/m2), and age (± 4 years); groups were also matched for race, with the exception of one subject in each control group. All subjects provided written informed consent; these studies were approved by the Institutional Review Board at the University of Maryland School of Medicine.
DXA
Dual-energy X-ray absorptiometry (DXA: DPX-IQ; LUNAR Radiation Corp., Madison, Wisconsin, USA) was used to measure percent body fat and lean tissue mass.
Oxygen Consumption
Peak oxygen consumption (Vo2peak) was measured by indirect calorimetry during a graded exercise test on a motorized treadmill, as previously described [22]. Briefly, subjects walked at a constant velocity throughout the protocol; grade was initially set to 0% and increased every two minutes thereafter to maximal effort. Vo2peak was defined as the highest oxygen consumption value obtained for a full 30-second increment.
Six-Minute Walk
The six-minute walk test was performed only in chronic stroke subjects, as previously described [8]. Subjects were instructed to cover as much distance as possible during six minutes while walking up and down a 30.5-meter hallway marked with cones.
Oral Glucose Tolerance Tests
Stroke and control subjects underwent a two-hour oral glucose tolerance test (OGTT) after a 12-hour overnight fast to categorize diabetes status [2]. A catheter was placed in an antecubital vein and blood samples were drawn before, and 30, 60, 90, and 120 minutes after, the ingestion of a 75-g glucose solution. Blood samples were centrifuged and plasma was separated and stored at −80°C until analysis. Plasma glucose levels were analyzed with a glucose analyzer (2300 STAT Plus; YSI, Yellow Springs, Ohio, USA). Plasma insulin levels were determined by radioimmunoassay (Millipore, St. Charles, Missouri, USA). Glucose and insulin total area under the curve (GAUC and IAUC, respectively) were calculated by using the trapezoidal method. The insulin sensitivity index (ISIM) was calculated by using the method of Matsuda and DeFronzo [23].
Muscle Biopsies
Percutaneous needle biopsies were obtained from the vastus lateralis, approximately 12−13 cm above the patella on the anterolateral aspect of the thigh using a Bergstrom needle (Stille, Solna, Sweden), as previously described [15]. Bilateral biopsies were performed in stroke subjects; unilateral biopsies were performed in control subjects. Muscle samples were rapidly embedded in OCT-tragacanth gum mixture, frozen, and stored at −80°C for histochemical analyses.
Histochemistry
Muscle was sectioned to a thickness of 14 μm on a cryostat. Fiber type was determined by using the myosin ATPase technique [4], and capillaries were identified in serial sections of muscle with the double-stain technique [28]. Muscle sections were viewed under a light microscope (Zeiss, Thornwood, NY, USA) and digital pictures (DXM1200; Nikon, Melville, New York, USA) were taken at a magnification of 125×. Quantification of capillarization was performed on at least 50 fibers for each sample (mean = 73.4 ± 2.8 fibers), as sampling a larger number of fibers does not improve the estimation of capillarization in human muscle [27]. Fiber cross-sectional area and perimeter were measured with software (SigmaScan Pro v5.0.0; SPSS, Inc., Chicago, Illinois, USA) calibrated to transform the number of pixels into micrometers. The following five indices of capillarization were measured, as described by Hepple et al. [16]: 1) capillary contacts (CC: the number of capillaries in contact with each muscle fiber), 2) share factor (SF: the number of fibers that share each capillary), 3) individual capillary-to-fiber ratio (C/Fi: the number of whole capillary equivalents in contact with each muscle fiber), 4) capillary density (CD: the number of capillaries per square millimeter of muscle cross-sectional area), and 5) capillary-to-fiber perimeter exchange index (CFPE: the number of capillaries per millimeter of muscle fiber perimeter). For each quantified fiber, the fiber type (I, IIa, or IIx) was determined from the myosin ATPase stain, and capillarization was expressed for the overall muscle and for each specific fiber type.
Statistical Analyses
The primary study measurements were skeletal muscle capillarization, Vo2peak, fasting plasma glucose (FPG), plasma glucose response to an OGTT, and ISIM. Secondary variables included fasting plasma insulin, plasma insulin response to an OGTT, lean body mass, and six-minute walk distance. Data are presented as mean ± SEM. The paired Student's t-tests were used to test for differences in capillarization between hemiparetic and nonparetic muscle in chronic stroke subjects. Analysis of variance (ANOVA) with contrasts planned a priori was used to test for differences in variables between chronic stroke subjects and controls. Bivariate Pearson correlation analyses were used to test for correlations between capillarization variables and other primary and secondary variables. Two-tailed probabilities are reported for all analyses.
Results
Subject Characteristics
Stroke, IGT control, and NGT control subjects were similar in age (62.1 ± 2.8 vs. 61.5 ± 2.5 vs. 61.4 ± 2.0 years, respectively; P = n.s.), BMI (29.1 ± 0.9 vs. 29.9 ± 0.6 vs. 29.5 ± 0.9 kg/m2, respectively; P = n.s.), percent body fat (39.7 ± 2.4 vs. 37.5 ± 1.9 vs. 37.8 ± 2.6%, respectively; P = n.s.), and lean body mass (46.4 ± 2.8 vs. 48.6 ± 3.0 vs. 50.9 ± 3.6 kg, respectively; P = n.s.). The mean stroke latency was 4.9 ± 1.2 years. Ten stroke subjects required assistive devices (four single-point canes, four quad canes, and two walkers).
Skeletal Muscle Fiber Characteristics
Skeletal muscle fiber data are presented in Table 1. Compared to NGT controls, nonparetic muscle from chronic stroke subjects had larger fiber cross-sectional area (P <0.05). No other significant differences in muscle fiber cross-sectional area or perimeter were observed among hemiparetic, nonparetic, or control muscle. The muscle fiber–type distribution in hemiparetic muscle was significantly different than nonparetic muscle and muscle from controls, whether expressed as proportion of fibers of specific fiber type (%) or the percent of total cross-sectional area represented by each fiber type (Table 1). Hemiparetic muscle had a lower proportion of type I muscle fibers (P <0.05) and a higher proportion of type IIx muscle fibers (P <0.05) than both nonparetic muscle and muscle from control subjects. No significant differences in fiber type proportion were observed between nonparetic muscle from stroke and control subjects.
Table 1.
Muscle fiber cross-sectional area in chronic stroke subjects and controls with normal (NGT) and impaired (IGT) glucose tolerance
| Stroke subjects (n = 12) | Controls | |||
|---|---|---|---|---|
| Hemiparetic | Nonparetic | IGT (n = 12) | NGT (n = 12) | |
| Overall fiber area (μm2) | 6831 ± 764 | 8071 ± 1135 | 61809512 | 5955 ± 488 |
| Type I | 7881 ± 966 | 9242 ± 1307† | 6969 ± 579 | 6139 ± 482 |
| Type IIa | 6163 ± 578 | 6330 ± 1056 | 5667 ± 499 | 5760 ± 548 |
| Type IIx | 5310 ± 743 | 4786 ± 839 | 4681 ± 741 | 4985 ± 662 |
| Overall fiber perimeter (μm) | 337 ± 19 | 358 ± 24 | 320 ± 12 | 319 ± 14 |
| Type I | 360 ± 22 | 383 ± 26 | 337 ± 13 | 323 ± 13 |
| Type IIa | 324 ± 15 | 320 ± 24 | 312 ± 12 | 314 ± 15 |
| Type IIx | 302 ± 20 | 286 ± 23 | 275 ± 20 | 295 ± 21 |
| Type I fibers (%) | 37.5 ± 6.4*,‡ | 63.9 ± 4.9 | 50.2 ± 5.3 | 61.2 ± 3.6 |
| Type IIa fibers (%) | 31.8 ± 3.7 | 24.0 ± 5.6 | 33.6 ± 4.7 | 27.9 ± 3.0 |
| Type IIx fibers (%) | 30.7 ± 5.6*, §,‖ | 12.0 ± 2.6 | 16.2 ± 3.6 | 10.9 ± 1.5 |
| Type I fiber area (%) | 41.4 ± 7.0*,‡ | 71.9 ± 4.5 | 56.8 ± 6.1 | 63.5 ± 4.0 |
| Type IIa fiber area (%) | 30.5 ± 4.8 | 19.9 ± 3.6 | 31.2 ± 4.8 | 27.5 ± 3.3 |
| Type IIx fiber area (%) | 28.1 ± 5.3*,§,‖ | 8.2 ± 2.1 | 12.0 ± 3.1 | 9.0 ± 1.6 |
Data are presented as mean ± SEM.
Significant difference, compared to nonparetic leg muscle (P <0.01);
significant difference, compared to NGT control leg muscle (†P <0.05, ‡P <0.01, ‖P <0.001);
significant difference, compared to IGT control leg muscle (P <0.05).
Skeletal Muscle Capillarization
Stroke subjects exhibited lower capillarization in hemiparetic muscle compared to nonparetic muscle and NGT control subjects (Tables 2 and 3). Capillary contacts (CC) and individual capillary-to-fiber ratio (C/Fi) were lower in overall muscle, type I, and type IIa fibers from hemiparetic muscle, compared to nonparetic muscle and muscle from NGT controls (Table 2; P <0.05). Within stroke subjects, CC and C/Fi were also lower in type IIx hemiparetic muscle, compared to nonparetic muscle (Table 2; P <0.05). Concordantly, share factor (SF) was higher in hemiparetic type I muscle fibers than in nonparetic muscle and muscle from NGT control subjects, and was higher in overall muscle in the hemiparetic versus nonparetic leg (Table 2; P <0.05). No differences in CC, C/Fi, or SF were found between stroke subjects (hemiparetic or nonparetic) and IGT controls.
Table 2.
Skeletal muscle capillarization in chronic stroke subjects and controls with normal (NGT) and impaired (IGT) glucose tolerance
| Stroke subjects(n = 12) | Controls | |||
|---|---|---|---|---|
| Hemiparetic | Nonparetic | IGT (n = 12) | NGT (n = 12) | |
| Overall share factor | 3.09 ± 0.04§ | 2.98 ± 0.05 | 3.05 ± 0.03 | 2.99 ± 0.05 |
| Type I | 3.12 ± 0.07§,† | 2.94 ± 0.04 | 3.01 ± 0.03 | 2.96 ± 0.05 |
| Type IIa | 3.07 ± 0.04 | 3.02 ± 0.05 | 3.08 ± 0.05 | 3.00±0.06 |
| Type IIx | 3.01 ± 0.04 | 3.09 ± 0.04 | 3.15 ± 0.05 | 3.14 ± 0.05 |
| Overall capillary contacts | 3.38 ± 0.24 ‖,‡ | 4.51 ± 0.36 | 4.02 ± 0.22 | 4.57 ± 0.23 |
| Type I | 3.83 ± 0.22§,† | 4.85 ± 0.38 | 4.47 ± 0.23 | 4.84 ± 0.25 |
| Type IIa | 3.23 ± 0.20*,‡ | 4.06 ± 0.41 | 3.72 ±.022 | 4.28 ± 0.25 |
| Type IIx | 2.97 ± 0.27* | 3.43 ± 0.38 | 3.17 ± 0.15 | 3.52 ± 0.23 |
| Overall individual capillary to fiber ratio | 1.16 ± 0.09‖,‡ | 1.62 ± 0.16 | 1.39 ± 0.09 | 1.63 ± 0.11 |
| Type I | 1.31 ± 0.09§,‡ | 1.76 ± 0.17 | 1.57 ± 0.10 | 1.74 ± 0.11 |
| Type IIa | 1.10 ± 0.08*,‡ | 1.44 ± 0.17 | 1.27 ± 0.10 | 1.50 ± 0.11 |
| Type IIx | 1.02 ± 0.10* | 1.16 ± 0.14 | 1.05 ± 0.10 | 1.19 ± 0.09 |
Data are presented as mean±SEM.
Significant difference, compared to nonparetic leg muscle (*P <0.05, §P <0.01, ‖P <0.001);
significant difference, compared to NGT control muscle (†P <0.05, ‡P <0.01).
Table 3.
Skeletal muscle capillary density (CD) and capillary-to-fiber perimeter exchange index (CFPE) in chronic stroke subjects and controls with normal (NGT) and impaired (IGT) glucose tolerance
| Stroke subjects (n = 12) | Controls | |||
|---|---|---|---|---|
| Hemiparetic | Nonparetic | IGT (n = 12) | NGT (n = 12) | |
| Overall CD (cap/mm2) | 207 ± 12§,‡ | 252 ± 22 | 242 ± 10† | 295 ± 11 |
| Type I | 203 ± 18*,‡ | 229 ± 23† | 241 ± 10† | 302 ± 12 |
| Type IIa | 216 ± 18*,‡ | 283 ± 21‖ | 232 ± 31† | 276 ± 41 |
| Type IIx | 212 ± 20*,† | 302 ± 34 | 273 ± 21 | 290 ± 29 |
| Overall CFPE (cap/mm) | 3.45 ± 0.17§,‖,‡ | 4.50 ± 0.22† | 4.29 ± 0.14‡ | 5.05 ± 0.15 |
| Type I | 3.69 ± 0.17§,‖,‡ | 4.58 ± 0.20† | 4.63 ± 0.15‡ | 5.34 ± 0.19 |
| Type IIa | 3.42 ± 0.17§,‖,‡ | 4.49 ± 0.27 | 3.97 ± 0.16‡ | 4.71 ± 0.16 |
| Type IIx | 3.07 ± 0.18*,‖,† | 4.06 ± 0.36 | 3.57 ± 0.26 | 4.08 ± 0.13 |
Data are presented as mean±SEM.
Significant difference, compared to nonparetic leg muscle (*P <0.05, §P <0.001);
significant difference, compared to IGT control muscle (P <0.05);
significant difference, compared to NGT control muscle (†P <0.05, ‡P <0.01).
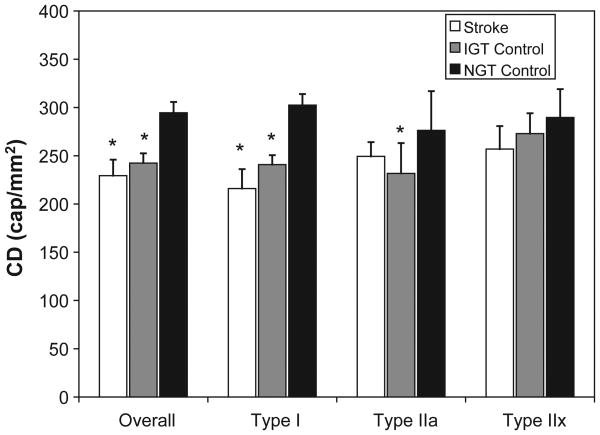
In overall muscle and all individual fiber types, capillary density (CD) was significantly lower in hemiparetic muscle than nonparetic muscle (11–32%) and NGT controls (22–35%) (Table 3; P <0.05). Similarly, CD was 16–20% lower in overall muscle, type I fibers, and IIa fibers from IGT controls, compared to NGT controls (Table 3; P <0.05). CD in hemiparetic muscle did not differ significantly from IGT controls (Table 3). Similar results were obtained for the capillary-to-fiber perimeter exchange index (CFPE), but while IGT controls had a 13–16% lower CFPE than NGT controls, CFPE in hemiparetic muscle was an additional 15–20% lower than IGT controls (Table 3; p <0.05). Using the average capillarization in the hemiparetic and nonparetic muscle to reflect average bilateral capillarization in stroke subjects, we found no difference between average CD in stroke subjects and IGT controls for any fiber type (Figure 1). Both stroke subjects and IGT controls had a lower average CD in overall and type I muscle fibers, compared to NGT controls (Figure 1; P <0.01). An analysis of CFPE yielded similar results (data not shown).
Figure 1.
Capillary density (CD) in chronic stroke subjects and controls. Data are presented as mean ± SEM. *Significant difference, compared to control subjects with normal glucose tolerance (NGT), P <0.01.
Metabolic and Functional Characteristics
As expected, Vo2peak was ∼40% lower in stroke subjects, compared to IGT control and NGT control subjects (1.1 ± 0.1 vs. 1.8 ± 0.2 and 1.9 ± 0.1 L/min, respectively; P <0.001). The mean distance achieved by stroke subjects during a six-minute walk was 146 ± 30 m. Neither CD nor CFPE significantly correlated with Vo2peak or six-minute walk distance in stroke subjects (r = 0.09 to 0.16; P = n.s.). In control subjects, CD in type I fibers correlated with Vo2peak (r = 0.46, P <0.05).
Data from OGTTs are presented in Table 4. Of the 12 stroke subjects in this study, six could be defined as having type 2 diabetes, four as having elevated fasting glucose and/or impaired glucose tolerance, and only two as having normal glucose tolerance. Compared to NGT control subjects, stroke subjects and IGT controls had higher 120-minute glucose (G120: P <0.001), GAUC (P <0.001), 120-minute insulin (P <0.001), and IAUC (P <0.05). Stroke subjects also had higher fasting insulin and FPG than NGT controls (P <0.05). Fasting insulin levels and FPG were higher in stroke subjects than in IGT controls (P <0.05), but these groups had similar plasma insulin and glucose responses to the OGTT. ISIM was lower in both stroke subjects and IGT controls compared to NGT controls (P <0.05).
Table 4.
Responses to an OGTT in chronic stroke subjects and controls with normal (NGT) and impaired (IGT) glucose tolerance
| Stroke subjects (n = 12) | IGT control (n = 12) | NGT control (n = 12) | |
|---|---|---|---|
| FPG (mmol/L) | 5.7 ± 0.15*, † | 5.2 ± 0.09 | 5.3 ± 0.13 |
| G120 (mmol/L) | 10.2 ± 0.76‖ | 9.5 ± 0.28‖ | 5.6 ± 0.36 |
| Glucose AUC (mmol/L/120min) | 1,158 ± 60‖ | 1,073 ± 31‖ | 807 ± 47 |
| Fasting Insulin (pmol/L) | 180 ± 27§, ‡ | 94 ± 9 | 82 ± 10 |
| I120 (pmol/L) | 1,125 ± 124‖ | 1,024 ± 183‖ | 383 ± 67 |
| Insulin AUC (pmol/L/120min) | 104,535 ± 10,573† | 107,947 ± 19,155† | 59,370 ± 7,212 |
| ISIM | 1.46 ± 0.21‖ | 2.26 ± 0.49† | 3.63 ± 0.40 |
Data are presented as mean±SEM.
Significant difference, compared to IGT controls (*P <0.05, §P <0.01);
significant difference, compared to NGT controls (†P <0.05, ‡P <0.01, ‖P <0.001).
FPG, fasting plasma glucose; G120, 120-minute glucose; AUC, area under the curve; I120, 120-minute insulin; ISIM, insulin sensitivity index calculated by using the method of Matsuda and DeFronzo [22].
In stroke subjects, overall CD in the hemiparetic leg inversely correlated with G120 (r = −0.70, P = 0.01) and GAUC (r = − 0.78, P <0.01). CFPE in overall hemiparetic muscle correlated with GAUC (r = −0.57, P <0.05). In hemiparetic type I fibers, CD correlated with G120 (r = −0.64, P <0.05) and GAUC (r = −0.75, P <0.01). Using the average capillarization in the hemiparetic and nonparetic muscle to reflect average bilateral capillarization in stroke subjects, similar correlations were observed (r = −0.59 to −0.72, P <0.05). In control subjects, overall CD inversely correlated with G120 (r = −0.55, P <0.01) and GAUC (r = −0.61, P = 0.001); CFPE in overall muscle correlated with G120 (r = −0.62, P = 0.001) and GAUC (r = − 0.47, P = 0.05); and both CD and CFPE in type I fibers correlated with G120 and GAUC (r = − 0.47 to − 0.69, P <0.05).
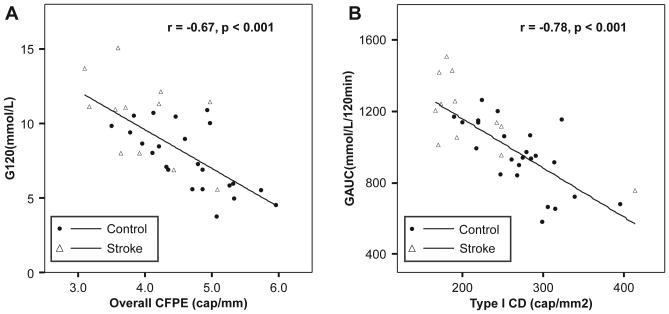
To determine whether the relationship between capillarization and glucose tolerance is maintained in subjects with and without stroke, we analyzed all subjects together, using the average capillarization in the hemiparetic and nonparetic muscle from stroke subjects. We found that overall CD inversely correlated with G120 (r = −0.70, P <0.001) and GAUC (r = −0.75, p <0.001) and directly correlated with ISIM (r = 0.56, p =0.001). Likewise, overall CFPE inversely correlated with G120 (Figure 2A: r = −0.67, p <0.001) and GAUC (r = −0.64, P <0.001). In fiber type–specific analyses, CD in type I fibers correlated with G120 (r= −0.74, P <0.001) and GAUC (Figure 2B: r = −0.78, P <0.001) and directly correlated with ISIM (r = 0.61, P <0.001). Similarly, we found that CFPE in type I fibers inversely correlated with G120 (r = −0.71, P <0.001) and GAUC (r = −0.72, P <0.001).
Figure 2.
Scatterplots depicting the correlations between (A) capillary-fiber perimeter exchange index (CFPE) in overall muscle and 120-minute glucose and (B) capillary density (CD) in type I muscle fibers and glucose area under the curve (GAUC) in all subjects. Capillarization measurements in chronic stroke subjects represent the average of hemiparetic and nonparetic muscle.
Discussion
The prevalence of type 2 diabetes and impaired glucose tolerance in chronic stroke patients is double that in older (> 60yrs) adults [5,18,19]. Persistent hemiparesis may result in changes in skeletal muscle morphology that impact function and metabolism. The major finding of the present study is that capillarization is reduced in hemiparetic skeletal muscle from chronic stroke subjects, and that reduced capillarization is associated with impaired glucose tolerance in these individuals. Further, we find that capillarization in overall muscle and type I muscle fibers is lower in control subjects with IGT, compared to those with NGT, and that average bilateral capillarization in stroke subjects is similar to capillarization in IGT control subjects.
To our knowledge, only one other report has addressed skeletal muscle capillarization in stroke subjects. Sunnerhagen et al. [36] reported ∼10% lower capillarization in hemiparetic vastus lateralis, compared to nonparetic muscle of stroke subjects. Similar to our findings, they report lower CC and CD in hemiparetic muscle; however, there was no difference in C/Fi [36]. Though our findings are similar, these researchers report values of CD that were approximately 20% higher than ours and found no differences in fiber-type proportion in hemiparetic vs. nonparetic muscle. These differences between studies are difficult to fully interpret; however, they may relate to differences in stroke latency or the degree of motor impairment in the subjects studied. Only one of the subjects in the previous report required the use of an assistive device and mean stroke latency was 1.2 years [36], whereas in the present report, 83% of the stroke subjects required assistive devices and stroke latency was four times greater. Thus, our findings may be more relevant in more disabled chronic stroke survivors.
Capillarization was lower in hemiparetic muscle compared to nonparetic muscle and muscle from NGT control subjects. Likewise, capillarization was lower in IGT control subjects compared to NGT controls. Differences between nonparetic muscle and muscle from control subjects were not as evident. CFPE was lower in overall and type I muscle fibers in nonparetic muscle from stroke subjects compared to NGT controls, as was CD in type I muscle fibers, yet CC and C/Fi were similar. These differences in CD and CFPE appear to be attributable to the larger fiber cross-sectional areas in nonparetic vs. control muscle, but still represent a functional reduction in diffusion capacity from capillary to skeletal muscle. The underlying cause of increased fiber cross-sectional area in nonparetic muscle is not established, but we speculate that long-term increased reliance on the nonparetic limb for ambulation and other activities may result in hypertrophy of muscle fibers.
In concordance with previous findings from our laboratory [18], we found a high prevalence of abnormal glucose tolerance in the chronic stroke subjects, with higher plasma glucose and insulin responses to the OGTT than controls. Recent studies, using microspheres to occlude capillaries in rodent models, demonstrate that an experimentally induced reduction in capillarization decreases insulin delivery to skeletal muscle and insulin-stimulated glucose uptake independent of changes in blood flow [30,38]. Given that hemiparetic muscle accounts for 48% of total appendicular lean mass in stroke subjects [33], reduced capillarization in paretic muscle may have a significant impact on whole-body glucose metabolism. In our stroke subjects, it appears that the hemiparetic limb experiences a reduction in capillarization, thereby lowering average skeletal muscle capillarization, with possible effects on whole-body glucose metabolism. In healthy men without type 2 diabetes, Lillioja et al. [21] previously identified a direct correlation between capillary density and insulin-stimulated glucose uptake. Our findings add that capillarization is associated with glucose metabolism in chronic hemiparetic stroke patients. The finding that capillarization is lower in IGT controls, compared to NGT controls, and is similar to average bilateral capillarization in stroke subjects, supports an association between reduced capillarization and glucose intolerance. The correlation of glucose tolerance and capillarization in all subjects shows that the relationship between skeletal muscle capillarization and whole-body glucose metabolism is present across a spectrum of healthy and chronic stroke subjects.
A reduction in skeletal muscle capillarization might also be expected to impact physical function, as limited blood flow and oxygen delivery to muscle could negatively impact Vo2peak or six-minute walk distance. While we did observe differences in Vo2peak between chronic stroke subjects and control subjects, as well as a direct correlation between capillarization and Vo2peak in control subjects, there were no correlations with capillarization in stroke subjects. It seems likely that restricted mobility may limit these functional outcomes in stroke subjects more than reduced capillarization.
The lower skeletal muscle capillarization we observed may have been the result of a variety of processes occurring after stroke. First, reduced physical activity may partially explain the observed reduction in capillary density. Skeletal muscle capillarization is lower in sedentary or inactive individuals compared to active or trained individuals [3,34,39], and ambulatory activity is lower in individuals with chronic hemiparetic stroke compared to healthy adults [11,25]. While hemiparetic muscle experiences a relative decrease in activity after stroke [13], compensation by the nonparetic leg during ambulation and other activities may explain the relatively higher maintenance of capillarization in the nonparetic leg muscle. Second, our laboratory has previously identified elevated tumor necrosis factor-α (TNF-α) in the skeletal muscle of chronic stroke subjects [12]. While elevated TNF-α is independently associated with insulin resistance [17,26], and may directly affect glucose tolerance in stroke subjects, TNF-α also induces apoptosis in human endothelial cells [35], which may serve to decrease angiogenesis and reduce capillarization in chronic stroke patients. Last, aberrant vascular endothelial growth factor (VEGF) expression after stroke may result in capillary rarefaction. VEGF is a potent angiogenic factor involved in angiogenesis and the maintenance of capillaries [9]. Tang et al. [37] show that inhibition of skeletal muscle VEGF expression decreases skeletal muscle capillarization in mice, indicating that decreased skeletal muscle VEGF expression may reduce capillarization in chronic stroke subjects. Currently, this remains speculative and the mechanisms underlying reduced capillarization in hemiparetic skeletal muscle remain to be determined.
Although we have identified a reduction in hemiparetic muscle capillarization and a relationship between hemiparetic muscle capillarization and whole-body glucose tolerance in chronic stroke subjects, the limitations and strengths of this study require discussion. First, it is possible that reduced physical activity could contribute to impaired glucose metabolism in chronic stroke subjects. As previously documented, our stroke subjects were sedentary [11,25], and our controls were specifically selected as being sedentary in order to minimize the influence of regular physical activity on our outcomes. In addition, we acknowledge that the presence of diabetes or IGT may contribute to the occurrence of stroke and partially explain the high prevalence of glucose intolerance in our stroke subjects. While we cannot rule out the possibility that some of our stroke subjects had undiagnosed diabetes prior to the occurrence of their stroke, none were diagnosed as having diabetes or IGT prior to participation in this study. The strength of our comparison of hemiparetic vs. nonparetic muscle is that chronic stroke subjects serve as their own controls. The use of bilateral muscle samples eliminates confounding by differences in physical activity levels and genetic variation that would otherwise exist. This model also provides an interesting possibility for future studies of limb-specific glucose uptake in the hemiparetic and nonparetic muscle in stroke subjects to better understand the relationship of reduced capillarization and glucose intolerance. The study is further strengthened by the comparison to well-matched control groups. The comparison of hemiparetic muscle to nonparetic muscle from stroke subjects and muscle from control subjects with IGT and NGT allows a better interpretation of changes in muscle morphology after stroke and the relationship of those changes to glucose intolerance. By studying stroke and control groups with similar age, BMI, and percent body fat, as well as the same proportion of race and sex in each group, we have minimized possible confounding by these variables.
Conclusion
In summary, the current study shows morphological differences in the vastus lateralis muscle of chronic stroke subjects, namely lower capillarization that may limit insulin, glucose, and oxygen supply to the hemiparetic muscle. This reduction in skeletal muscle capillarization is associated with impairments in whole-body glucose metabolism in chronic stroke. The development of pharmacological interventions, or physical activity and aerobic exercise training recommendations to increase skeletal muscle capillarization in chronic stroke patients may prove beneficial for improving glucose metabolism and functional outcomes. This may translate to reduced risk of type 2 diabetes, as well as reduced risk of developing secondary stroke and cardiovascular disease in chronic stroke patients.
Acknowledgments
The authors' appreciation is extended to the men and women who participated in this study. They are grateful to the clinicians, students, nurses, laboratory technicians, and exercise physiologists who assisted with this study. This research was supported, in part, by the Baltimore Veterans Affairs Maryland Exercise and Robotics Center of Excellence (R.F.M.), the Baltimore Veterans Affairs Research Enhancement Award Program (R.F.M.), Veterans Affairs Merit Awards (C.E.H.-M. and A.S.R.), the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC), the University of Maryland Claude D. Pepper Center (P30-AG-12583; A.P.G.), and NIH grant R01-AG-019310 (A.S.R.). Dr. Prior is supported by NIH T32-AG-000219, Dr. Joseph is supported by NIH K01-AG-021457, and Dr. Ryan is supported by a VA Research Career Scientist Award.
References
- 1.Ader M, Bergman RN. Importance of transcapillary insulin transport to dynamics of insulin action after intravenous glucose. Am J Physiol. 1994;266:E17–E25. doi: 10.1152/ajpendo.1994.266.1.E17. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2008;31(Suppl 1):S55–S60. doi: 10.2337/dc08-S055. [DOI] [PubMed] [Google Scholar]
- 3.Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977;232:H705–H712. doi: 10.1152/ajpheart.1977.232.6.H705. [DOI] [PubMed] [Google Scholar]
- 4.Brooke MH, Kaiser KK. Three “myosin adenosine triphosphatase” systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- 5.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 6.Coyle EF, Feltner ME, Kautz SA, Hamilton MT, Montain SJ, Baylor AM, Abraham LD, Petrek GW. Physiological and biomechanical factors associated with elite endurance cycling performance. Med Sci Sports Exerc. 1991;23:93–107. [PubMed] [Google Scholar]
- 7.De Deyne PG, Hafer-Macko CE, Ivey FM, Ryan AS, Macko RF. Muscle molecular phenotype after stroke is associated with gait speed. Muscle Nerve. 2004;30:209–215. doi: 10.1002/mus.20085. [DOI] [PubMed] [Google Scholar]
- 8.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–785. [PubMed] [Google Scholar]
- 9.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 10.Gudbjornsdottir S, Sjostrand M, Strindberg L, Wahren J, Lonnroth P. Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab. 2003;88:4559–4564. doi: 10.1210/jc.2003-030434. [DOI] [PubMed] [Google Scholar]
- 11.Haeuber E, Shaughnessy M, Forrester LW, Coleman KL, Macko RF. Accelerometer monitoring of home- and community-based ambulatory activity after stroke. Arch Phys Med Rehabil. 2004;85:1997–2001. doi: 10.1016/j.apmr.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Hafer-Macko CE, Yu S, Ryan AS, Ivey FM, Macko RF. Elevated tumor necrosis factor-alpha in skeletal muscle after stroke. Stroke. 2005;36:2021–2023. doi: 10.1161/01.STR.0000177878.33559.fe. [DOI] [PubMed] [Google Scholar]
- 13.Harris-Love ML, Forrester LW, Macko RF, Silver KH, Smith GV. Hemiparetic gait parameters in overground versus treadmill walking. Neurorehabil Neural Repair. 2001;15:105–112. doi: 10.1177/154596830101500204. [DOI] [PubMed] [Google Scholar]
- 14.Hedman A, Berglund L, Essen-Gustavsson B, Reneland R, Lithell H. Relationships between muscle morphology and insulin sensitivity are improved after adjustment for intra-individual variability in 70-year-old men. Acta Physiol Scand. 2000;169:125–132. doi: 10.1046/j.1365-201x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 15.Hennessey JV, Chromiak JA, Della VS, Guertin J, MacLean DB. Increase in percutaneous muscle biopsy yield with a suction-enhancement technique. J Appl Physiol. 1997;82:1739–1742. doi: 10.1152/jappl.1997.82.6.1739. [DOI] [PubMed] [Google Scholar]
- 16.Hepple RT. A new measurement of tissue capillarity: the capillary-to-fibre perimeter exchange index. Can J Appl Physiol. 1997;22:11–22. doi: 10.1139/h97-002. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 18.Ivey FM, Ryan AS, Hafer-Macko CE, Garrity BM, Sorkin JD, Goldberg AP, Macko RF. High prevalence of abnormal glucose metabolism and poor sensitivity of fasting plasma glucose in the chronic phase of stroke. Cerebrovasc Dis. 2006;22:368–371. doi: 10.1159/000094853. [DOI] [PubMed] [Google Scholar]
- 19.Kernan WN, Inzucchi SE, Viscoli CM, Brass LM, Bravata DM, Shulman GI, McVeety JC, Horwitz RI. Impaired insulin sensitivity among nondiabetic patients with a recent TIA or ischemic stroke. Neurology. 2003;60:1447–1451. doi: 10.1212/01.wnl.0000063318.66140.a3. [DOI] [PubMed] [Google Scholar]
- 20.LeBrasseur NK, Sayers SP, Ouellette MM, Fielding RA. Muscle impairments and behavioral factors mediate functional limitations and disability following stroke. Phys Ther. 2006;86:1342–1350. doi: 10.2522/ptj.20050162. [DOI] [PubMed] [Google Scholar]
- 21.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80:415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macko RF, DeSouza CA, Tretter LD, Silver KH, Smith GV, Anderson PA, Tomoyasu N, Gorman P, Dengel DR. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients. A preliminary report. Stroke. 1997;28:326–330. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 24.McGuire BJ, Secomb TW. Estimation of capillary density in human skeletal muscle based on maximal oxygen consumption rates. Am J Physiol Heart Circ Physiol. 2003;285:H2382–H2391. doi: 10.1152/ajpheart.00559.2003. [DOI] [PubMed] [Google Scholar]
- 25.Michael KM, Allen JK, Macko RF. Reduced ambulatory activity after stroke: the role of balance, gait, and cardiovascular fitness. Arch Phys Med Rehabil. 2005;86:1552–1556. doi: 10.1016/j.apmr.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 26.Nilsson J, Jovinge S, Niemann A, Reneland R, Lithell H. Relation between plasma tumor necrosis factor-alpha and insulin sensitivity in elderly men with non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1998;18:1199–1202. doi: 10.1161/01.atv.18.8.1199. [DOI] [PubMed] [Google Scholar]
- 27.Porter MM, Koolage CW, Lexell J. Biopsy sampling requirements for the estimation of muscle capillarization. Muscle Nerve. 2002;26:546–548. doi: 10.1002/mus.10221. [DOI] [PubMed] [Google Scholar]
- 28.Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol. 2002;92:1451–1457. doi: 10.1152/japplphysiol.00744.2001. [DOI] [PubMed] [Google Scholar]
- 29.Potempa K, Braun LT, Tinknell T, Popovich J. Benefits of aerobic exercise after stroke. Sports Med. 1996;21:337–346. doi: 10.2165/00007256-199621050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Rattigan S, Clark MG, Barrett EJ. Hemodynamic actions of insulin in rat skeletal muscle: evidence for capillary recruitment. Diabetes. 1997;46:1381–1388. doi: 10.2337/diab.46.9.1381. [DOI] [PubMed] [Google Scholar]
- 31.Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O(2) consumption in trained human skeletal muscle. J Appl Physiol. 1999;87:325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- 32.Ryan AS, Dobrovolny CL, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: role of muscle mass and gait deficit severity. J Stroke Cerebrovasc Dis. 2000;9:185–191. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 33.Ryan AS, Dobrovolny CL, Smith GV, Silver KH, Macko RF. Hemiparetic muscle atrophy and increased intramuscular fat in stroke patients. Arch Phys Med Rehabil. 2002;83:1703–1707. doi: 10.1053/apmr.2002.36399. [DOI] [PubMed] [Google Scholar]
- 34.Saltin B, Rowell LB. Functional adaptations to physical activity and inactivity. Fed Proc. 1980;39:1506–1513. [PubMed] [Google Scholar]
- 35.Spyridopoulos I, Brogi E, Kearney M, Sullivan AB, Cetrulo C, Isner JM, Losordo DW. Vascular endothelial growth factor inhibits endothelial cell apoptosis induced by tumor necrosis factor-alpha:balance between growth and death signals. J Mol Cell Cardiol. 1997;29:1321–1330. doi: 10.1006/jmcc.1996.0365. [DOI] [PubMed] [Google Scholar]
- 36.Sunnerhagen KS, Svantesson U, Lonn L, Krotkiewski M, Grimby G. Upper motor neuron lesions: their effect on muscle performance and appearance in stroke patients with minor motor impairment. Arch Phys Med Rehabil. 1999;80:155–161. doi: 10.1016/s0003-9993(99)90113-2. [DOI] [PubMed] [Google Scholar]
- 37.Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genom. 2004;18:63–69. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- 38.Vollus GC, Bradley EA, Roberts MK, Newman JM, Richards SM, Rattigan S, Barrett EJ, Clark MG. Graded occlusion of perfused rat muscle vasculature decreases insulin action. Clin Sci (Lond) 2007;112:457–466. doi: 10.1042/CS20060311. [DOI] [PubMed] [Google Scholar]
- 39.Westerterp KR. Daily physical activity and ageing. Curr Opin Clin Nutr Metab Care. 2000;3:485–488. doi: 10.1097/00075197-200011000-00011. [DOI] [PubMed] [Google Scholar]