Abstract
Background
The presence of central nervous system (CNS) disease in pediatric acute myeloid leukemia (AML) is often thought to confer a worse prognosis. This study examined the outcome of children with AML who had CNS disease at diagnosis.
Methods
Patients enrolled on Children's Cancer Group protocols 2861, 2891, 2941 and 2961 being treated for de novo AML were classified for the presence of CNS disease at diagnosis as CNS1 (< 5 WBC in the CSF without blasts), CNS2 (< 5 WBC in the CSF with blasts) or CNS3 (≥5 WBC in the CSF with blasts). CNS disease at diagnosis was then analysed regarding patient characteristics and outcome.
Results
There was an incidence of CNS disease (i.e., CNS3 status) of 11% in the 1459 patients analyzed in this study. The risk factors found are young age, high white cell count, hepatomegaly or splenomegaly at diagnosis, M4 subtype, chromosome 16 abnormalities and hyperdiploid cytogenetics. There were no significant differences in overall survival, event free survival, or remission rates between the groups; however, a significant difference was seen between the CNS1 and CNS3 groups in disease free survival and isolated CNS relapse risk.
Conclusions
Patients with CNS disease at diagnosis have similar survival to those without CNS disease, although they have an increased incidence of isolated CNS relapse. Patients with CNS disease at diagnosis may warrant more aggressive CNS directed therapy.
Keywords: central nervous system, leukemia, AML, pediatric
Introduction
The presence of CNS disease at diagnosis in pediatric acute myeloid leukemia (AML) is fairly common, with a published incidence of 6–29%1–6 and is often thought to confer a worse prognosis7–9. Some studies though have shown that the presence of CNS disease at diagnosis does not affect survival1,3,10,11 and another showed that it improved survival4. The presence of CNS disease at diagnosis has been associated with a high white cell count at diagnosis and age less than 2 years3,5, as well as M4 and M5 FAB morphology, inversion (16), t(9;11) and t(8;21)4,8. Other studies have shown a lack of correlation with race, hemoglobin, spleen size, sex, FAB subtype, coagulation abnormality, liver size or platelet and white cell count at diagnosis3,4. Thus, there are conflicting data as to the effect of CNS disease at diagnosis on outcome, as well as associated risk factors.
This study examined the survival and clinical features of children who had CNS disease at the time of diagnosis of de novo AML. Children included in this study were those with de novo AML treated on Children’s Cancer Group (CCG) protocols 2861, 2891 (intensive-timing arm), 2941 and 2961. These patients were all treated in a similar fashion with intensive-timing induction therapy followed by high-dose cytarabine-based chemotherapy or stem cell transplantation. This analysis represents the largest cohort of patients with CNS disease at diagnosis and describes features we found to be predictive for this occurrence as well as the outcome for these patients.
Methods
Patients and Therapy
Information regarding the presence of CNS disease at diagnosis was obtained from the data submitted by institutions for patients enrolled on protocol. Only patients with de novo AML were included in the analysis; patients with myelodysplastic syndrome, Down syndrome, FAB M3 APL, t(15;17), secondary AML and isolated extramedullary leukemia without bone marrow (BM) involvement at diagnosis were excluded. There are 142 eligible patients from CCG 2861, 1184 from CCG 2891, 93 from CCG 2941 and 987 from CCG 2961. Only patients on CCG 2891 who received intensive-timing treatment were included to ensure similar therapy for all patients analyzed. As a result, there are a total of 106, 514, 86 and 901 eligible patients on CCG 2861, 2891, 2941 and 2961 respectively who were used for these analyses (total N=1607).
Patients were classified into 3 groups (CNS1, CNS2, CNS3) with the following definitions: CNS1=patients with white blood cell (WBC) count in CSF <5 and having no blasts in the CSF, CNS2=patients with WBC count in CSF <5 and having blasts in the CSF, CNS3=patients with WBC count in CSF ≥5 and having blasts in the CSF. A group of patients were not classified due to either missing data or WBC in the CSF ≥5 with no blasts. As a result, there were a total of 1113 patients in the CNS1 category, 192 in the CNS2 category, 154 in the CNS3 category, and 148 patients that were not classified (94 of whom had ≥5 WBC in the CSF but no blasts). Patients who had blood contaminated CSF had the Steinherz/Bleyer algorithm applied to determine if it was true CNS disease. Patients with CNS myeloid sarcoma were analyzed in a separate study. All patients underwent central cytogenetic and pathology review.
Patients enrolled on CCG 2861 and 2891 (intensive-timing arm), were treated as previously reported9,12. A summary of their treatment is as follows: induction chemotherapy consisted of five drugs (dexamethasone, cytarabine, 6-thioguanine, etoposide, and daunomycin (DCTER)) given at diagnosis and then repeated after 6-days. Patients received intrathecal cytarabine at the start of each DCTER cycle for a total of 4 doses. CNS involvement with leukemia was diagnosed if the patient had CNS3 status and these patients received additional twice-weekly intrathecal cytarabine for 6 doses, and if this failed to clear the leukemia cells they then received twice-weekly triple intrathecal therapy for 6 doses. All patients randomized to chemotherapy also received intrathecal chemotherapy with each post consolidation cycle, except Capizzi II treatment, for another 3 doses. Radiation therapy was given to patients who had CNS leukemia that did not clear after 6 doses of intrathecal chemotherapy (dose 2400 cGy to cranial contents and 1200 cGy to spinal axis) or had CNS chloromas (dose 2000 cGy). Postremission patients with a HLA matched family donor were allocated to an allogeneic bone marrow transplant (BMT) with busulfan/cyclophosphamide conditioning. The remaining patients received autologous BMT with busulfan/cyclophosphamide conditioning on 2861 and on 2891 were randomized to intensification with autologous BMT versus intensive-timing high-dose cytarabine.
Patients enrolled on CCG protocols 2941 and 2961 were treated as previously reported13,14. On protocol 2941 treatment was as follows: induction chemotherapy consisted of a 4 day cycle of 5 drugs (dexamethasone, cytarabine, 6-thioguanine, etoposide, and either daunomycin or idarubicin) which was repeated 6 days later. This was repeated and patients with a matched related donor received a BMT, while the remaining patients received Capizzi II intensification. They then received interleukin-2 therapy. On protocol 2961 treatment was similar with induction therapy consisting of a 4 day cycle of 5 drugs (dexamethasone, cytarabine, 6-thioguanine, etoposide, and idarubicin) followed by a similar cycle 6 days later using daunomycin instead of idarubicin. Patients were then randomized to a second 2 cycles of chemotherapy the same as the first 2 cycles or a course of fludarabine, cytarabine and idarubicin. Following this, patients with a matched related donor received a BMT, while the others received Capizzi II intensification and were then randomized to interleukin-2 therapy. CNS prophylaxis consisted of 8 doses of intrathecal cytarabine, and chloromas were irradiated at the investigators option. Patients with CNS leukemia at diagnosis (CNS3 status) received additional intrathecal cytarabine on days 5 and 7, and if this failed to clear the leukemia cells they then received twice-weekly triple intrathecal therapy beginning day 10 until the CSF was clear of leukemia cells (for a maximum of 6 doses), and if this failed to clear the leukemia cells they were removed from protocol.
Statistical Method
This report analyzes data collected on CCG 2861 through September 21, 2001, on CCG 2891 through January 14, 2004, on CCG 2941 through April 14, 2005, and on CCG 2961 through October 30, 2006. The significance of observed differences in proportions was tested using the Chi-squared test and Fishers exact test when data were sparse. The Mann-Whitney test was used to determine the significance between differences in medians. The Kaplan-Meier method was used to calculate estimates of OS, EFS, and DFS. Overall survival is defined as time from study entry to death. Event free survival is defined as time from study entry to failure at course 1 or course 2, relapse or death. Disease free survival is defined as time from end of course 2 for patients in remission or who have residual leukemia to relapse or death. Differences between all three groups of patients were tested for significance using the log-rank statistic for OS, EFS and DFS analyses. Confidence intervals were calculated using Greenwood’s estimate of the standard error. Relapse risk was defined as the cumulative incidence of relapse and was estimated by considering deaths due to non-progressive disease as competing events. Isolated BM or CNS relapse were defined as relapse in the BM or CNS respectively with no evidence of disease relapse elsewhere within 30 days of the relapse. Concurrent relapse was defined as a relapse of both the BM and CNS at the same time, or within 30 days of the first relapse. The cumulative incidence of isolated BM relapse was estimated by considering concurrent BM and CNS relapses, isolated CNS relapses and first event deaths as competing events. The cumulative incidence of isolated CNS relapse was estimated by considering concurrent BM and CNS relapses, isolated BM relapses and first event deaths as competing events. Significant differences in cumulative incidence were determined by Gray’s test. Cox proportional hazards models were used for multivariate analyses. Children lost to follow-up were censored at their date of last known contact or at a cutoff 6 months prior to September 2001, January 2004, April 2005, and October 2006 for CCG 2861, CCG 2891, CCG 2941 and CCG 2961, respectively.
Patients in the 3 CNS categories were compared with respect to gender, race, age, FAB leukemia subtype, organ enlargement, WBC at diagnosis, hemoglobin level at diagnosis, platelet count at diagnosis, and cytogenetics.
Results
Patient Characteristics
A total of 1459 patients treated with intensive-timing chemotherapy were analyzed in this study. At diagnosis, 76% were CNS1, 13% were CNS2, and 11% were CNS3. Thus 11% of patients had CNS disease (CNS3 status) at diagnosis. Table I presents a summary of data for these patients. In comparing patient characteristics, there was an equal male to female distribution among the 3 groups and no significant difference comparing race. The median age of patients at diagnosis with CNS3 status was 4.3 years which was significantly lower than the CNS1 and CNS2 patients. A significantly higher proportion of patients with CNS3 status were in the 0–2 year age range and lower proportion in the 3–10 year age range compared to the CNS1 and CNS2 groups. There was also a significantly higher incidence of hepatic or splenic enlargement at diagnosis in patients with CNS3 status compared to those with CNS1 and CNS2 status, as well as a significantly higher WBC count at diagnosis in CNS3 patients. In comparing FAB subtypes among the patient groupings, significantly more patients with M4 AML had CNS3 status, while significantly more patients with M2 and M7 AML had CNS1 status at diagnosis.
Table I.
Patient Characteristics for CNS1, CNS2 and CNS3 patients
| CNS 1 (N=1113) | CNS2 (N=192) | CNS3 (N=154) | CNS1 vs CNS2 vs CNS3 |
Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | p-value | |||
| Gender | |||||||||
| Male | 552 | 50% | 104 | 54% | 85 | 55% | 0.258 | 741 | |
| Female | 561 | 50% | 88 | 46% | 69 | 45% | 718 | ||
| Age (yrs) | |||||||||
| Median | 8.9 | 8.4 | 4.3 | <0.001 | |||||
| 0–2 y | 285 | 26% | 63 | 33% | 70 | 45% | <0.001 | 418 | |
| 3–10 y | 375 | 34% | 54 | 28% | 36 | 23% | 0.018 | 465 | |
| 11–21 y | 453 | 41% | 75 | 39% | 48 | 31% | 0.076 | 576 | |
| Race | |||||||||
| White | 753 | 68% | 126 | 67% | 102 | 67% | 0.946 | 981 | |
| Black | 104 | 9% | 14 | 7% | 10 | 7% | 0.398 | 128 | |
| Hispanic | 157 | 14% | 38 | 20% | 23 | 15% | 0.098 | 218 | |
| Asian | 40 | 4% | 2 | 1% | 5 | 3% | 0.188 | 47 | |
| Other | 50 | 5% | 7 | 4% | 12 | 8% | 0.147 | 69 | |
| Unknown | 9 | 5 | 2 | 16 | |||||
| Enlarged liver | |||||||||
| Normal | 757 | 68% | 117 | 61% | 65 | 42% | <0.001 | 939 | |
| Enlarged | 354 | 32% | 74 | 39% | 89 | 58% | 517 | ||
| Unknown | 2 | 1 | 0 | 3 | |||||
| Enlarged spleen | |||||||||
| Normal | 770 | 69% | 117 | 61% | 71 | 46% | <0.001 | 958 | |
| Enlarged | 354 | 32% | 74 | 39% | 89 | 58% | 517 | ||
| Unknown | 4 | 1 | 0 | 5 | |||||
| WBC (×103/µL) | |||||||||
| (median) | 14.9 | 39.0 | 68.6 | <0.001 | |||||
| Platelets (×103/µL) | 51 | ||||||||
| (median) | 51 | 49.5 | 0.762 | ||||||
| Hemoglobin (gm/dL) | 7.7 | ||||||||
| (median) | 8.2 | 8.1 | 0.045 | ||||||
| FAB | |||||||||
| Classification | |||||||||
| M0 | 61 | 6% | 7 | 4% | 4 | 3% | 0.213 | 72 | |
| M1 | 181 | 17% | 38 | 21% | 22 | 15% | 0.338 | 241 | |
| M2 | 313 | 30% | 44 | 24% | 22 | 15% | 0.001 | 379 | |
| M4 | 223 | 21% | 48 | 27% | 63 | 44% | <0.001 | 334 | |
| M5 | 162 | 15% | 37 | 21% | 27 | 19% | 0.167 | 226 | |
| M6 | 29 | 3% | 2 | 1% | 2 | 1% | 0.399 | 33 | |
| M7 | 82 | 8% | 4 | 2% | 4 | 3% | 0.003 | 90 | |
| Other/no data | 62 | 12 | 10 | 84 | |||||
| Cytogenetics (Data for 825 Patients) | |||||||||
| Normal | 149 | 24% | 20 | 16% | 19 | 22% | 0.162 | 188 | |
| t(8;21) | 97 | 16% | 21 | 17% | 2 | 2% | 0.003 | 120 | |
| Abnormal 16 | 28 | 5% | 20 | 16% | 17 | 20% | <0.001 | 65 | |
| Abnormal 11 | 124 | 20% | 31 | 25% | 21 | 25% | 0.327 | 176 | |
| t(6;9)(p23;q34) | 9 | 1% | 1 | 1% | 1 | 1% | 1.000 | 11 | |
| −7/7q- | 34 | 6% | 5 | 4% | 2 | 2% | 0.485 | 41 | |
| −5/5q- | 8 | 1% | 0 | 0% | 1 | 1% | 0.516 | 9 | |
| +8 | 43 | 7% | 9 | 7% | 3 | 4% | 0.468 | 55 | |
| +21 | 18 | 3% | 0 | 0% | 0 | 0% | 0.041 | 18 | |
| Pseudodiploid | 82 | 13% | 10 | 8% | 9 | 11% | 0.249 | 101 | |
| Hyperdiploid* | 13 | 2% | 3 | 2% | 9 | 11% | <0.001 | 25 | |
| Hypodiploid | 12 | 2% | 3 | 2% | 1 | 1% | 0.831 | 16 | |
| No data | 496 | 69 | 69 | 634 | |||||
Hyperdiploid patients are those patients with greater than 46 chromosomes which are not classified in other groupings (ie not +8, +21, t(8;21), etc).
Fifty-seven percent of patients had cytogenetic data available; 77% were abnormal. CNS3 patients had a significantly higher incidence of abnormal chromosome 16 and hyperdiploidy, while CNS1 patients had a significantly higher incidence of +21 and t(8;21). In comparing all patients with and without cytogenetic data available, there was no significant difference with respect to gender, median age, hepatosplenomegaly, WBC, response to therapy or outcome (data not shown). There were significantly more patients with FAB M4 in those with cytogenetic data available compared to those without data, and significantly less patients with FAB M1. A significant number of patients with chromosome 16 abnormality had M4 morphology (72% vs 28%, p<0.001).
Outcome
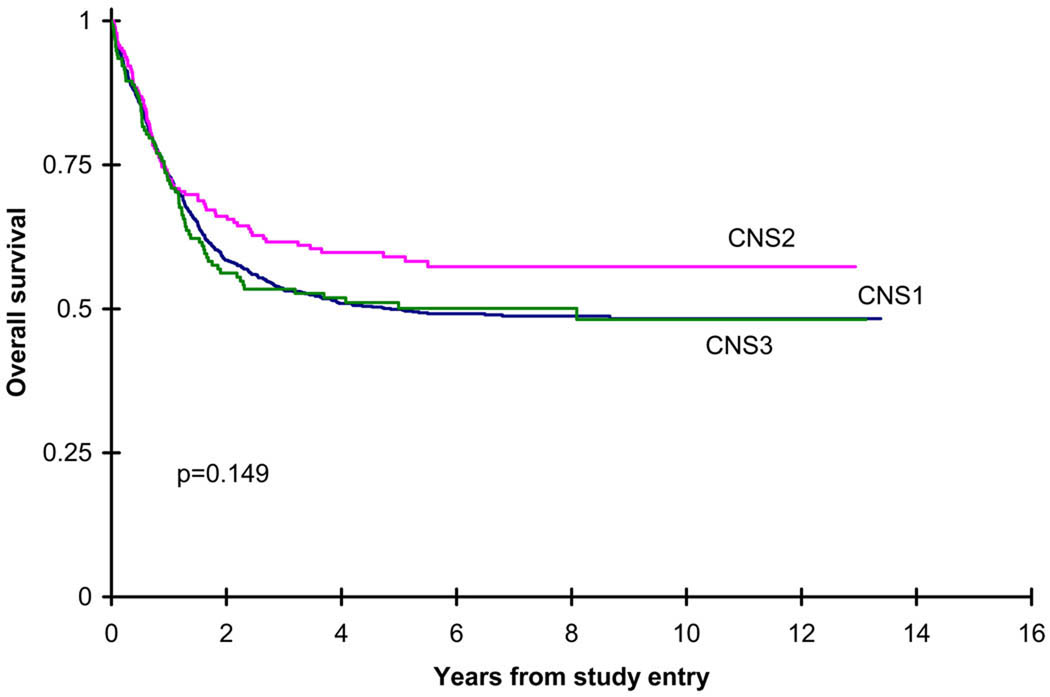
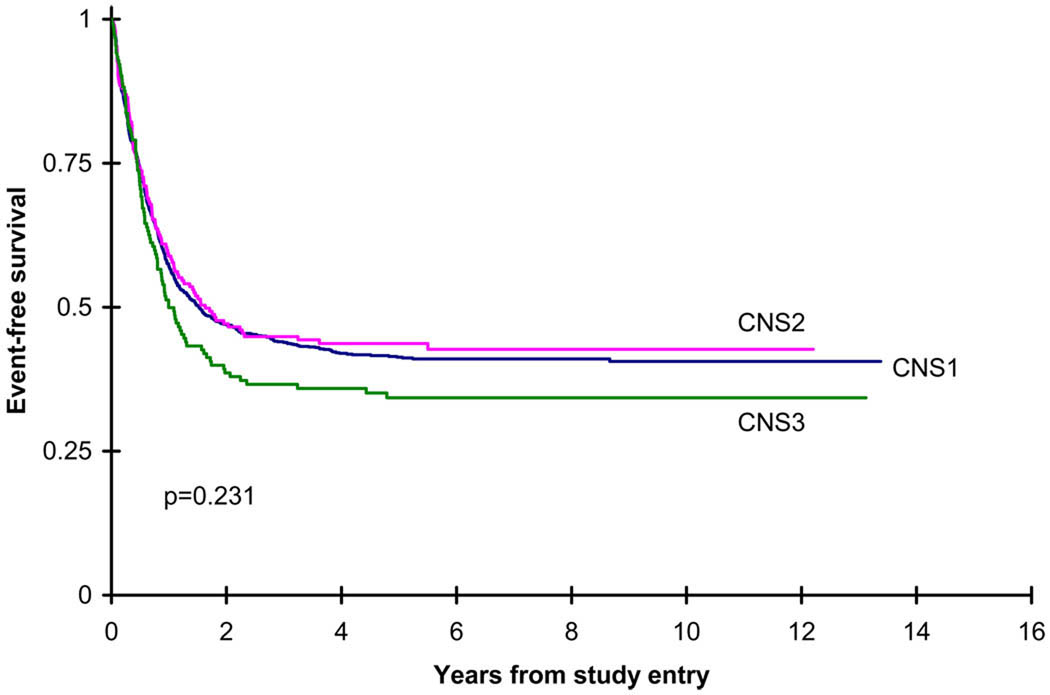
The response of patients showed no significant difference in remission, progressive disease or death among the CNS1, CNS2 and CNS3 patients. As well, the 94 patients who had more than 5 WBC in the CSF without blasts had no difference in response compared to the CNS1 patients (phase I remission 86% vs 87%, p=0.889). The outcomes of the CNS1, CNS2 and CNS3 patients are shown in Table II and in Figures 1–2. There was no significant difference in overall survival measured both from study entry among the 3 groups (CNS2 vs CNS1 (p=0.054), CNS2 vs CNS3 (p=0.142), CNS2 vs CNS1/CNS3 (p=0.051)), as well as from the end of induction course 2 (CNS2 vs CNS1 (p=0.386), CNS2 vs CNS3 (p=0.170), CNS2 vs CNS1/CNS3 (p=0.317)). As well, the incidence of isolated BM relapse from the end of course 2 was not significantly different among the 3 groups. The EFS between CNS1/CNS2 versus CNS3 was 42±3% vs 34±8%, which was not significantly different (p=0.113). There was, however, a significant difference in disease free survival and relapse risk from the end of induction course 2 among the 3 groups, with the CNS3 patients having a worse outcome. The incidence of an isolated CNS relapse was significantly higher among the CNS3 patients, and the incidence of a concurrent CNS and BM relapse from the end of induction course 2 was significantly lower in the CNS1 patients. Multivariate Cox models indicate that CNS groups do not differ significantly in OS from study entry, EFS from study entry, OS from end of course 2, and DFS from end of course 2 after adjusting for age, WBC at diagnosis, race, and cytogenetic risk group (Table III and Table IV).
Table II.
Outcome for CNS1, CNS2 and CNS3 patients
| CNS1 | CNS2 | CNS3 | CNS1 vs CNS2 vs CNS3 |
||||
|---|---|---|---|---|---|---|---|
| N | 5 yr%±2SE% | N | 5 yr%±2SE% | N | 5 yr%±2SE% | p-value | |
| OS from study entry | 1113 | 50±3% | 192 | 59±7% | 154 | 50±8% | 0.149 |
| EFS from study entry | 1113 | 41±3% | 192 | 44±7% | 154 | 34±8% | 0.231 |
| OS from end course 2 | 807 | 61±4% | 146 | 67±8% | 120 | 58±9% | 0.343 |
| DFS from end course 2 | 807 | 53±4% | 146 | 50±8% | 120 | 42±9% | 0.028 |
| RR from end course 2 | 807 | 40±4% | 146 | 44±8% | 120 | 51±9% | 0.036 |
| BM relapse from end course 2 | 807 | 34±3% | 146 | 29±8% | 120 | 35±9% | 0.498 |
| Isolated CNS relapse from end course 2 | 807 | 1±1% | 146 | 4±3% | 120 | 9±5% | <0.001 |
| Concurrent BM and CNS relapse from end of course 2 | 807 | 2±1% | 146 | 5±4% | 120 | 5±4% | 0.017 |
Figure 1.
Kaplan-Meier figure for overall survival (OS) from study entry for de novo patients by CNS groups. CNS1 five year OS= 50±3%, CNS2 five year OS=59±7%, CNS3 five year OS=50±8%.
Figure 2.
Kaplan-Meier figure for event free survival (EFS) from study entry for de novo patients by CNS groups. CNS1 five year EFS=41±3%, CNS2 five year EFS=44±7%, CNS3 five year EFS=34±8%.
Table III.
Univariate Cox analyses from study entry
| Univariate cox analyses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry | EFS from study entry | Relapse risk from study entry | |||||||||
| N | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| CNS groups | |||||||||||
| CNS2 | 192 | 1 | 1 | 1 | |||||||
| CNS1 | 1113 | 1.26 | 1.00 – 1.60 | 0.055 | 1.04 | 0.84 – 1.27 | 0.744 | 0.92 | 0.74 – 1.16 | 0.495 | |
| CNS3 | 154 | 1.28 | 0.93 – 1.76 | 0.129 | 1.22 | 0.93 – 1.60 | 0.155 | 1.13 | 0.83 – 1.53 | 0.452 | |
| Age group | |||||||||||
| 3–10y | 507 | 1 | 1 | 1 | |||||||
| 0–2y | 463 | 1.11 | 0.92 – 1.33 | 0.270 | 1.19 | 1.01 – 1.41 | 0.035 | 1.36 | 1.13 – 1.63 | 0.001 | |
| 11–21 y | 637 | 1.24 | 1.05 – 1.47 | 0.013 | 1.09 | 0.93 – 1.27 | 0.285 | 0.92 | 0.76 – 1.10 | 0.351 | |
| WBC | |||||||||||
| <100,000 | 1338 | 1 | 1 | 1 | |||||||
| ≥100,000 | 267 | 1.47 | 1.23 – 1.74 | <0.001 | 1.60 | 1.37 – 1.88 | <0.001 | 1.79 | 1.50 – 2.14 | <0.001 | |
| Cytogenetic risk group** | |||||||||||
| Standard | 639 | 1 | 1 | 1 | |||||||
| Low | 202 | 0.50 | 0.38 – 0.65 | <0.001 | 0.50 | 0.39 – 0.63 | <0.001 | 0.36 | 0.27 – 0.49 | <0.001 | |
| High | 53 | 1.15 | 0.80 – 1.66 | 0.462 | 1.20 | 0.85 – 1.68 | 0.297 | 1.14 | 0.77 – 1.68 | 0.523 | |
| Race | |||||||||||
| White race | 1076 | 1 | 1 | 1 | |||||||
| Not white race | 513 | 1.20 | 1.04 – 1.40 | 0.016 | 1.11 | 0.97 – 1.27 | 0.144 | 1.01 | 0.86 – 1.19 | 0.919 | |
Cytogenetic low risk group is defined as patients with the cytogenetic abnormality of t(8;21) or inv(16). Cytogenetic high risk group is defined as patients with the cytogenetic abnormality −7/7q- or −5/5q-. Cytogenetic standard risk is all other cytogenetic abnormalities not in the high or low risk groups.
Table IV.
Multivariate Cox analyses from study entry
| Multivariate cox analyses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OS from study entry | EFS from study entry | Relapse risk from study entry | |||||||||
| N | HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | ||
| CNS groups | |||||||||||
| CNS2 | 119 | 1 | 1 | 1 | |||||||
| CNS1 | 611 | 1.29 | 0.94 – 1.77 | 0.112 | 1.14 | 0.86 – 1.50 | 0.371 | 1.04 | 0.76 – 1.42 | 0.809 | |
| CNS3 | 83 | 1.37 | 0.90 – 2.08 | 0.137 | 1.31 | 0.90 – 1.90 | 0.154 | 1.13 | 0.74 – 1.74 | 0.575 | |
| Age group | |||||||||||
| 3–10y | 243 | 1 | 1 | 1 | |||||||
| 0–2y | 226 | 1.00 | 0.76 – 1.32 | 0.993 | 0.97 | 0.76 – 1.24 | 0.828 | 1.07 | 0.82 – 1.41 | 0.606 | |
| 11–21 y | 344 | 1.52 | 1.20 – 1.94 | 0.001 | 1.24 | 1.00 – 1.54 | 0.054 | 1.05 | 0.81 – 1.36 | 0.714 | |
| WBC | |||||||||||
| <100,000 | 674 | 1 | 1 | 1 | |||||||
| ≥100,000 | 139 | 1.52 | 1.18 – 1.96 | 0.001 | 1.59 | 1.25 – 2.01 | <0.001 | 1.72 | 1.31 – 2.26 | <0.001 | |
| Cytogenetic risk group** | |||||||||||
| Standard | 581 | 1 | 1 | 1 | |||||||
| Low | 182 | 0.48 | 0.36 – 0.64 | <0.001 | 0.50 | 0.39 – 0.64 | <0.001 | 0.37 | 0.26 – 0.51 | <0.001 | |
| High | 50 | 1.24 | 0.85 – 1.83 | 0.266 | 1.32 | 0.92 – 1.88 | 0.131 | 1.22 | 0.80 – 1.86 | 0.353 | |
| Race | |||||||||||
| White race | 564 | 1 | 1 | 1 | |||||||
| Not white race | 249 | 1.28 | 1.04 – 1.57 | 0.022 | 1.19 | 0.98 – 1.44 | 0.079 | 1.13 | 0.90 – 1.42 | 0.282 | |
Cytogenetic low risk group is defined as patients with the cytogenetic abnormality of t(8;21) or inv(16). Cytogenetic high risk group is defined as patients with the cytogenetic abnormality −7/7q- or −5/5q-. Cytogenetic standard risk is all other cytogenetic abnormalities not in the high or low risk groups.
Discussion
The presence of CNS disease at was 11% on recent CCG AML studies, within the 6–29% range reported previously1–6. Previous studies conflict regarding the effect of CNS disease on outcome1,3,4,7,8,10,11, and we found no difference in overall survival among the 3 CNS groups.
Previous studies have not consistantly shown the prognostic value of CNS disease at diagnosis. Some have found that the presence of CNS disease at diagnosis is not an adverse prognostic factor1,3,4,10,11. Conversely, others have found a decrease in survival7,8 and an increased risk of marrow and CNS relapse2. A Pediatric Oncology Group study found that patients with CNS disease at diagnosis were more likely to experience a CNS relapse15. We found that the presence of CNS disease at diagnosis did not affect remission rates, overall survival, event free survival or risk of isolated BM relapse. However, CNS disease at diagnosis affected the disease free survival, relapse risk, risk of a concurrent BM and CNS relapse, and the risk of isolated CNS relapse. A previous study of isolated CNS relapse showed that the presence of CNS disease at diagnosis was predictive of isolated CNS relapse and these patients had an 8 year OS of 26%16 compared to 54% for all patients treated on CCG-289112. Our current finding of increased risk of isolated relapse in patients with CNS disease at diagnosis is not surprising. The fact that overall survival and event free survival were not affected by the presence of CNS disease is surprising given our previous results. However, it may reflect small differences in the patient populations with CNS disease in these studies.
An interesting finding of this study was an apparent absence of CNS disease continuous variable effect of CNS disease on outcome. Patients with CNS2 disease had an increased (although not significant − 59% vs 50%) overall survival and event free survival than CNS1 patients. Patients with CNS2 disease did not receive the extra intrathecal therapy that CNS3 patients received, but were treated identically to CNS1 patients. The practice of treating CNS2 patients in the same fashion as CNS1 patients is done in some studies1,2,5,6, while others treat all patients with blasts in the CNS (CNS2 and CNS3) as CNS positive3,4,15. The current Children’s Oncology Group AML protocol treats all patients with blasts in the CNS as CNS positive. This current study confirms though that extra therapy is unlikely to be needed for CNS2 patients, but in contrast is needed in CNS3 patients.
Patients with CNS3 status in this study received extra intrathecal therapy, as is the case in most studies1–6. A study from St. Jude Children’s Research Hospital found patients with CNS3 status had a superior outcome, but they utilized radiation therapy in over half of these patients4. Radiation therapy is known to have significant side effects17, and its use no longer standard in pediatric patients because of the excellent CNS penetration of high dose cytarbine containing regimens. Given that the patients in this current study with CNS3 status had an increased incidence of CNS relapse, perhaps an increase in the number of intrathecal therapies given may be warranted. This question cannot be easily addressed in future randomized pediatric AML studies, since numbers of patients exceeds those available in COG and other cooperative group trials.
Studies conflict about factors associated with the presence of CNS disease3,4,8. This large study confirmed that the presence of CNS disease was significantly associated with young age, hepatosplenomegaly, high WBC, M4 morphology, abnormal chromosome 16, and hyperdiploid cytogenetics. Young age has previously been demonstrated to be a risk factor for the presence of CNS disease1,3,4,5,8,18, and we also found this to be the case. This risk may be due to a greater proportion of vasculature in the leptomeninges in infants and preschool children19. Certain FAB subtypes or cytogenetic abnormalities, which are more common in young children, may lead to more extramedullary leukemia. There is a higher incidence of M5 disease in infants under the age of 2 years9, but we did not find a higher incidence of CNS disease in patients with M5 leukemia, contrary to previous studies1,4,8. Also, infants under the age of 1 year with 11q23 abnormality have been reported to have an increased incidence of CNS disease20, but in this study, chromosome 11 abnormalities were not associated with CNS disease, thus again not explaining the increased incidence of CNS disease in these young patients.
Patients with FAB M2 and M7 were significantly less likely to have CNS disease at diagnosis. Previous studies have shown that patients with constitutional trisomy 21 (Down syndrome) more commonly display M7 AML morphology, but M7 in the absence of trisomy 21 has a worse overall prognosis in pediatric AML11,21–23. Patients with Down syndrome were excluded from this analysis but are known to have a lower incidence of CNS disease2,24,25, and this may partially explain the lower incidence of CNS disease in patients with clonal trisomy 21 cytogenetics.
Cytogenetics affect survival in AML. There is a favourable prognosis in patients with chromosome 16 inversion and chromosome (8;21) translocation26–29. We found that patients with AML with abnormalities of chromosome 16 had an increased incidence of CNS2 and CNS3 disease, as did those with AML with hyperdiploid cytogenetics. Our previous study found that these patients did not have an increased incidence of CNS relapse; only 1 of 26 patients with chromosome 16 abnormality had a CNS relapse, while none of those with t(8;21) or hyperdiploidy had a CNS relapse16. The finding of increased incidence of chromosome 16 abnormalities has previously been found to be associated with CNS disease at diagnosis and this is likely secondary to an increase in M4 morphology in these patients4,8,30. Hyperdiploidy has not previously been reported as a risk factor for CNS disease, but was a significant finding in this large study. On the other hand, patients with t(8;21) had a significantly higher incidence of CNS1 and CNS2 status. The decreased incidence of t(8;21) in patients with CNS3 disease contradicts previous studies which found this abnormality to be associated with extramedullary leukemia4,31. The POG 8821 study showed that extramedullary leukemia, including in the CNS, significantly decreased the EFS in patients with the favourable cytogenetic findings of t(8;21) and inv(16)32. Previous studies also have shown an increased incidence of 11q23 abnormalities8 and chromosome 11 abnormalities in patients with extramedullary leukemia30, as well as a significant risk for isolated CNS relapse in patients with chromosome 11 abnormalities16. In our analysis, abnormalities of chromosome 11, including 11q23, were not found to be predictive of CNS disease.
Patients with an elevated WBC at diagnosis have been shown to have a higher incidence of extramedullary involvement of leukemia3,7,8 and we also found this. Previous studies have found that a low WBC at diagnosis confers a better prognosis15,18, but other reports have found that high WBC was a significant predictor of survival10. Results of CCG 2861 and 2891 found that an elevated WBC at diagnosis was associated with a lower rate of remission following induction therapy and lower overall survival rate compared to low WBC at diagnosis9. We found that the WBC was significantly higher at diagnosis in patients with CNS disease but this did not affect the overall survival rates.
In summary, this study represents a large cohort of pediatric patients with AML examined for the presence of CNS disease at diagnosis. It confirms that the presence of CNS disease in pediatric AML is associated with young age, high WBC, hepatomegaly or splenomegaly at diagnosis, M4 morphology, chromosome 16 abnormalities and hyperdiploid cytogenetics. A lack of CNS disease at diagnosis was significantly associated with M2 and M7 morphology, as well as (8;21) chromosome translocation. Patients with CNS disease at diagnosis had no difference in overall survival compared to those without CNS disease, but they were at an increased risk of isolated CNS relapse, indicating a need to investigate more aggressive CNS directed therapy for these patients.
Acknowledgements
Funding for CCG 2861, 2891, 2941 and 2961 was provided by Chair’s Grant U10 CA98543 and U10 CA98413.
Footnotes
Presented at the American Society of Hematology Meeting, Atlanta, GA, December 9, 2007
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Bisschop MM, Revesz T, Bierings M, et al. Extramedullary infiltrates at diagnosis have no prognostic significance in children with acute myeloid leukemia. Leukemia. 2001;15:46–49. doi: 10.1038/sj.leu.2401971. [DOI] [PubMed] [Google Scholar]
- 2.Creutzig U, Zimmermann M, Ritter J, et al. Treatment strategies and long-term results in paediatric patients treated in four consecutive AML-BFM trials. Leukemia. 2005;19:2030–2042. doi: 10.1038/sj.leu.2403920. [DOI] [PubMed] [Google Scholar]
- 3.Pui C-H, Dahl GV, Kalwinsky DK, Look AT, Mirro J, Dodge RK, et al. Central nervous system leukemia in children with acute nonlymphoblastic leukemia. Blood. 1985;66:1062–1067. [PubMed] [Google Scholar]
- 4.Abbott BL, Rubnitz JE, Tong X, et al. Clinical significance of central nervous system involvement at diagnosis of pediatric acute myeloid leukemia: a single institution’s experience. Leukemia. 2003;17:2090–2096. doi: 10.1038/sj.leu.2403131. [DOI] [PubMed] [Google Scholar]
- 5.Webb DH, Harrison G, Stevens RF, et al. Relationships between age at diagnosis, clinical features, and outcome of therapy in children treated in the Medical Research Council AML 10 and 12 trials for acute myeloid leukemia. Blood. 2001;98:1714–1720. doi: 10.1182/blood.v98.6.1714. [DOI] [PubMed] [Google Scholar]
- 6.Gibson BES, Wheatley K, Hann IM, et al. Treatment stragegy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi R, Tawa A, Hanada R, et al. Extramedullary infiltration at diagnosis and prognosis in children with acute myelogenous leukemia. Pediatr Blood Cancer. 2007;48:393–398. doi: 10.1002/pbc.20824. [DOI] [PubMed] [Google Scholar]
- 8.Chang H, Brandwein J, Yi Q, et al. Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leukemia Research. 2004;28:1007–1011. doi: 10.1016/j.leukres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Woods WG, Kobrinsky N, Buckley J, et al. Intensively timed induction therapy followed by autologous or allogeneic bone marrow transplantation for children with acute myeloid leukemia or myelodysplastic syndrome: A Childrens Cancer Group pilot study. J Clin Oncol. 1993;11:1448–1457. doi: 10.1200/JCO.1993.11.8.1448. [DOI] [PubMed] [Google Scholar]
- 10.Grier HE, Gelber RD, Camitta BM, et al. Prognostic factors in childhood acute myelogenous leukemia. J Clin Oncol. 1987;5:1026–1032. doi: 10.1200/JCO.1987.5.7.1026. [DOI] [PubMed] [Google Scholar]
- 11.Rubintz JE, Razzouk BI, Lensing S, et al. Prognostic factors and outcome of recurrence in childhood acute myeloid leukemia. Cancer. 2007;109:157–163. doi: 10.1002/cncr.22385. [DOI] [PubMed] [Google Scholar]
- 12.Woods WG, Neudorf S, Gold S, et al. A comparison of allogeneic bone marrow transplantation, autologous bone marrow transplantation, and aggressive chemotherapy in children with acute myeloid leukemia in remission. Blood. 2001;97:56–62. doi: 10.1182/blood.v97.1.56. [DOI] [PubMed] [Google Scholar]
- 13.Lange BJ, Dinndorf P, Smith FO, et al. Pilot study of idarubicin-based intensive-timing induction therapy for children with previously untreated acute myeloid leukemia: Children’s Cancer Group study 2941. J Clin Oncol. 2004;22:150–156. doi: 10.1200/JCO.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children’s Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ravindranath Y, Steuber CP, Krischer J, et al. High-dose cytarabine for intensification of early therapy of childhood acute myeloid leukemia: a pediatric oncology group study. J Clin Oncol. 1991;9:572–580. doi: 10.1200/JCO.1991.9.4.572. [DOI] [PubMed] [Google Scholar]
- 16.Johnston DL, Alonzo TA, Gerbing RB, et al. Risk factors and therapy for isolated central nervous system relapse of pediatric acute myeloid leukemia. J Clin Oncol. 2005;23:9172–9178. doi: 10.1200/JCO.2005.02.7482. [DOI] [PubMed] [Google Scholar]
- 17.Pui C-H, Howard SC. Current management and challenges of malignant disease in the CNS in paediatric leukemia. Lancet Oncol. 2008;9:257–268. doi: 10.1016/S1470-2045(08)70070-6. [DOI] [PubMed] [Google Scholar]
- 18.Pui C-H, Raimondi SC, Srivastava DK, et al. Prognostic factors in infants with acutemyeloid leukemia. Leukemia. 2000;14:684–687. doi: 10.1038/sj.leu.2401725. [DOI] [PubMed] [Google Scholar]
- 19.Pinkel D, Woo S. Prevention and treatment of meningeal leukemia in children. Blood. 1994;84:355–366. [PubMed] [Google Scholar]
- 20.Martinez-Climent JA, Garcia-Conde J. Chromosomal rearrangements in childhood acute myeloid leukemia and myelodysplatic syndromes. J Pediatr Hematol Oncol. 1999;21:91–102. doi: 10.1097/00043426-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Barnard DR, Alonzo TA, Gerbing RB, et al. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: Report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49:17–22. doi: 10.1002/pbc.20951. [DOI] [PubMed] [Google Scholar]
- 22.Lange BJ, Kobrinsky N, Barnard DR, et al. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplasic syndrome in children with Down Syndrome: Children’s Cancer Group studies 2861 and 2891. Blood. 1998;91:608–615. [PubMed] [Google Scholar]
- 23.Creutzig U, Reinhardt D, Diekamp S, et al. AML patients with Down syndrome have a high cure rate with AML-BFM therapy with reduced dose intensity. Leukemia. 2005;19:1355–1360. doi: 10.1038/sj.leu.2403814. [DOI] [PubMed] [Google Scholar]
- 24.Gamis AS, Alonzo TA, Hilden JM, et al. Outcome of Down Syndrome (DS) children with acute myeloid leukemia (AML) or myelodysplasia (MDS) treated with a uniform prospective clinical trial – Initial repot of the COG trial A2971. Blood. 108:9a. 206. [Google Scholar]
- 25.Gamis AS, Woods WG, Alonzo TA, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: A report from the Children’s Cancer Group study 2891. J Clin Oncol. 2003;21:3415–3422. doi: 10.1200/JCO.2003.08.060. [DOI] [PubMed] [Google Scholar]
- 26.Chen AR, Alonzo TA, Woods WG, Arceci RJ. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?--an American view. Br J Haematol. 2002;118:378–384. doi: 10.1046/j.1365-2141.2002.03701.x. [DOI] [PubMed] [Google Scholar]
- 27.Creutzig U, Reinhardt D. Current controversies: which patients with acute myeloid leukaemia should receive a bone marrow transplantation?--a European view. Br J Haematol. 2002;118:365–377. doi: 10.1046/j.1365-2141.2002.03697.x. [DOI] [PubMed] [Google Scholar]
- 28.Raimondi SC, Chang MN, Ravindranath Y, et al. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study – POG 8821. Blood. 1999;94:3707–3716. [PubMed] [Google Scholar]
- 29.Meshinchi S, Arceci RJ. Prognostic factors and risk-based therapy in pediatric acute myeloid leukemia. Oncologist. 2007;12:341–355. doi: 10.1634/theoncologist.12-3-341. [DOI] [PubMed] [Google Scholar]
- 30.Kalwinsky DK, Raimondi SC, Schell MJ, et al. Prognostic importance of cytogenetic subgroups in de novo pediatric acute nonlymphocytic leukemia. J Clin Oncol. 1990;8:75–83. doi: 10.1200/JCO.1990.8.1.75. [DOI] [PubMed] [Google Scholar]
- 31.Dusenbery KE, Howells WB, Arthur DC, et al. Extramedullary leukemia in children with newly diagnosed acute myeloid leukemia. J Pediatr Hematol Oncol. 2003;25:760–768. doi: 10.1097/00043426-200310000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Chang M, Raimondi SC, Ravindranath Y, et al. Prognostic factors in children and adolescents with acute myeloid leukemia (excluding children with Down syndrome and acute promyelocytic leukemia) : univariate and recursive partitioning analysis of patients treated on Pediatric Oncology Group (POG) Study 8821. Leukemia. 2000;14:1201–1207. doi: 10.1038/sj.leu.2401832. [DOI] [PubMed] [Google Scholar]