Abstract
Conditioned drug craving and withdrawal elicited by cues paired with drug use or acute withdrawal are among the many factors contributing to compulsive drug taking. Understanding how to stop these cues from having these effects is a major goal of addiction research. Extinction is a form of learning in which associations between cues and the events they predict are weakened by exposure to the cues in the absence of those events. Evidence from animal models suggests that conditioned responses to drug cues can be extinguished, although the degree to which this occurs in humans is controversial. Investigations into the neurobiological substrates of extinction of conditioned drug craving and withdrawal may facilitate the successful use of drug cue extinction within clinical contexts. While this work is still in the early stages, there are indications that extinction of drug- and withdrawal-paired cues shares neural mechanisms with extinction of conditioned fear. Using the fear extinction literature as a template, it is possible to organize the observations on drug cue extinction into a cohesive framework.
Keywords: Extinction, Addiction, Craving, Withdrawal, Drug-Paired Cue, Withdrawal-Paired Cue, Cue Exposure Therapy, Conditioned Response
I. Introduction
Addiction is characterized as a compulsively relapsing pattern of drug use that persists in the face of negative consequences and a desire to stop using. It is a complex and multifaceted disorder that has resisted simple solutions, and current therapies are disappointing in that many addicts have difficulty maintaining abstinence.
Conditioned drug craving and withdrawal elicited by drug-paired cues are among the many factors contributing to compulsive drug use. It is known that addicts respond to cues such as drugs, drug paraphernalia, people using drugs, and environments where drugs were consumed, with subjective reports of craving and withdrawal as well as physiological indices of arousal, anxiety, and discomfort (Childress et al., 1986; Ehrman et al., 1992; Foltin and Haney, 2000; Herman, 1974; Hugdahl and Ternes, 1981; O’Brien et al., 1977; Pomerleau et al., 1983; Sideroff and Jarvik, 1980; Wikler, 1948). When asked to consider factors contributing to relapse, addicts identify drug-paired cues as being equally or more powerful than other influences including moods or impulsive choices (Heather et al., 1991).
Understanding how to stop these cues from having these effects is a major goal of addiction research. In this review we focus on one way in which this might be accomplished: extinction, a form of learning in which associations between cues and the events they predict are weakened by exposure to the same cues in the absence of those events. Accumulating evidence indicates that conditioned drug craving and withdrawal in animals can be extinguished through exposure to drug- or withdrawal-paired cues in the absence of drug administration or acute withdrawal. The degree to which this occurs in human addicts is less clear, but numerous laboratories (including our own) are engaged in studies designed to identify the behavioral and neurobiological mechanisms of drug- or withdrawal-paired cue extinction in the hope that increased understanding of those mechanisms will facilitate successful application of extinction procedures in therapeutic settings.
The organization of this review is as follows: we describe what has been learned about the behavioral features of extinction of drug- and withdrawal-paired cues in animals, the outcome of clinical studies of extinction of drug cues in people, and the emerging literature on the neurobiological substrates of extinction of conditioned drug craving and withdrawal. In our discussion of extinction we draw substantially from the fear extinction literature to provide a framework within which to consider the relatively modest literature on extinction of drug- and withdrawal-paired cues. To provide background before turning to these issues, we begin by discussing the concept of drug administration as an associative learning episode, describing paradigms for studying the acquisition and expression of conditioned responses to drug- and withdrawal-paired cues in laboratory animals, and presenting some of the major findings on the neurobiological mechanisms of conditioned drug craving and withdrawal.
II. Drug Exposure as an Associative Learning Process
Craving and withdrawal elicited by drug-paired cues can be conceptualized as conditioned responses (CRs) that develop through a Pavlovian conditioning process, such that over the course of an addict’s drug use history, these cues become signals for impending drug use. Pavlov (1927) observed that dogs injected repeatedly with morphine or apomorphine exhibited salivation, restlessness, and vomiting upon exposure to what he called “the preliminaries of injection” (e.g., the appearance of the experimenter or being brought into the laboratory). He proposed that the situation was analogous to the conditioning of salivation to the sound of a metronome paired with food, such that the responses were preparatory responses to a pre-injection ritual that preceded and predicted the onset of drug effects.
That drug exposure engages an associative learning process is corroborated by decades of research on conditioned tolerance to the analgesic effects of morphine in rats. Rats administered morphine for the first time show analgesia as assessed by tail flick or hot plate tests, but this analgesia undergoes tolerance such that it diminishes with repeated morphine administrations. Siegel and colleagues (1976) discovered that this tolerance was context-specific, such that rats injected repeatedly with morphine in one context exhibited renewed analgesia when injected with morphine in a different context. This situational specificity suggests that tolerance is not just a physiological response to repeated drug administration, but that it also involves a learning component. Siegel proposed that the rats learned to associate morphine with the context in which it is administered, and as a function of this association, the context acquired the ability to elicit a conditioned physiological response to oppose the effects of the drug. Consistent with this, rats administered morphine repeatedly and then tested in the same context following an injection of saline exhibit hyperalgesia (Siegel, 1975). The hyperalgesic response is a type of CR called a conditioned compensatory response (CCR), defined as a conditioned homeostatic mechanism triggered by drug-paired cues that serves to maintain the system at or close to its normal set point in the presence of drugs that otherwise would perturb this balance (Siegel et al., 2000). CCRs are elicited by cues paired with a variety of drugs in addition to morphine, and show all of the features and effects associated with other types of Pavlovian CRs including external inhibition, latent inhibition, extinction, conditioned inhibition, overshadowing, blocking, and sensory preconditioning (for review see Siegel et al., 2000). CCRs are observed in humans as well and may contribute to incidences of overdose, particularly in people who are found to have what should be non-lethal quantities of drug in their system (Siegel et al., 1982). Theoretically, an experienced user who takes drugs in a new environment or otherwise in the absence of cues that normally accompanied drug administration may be vulnerable because no CCR is mounted to counteract the effects of the drug.
CCRs also are relevant to the maintenance of drug use in addicts because they can be unpleasant and involve physical and psychological signs of withdrawal (Childress et al., 1986; Kenny et al., 2006; Siegel and Ramos, 2002; Wheeler et al., 2008). These responses can motivate progressively more aggressive drug seeking because drug use is negatively reinforced by alleviating withdrawal (Kenny et al., 2006; Wikler, 1948) and because the drug is more rewarding when consumed in the presence of a withdrawal state (Hellemans et al., 2006b; Hutcheson et al., 2001; Robinson and Berridge, 1993).
II.A. Paradigms for the study of conditioned responses to drug- and withdrawal-paired cues in animals
The neurobiological mechanisms underlying the acquisition and expression of conditioned drug craving and withdrawal have been the subject of intensive study. Much of this work has been done using animal models, where the experimental conditions can be controlled with precision. Two paradigms are used most often in this type of research: the drug self-administration paradigm and the place conditioning paradigm. In both, initially neutral cues are paired with drug administration or acute withdrawal, but they differ in how conditioned responses to those cues are assessed.
II.A.1. Drug self-administration paradigm
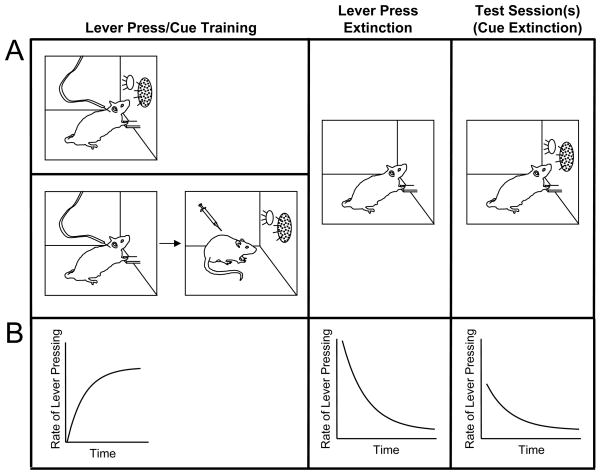
In the drug self-administration paradigm, animals (nonhuman primates, rats, mice) learn to emit an operant response (typically a lever press) that is reinforced by an intravenous infusion of a drug of abuse (Figure 1). Drug cues are introduced into this paradigm in one of two ways. In the more common method (e.g., Grimm and See, 2000), a cue such as a multi-modal stimulus consisting of visual (light) and auditory (tone) components occurs simultaneously with drug infusions. That is, when an animal completes a required schedule of responding, the cue is triggered at the same time that the drug is administered. In the second method (e.g., Kruzich and See, 2001), Pavlovian cue-drug pairing sessions are conducted separately from drug self-administration sessions. Animals first acquire lever pressing, then in a separate session in which the lever is unavailable, they are presented several times with cues followed by drug administered by the experimenter. In both methods, following this initial training phase, lever pressing is extinguished over the course of one or more sessions in which lever presses produce neither drugs nor cues. The purpose of lever press extinction is to reduce the rate of lever pressing so as to be able to detect modulation of drug-seeking behavior during subsequent test sessions. During these tests, the cues are reintroduced, either by being presented periodically independent of the animal’s responding or by being made contingent on lever pressing. The typical outcome is an increase in the rate of lever pressing after exposure to the cues as compared to before. It is presumed that the cues enhance drug-seeking behavior by eliciting drug craving, and the renewed lever pressing is considered a model of relapse.
Figure 1.
Schematic representation of the drug self-administration paradigm. Panel A, left. In this paradigm, animals (often rats) learn to emit an operant response (typically a lever press) that is reinforced by an intravenous infusion of a drug of abuse. Drug cues can introduced into this paradigm in two ways. In the more common method (top), a discrete cue such as a multi-modal stimulus consisting of visual (light) and auditory (tone) components is triggered simultaneously with drug infusions when the animal completes a required schedule of responding. In the second, less common method (bottom), animals first are trained to lever press for drug administration only (no cues), and then in a separate session in which the manipulandum (lever) is not available, the animal is presented several times with cues followed by drug administered by the experimenter. Panel A, center. Following lever press/cue training, lever pressing is extinguished by allowing the animal to lever press but withholding both the drug and the cues. Panel A, right. Finally, the response-reinstating value of the cues is assessed in test sessions in which the cues are reintroduced in the absence of drug. At the outset of testing, there is typically a transient increase in the rate of lever pressing following exposure to the cues. Cue extinction occurs over the course of multiple cue presentations and is assessed as a lessening of the ability of the cues to maintain responding in the continued absence of drug. Panel B. The rate of lever pressing tends to increase during lever press training until it reaches a maximal value, and then tends to decline during lever press extinction (after an initial “extinction burst”). During a test, lever pressing is reinstated when the drug-paired cues are reintroduced. This response-reinstating ability of the cues extinguishes over the course of the test in the continued absence of drug.
In humans, drug-paired cues elicit not only drug craving but also conditioned withdrawal, likely through different neural mechanisms (Robbins et al., 1997). To isolate the withdrawal component of the conditioned response, it is possible to use a modified version of the drug self-administration paradigm in which cues are paired not with drug administration but with drug abstinence. This method is not often used, but it may be valuable as a complement to studies of conditioned withdrawal in the place conditioning paradigm (discussed below in section II.A.2.). Establishing withdrawal-paired cues is accomplished most easily by using an opiate receptor antagonist such as naloxone to precipitate withdrawal in opiate-dependent animals. This has been done in two different ways, which, paradoxically, produce opposite results. In one approach (Goldberg et al., 1969; Kenny et al., 2006), animals are trained to self-administer an opiate over multiple sessions and in the process become dependent on the drug. In the case of opiates, dependence is reflected by well-characterized physical and motivational signs during periods of drug abstinence or, alternatively, antagonist-precipitated withdrawal (see Gracy et al., 2001; Koob and Le Moal, 2008). Withdrawal then is precipitated prior to self-administration sessions into which an extended cue (such as a continuous tone) is introduced. Importantly, the drug is available to the animal during these sessions under the same contingency that was in place during the initial training phase. In a test session conducted after the completion of training, the cue is present but the animal is not administered the opiate receptor antagonist. Similar to drug-paired cues, withdrawal-paired cues trained in this way increase drug-seeking behavior (Goldberg et al., 1969; Kenny et al., 2006). The second approach involves pairing a cue with precipitated withdrawal in Pavlovian conditioning sessions conducted separately from self-administration sessions (Hellemans et al., 2006b). When trained in this way, a withdrawal-paired cue actually decreases drug-seeking behavior in test (Hellemans et al., 2006b). One possible explanation for the discrepancy is that the opiate is more rewarding in the first approach because it is self-administered during withdrawal (Robinson and Berridge, 1993), giving it both positive- and negative-reinforcing effects that can overcome a general behavioral suppressive effect of conditioned withdrawal (Hellemans et al., 2006b).
The self-administration paradigm is advantageous in that it models human drug-taking and drug-seeking behavior very closely. However, it is difficult and time-consuming because of the need to surgically implant intravenous catheters, maintain the patency of those catheters over time, and train animals to self-administer drug over multiple training sessions. Because of the time and degree of expertise required to do these kinds of experiments, many labs opt for a simpler alternative: place conditioning.
II.A.2. Place conditioning
Contrary to the drug self-administration paradigm, in the place conditioning paradigm administration of the drug is under the control of the experimenter rather than the subject, and measurement of the conditioned motivational effects of drug- or withdrawal-paired cues is indirect. Place conditioning most commonly involves a 2- or 3-chambered apparatus in which the chambers are distinguished by wall color, floor texture, or both. Training proceeds in several stages. First, animals (generally rats or mice) are given a pre-test in which they are exposed to the apparatus for a short period of time (10–30 min) and their exploration of the chambers is recorded. Typically the apparatus is configured such that a population of subjects show no inherent preference for one or the other chamber; individual animals that show a strong bias can be eliminated from the experiment on the basis of the pre-test, before time- or labor-intensive aspects of the studies begin. Training involves administering a drug or precipitating drug withdrawal and then confining an animal in one of the chambers for a period of time (usually 30–60 min). Animals also are confined in the other chamber for the same amount of time following an injection of saline to equate exposure to the two contexts. The number and spacing of confinements varies; for example, with precipitated withdrawal, a single context-withdrawal pairing generally is sufficient, whereas with cocaine, multiple context-drug pairings typically are required. At least 24 hrs after the completion of training, animals once again are given the opportunity to explore the apparatus freely for the same 10–30 min period as was done in the pre-test. The post-test almost always is conducted while the animals are in a drug-free state. Animals trained in this way come to prefer a drug-paired compartment or avoid a withdrawal-paired compartment, where preference or avoidance is measured in terms of time spent in each compartment in the post-test. Animals showing conditioned place preference (CPP) spend more time in the drug-paired compartment than in the saline-paired compartment, whereas animals showing conditioned place avoidance (CPA) spend less time in the withdrawal-paired compartment than in the saline-paired compartment. The change in time spent in the paired compartment from pre-test to post-test is taken as a measure of the motivational effects of cues (environments) paired with drugs or withdrawal – motivational effects that in humans lead to drug-seeking behavior.
Although it might be proposed that the place conditioning paradigm and the drug self-administration paradigm are different ways of modeling the same process, several caveats are important. First, in place conditioning, the drug- or withdrawal-paired cues are contexts, whereas in the drug self-administration paradigm, discrete cues such as lights and tones are employed. This is significant because contextual cues and discrete cues engage somewhat divergent neural mechanisms (Kim and Fanselow, 1992). Second, place conditioning involves passive drug administration, which engages different neural mechanisms than does self-administration (Jacobs et al., 2003). Finally, indirect measures of the motivational effects of drug cues as they relate to relapse (as in the place conditioning paradigm) may not map perfectly onto direct measures of drug-seeking behavior (as in the drug self-administration paradigm). Nevertheless, despite their fundamental differences, the two paradigms produce concordant results the large majority of the time (Bardo and Bevins, 2000).
II.B. Classes of conditioned responses to drug- and withdrawal-paired cues
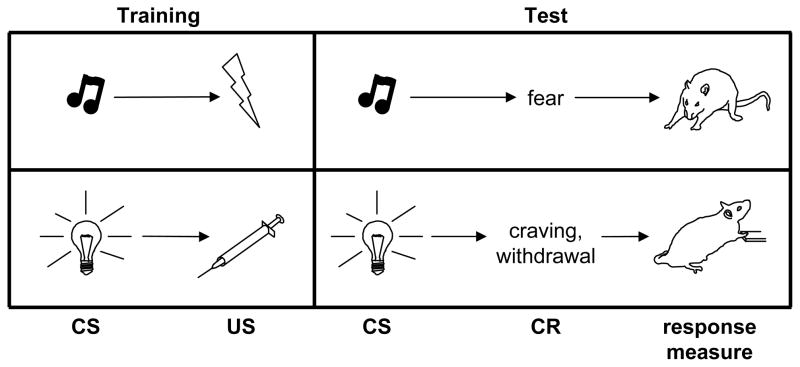
It is important to note that drug cues can elicit conditioned responses or modulate behavior through more than one mechanism. As described above, drug- or withdrawal-paired cues can be Pavlovian conditioned stimuli (CSs), defined as elements of a contingency in which CS presentation predicts either drug administration or acute withdrawal (Figure 2). However, drug-paired cues also can serve as discriminative stimuli or occasion setters, which signify that a contingency between other events is in effect. A discriminative stimulus (SD) signals that a response (operant) will be followed by an outcome (reinforcer), and an occasion setter signals that a different cue (a CS) will be followed by an outcome (an unconditioned stimulus or US). Sometimes it can be difficult to make these distinctions, particularly with people in real-life situations, and it is possible under some circumstances for a cue to serve more than one role; however, in animal models the roles played by drug- or withdrawal-paired cues generally are clear-cut. CSs can be discrete cues (e.g., a light, a tone, or the sound of a syringe pump) triggered at the same time as drug self-administration, or environments paired with passive drug administration or precipitated withdrawal. An example of an SD would be a signal such as houselight illumination, the presence of which indicates that lever pressing will lead to drug administration, and the absence of which indicates that lever pressing will have no programmed consequence. An example of an occasion setter would be a context in which Pavlovian pairings are conducted; if an animal receives cue-drug pairings in context A but cue alone (without drug) in context B, the significance of the cue will depend on the context in which it is presented, and the ability of that cue to elicit conditioned responses likewise will be modulated by the context in which a test occurs.
Figure 2.
Simplified depiction of how conditioning contributes to the development and maintenance of fear- and addiction-related behaviors. A Pavlovian conditioned stimulus (CS) is a cue that signals impending delivery of an innately appetitive or aversive unconditioned stimulus (US). In the fear conditioning paradigm, a CS such as a tone is paired with a US such as foot shock. After training, the tone elicits a conditioned response (CR), fear, which can be defined operationally in rodents as freezing (cessation of all bodily movements except those required for respiration). In an analogous manner, a CS such as a light that is paired with administration of a drug such as morphine (US) acquires the ability to elicit drug craving and withdrawal CRs, which can be defined operationally as drug-seeking behavior (e.g., pressing a lever for delivery of morphine).
It is important to maintain these distinctions because CSs, SDs, and occasion setters have different behavioral features and almost certainly rely on different neurobiological mechanisms. For example, CSs extinguish readily when the CS is no longer followed by the US, whereas occasion setters are very resistant to extinction even following extensive exposure to the occasion setter in the absence of the US (Bouton and Swartzentruber, 1986; Rescorla, 1986). Because our major interest is in extinction of Pavlovian conditioned responses, in this review we focus on drug- and withdrawal-paired cues as CSs.
III. Neurobiology of Conditioned Responses to Drug- and Withdrawal-Paired Cues
There has been considerable interest in determining the neurobiological underpinnings of conditioned drug craving and withdrawal. A full review of this literature is beyond the scope of this paper, but because some familiarity with it is essential for understanding the evolving literature on the neurobiology of drug cue extinction, in this section we describe some of the most significant findings.
III.A. Human imaging studies
Several studies have used brain imaging techniques including positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) to examine brain activation in addicts in response to drug-paired cues. Even though the studies vary in terms of the participants’ drug of choice (cocaine, heroin, crack cocaine, alcohol, or nicotine) and the manner of presenting drug-paired cues (viewing images or videos, handling paraphernalia, engaging in script-guided imagery, or some combination of these), the results have been remarkably consistent. Most report activation of prefrontal cortical areas, particularly anterior cingulate (Brody et al., 2002; Childress et al., 1999; Grusser et al., 2004; Kilts et al., 2004; Kilts et al., 2001; Maas et al., 1998; McBride et al., 2006; McClernon et al., 2007; Sell et al., 1999; Wexler et al., 2001; Wilson et al., 2005), but also dorsolateral prefrontal cortex (Brody et al., 2002; Grant et al., 1996; Maas et al., 1998; Sell et al., 1999; Wilson et al., 2005; Yang et al., 2009) and orbitofrontal cortex (Bonson et al., 2002; Childress et al., 1999; Grant et al., 1996; Kilts et al., 2001; McClernon et al., 2007; Yang et al., 2009). The prefrontal cortex is involved in response inhibition, decision making, and emotional regulation, among other functions, and is highly interconnected with limbic and reward circuitries (Miller and Cohen, 2001). Somewhat less consistent are reports of activation of the amygdala (Bonson et al., 2002; Childress et al., 1999; Grant et al., 1996; Kilts et al., 2001; McClernon et al., 2007; Yang et al., 2009) and the ventral striatum (Braus et al., 2001; Kareken et al., 2004; Sell et al., 1999), as might be expected from the emotionally charged and motivationally significant nature of drug cues. In several studies, activity in one or more of these areas correlated significantly with subjective reports of drug craving (Bonson et al., 2002; Brody et al., 2002; Grant et al., 1996; Kilts et al., 2004; Kilts et al., 2001; Maas et al., 1998; Sell et al., 2000; Smolka et al., 2006).
III.B. Animal studies
Studies in laboratory animals have examined both acquisition and expression of conditioned responses to drug- and withdrawal-paired cues. Here we focus on expression rather than acquisition, because in many cases acquisition studies are complicated by the possibility that an experimental manipulation could modulate the intensity of acute drug effects or acute withdrawal as opposed to modulating learning about the relationship between cues and the drug or withdrawal state. In other words, it can be difficult to determine whether an apparent impairment or facilitation of learning about drug- or withdrawal-paired cues is a secondary effect of increasing or decreasing the salience of the unconditioned stimulus (US) rather than an effect on the associative learning process per se.
III.B.1. Cues paired with drug administration
Consistent with the human imaging literature, studies using Fos or other immediate-early genes as markers of neuronal activity have reported activation of cortical, limbic, and reward circuitries following exposure to drug-paired cues. In most of these studies, rats were exposed to pairings of an environment with a drug administered by the experimenter, then some later time were re-exposed to the environment in the absence of the drug. Activated prefrontal areas include anterior cingulate (Brown et al., 1992; Franklin and Druhan, 2000; Honsberger and Leri, 2008; Neisewander et al., 2000; Schmidt et al., 2005; Schroeder et al., 2000; Schroeder and Kelley, 2002; Zavala et al., 2008), prelimbic cortex (Franklin and Druhan, 2000; Miller and Marshall, 2004, 2005b; Schmidt et al., 2005; Schroeder et al., 2000; Schroeder and Kelley, 2002; Zavala et al., 2008), infralimbic cortex (Franklin and Druhan, 2000; Schroeder et al., 2000; Schroeder and Kelley, 2002) (but see Miller and Marshall, 2004, 2005b), claustrum (Brown et al., 1992; Franklin and Druhan, 2000), and orbitofrontal cortex (Koya et al., 2006; Zavala et al., 2008). Activation of the basolateral amygdala (BLA) has been reported by several groups (Baker et al., 1999; Brown et al., 1992; Franklin and Druhan, 2000; Miller and Marshall, 2004, 2005b; Neisewander et al., 2000) but not all (Schroeder et al., 2000; Schroeder and Kelley, 2002; Zavala et al., 2008), whereas conditioned activation of the central amygdala (CeA) has not been observed (Baker et al., 1999; Franklin and Druhan, 2000; Miller and Marshall, 2004, 2005b; Neisewander et al., 2000; Schroeder et al., 2000; Schroeder and Kelley, 2002; Zavala et al., 2008). Finally, activation of the nucleus accumbens (NAcc), including core, shell, or both, is a common observation (Franklin and Druhan, 2000; Koya et al., 2006; Miller and Marshall, 2004; Neisewander et al., 2000; Schmidt et al., 2005; Schroeder et al., 2000; Schroeder and Kelley, 2002) (but see Brown et al., 1992).
The contribution of each of these areas to the expression of conditioned responses to drug cues has been investigated further using lesion, inactivation, and pharmacological techniques. Because of the central role it plays in responding to drugs of abuse, NAcc has been the focus of many of these studies. Single units in NAcc respond selectively with increased firing rates to cocaine-paired cues (Carelli, 2000; Carelli and Deadwyler, 1996). Inactivation of NAcc core (but not shell) blocks cue-induced reinstatement of cocaine-seeking (Di Ciano et al., 2008; Fuchs et al., 2004; Ito et al., 2004) and heroin-seeking (Rogers et al., 2008; Zhou et al., 2007); similarly, infusion of a mitogen-activated protein kinase (MAPK) inhibitor into NAcc core blocks expression of cocaine CPP (Miller and Marshall, 2005a) (but see Gremel and Cunningham, 2008). These effects are mediated at least in part by glutamate release within NAcc, as cue-induced reinstatement of heroin seeking is associated with increased glutamate release in NAcc core (Hotsenpiller et al., 2001; LaLumiere and Kalivas, 2008), and targeted infusion of AMPA receptor antagonists into NAcc core blunts cue-induced reinstatement of cocaine- and heroin-seeking (Backstrom and Hyytia, 2007; Di Ciano and Everitt, 2001, 2004; LaLumiere and Kalivas, 2008) and expression of cocaine and ethanol CPP (Gremel and Cunningham, 2009; Kaddis et al., 1995). By contrast, the role of dopamine release within NAcc is controversial. Increased dopamine release following exposure to drug-paired cues has been reported by some (Di Ciano et al., 1998; Fontana et al., 1993; Gerasimov et al., 2001; Ito et al., 2000; Phillips et al., 2003) but not all investigators (Brown and Fibiger, 1992). Likewise, impairment of cue-induced reinstatement of drug-seeking or expression of CPP following localized infusions of dopamine receptor antagonists has been shown in some studies (Bossert et al., 2007; Hiroi and White, 1990, 1991b; Kaddis et al., 1995; LaLumiere and Kalivas, 2008) but not others (Di Ciano and Everitt, 2004; Gremel and Cunningham, 2009).
The dorsomedial prefrontal cortex (dmPFC; anterior cingulate and prelimbic cortices) is a major source of glutamatergic input to NAcc (Christie et al., 1985) and has been implicated in relapse to drug-seeking by priming drug administrations and stress (McFarland et al., 2003, 2004; McFarland and Kalivas, 2001). This area seems to play a critical role in responding to drug cues as well, as dmPFC lesions or inactivation diminish cue-induced reinstatement of cocaine-seeking (Di Ciano et al., 2007; Di Pietro et al., 2006; McLaughlin and See, 2003; Weissenborn et al., 1997) and heroin-seeking (Rogers et al., 2008; Van den Oever et al. 2008; but see Schmidt et al. 2005). Dopamine is released within dmPFC following exposure to a previously methamphetamine-paired context (Lin et al., 2007), and targeted administration of a D1 dopamine receptor antagonist into prelimbic cortex blocks cue-induced reinstatement of heroin-seeking (See, 2009).
The amygdala is another major source of glutamatergic input to NAcc (Christie et al., 1987) and is reciprocally connected with the dorsomedial PFC (Llamas et al., 1977; Room et al., 1985). There is a great deal of evidence that the amygdala, and particularly the basolateral complex of the amygdala (BLA; lateral and basal nuclei), is required for expression of conditioned responses to drug-paired cues. BLA lesions or inactivation attenuate cue-induced reinstatement of cocaine-seeking (Kruzich and See, 2001; McLaughlin and See, 2003; Meil and See, 1997; Whitelaw et al., 1996) (but see Fuchs et al., 2002) and heroin-seeking (Fuchs and See, 2002; Grimm and See, 2000; Rogers et al., 2008) and can impair expression of amphetamine and ethanol CPP (Gremel and Cunningham, 2008; Hiroi and White, 1991a). Conversely, administration of D-amphetamine into BLA potentiates cue-induced reinstatement of cocaine-seeking (Ledford et al., 2003). Dopamine, but not glutamate, release within BLA seems to be critical, as targeted infusions of dopamine receptor antagonists into BLA block cue-induced reinstatement of cocaine-seeking (Di Ciano and Everitt, 2004; See et al., 2001) and expression of ethanol CPP (Gremel and Cunningham, 2009), whereas intra-BLA infusions of AMPA and/or NMDA glutamate receptor antagonists have no effect on cue-induced reinstatement of cocaine-seeking (Di Ciano and Everitt, 2004; See et al., 2001). BLA single units respond with increased firing rates to cocaine-paired cues (Carelli et al., 2003), suggesting that amygdalar input may contribute to the similar cocaine cue-responsivity of single units in NAcc. Serial processing of drug cue-related information from BLA to NAcc is supported by the finding that “disconnection” of these two structures via unilateral infusion of a dopamine receptor antagonist into BLA and contralateral infusion of an AMPA receptor antagonist into NAcc core impairs the ability of a cocaine-paired cue to maintain cocaine-seeking behavior under an extended chain schedule of reinforcement (Di Ciano and Everitt, 2004).
III.B.2. Cues paired with acute withdrawal
The literature on the neurobiological mechanisms underlying the expression of conditioned withdrawal is relatively modest, but some important information is available. Frenois and colleagues (2005; Lucas et al., 2008) used Fos expression to map brain activation patterns in rats undergoing acute (precipitated) opiate withdrawal or conditioned withdrawal elicited by re-exposure to a previously withdrawal-paired context. Interestingly, largely overlapping circuitries were engaged: acute withdrawal was associated with activation of the extended amygdala (CeA, bed nucleus of the stria terminalis or BNST, and NAcc shell) and some of its major afferents (VTA and hippocampus), as well as a number of brainstem structures and nuclei (periaqueductal gray, nucleus of the solitary tract, and locus coeruleus or LC). Likewise, conditioned withdrawal was associated with activation of elements of extended amygdala (BNST and NAcc shell, but not CeA), as well as BLA, regions of hippocampus, VTA, and LC. BLA and the extended amygdala have been implicated in fear, anxiety, stress, and aversive motivation (Cardinal et al., 2002; Walker et al., 2003), and NAcc is a site of neuroplastic changes occurring during the development of drug dependence that are associated with dysphoria and anhedonia (Carlezon et al., 1998). Recruitment of this “anti-reward” system (Koob and Le Moal, 2005) is a hallmark of the progression of addiction (Koob and Le Moal, 1997) and may be responsible for the experience of withdrawal (acute or conditioned) as a stressful, anxiogenic, dysphorogenic state that triggers an intense desire to take drugs.
Despite activating similar circuitries, acute withdrawal and conditioned withdrawal are not identical mechanistically. The adrenergic a2 receptor antagonist clonidine blocks both acute withdrawal symptoms and withdrawal-associated activation of LC (Aghajanian, 1978; Tseng et al., 1975), but clonidine does not block conditioned withdrawal, as it has no effect on suppression of bar pressing by a previously withdrawal-paired CS and actually enhances the expression of CPA to a previously withdrawal-paired environment (Schulteis et al., 1998).
In two studies the role of the amygdala in mediating conditioned withdrawal has been examined, and consistent with the literature on conditioned craving, this region seems to play an important role. In one study (Hellemans et al. 2006a), intra-BLA infusions of antisense oligonucleotides to Zif268 (an immediate early gene) prior to a brief re-exposure to a previously withdrawal-paired CS impaired later expression of conditioned withdrawal (measured as suppression of bar pressing) to that same CS, presumably by blocking memory reconsolidation. In the other study (Heinrichs et al. 1995), destruction of CeA corticotropin-releasing factor (CRF) – containing neurons by localized infusion of immunotoxins attenuated the expression of conditioned withdrawal as assessed via response suppression in the presence of a previously withdrawal-paired CS.
IV. Extinction of Conditioned Responses to Drug- and Withdrawal-Paired Cues
Conditioned responses (CRs) to drug- and withdrawal-paired cues, like all other types of Pavlovian conditioned responses, can be reduced through extinction. Extinction involves repeated presentations of a CS in the absence of the unconditioned stimulus (US) with which it was paired previously. The outcome of exposure to this type of training (“extinction training”) is a decline in the magnitude and/or frequency of the CR (“extinction”). Because there is considerable evidence that they are mediated by different mechanisms, a distinction is made between within-session extinction, defined as extinction occurring during extinction training, and extinction retention, defined as extinction memory assessed at some point after the completion of extinction training (typically at least 24 hrs).
In the drug self-administration paradigm, cue extinction training involves conducting several test sessions (after the completion of lever press extinction) in which no drug is administered but the cues are present (Figure 1). Extinction of the response-reinstating ability of the cues occurs over the course of the test sessions and is measured as a lessening of their ability to modulate drug-seeking behavior. It is important to maintain the distinction between operant (i.e., lever press) extinction and cue extinction, which are different processes that likely engage different neural mechanisms. In this review we are concerned with cue extinction and do not discuss lever press extinction in any detail.
In the place conditioning paradigm, extinction training can occur in either of two ways: animals can be given free access to the place conditioning apparatus in repeated test sessions, or animals can be confined in the formerly drug- or withdrawal-paired context in the absence of drug administration or precipitated withdrawal (i.e., following an injection of saline) and subsequently given free access tests to assess extinction. When extinction has occurred, there no longer is a preference for or aversion to the previously drug- or withdrawal-paired context; that is, animals spend approximately equal amounts of time in each.
IV.A. Response recovery after extinction
Extinction is not due to forgetting or “unlearning” of the significance of the CS. In most paradigms Pavlovian conditioned memories are long-lasting and resistant to forgetting, such that without exposure to the CS in the absence of the US, the conditioned response does not disappear (Quirk, 2002; Stinus et al. 2000). On the other hand, extinction memory is fragile in that extinguished CRs are subject to recovery under several circumstances. For example, extinction typically is expressed selectively within the context in which extinction training occurred (the renewal effect) (Bouton and Bolles, 1979). Likewise, extinguished CRs re-emerge over time following extinction training, an effect called spontaneous recovery (Pavlov, 1927). These effects indicate that extinction does not undo original learning, but is itself a form of new learning in which the CR comes to be suppressed in a context- and time-dependent manner (Bouton, 1993).
Various types of CRs to drug-paired cues undergo renewal and spontaneous recovery. In animals, spontaneous recovery of tolerance to the analgesic effects of morphine has been observed (Millin and Riccio, 2002). Renewal of cocaine and morphine CPP in a variant of the CPP paradigm in which a distinctive floor texture is the drug-paired cue (Parker et al., 2006), and of nose poking for nicotine- and ethanol- paired cues (Chaudhri et al., 2008; Diergaarde et al., 2008), has also been reported. Interestingly, and similar to other types of CRs (Chelonis et al., 1999; Gunther et al., 1998; but see Bouton et al., 2006), renewal of CRs to drug-paired cues is attenuated when extinction training is conducted in multiple contexts (Chaudhri et al., 2008). Human studies have been less consistent, with two groups reporting renewal of craving induced by nicotine-and alcohol-paired cues in smokers and moderate to heavy social drinkers, respectively (Collins and Brandon, 2002; Thewissen et al., 2006), and two groups finding no evidence of renewal of craving or salivation elicited by alcohol-paired cues in heavy social drinkers and alcoholics (MacKillop and Lisman, 2008; Stasiewicz et al., 2007). Both renewal (Collins and Brandon, 2002) and spontaneous recovery (Brooks et al., 2004) of CRs to drug-paired cues are attenuated when an extinction cue, defined as an incidental cue that is present during extinction training, is present during testing as well, similar to other forms of extinction (Brooks and Bouton, 1993, 1994; Brooks et al., 1999).
IV.B. Drug cue exposure therapy
Extinction can be employed in a clinical context as part of treatment programs for psychiatric conditions that involve a significant learned component. For example, post-traumatic stress disorder (PTSD) is characterized by extreme fearfulness, intrusive thoughts, nightmares, and increased arousal, which develop following exposure to a severe stressor. Many of these responses are triggered by cues that were present at the time of the stressor, suggesting that they acquired significance through a Pavlovian conditioning mechanism in which the stressor acted as a US. One form of PTSD treatment is exposure therapy, an extinction-based protocol in which the patient is exposed to fear-triggering cues in a safe environment until fear responses decline (Rothbaum and Davis, 2003). A challenge inherent in using extinction clinically is overcoming its context dependence and general fragility, but techniques are being developed to make extinction memory stronger and more permanent (Walker et al., 2002). Some of these techniques have been applied successfully in clinical studies (Ressler et al., 2004).
As described above, addiction also involves a learning component, and as such it may be amenable to extinction-based interventions. Typically, however, addiction treatment programs emphasize avoidance of drug cues rather than nonreinforced exposure to them, as recovering addicts are advised not to return to drug-paired environments or associate with people with whom they consumed drugs. This is an effective strategy to the extent that avoidance is possible, but it is not optimal because there often are instances of unintended exposure to cues that once signaled drug procurement or use (e.g., money). A better approach would be to reduce the ability of drug cues to provoke craving and withdrawal so that if they are encountered they will be less powerful.
Several groups have examined the utility of exposing drug users to drug cues in the absence of drug effects. These studies differ in a number of respects, including the characteristics of the participants, their drug of choice (opiates, cocaine, nicotine, or alcohol), extent of drug use (addict, heavy user, or user looking to moderate intake), and inpatient or outpatient status. Different types of cues and methods of presenting them have been employed, including images of drugs or people using drugs; videos of people preparing or using drugs; audio tapes of drug deals; and “in vivo” exposure involving handling of drugs or drug paraphernalia, preparing to self-administer drugs, and in some cases, self-administering either saline or a drug itself with a drug antagonist “on board” to block physiological drug effects. A few studies have involved virtual reality technology to simulate environments associated with drug use, such as “crack houses” (Saladin et al., 2006) or bars (Lee et al., 2007). The details of the exposure protocol, including the number and duration of exposures and number and spacing of exposure sessions, vary widely; moreover, in some studies cue exposure was conducted in conjunction with other types of therapy, such as cognitive-behavioral or relaxation therapy, whereas in others cue exposure was used alone. Response measures include subjective reports of craving, withdrawal, and emotional states; physiological or autonomic responses such as salivation or skin conductance; and outcome measures such as relapse rates. Finally, some but not all studies include untreated or alternatively treated control groups and/or follow-up assessments after the completion of therapy.
Overall, the results have been disappointing. There have been some reports of reductions in craving and withdrawal following drug cue exposure, but the effects tend to be modest and time-limited (Childress et al., 1986; Drummond and Glautier, 1994; Lee et al., 2004; Lee et al., 2007; Loeber et al., 2006; Monti et al., 1993; Monti et al., 2001; O’Brien et al., 1990; Powell et al., 1993; Rohsenow et al., 2001; Sitharthan et al., 1997). Several studies observed no benefit (Corty and McFall, 1984; Dawe et al., 1993; Kasvikis et al., 1991; Kavanagh et al., 2006; McCusker and Brown, 1995; Niaura et al., 1999; Raw and Russell, 1980) and one reported detrimental effects in terms of increased dropout and relapse among participants receiving drug cue exposure (Marissen et al., 2007). A meta-analysis of the literature through 2001 revealed no statistically significant benefit of drug cue exposure therapy (Conklin and Tiffany, 2002). There are some indications that cue exposure may be more efficacious with certain kinds of drugs than with others; for example, alcohol users seem to respond particularly well, as many of the studies reporting positive effects involved exposure to alcohol cues in alcoholics or heavy drinkers looking to moderate intake (Drummond and Glautier, 1994; Lee et al., 2007; Loeber et al., 2006; Monti et al., 1993; Monti et al., 2001; Rohsenow et al., 2001; Sitharthan et al., 1997). In this regard it is potentially interesting that the two failures to observe renewal in cue exposure studies both involved alcohol-paired cues (MacKillop and Lisman, 2008; Stasiewicz et al., 2007), suggesting that extinction of CRs to alcohol cues may generalize across contexts particularly well. Nevertheless, collectively these studies fail to substantiate the efficacy of drug cue exposure therapy.
It is unclear why the results of these studies have been so discouraging thus far. Several authors speculate that incorporating advances in the understanding of extinction in laboratory animals would improve the outcome (Conklin, 2006; Conklin and Tiffany, 2002; Havermans and Jansen, 2003; Powell, 1995; Siegel and Ramos, 2002). For example, since renewal is attenuated following extinction in multiple contexts, exposure therapy conducted in multiple environments might promote generalization of extinction (Conklin and Tiffany, 2002; Havermans and Jansen, 2003; Siegel and Ramos, 2002). Likewise, since both renewal and spontaneous recovery are lessened in the presence of extinction cues, incorporating a portable token that can be taken home (such as a distinctive coin or rock) into cue exposure sessions might be beneficial (Conklin and Tiffany, 2002; Havermans and Jansen, 2003; Siegel and Ramos, 2002). It also has been proposed that discrete, tangible drug cues such as drug paraphernalia represent only a subset of cues associated with drug use and may not be the most salient ones. Interoceptive cues related to drug self-administration and initial drug effects are powerful, but with few exceptions (e.g., O’Brien et al., 1984) they have not been incorporated into exposure therapy sessions (Siegel and Ramos, 2002). Similarly, the perception of drug availability is itself a drug cue, and its absence during cue exposure sessions may affect both the degree to which other drug-paired cues elicit craving and withdrawal responses (Droungas et al., 1995; McBride et al., 2006; Thewissen et al., 2005; Wilson et al., 2005) and the generalization of extinction to environments in which drugs are accessible (Powell, 1995). Finally, since drug users are often exposed to hundreds if not thousands of pairings of cues with drug effects over the course of their addiction, successful cue exposure therapy may require many exposures to extinguish entrenched associations (Conklin and Tiffany, 2002).
Another possibility is that if the neural underpinnings of drug cue extinction were clearer then it might be more evident why current drug cue exposure protocols have had limited success. In other contexts, basic neuroscience research has led to more efficacious clinical interventions for psychiatric conditions; analogous studies of the neurobiology of drug cue extinction might have a similar impact.
V. Neurobiological Mechanisms of Extinction
As yet little is known about the neurobiological mechanisms of extinction of conditioned drug craving and withdrawal. There have been few systematic investigations; in fact, some of the relevant findings were incidental observations in studies designed to characterize other processes. Different groups have employed cues paired with different drugs or classes of drugs, which may engage different mechanisms, and the vast majority of the data comes from models of conditioned drug craving, such that almost nothing is known about extinction of conditioned withdrawal. Clearly, there is much work to be done in this area, although the topic is attracting increased interest.
Without any theoretical framework within which to organize this fragmentary literature, it can be difficult to piece together a cohesive picture or apply what has been learned clinically. However, there is relevant information available from another line of research. Extinction of a different type of Pavlovian conditioned response – conditioned fear – has been the subject of intensive study for a number of years and a great deal has been learned about its underlying neural circuitry, neuropharmacology, and molecular mechanisms. At the core of the fear extinction process is a recruitment of circuitry in the ventromedial prefrontal cortex that inhibits conditioned fear responses arising from limbic structures, particularly the amygdala. Accumulating evidence indicates that this same basic process is engaged by extinction of other kinds of Pavlovian CRs (Akirav et al., 2006) and operant responses (LaLumiere et al., 2008a) as well.
In many cases parallels can be drawn between observations in the fear and drug cue extinction literatures, suggesting that extinction of conditioned drug craving and withdrawal may be mediated by a similar cortico-limbic mechanism. For this reason, It is helpful to describe some of the major features of fear extinction before turning to a discussion of the neurobiology of extinction of drug- and withdrawal-paired cues. This will facilitate use of the fear extinction literature as a template to organize the observations on drug cue extinction into a cohesive framework.
The fear extinction literature is too large to be reviewed in its entirety, and interested readers are referred to other sources (Myers and Davis, 2007; Quirk and Mueller, 2008). Here we emphasize major substrates and mechanisms, focusing in particular on those that may be shared with extinction of conditioned drug craving and withdrawal.
V.A. Extinction of conditioned fear
In the fear conditioning paradigm, an organism (often a rat or mouse) is exposed to pairings of an initially neutral cue, such as a light, tone, or context, with an aversive stimulus such as foot shock, and comes to exhibit conditioned fear responses in the presence of that cue. In rodents fear is defined operationally in any of several ways; the most common measures are freezing (cessation of all movements except those required for respiration) and fear-potentiated startle (an increase in the amplitude of the acoustic startle response in the presence of a fear-eliciting cue vs. in its absence). Extinction of conditioned fear occurs readily when the CS is presented repeatedly in the absence of the aversive US.
The neurobiology of conditioned fear acquisition has been studied extensively. The amygdala in particular is critical: sensory information about the CS and US is transmitted via cortical and subcortical pathways to BLA, where essential plasticity underlying the formation of the CS-US association occurs. BLA projects to CeA, which in turn innervates hypothalamic and brainstem regions responsible for triggering autonomic and behavioral fear responses (for reviews see Davis and Shi, 2000; Rodrigues et al., 2004). Fear extinction involves this circuitry as well, but also recruits additional structures, particularly the medial prefrontal cortex (mPFC). Consistent with its general role in emotional regulation and behavioral inhibition, in extinction mPFC plays an active role in inhibiting conditioned responses by suppressing neural activity within the amygdala. Both the amygdala and mPFC are sites of essential synaptic plasticity underlying extinction, including NMDA receptor-mediated forms of plasticity, although each structure plays a somewhat different role in the encoding, consolidation, and expression of extinction memory. In what follows we describe what has been learned about each of these components in more detail.
V.B.1. Amygdala
Evidence for a role of the amygdala in fear extinction is extensive. The amygdala is activated during fear extinction training (Herry and Mons, 2004; LaBar et al., 1998; Phelps et al., 2004). Individual BLA neurons fire selectively to extinguished cues both as extinction training progresses (Herry et al., 2008) and after extinction training is complete, in a context-dependent manner (Herry et al., 2008; Hobin et al., 2003). Pre-extinction training inactivation of the basal nucleus (Herry et al., 2008) or post-extinction training lesions of the intercalated cell masses (ICMs) lying between BLA and CeA (Likhtik et al., 2008) impair within-session extinction and extinction retention, respectively. Both encoding and consolidation of extinction memory can be modulated by intra-BLA infusions of a wide variety of drugs including neurotransmitter receptor agonists and antagonists (Berlau and McGaugh, 2006; Falls et al., 1992; Harris and Westbrook, 1998; Hikind and Maroun, 2008; Kim et al., 2007a; Laurent and Westbrook, 2008; Lee and Kim, 1998; Roche et al., 2007; Yang et al., 2006) as well as modulators of downstream second messengers, transcription, and translation (Lin et al., 2003c; Lin et al., 2003b; Lu et al., 2001). The phosphorylation state of second messengers and gene expression patterns within BLA are altered following extinction training (Cannich et al., 2004; Chhatwal et al., 2005b; Chhatwal et al., 2006; Heldt and Ressler, 2007; Herry and Mons, 2004; Lin et al., 2003c; Lin et al., 2003b), and manipulation of gene expression within BLA prior to extinction training modulates extinction memory consolidation (Chhatwal et al., 2006).
The contribution of GABAergic neurotransmission within the amygdala has been a topic of particular interest. Based on findings of recovery of fear CRs following extinction (e.g., the renewal effect), it has been proposed that amygdalar plasticity underlying fear memory remains at least partially intact following extinction but that the expression of that memory is suppressed. One way that this might occur is via recruitment of GABAergic neurotransmission within the amygdala to dampen amygdalar throughput (Davis and Myers, 2002) (for a different view, see Dalton et al., 2008; Kim et al., 2007b; Lin et al., 2003a; Mao et al., 2008). There is an extensive network of GABAergic interneurons within BLA (Muller et al., 2003; Rainnie et al., 1991), as well as GABAergic ICMs lying between BLA and CeA that gate impulse traffic from one to the other (Royer et al., 1999), and either or both of these could be critical (Jungling et al., 2008; Rosenkranz and Grace, 2001; Rosenkranz et al., 2003; Royer and Pare, 2002). Straightforward pharmacological manipulations of GABAergic tone in the amygdala are problematic due to the potential for either sedation or epileptogenesis; however, it has been shown that extinction induces changes in GABA-related genes and modulates GABAA receptor binding in BLA (Chhatwal et al., 2005b; Heldt and Ressler, 2007), and that selective lesions of ICMs impair extinction retention (Likhtik et al., 2008). Hence, modulation of the activity of GABAergic systems within BLA likely plays a key role in extinction of conditioned fear.
V.A.2. Medial prefrontal cortex
The medial prefrontal cortex (mPFC) is a major source of input to amygdalar GABAergic neurons. Stimulation of mPFC induces firing in BLA interneurons (Rosenkranz and Grace, 2001) and inhibits conditioned responses of BLA principal neurons to a previously fear conditioned cue (Rosenkranz et al., 2003); it also induces Fos expression in ICMs (Berretta et al., 2005) and blunts responses of CeA neurons triggered by stimulation of BLA, presumably via activation of ICMs (Quirk et al., 2003). Hence, mPFC is in a position to exert inhibitory control over the amygdala. Consistent with this, there is a great deal of evidence indicating that it plays a central role in extinction.
The first studies implicating mPFC in fear extinction demonstrated that pre-training, electrolytic lesions of this region produce an impairment (Morgan and LeDoux, 1995; Morgan et al., 1993). Later studies employing more focal lesions (Quirk et al., 2000) or localized inactivation (Sierra-Mercado et al., 2006) identified a prominent role for the ventral medial or infralimbic (IL) region of mPFC, specifically. Medial PFC is activated during fear extinction training (Barrett et al., 2003; Burgos-Robles et al., 2007; Herry and Mons, 2004; Kalisch et al., 2006; Knapska and Maren, 2009; Milad et al., 2007; Phelps et al., 2004; Santini et al., 2004; Santini et al., 2008), and subnormal mPFC activation is associated with poor extinction retention (Burgos-Robles et al., 2007) and is seen in disorders such as PTSD that are thought to involve an extinction deficit (Bremner et al., 2005). Medial PFC thickness is associated positively with retention of extinction memory (Milad et al., 2005), whereas manipulations that adversely affect mPFC circuitry, such as acute or chronic stress and associated dendritic retraction, impair extinction retention (Izquierdo et al., 2006; Miracle et al., 2006).
Medial PFC seems to be involved specifically in consolidation and expression of extinction memory. Medial PFC lesions impair extinction retention while sparing within-session extinction (Quirk et al., 2000). Single units within IL fire selectively to presentations of a previously fear-conditioned cue during an extinction retention test 24 hrs after extinction training but not during the extinction training session itself (Milad and Quirk, 2002). When IL microstimulation is paired with presentations of a previously fear conditioned cue in nonextinguished animals, freezing to those cues is attenuated (Milad and Quirk, 2002; Milad et al., 2004), consistent with the putative role of this region in the expression of extinction. Pre-extinction training, intra-mPFC or intra-IL infusions of neurotransmitter receptor agonists or antagonists (Burgos-Robles et al., 2007; Hikind and Maroun, 2008; Laurent and Westbrook, 2008; Mueller et al., 2008; Pfeiffer and Fendt, 2006; Sotres-Bayon et al., 2009; Zushida et al., 2007), as well as modulators of downstream second messengers, transcription, and translation (Hugues et al., 2006; Hugues et al., 2004; Mueller et al., 2008; Santini et al., 2004), modulate extinction retention without affecting within-session extinction. Immediate post-extinction training administration of many of these agents has a similar effect.
V.A.3. NMDA receptors
Like other forms of learning, extinction involves NMDA receptor-mediated synaptic plasticity. Studies involving systemic administration of NMDA receptor antagonists prior to extinction training report dose-dependent impairments of both within-session extinction and extinction retention (Baker and Azorlosa, 1996; Dalton et al., 2008; Santini et al., 2001; Sotres-Bayon et al., 2007; Sotres-Bayon et al., 2009). Systemic NMDA receptor antagonists also impair extinction retention when administered immediately after extinction training (Burgos-Robles et al., 2007; Laurent and Westbrook, 2008; Santini et al., 2004), indicating that NMDA receptors are involved in consolidation as well as encoding of extinction memory.
NMDA receptors within BLA and IL contribute to different aspects or phases of extinction learning and memory. Microinfusions of NMDA receptor antagonists into BLA prior to fear extinction training impair both within-session extinction and extinction retention (Falls et al., 1992; Laurent et al., 2008; Laurent and Westbrook, 2008; Lee and Kim, 1998; Lin et al., 2003b), whereas immediate post-extinction training intra-BLA infusions of the NR2B-specific antagonist ifenprodil have no effect on subsequent extinction retention (Laurent and Westbrook, 2008; Sotres-Bayon et al., 2009). This suggests that NMDA receptor-dependent synaptic plasticity within BLA is involved in encoding of extinction memory, but that NR2B-containing NMDA receptors within BLA are not required for consolidation. Conversely, infusions of NMDA receptor antagonists into IL prior to extinction training have no effect on within-session extinction but impair later extinction retention (Burgos-Robles et al., 2007; Laurent and Westbrook, 2008; Sotres-Bayon et al., 2009). Intra-IL infusions immediately after extinction training impair extinction retention as well (Burgos-Robles et al., 2007; Laurent and Westbrook, 2008). This suggests that NMDA receptor-dependent synaptic plasticity within IL is involved primarily in consolidation of extinction memory.
Whereas antagonism of NMDA receptors impairs extinction, enhancement of NMDA receptor function by the NMDA receptor partial agonist D-cycloserine (DCS) facilitates extinction. DCS acts at the glycine modulatory site on the NR1 NMDA receptor subunit to increase calcium influx without causing damage due to neurotoxicity (Sheinin et al., 2001). Systemic administration of DCS either before (Bouton et al., 2008; Ledgerwood et al., 2003, 2005; Lee et al., 2006; Mao et al., 2008; Walker et al., 2002; Weber et al., 2007; Woods and Bouton, 2006; Yang and Lu, 2005) or immediately after (Ledgerwood et al., 2003) extinction training facilitates extinction, suggesting that DCS modulates consolidation of extinction memory. To date no studies have examined the effect of infusions of DCS directly into IL, but local infusion of DCS into BLA prior to (Mao et al., 2006; Walker et al., 2002) or immediately after (Ledgerwood et al., 2003) fear extinction training mimics the effects of systemic administration.
V.A.4. Mechanistic view of fear extinction
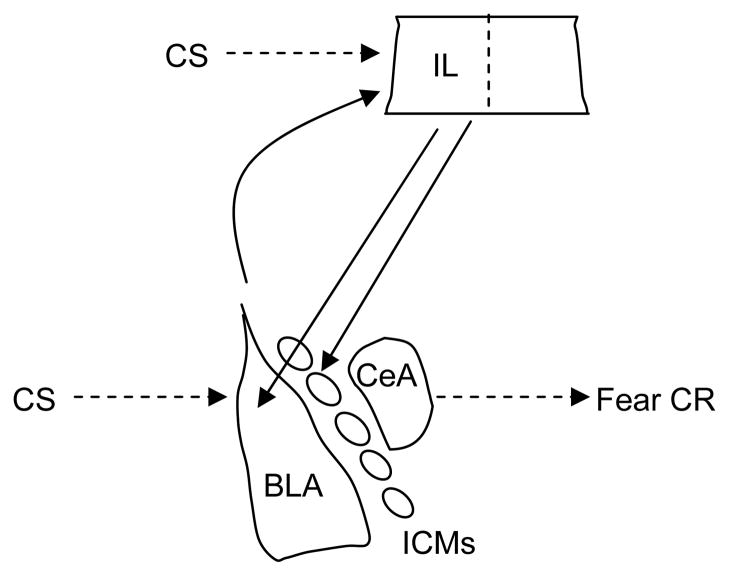
The findings on amygdala and mPFC involvement in fear extinction suggest a model in which the two structures interact dynamically and contribute to different aspects of extinction learning and memory (Figure 3) (Quirk and Mueller, 2008). When a CS is presented in the absence of the US with which it had been paired previously, the contingency change is detected through a mechanism that is not well understood (McNally et al., 2004). NMDA receptor-dependent synaptic plasticity occurs in BLA and contributes to within-session extinction; IL also is recruited and NMDA receptor-dependent synaptic plasticity occurring here is involved in consolidation of extinction memory. Later, at the time of an extinction retention test, IL is activated and inhibits the expression of fear by inhibiting the amygdala via innervation of GABAergic interneurons in BLA and/or ICMs. The nature of the synaptic plasticity occurring at each site is as yet unclear, but may include long-term potentiation (LTP), long-term depression (LTD), synaptic depotentiation, or a combination of these (Herry and Garcia, 2002; Kim et al., 2007b; Li et al., 2009; Royer and Pare, 2002; Santini et al., 2001).
Figure 3.
Highly simplified schematic depicting hypothetical mechanisms by which extinction of conditioned fear relies on interactions between the amygdala and the infralimbic (IL) region of medial prefrontal cortex. The basolateral complex of the amygdala (BLA) is a site of essential plasticity underlying conditioned fear memories. BLA triggers fear responses via its projections to the central nucleus of the amygdala (CeA), and also projects to IL. In extinction, the omission of the US is detected through a mechanism that is not well understood. CS-related information is relayed to both BLA and IL and NMDA receptor-dependent synaptic plasticity occurs at both sites. After extinction training is complete, IL contributes to the suppression of fear CRs by inhibiting amygdalar throughput, likely by activating GABAergic interneurons within BLA, GABAergic intercalated cell masses (ICMs) lying between BLA and CeA, or both. Other brain areas (including the hippocampus; not pictured) likely also play important roles in extinction. There is accumulating evidence that analogous mechanisms are involved in extinction of drug-related cues.
V.B. Extinction of conditioned drug craving and conditioned withdrawal
Many of the findings on extinction of drug- or withdrawal-paired cues also implicate these three major substrates: amygdala, mPFC, and NMDA receptor-mediated synaptic plasticity. In light of this, it can reasonably be anticipated that some of the mechanisms of fear extinction outlined above may overlap with the mechanisms of extinction of drug-associated cues. There also are some findings involving additional mechanisms whose significance is as yet unclear.
V.B.1. Amygdala
Studies using the immediate early gene Fos as a marker of neuronal activity suggest that a broad network of structures engaged by drug cue acquisition is re-engaged by extinction. Among these structures is BLA, where Fos expression is increased following exposure to a nonextinguished drug-paired cue or context, but is unchanged relative to that seen in drug-naïve controls following exposure to an extinguished drug-paired cue or context (Baker et al., 1999; Neisewander et al., 2000). Perhaps similarly, in smokers, amygdala activation by smoking-related cues (as assessed by fMRI) diminished over the course of an extinction-like smoking cessation program in which participants smoked reduced nicotine content cigarettes in order to weaken the association between the physical act of smoking and the delivery of a bolus of nicotine (McClernon et al., 2007).
Studies involving BLA manipulations indicate that the plasticity reflected in altered amygdala reactivity following extinction is critical. In the drug self-administration paradigm, rats with excitotoxic lesions of BLA induced after acquisition of cocaine-seeking take longer to cease lever pressing during test sessions in which cocaine delivery is discontinued but previously response-contingent, drug-paired cues are presented noncontingently (Fuchs et al., 2002). In the same paradigm, inactivation of BLA via localized infusions of the voltage-gated sodium channel blocker tetrodotoxin (TTX) immediately following each of several test sessions has a similar effect (Fuchs et al., 2006). One interpretation of these findings is that extinction of the response-reinstating value of drug-paired cues is impaired by disruption of BLA function. Consistent with this interpretation, excitotoxic BLA lesions induced after acquisition of cocaine conditioned place preference (CPP) impair CPP extinction over repeated test sessions (Fuchs et al., 2002). Finally, enhancement of BLA function via localized infusions of glucose (Schroeder and Packard, 2003) or the cholinergic agonist oxotremorine (Schroeder and Packard, 2004) prior to or immediately after nonreinforced confinements in a previously amphetamine-paired environment facilitates extinction of amphetamine CPP, as assessed off-drug in a separate test session.
V.B.2. Medial prefrontal cortex
Only two behavioral studies have examined the role of mPFC in drug cue extinction, but the data suggest that intact function of this region is important as well. In one study, temporary inactivation of mPFC (prelimbic and infralimbic cortices) via localized infusion of the anesthetic bupivacaine prior to nonreinforced confinements in a previously amphetamine-paired context impaired extinction of amphetamine CPP (Hsu and Packard, 2008). Localized infusion of the NMDA receptor antagonist AP5 prior to extinction training had similar effects (Hsu and Packard, 2008). By contrast, in the second study, excitotoxic mPFC lesions had no effect on either the acquisition or extinction of cocaine CPP (Zavala et al., 2003). However, in this study the lesions were targeted to the prelimbic region of mPFC, which does not seem to be required for extinction of conditioned fear (Burgos-Robles et al., 2009; Vidal-Gonzalez et al., 2006). Moreover, because these permanent lesions were made before all phases of training, the data may also reflect some aspects of recovery of function or other compensatory neuroadaptations.
Zavala et al. (2007), using Fos as a marker of neuronal activity, found that the prelimbic and infralimbic regions of mPFC were activated significantly by nonextinguished cocaine-paired cues but were not activated at all by extinguished cocaine-paired cues. Significant behavioral differences between the extinguished and nonextinguished groups may, however, have contributed to the differences in Fos expression, which complicates interpretation of these findings. A previous study from the same group (Neisewander et al. 2000) included conditions that controlled for these differences in behavior, but Fos expression was not examined in prelimbic or infralimbic cortex.
V.B.3. NMDA receptors
NMDA receptor antagonists impair extinction of drug-paired cues. As described above, infusions of AP5 into mPFC impair extinction of amphetamine CPP (Hsu and Packard, 2008). In the drug self-administration paradigm, infusions of AP5 into BLA immediately after multiple test sessions are associated with more persistent cue-maintained drug-seeking behavior, perhaps because extinction of the response-reinstating value of the cues was impaired (Feltenstein and See, 2007). Finally, pre-extinction training, systemic administration of the NMDA receptor antagonist MK-801 blocks facilitation of cocaine CPP extinction by CDPPB, a modulator of mGluR5 receptors, which are functionally linked to NMDA receptors (Gass and Olive, 2009).
Conversely, extinction of drug- and withdrawal-paired cues is facilitated by DCS. Extinction of cocaine CPP is facilitated when DCS is administered systemically either prior to or immediately after repeated place preference tests, or when infused into BLA immediately after repeated tests (Botreau et al., 2006; Paolone et al., 2009). Likewise, extinction of naloxone-induced conditioned place aversion (CPA) in morphine-dependent rats is facilitated by systemic administration of DCS immediately prior to confinement in the formerly naloxone-paired environment in the absence of acute withdrawal (Myers and Carlezon, 2010). DCS does not enhance the rate of extinction of ethanol CPP but does retard subsequent reconditioning, suggesting a facilitation of extinction that may have been obscured by floor or performance effects (Groblewski et al., 2009). Finally, in an ethanol self-administration paradigm, rats receiving DCS prior to sessions in which ethanol delivery was discontinued but response-contingent cues continued to occur stopped responding more rapidly than did rats receiving vehicle, presumably because DCS facilitated extinction of the secondary reinforcing value of the cues (Vengeliene et al., 2008).
V.B.4. Other observations
There are a few studies examining the contribution of neuromodulator systems and molecular mechanisms to extinction of drug-paired cues.
Using a place conditioning paradigm in mice in which ethanol conditions either CPP (when administered before confinement in the paired environment) or CPA (when administered after confinement in the paired environment), Cunningham et al. (1998) found that systemic administration of the opiate receptor antagonist naloxone prior to each of several test sessions facilitates extinction of CPP but retards extinction of CPA. Naloxone had no effect on extinction recall when administered prior to an extinction retention test conducted after extinction training was complete. Similar findings have been reported with fear extinction: administration of naloxone prior to extinction training sessions impairs both within-session extinction and extinction retention, whereas administration immediately after extinction training sessions or immediately prior to an extinction retention test has no effect (McNally and Westbrook, 2003). The fear extinction observations are part of a larger literature on the role of endogenous opioids in conditioned fear acquisition, extinction, and inhibition, which when taken as a whole suggests that endogenous opioids might serve as an “error signal” driving associative learning (for discussion see McNally et al., 2004; Myers and Davis, 2007). It is possible that naloxone modulates this error signal similarly for drug cues and fear cues, albeit in opposite directions for cues with appetitive and aversive motivational valences. In this regard endogenous opioid signaling could be viewed as a general associative learning mechanism.
A similar bidirectional, valence-specific effect on extinction of place conditioning has been observed in mice lacking the gene for Narp, an AMPA receptor aggregating protein. Narp knockout mice show delayed extinction of morphine CPP (Crombag et al., 2009) and enhanced extinction of morphine withdrawal-induced CPA (Reti et al., 2008). It is not immediately obvious how to interpret these findings, particularly since the Narp knockout was constitutive, but one possibility is that Narp contributes to error signaling, similarly (perhaps) to endogenous opioids.
Endogenous cannabinoids also have been implicated in drug cue extinction. Parker et al. (2004) found that systemic administration of Δ9-tetrahydrocannabinol (THC) or cannabidiol (CBD) – cannabinoids found in marijuana – prior to confinement in a previously cocaine- or amphetamine-paired environment facilitated extinction of cocaine and amphetamine CPP, at doses where they themselves conditioned neither CPP nor CPA. These effects were not blocked by co-administration of a CB1 cannabinoid receptor antagonist, suggesting that they are mediated through other mechanisms. Perhaps similarly, systemic administration of a cannabinoid reuptake inhibitor prior to fear extinction training facilitates extinction of conditioned fear (Chhatwal et al., 2005a), although it is thought that modulation of fear extinction by endogenous cannabinoids occurs via a CB1 receptor-dependent mechanism (Marsicano et al., 2002).
Finally, in an investigation of the molecular mechanisms underlying acquisition and extinction of conditioned responses to cocaine-paired cues, Zhang et al. (2006) found that transgenic mice lacking the c-fos gene selectively in D1 dopamine receptor-expressing neurons show delayed extinction of cocaine CPP. This impairment may be secondary to other gene expression changes seen in these mice, most notably a lack of increased expression of the NR1 NMDA receptor subunit and the GluR2 AMPA receptor subunit within the nucleus accumbens and caudate putamen in response to repeated cocaine administrations.
VI. Towards a Mechanistic View of Extinction of Conditioned Responses to Drug- and Withdrawal-Paired Cues
There is much work to be done before the mechanisms underlying extinction of conditioned drug craving and withdrawal will be understood, but progress is being made. Immediate, major goals of this work include developing a map of the underlying circuitries, characterizing the interactions among the structures that are involved, and identifying the relevant synaptic plasticity and molecular mechanisms. Given the evidence outlined above, it seems likely that extinction of drug- and withdrawal-paired cues involves similar cortico-limbic mechanisms as have been implicated in fear extinction, but more evidence is needed to solidify that view.
For example, the contribution of mPFC to extinction of conditioned craving or conditioned withdrawal remains unclear. There is little in the way of direct evidence for a role of mPFC, with the exception of the Hsu and Packard (2008) study mentioned above. In addition, the evidence for an involvement of mPFC in fear extinction is suggestive but not necessarily indicative of an involvement in extinction of drug- and withdrawal-paired cues. However, when considered in the context of emerging evidence that prefrontal regions contribute to conditioned fear and drug-seeking behavior in a parallel manner (for review see Peters et al., 2009), a stronger case can be made. Prelimbic (PL) and infralimbic (IL) cortices play complimentary roles with regard to conditioned fear, such that PL contributes to the expression of learned fear whereas IL is critical for its suppression (Corcoran and Quirk, 2007; Knapska and Maren, 2009; Milad and Quirk, 2002; Vidal-Gonzalez et al., 2006). There seems to be a similar regionally-specific contribution of mPFC to the expression and suppression of drug-seeking behavior: dmPFC (anterior cingulate and prelimbic cortices) mediates reinstatement of drug-seeking by drug cues, drug primes, and stress (Capriles et al., 2003; Di Pietro et al., 2006; McFarland et al., 2004; McFarland and Kalivas, 2001; Park et al., 2002), whereas IL is involved in inhibiting drug-seeking following drug primes or extinction of operant responding (McFarland et al., 2004; McLaughlin and See, 2003; Ovari and Leri, 2008; Peters et al., 2008a; Peters et al., 2008b). While no studies have looked at the role of IL specifically in extinction of conditioned responses to drug- and withdrawal-paired cues, it would fit well within this overall framework for IL to be a contributor. Future studies that explicitly examine regional specificity and target prefrontal subregions selectively are a priority.
The amygdala is a potential downsteam target of mPFC actions in extinction, considering that BLA is required for expression of conditioned craving and withdrawal responses and involved importantly in their extinction. Similar to the scenario that has been proposed to occur with fear extinction, mPFC might exert top-down inhibition of the amygdala following extinction of drug- and withdrawal-paired cues via projections to BLA interneurons, ICMs lying between BLA and CeA, or both, and thereby suppress conditioned drug craving and withdrawal. Future work could explore this possibility by (for example) inactivating IL with localized infusions of tetrodotoxin during extinction of drug-paired cues, and determining if this would block the reduction in cue-induced Fos expression observed in BLA following extinction training.
The amygdala is not the only candidate downstream target of mPFC, however. Drug-and withdrawal-paired cues activate a broad network of structures including extended amygdala and reward circuitries, and Fos studies indicate that many of these areas, like BLA, are activated less by extinguished than by nonextinguished drug-paired cues (Neisewander et al., 2000; Zavala et al., 2007). This coordinated response is suggestive of top-down inhibition occurring in a global fashion over a distributed network of plasticity. It could be that this inhibition is orchestrated by IL, whose projections are widespread (Takagishi and Chiba, 1991), and that IL interaction with different “nodes” in the network independently modulates different kinds of CRs to drug- and withdrawal-paired cues. It will be important in future work to determine the contribution of structures other than the amygdala and mPFC to drug cue extinction and to build a case for interactions among them, should they exist.
Finally, the nature of the synaptic plasticity underlying extinction of drug- and withdrawal-paired cues remains to be elucidated. Identifying the sites and types of extinction-related NMDA receptor-dependent synaptic plasticity, and determining which phases of extinction learning and memory it mediates within each site, will be an important step. Based on the evidence reviewed above, it appears that NMDA receptors within BLA are involved in the encoding and consolidation of drug cue extinction memory, but it is not clear which phase(s) of extinction learning are mediated by NMDA receptors in mPFC because of the limited data available.
VII. Clinical implications
As alluded to earlier, basic research on fear extinction is beginning to have an impact clinically. For example, neuroimaging studies have found consistently that PTSD is associated with amygdala hyperactivity and medial prefrontal cortex hypoactivity during exposure to trauma-related cues (Etkin and Wager, 2007), which, together with the animal data, suggests that dysfunction of inhibitory cortical control over the amygdala contributes to inappropriate triggering and diminished extinction of fear responses in PTSD patients (Milad et al., 2008; Shin et al., 2004; Shin et al., 2005). To restore functionality in cases of intractable and debilitating PTSD, it has been proposed that techniques such as deep brain stimulation could be used to correct the balance of amygdalar and prefrontal cortical activity (Koenigs and Grafman, 2009). Another clinical strategy for patients with less incapacitating symptoms is to couple standard cue exposure therapy with treatments that enhance NMDA receptor functionality. DCS, which is FDA-approved for the treatment of tuberculosis, has been used in several clinical studies showing that it can facilitate cue exposure therapy for phobias, social anxiety disorder, and obsessive-compulsive disorder in people in much the same way that it facilitates fear extinction in animal models (Hofman et al., 2006; Kushner et al., 2007; Ressler et al., 2004). While there have been some failures to observe DCS effects, particularly with subclinical populations (Guastella et al., 2006), the use of this drug represents a major advance in this area and is considered a prototypical example of bench-to-bedside translational research.
Similar clinical insights and advances may emerge from an advancing understanding of drug cue extinction. For example, addicts perform poorly on neuropsychological tasks that reflect prefrontal function — in some cases exhibiting deficits comparable to those seen in patients with prefrontal cortex lesions (Bechara et al., 2001) — and show reduced (Adams et al., 1993; Fishbein et al., 2005; Forman et al., 2004; Fu et al., 2008; Hester and Garavan, 2004; Kaufman et al., 2003; Kubler et al., 2005; Lee et al., 2005; Li et al., 2008) or altered (Ersche et al., 2005; Goldstein et al., 2001; Yucel et al., 2007) prefrontal responsivity while engaging in them. Moreover, addiction is associated with reduced prefrontal volume and gray matter density (Franklin et al., 2002; Jernigan et al., 1991; Kim et al., 2006; Liu et al., 1998). These findings might explain why drug cue exposure therapy has been relatively unsuccessful; perhaps more intensive therapy than has been tried to date will be required to compensate for limited prefrontal function in addicts. Alternatively, combining drug cue exposure therapy with “talk” therapies designed to maximize skills based on prefrontal functionality, such as behavioral inhibition and gratification delay, may be helpful.
Coupling drug cue exposure therapy with D-cycloserine (DCS) is another potentially useful approach. The demonstrated efficacy of DCS in facilitating both extinction of drug- and withdrawal-paired cues in animals and cue exposure therapy for fear and anxiety disorders in people argues strongly for investigating its utility as an adjunct to drug cue exposure therapy in addicts. It could be that DCS-induced facilitation of extinction in addicts will compensate for extinction deficits and improve the course of therapy. Consistent with this idea, it very recently was reported that DCS facilitates extinction of conditioned craving and skin conductance responses to smoking-related cues in nicotine-dependent smokers (Santa Ana et al., 2009). This important finding provides a strong rationale for further clinical research. At the present time there are several ongoing clinical studies involving pairing DCS with exposure to drug-paired cues (National Clinical Trial number NCT00827281, NCT00430573, NCT00926900, NCT00759473); their outcome may substantiate the utility of using pharmacological interventions to weaken conditioned responses to cues that trigger addictive behavior.
VIII. Concluding Remarks
As basic research on extinction of drug- and withdrawal-paired cues progresses, additional clinical insights will emerge. While far in the future, it is not difficult to envision a scenario in which pharmacotherapeutics or other tools will be developed to target specific brain regions or synaptic plasticity mechanisms in order to facilitate and maximize the generalizability of drug cue extinction. The exact direction that this will take remains to be seen. Regardless, it is important to remember that extinction likely is a process that is adaptive within a narrow range of function, and treatments designed to non-specifically boost or retard its pace may have unintended consequences on cognitive function and the quality of life.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (DA012736 to W.A.C. and DA027752 to K.M.M.). We thank Dr. Danielle Graham and Dr. John Muschamp for their careful reading of the manuscript and many helpful comments.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, Berent S, Kroll PD. Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res. 1993;17:205–210. doi: 10.1111/j.1530-0277.1993.tb00750.x. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Akirav I, Khatsrinov V, Vouimba RM, Merhav M, Ferreira G, Rosenblum K, Maroun M. Extinction of conditioned taste aversion depends on functional protein synthesis but not on NMDA receptor activation in the ventromedial prefrontal cortex. Learn Mem. 2006;13:254–258. doi: 10.1101/lm.191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer AJ, Marshall JF, McPherson RJ, Neisewander JL. Cocaine-seeking behavior and Fos expression in the amygdala produced by cocaine or a cocaine self-administration environment. Ann N Y Acad Sci. 1999;877:796–799. doi: 10.1111/j.1749-6632.1999.tb09325.x. [DOI] [PubMed] [Google Scholar]
- Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci. 1996;110:618–620. doi: 10.1037//0735-7044.110.3.618. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J Neurosci. 2003;23:5740–5749. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: The role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Berretta S, Pantazopoulos H, Caldera M, Pantazopoulos P, Pare D. Infralimbic cortex activation increases c-Fos expression in intercalated neurons of the amygdala. Neuroscience. 2005;132:943–953. doi: 10.1016/j.neuroscience.2005.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botreau F, Paolone G, Stewart J. D-cycloserine facilitates extinction of a cocaine-induced conditioned place preference. Behav Brain Res. 2006;172:173–178. doi: 10.1016/j.bbr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:455–466. [Google Scholar]
- Bouton ME, Swartzentruber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. J Exp Psychol Anim Behav Proc. 1986;12:333–350. [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Garcia-Gutierrez A, Zilski J, Moody EW. Extinction in multiple contexts does not necessarily make extinction less vulnerable to relapse. Behav Res Ther. 2006;44:983–994. doi: 10.1016/j.brat.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Braus DF, Wrase J, Grusser S, Hermann D, Ruf M, Flor H, Mann K, Heinz A. Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm. 2001;108:887–894. doi: 10.1007/s007020170038. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Lee GS, Bota RG, Ho ML, Saxena S, Baxter LR, Jr, Madsen D, Jarvik ME. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates spontaneous recovery. J Exp Psychol Anim Behav Proc. 1993;19:77–89. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psychol Anim Behav Proc. 1994;20:366–379. doi: 10.1037//0097-7403.19.1.77. [DOI] [PubMed] [Google Scholar]
- Brooks DC, Palmtier MI, Garcia EO, Johnson JL. An extinction cue reduces spontaneous recovery of a conditioned taste aversion. An Learn Behav. 1999;27:77–88. [Google Scholar]
- Brooks DC, Vaughn JM, Freeman AJ, Woods AM. An extinction cue reduces spontaneous recovery of ataxic ethanol tolerance in rats. Psychopharmacology (Berl) 2004;176:256–265. doi: 10.1007/s00213-004-1882-y. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fibiger HC. Cocaine-induced conditioned locomotion: absence of associated increases in dopamine release. Neuroscience. 1992;48:621–629. doi: 10.1016/0306-4522(92)90406-r. [DOI] [PubMed] [Google Scholar]
- Brown EE, Robertson GS, Fibiger HC. Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci. 1992;12:4112–4121. doi: 10.1523/JNEUROSCI.12-10-04112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J Neurosci. 2009;29:8474–8482. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cannich A, Wotjak CT, Kamprath K, Hermann H, Lutz B, Marsicano G. CB1 cannabinoid receptors modulate kinase and phosphatase activity during extinction of conditioned fear in mice. Learn Mem. 2004;11:625–632. doi: 10.1101/lm.77904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Carelli RM. Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse. 2000;35:238–242. doi: 10.1002/(SICI)1098-2396(20000301)35:3<238::AID-SYN10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Deadwyler SA. Dual factors controlling activity of nucleus accumbens cell-firing during cocaine self-administration. Synapse. 1996;24:308–311. doi: 10.1002/(SICI)1098-2396(199611)24:3<308::AID-SYN14>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Williams JG, Hollander JA. Basolateral amygdala neurons encode cocaine self-administration and cocaine-associated cues. J Neurosci. 2003;23:8204–8211. doi: 10.1523/JNEUROSCI.23-23-08204.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, Duman RS, Neve RL, Nestler EJ. Regulation of cocaine reward by CREB. Science. 1998;282:2272–2275. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Sahuque LL, Janak PH. Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry. 2008;64:203–210. doi: 10.1016/j.biopsych.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelonis JJ, Calton JL, Hart JA, Schachtman TR. Attenuation of the renewal effect by extinction in multiple contexts. Learning & Motivation. 1999;30:1–14. [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005a;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Myers KM, Ressler KJ, Davis M. Regulation of gephyrin and GABAA receptor binding within the amygdala after fear acquisition and extinction. J Neurosci. 2005b;25:502–506. doi: 10.1523/JNEUROSCI.3301-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, O’Brien CP. Abstinent opiate abusers exhibit conditioned craving, conditioned withdrawal and reductions in both through extinction. Br J Addict. 1986;81:655–660. doi: 10.1111/j.1360-0443.1986.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie MJ, James LB, Beart PM. An excitant amino acid projection from the medial prefrontal cortex to the anterior part of nucleus accumbens in the rat. J Neurochem. 1985;45:477–482. doi: 10.1111/j.1471-4159.1985.tb04013.x. [DOI] [PubMed] [Google Scholar]
- Christie MJ, Summers RJ, Stephenson JA, Cook CJ, Beart PM. Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience. 1987;22:425–439. doi: 10.1016/0306-4522(87)90345-9. [DOI] [PubMed] [Google Scholar]
- Collins BN, Brandon TH. Effects of extinction context and retrieval cues on alcohol cue reactivity among nonalcoholic drinkers. J Consult Clin Psychol. 2002;70:390–397. [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–19. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–167. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corty E, McFall RM. Response prevention in the treatment of cigarette smoking. Addict Behav. 1984;9:405–408. doi: 10.1016/0306-4603(84)90042-x. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Dickson M, Dinenna M, Johnson AW, Perin MS, Holland PC, Baraban JM, Reti IM. Narp deletion blocks extinction of morphine place preference conditioning. Neuropsychopharmacology. 2009;34:857–866. doi: 10.1038/npp.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology (Berl) 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Dalton GL, Wang YT, Floresco SB, Phillips AG. Disruption of AMPA receptor endocytosis impairs the extinction, but not acquisition of learned fear. Neuropsychopharmacology. 2008;33:2416–2426. doi: 10.1038/sj.npp.1301642. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C-J. The amygdala. Curr Biol. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and Gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biological Psychiatry. 2002;52:998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J, Gray JA. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88:1233–1245. doi: 10.1111/j.1360-0443.1993.tb02146.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci. 2004;24:7167–7173. doi: 10.1523/JNEUROSCI.1581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Blaha CD, Phillips AG. Conditioned changes in dopamine oxidation currents in the nucleus accumbens of rats by stimuli paired with self-administration or yoked-administration of d-amphetamine. Eur J Neurosci. 1998;10:1121–1127. doi: 10.1046/j.1460-9568.1998.00125.x. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology. 2008;33:1413–1425. doi: 10.1038/sj.npp.1301522. [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Benham-Hermetz J, Fogg AP, Osborne GE. Role of the prelimbic cortex in the acquisition, re-acquisition or persistence of responding for a drug-paired conditioned reinforcer. Neuroscience. 2007;150:291–298. doi: 10.1016/j.neuroscience.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur J Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, de Vries W, Raaso H, Schoffelmeer AN, De Vries TJ. Contextual renewal of nicotine seeking in rats and its suppression by the cannabinoid-1 receptor antagonist Rimonabant (SR141716A) Neuropharmacology. 2008;55:712–716. doi: 10.1016/j.neuropharm.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Drummond DC, Glautier S. A controlled trial of cue exposure treatment in alcohol dependence. J Consult Clin Psychol. 1994;62:809–817. doi: 10.1037//0022-006x.62.4.809. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. NMDA receptor blockade in the basolateral amygdala disrupts consolidation of stimulus-reward memory and extinction learning during reinstatement of cocaine-seeking in an animal model of relapse. Neurobiol Learn Mem. 2007;88:435–444. doi: 10.1016/j.nlm.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, Kurian V, Kimes AS, Breeden A, Grant S. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Brain Res Cogn Brain Res. 2005;23:119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Foltin RW, Haney M. Conditioned effects of environmental stimuli paired with smoked cocaine in humans. Psychopharmacology (Berl) 2000;149:24–33. doi: 10.1007/s002139900340. [DOI] [PubMed] [Google Scholar]
- Fontana DJ, Post RM, Pert A. Conditioned increases in mesolimbic dopamine overflow by stimuli associated with cocaine. Brain Res. 1993;629:31–39. doi: 10.1016/0006-8993(93)90477-5. [DOI] [PubMed] [Google Scholar]
- Forman SD, Dougherty GG, Casey BJ, Siegle GJ, Braver TS, Barch DM, Stenger VA, Wick-Hull C, Pisarov LA, Lorensen E. Opiate addicts lack error-dependent activation of rostral anterior cingulate. Biol Psychiatry. 2004;55:531–537. doi: 10.1016/j.biopsych.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Druhan JP. Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci. 2000;12:2097–2106. doi: 10.1046/j.1460-9568.2000.00071.x. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Frenois F, Stinus L, Di Blasi F, Cador M, Le Moine C. A specific limbic circuit underlies opiate withdrawal memories. J Neurosci. 2005;25:1366–1374. doi: 10.1523/JNEUROSCI.3090-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu LP, Bi GH, Zou ZT, Wang Y, Ye EM, Ma L, Ming F, Yang Z. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett. 2008;438:322–326. doi: 10.1016/j.neulet.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology. 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Feltenstein MW, See RE. The role of the basolateral amygdala in stimulus-reward memory and extinction memory consolidation and in subsequent conditioned cued reinstatement of cocaine seeking. Eur J Neurosci. 2006;23:2809–2813. doi: 10.1111/j.1460-9568.2006.04806.x. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Weber SM, Rice HJ, Neisewander JL. Effects of excitotoxic lesions of the basolateral amygdala on cocaine-seeking behavior and cocaine conditioned place preference in rats. Brain Res. 2002;929:15–25. doi: 10.1016/s0006-8993(01)03366-2. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Parker MC, See RE. Differential involvement of the core and shell subregions of the nucleus accumbens in conditioned cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2004;176:459–465. doi: 10.1007/s00213-004-1895-6. [DOI] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Positive allosteric modulation of mGluR5 receptors facilitates extinction of a cocaine contextual memory. Biol Psychiatry. 2009;65:717–720. doi: 10.1016/j.biopsych.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimov MR, Schiffer WK, Gardner EL, Marsteller DA, Lennon IC, Taylor SJ, Brodie JD, Ashby CR, Jr, Dewey SL. GABAergic blockade of cocaine-associated cue-induced increases in nucleus accumbens dopamine. Eur J Pharmacol. 2001;414:205–209. doi: 10.1016/s0014-2999(01)00800-7. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Woods JH, Schuster CR. Morphine: conditioned increases in self-administration in rhesus monkeys. Science. 1969;166:1306–1307. doi: 10.1126/science.166.3910.1306. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND, Wang GJ, Fowler JS, Rajaram S. Addiction changes orbitofrontal gyrus function: involvement in response inhibition. Neuroreport. 2001;12:2595–2599. doi: 10.1097/00001756-200108080-00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Koob GF. Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology. 2001;24:152–160. doi: 10.1016/S0893-133X(00)00186-X. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Roles of the nucleus accumbens and amygdala in the acquisition and expression of ethanol-conditioned behavior in mice. J Neurosci. 2008;28:1076–1084. doi: 10.1523/JNEUROSCI.4520-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremel CM, Cunningham CL. Involvement of amygdala dopamine and nucleus accumbens NMDA receptors in ethanol-seeking behavior in mice. Neuropsychopharmacology. 2009;34:1443–1453. doi: 10.1038/npp.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, See RE. Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology. 2000;22:473–479. doi: 10.1016/S0893-133X(99)00157-8. [DOI] [PubMed] [Google Scholar]
- Groblewski PA, Lattal KM, Cunningham CL. Effects of d-Cycloserine on Extinction and Reconditioning of Ethanol-Seeking Behavior in Mice. Alcohol Clin Exp Res. 2009;33:772–782. doi: 10.1111/j.1530-0277.2009.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grusser SM, Wrase J, Klein S, Hermann D, Smolka MN, Ruf M, Weber-Fahr W, Flor H, Mann K, Braus DF, Heinz A. Cue-induced activation of the striatum and medial prefrontal cortex is associated with subsequent relapse in abstinent alcoholics. Psychopharmacology (Berl) 2004;175:296–302. doi: 10.1007/s00213-004-1828-4. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, Mitchell P, Richardson R. A randomized controlled trial of the effect of d-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2006;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Gunther LM, Denniston JC, Miller RR. Conducting exposure treatment in multiple contexts can prevent relapse. Behav Res Ther. 1998;36:75–91. doi: 10.1016/s0005-7967(97)10019-5. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–115. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Havermans RC, Jansen AT. Increasing the efficacy of cue exposure treatment in preventing relapse of addictive behavior. Addict Behav. 2003;28:989–994. doi: 10.1016/s0306-4603(01)00289-1. [DOI] [PubMed] [Google Scholar]
- Heather N, Stallard A, Tebbutt J. Importance of substance cues in relapse among heroin users: comparison of two methods of investigation. Addict Behav. 1991;16:41–49. doi: 10.1016/0306-4603(91)90038-j. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, Menzaghi FF, Schulteis GG, Koob GF, Stinus LL. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behavioral Pharmacology. 1995;6:74–80. [PubMed] [Google Scholar]
- Heldt SA, Ressler KJ. Training-induced changes in the expression of GABAA-associated genes in the amygdala after the acquisition and extinction of Pavlovian fear. Eur J Neurosci. 2007;26:3631–3644. doi: 10.1111/j.1460-9568.2007.05970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci. 2006a;26:12694–12699. doi: 10.1523/JNEUROSCI.3101-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Dickinson A, Everitt BJ. Motivational control of heroin seeking by conditioned stimuli associated with withdrawal and heroin taking by rats. Behav Neurosci. 2006b;120:103–114. doi: 10.1037/0735-7044.120.1.103. [DOI] [PubMed] [Google Scholar]
- Herman CP. External and internal cues as determinants of the smoking behavior of light and heavy smokers. J Pers Soc Psychol. 1974;30:664–672. doi: 10.1037/h0037440. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci. 2002;22:577–583. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Mons N. Resistance to extinction is associated with impaired immediate early gene induction in medial prefrontal cortex and amygdala. Eur J Neurosci. 2004;20:781–790. doi: 10.1111/j.1460-9568.2004.03542.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikind M, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol Learn Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The reserpine-sensitive dopamine pool mediates (+)amphetamine-conditioned reward in the place preference paradigm. Brain Res. 1990;510:33–42. doi: 10.1016/0006-8993(90)90724-p. [DOI] [PubMed] [Google Scholar]
- Hiroi N, White NM. The lateral nucleus of the amygdala mediates expression of the amphetamine-produced conditioned place preference. J Neurosci. 1991a;11:2107–2116. doi: 10.1523/JNEUROSCI.11-07-02107.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiroi N, White NM. The amphetamine conditioned place preference: differential involvement of dopamine receptor subtypes and two dopaminergic terminal areas. Brain Res. 1991b;552:141–152. doi: 10.1016/0006-8993(91)90672-i. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Goosens KA, Maren S. Context-dependent neuronal activity in the lateral amygdala represents fear memories after extinction. J Neurosci. 2003;23:8410–8416. doi: 10.1523/JNEUROSCI.23-23-08410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, Shiekh M, Otto MW. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Honsberger MJ, Leri F. Fos expression in mesocorticolimbic areas during heroin place conditioning. Neuroreport. 2008;19:63–67. doi: 10.1097/WNR.0b013e3282f31d82. [DOI] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Hsu E, Packard MG. Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem. 2008;89:504–512. doi: 10.1016/j.nlm.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Ternes JW. An electrodermal measure of arousal in opiate addicts to drug-related stimuli. Biol Psychol. 1981;12:291–298. doi: 10.1016/0301-0511(81)90002-8. [DOI] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn Mem. 2004;11:540–543. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Hugues S, Chessel A, Lena I, Marsault R, Garcia R. Prefrontal induction of PD098059 immediately after fear extinction training blocks extinction-associated prefrontal synaptic plasticity and decreases prefrontal ERK2 phosphorylation. Synapse. 2006;60:280–287. doi: 10.1002/syn.20291. [DOI] [PubMed] [Google Scholar]
- Hutcheson DM, Everitt BJ, Robbins TW, Dickinson A. The role of withdrawal in heroin addiction: enhances reward or promotes avoidance? Nat Neurosci. 2001;4:943–947. doi: 10.1038/nn0901-943. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Everitt BJ. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat Neurosci. 2004;7:389–397. doi: 10.1038/nn1217. [DOI] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Howes SR, Robbins TW, Everitt BJ. Dissociation in conditioned dopamine release in the nucleus accumbens core and shell in response to cocaine cues and during cocaine-seeking behavior in rats. J Neurosci. 2000;20:7489–7495. doi: 10.1523/JNEUROSCI.20-19-07489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, Schafer K, Smith T, Irwin M, Grant I, Schuckit M, Cermak LS. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaddis FG, Uretsky NJ, Wallace LJ. DNQX in the nucleus accumbens inhibits cocaine-induced conditioned place preference. Brain Res. 1995;697:76–82. doi: 10.1016/0006-8993(95)00786-p. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareken DA, Claus ED, Sabri M, Dzemidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Clin Exp Res. 2004;28:550–557. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- Kasvikis Y, Bradley B, Powell J, Marks I, Gray JA. Postwithdrawal exposure treatment to prevent relapse in opiate addicts: a pilot study. Int J Addict. 1991;26:1187–1195. doi: 10.3109/10826089109062154. [DOI] [PubMed] [Google Scholar]
- Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23:7839–7843. doi: 10.1523/JNEUROSCI.23-21-07839.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh DJ, Sitharthan G, Young RM, Sitharthan T, Saunders JB, Shockley N, Giannopoulos V. Addition of cue exposure to cognitive-behaviour therapy for alcohol misuse: a randomized trial with dysphoric drinkers. Addiction. 2006;101:1106–1116. doi: 10.1111/j.1360-0443.2006.01488.x. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci. 2006;26:5894–5900. doi: 10.1523/JNEUROSCI.0740-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Gross RE, Ely TD, Drexler KP. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, Shin K, Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007a;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci U S A. 2007b;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Lyoo IK, Hwang J, Chung A, Hoon Sung Y, Kim J, Kwon DH, Chang KH, Renshaw PF. Prefrontal grey-matter changes in short-term and long-term abstinent methamphetamine abusers. Int J Neuropsychopharmacol. 2006;9:221–228. doi: 10.1017/S1461145705005699. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn Mem. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic Stress Disorder: The Role of Medial Prefrontal Cortex and Amygdala. Neuroscientist. 2009 doi: 10.1177/1073858409333072. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer AN, De Vries TJ, Smit AB. Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem. 2006;98:905–915. doi: 10.1111/j.1471-4159.2006.03917.x. [DOI] [PubMed] [Google Scholar]
- Kruzich PJ, See RE. Differential contributions of the basolateral and central amygdala in the acquisition and expression of conditioned relapse to cocaine-seeking behavior. J Neurosci. 2001;21:RC155. doi: 10.1523/JNEUROSCI.21-14-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubler A, Murphy K, Garavan H. Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci. 2005;21:1984–1992. doi: 10.1111/j.1460-9568.2005.04027.x. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: a Mixed-Trial fMRI Study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/lm.1080108. [DOI] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn Mem. 2008;15:304–314. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford CC, Fuchs RA, See RE. Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology. 2003;28:1721–1729. doi: 10.1038/sj.npp.1300249. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci. 2003;117:341–349. doi: 10.1037/0735-7044.117.2.341. [DOI] [PubMed] [Google Scholar]
- Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Lim Y, Graham SJ, Kim G, Wiederhold BK, Wiederhold MD, Kim IY, Kim SI. Nicotine craving and cue exposure therapy by using virtual environments. Cyberpsychol Behav. 2004;7:705–713. doi: 10.1089/cpb.2004.7.705. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kwon H, Choi J, Yang BH. Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol Behav. 2007;10:617–623. doi: 10.1089/cpb.2007.9978. [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Zhou WH, Luo XJ, Yuen KS, Ruan XZ, Weng XC. Neural activity associated with cognitive regulation in heroin users: A fMRI study. Neurosci Lett. 2005;382:211–216. doi: 10.1016/j.neulet.2005.03.053. [DOI] [PubMed] [Google Scholar]
- Li CS, Huang C, Yan P, Bhagwagar Z, Milivojevic V, Sinha R. Neural correlates of impulse control during stop signal inhibition in cocaine-dependent men. Neuropsychopharmacology. 2008;33:1798–1806. doi: 10.1038/sj.npp.1301568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003a;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lu HY, Gean PW. The similarities and diversities of signal pathways leading to consolidation of conditioning and consolidation of extinction of fear memory. J Neurosci. 2003b;23:8310–8317. doi: 10.1523/JNEUROSCI.23-23-08310.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003c;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SK, Pan WH, Yeh PH. Prefrontal dopamine efflux during exposure to drug-associated contextual cues in rats with prior repeated methamphetamine. Brain Res Bull. 2007;71:365–371. doi: 10.1016/j.brainresbull.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Liu X, Matochik JA, Cadet JL, London ED. Smaller volume of prefrontal lobe in polysubstance abusers: a magnetic resonance imaging study. Neuropsychopharmacology. 1998;18:243–252. doi: 10.1016/S0893-133X(97)00143-7. [DOI] [PubMed] [Google Scholar]
- Llamas A, Avendano C, Reinoso-Suarez F. Amygdaloid projections to prefrontal and motor cortex. Science. 1977;195:794–796. doi: 10.1126/science.836591. [DOI] [PubMed] [Google Scholar]
- Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol. 2006;45:515–529. doi: 10.1348/014466505X82586. [DOI] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Frenois F, Vouillac C, Stinus L, Cador M, Le Moine C. Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: differential expression of c-fos and arc immediate early genes. Neuroscience. 2008;154:1021–1033. doi: 10.1016/j.neuroscience.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Lisman SA. Effects of a context shift and multiple context extinction on reactivity to alcohol cues. Exp Clin Psychopharmacol. 2008;16:322–331. doi: 10.1037/a0012686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Hsiao YH, Gean PW. Extinction training in conjunction with a partial agonist of the glycine site on the NMDA receptor erases memory trace. J Neurosci. 2006;26:8892–8899. doi: 10.1523/JNEUROSCI.0365-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao SC, Lin HC, Gean PW. Augmentation of fear extinction by D-cycloserine is blocked by proteasome inhibitors. Neuropsychopharmacology. 2008;33:3085–3095. doi: 10.1038/npp.2008.30. [DOI] [PubMed] [Google Scholar]
- Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM. Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom. 2007;76:97–105. doi: 10.1159/000097968. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McBride D, Barrett SP, Kelly JT, Aw A, Dagher A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- McClernon FJ, Hiott FB, Liu J, Salley AN, Behm FM, Rose JE. Selectively reduced responses to smoking cues in amygdala following extinction-based smoking cessation: results of a preliminary functional magnetic resonance imaging study. Addict Biol. 2007;12:503–512. doi: 10.1111/j.1369-1600.2007.00075.x. [DOI] [PubMed] [Google Scholar]
- McCusker CG, Brown K. Cue-exposure to alcohol-associated stimuli reduces autonomic reactivity, but not craving and anxiety, in dependent drinkers. Alcohol Alcohol. 1995;30:319–327. [PubMed] [Google Scholar]
- McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- McNally GP, Westbrook RF. Opioid receptors regulate the extinction of Pavlovian fear conditioning. Behav Neurosci. 2003;117:1292–1301. doi: 10.1037/0735-7044.117.6.1292. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G. Blocking, unblocking, and overexpectation of fear: a role for opioid receptors in the regulation of Pavlovian association formation. Behav Neurosci. 2004;118:111–120. doi: 10.1037/0735-7044.118.1.111. [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered prelimbic cortex output during cue-elicited drug seeking. J Neurosci. 2004;24:6889–6897. doi: 10.1523/JNEUROSCI.1685-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005a;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci. 2005b;21:1385–1393. doi: 10.1111/j.1460-9568.2005.03974.x. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Millin PM, Riccio DC. Spontaneous recovery of tolerance to the analgesic effect of morphine. Physiol Behav. 2002;75:465–471. doi: 10.1016/s0031-9384(02)00651-0. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Rubonis AV, Niaura RS, Sirota AD, Colby SM, Goddard P, Abrams DB. Cue exposure with coping skills treatment for male alcoholics: a preliminary investigation. J Consult Clin Psychol. 1993;61:1011–1019. doi: 10.1037//0022-006x.61.6.1011. [DOI] [PubMed] [Google Scholar]
- Monti PM, Rohsenow DJ, Swift RM, Gulliver SB, Colby SM, Mueller TI, Brown RA, Gordon A, Abrams DB, Niaura RS, Asher MK. Naltrexone and cue exposure with coping and communication skills training for alcoholics: treatment process and 1-year outcomes. Alcohol Clin Exp Res. 2001;25:1634–1647. [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JF, Mascagni F, McDonald AJ. Synaptic connections of distinct interneuronal subpopulations in the rat basolateral amygdalar nucleus. J Comp Neurol. 2003;456:217–236. doi: 10.1002/cne.10435. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA., Jr D-cycloserine facilitates extinction of naloxone-induced conditioned place aversion in morphine-dependent rats. Biol Psychiatry. 2010;67:85–87. doi: 10.1016/j.biopsych.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, Shadel WG, Rohsenow DJ, Monti PM, Sirota AD. Cue exposure treatment for smoking relapse prevention: a controlled clinical trial. Addiction. 1999;94:685–695. doi: 10.1046/j.1360-0443.1999.9456856.x. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan T, Ehrman R. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav. 1990;15:355–365. doi: 10.1016/0306-4603(90)90045-y. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Testa T, O’Brien TJ, Brady JP, Wells B. Conditioned narcotic withdrawal in humans. Science. 1977;195:1000–1002. doi: 10.1126/science.841320. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, McLellan AT, Ternes J, Ehrman RN. Use of naltrexone to extinguish opioid-conditioned responses. J Clin Psychiatry. 1984;45:53–56. [PubMed] [Google Scholar]
- Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci Lett. 2008;444:52–55. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Paolone G, Botreau F, Stewart J. The facilitative effects of D-cycloserine on extinction of a cocaine-induced conditioned place preference can be long lasting and resistant to reinstatement. Psychopharmacology (Berl) 2009;202:403–409. doi: 10.1007/s00213-008-1280-y. [DOI] [PubMed] [Google Scholar]
- Park WK, Bari AA, Jey AR, Anderson SM, Spealman RD, Rowlett JK, Pierce RC. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker LA, Limebeer CL, Slomke J. Renewal effect: context-dependent extinction of a cocaine- and a morphine-induced conditioned floor preference. Psychopharmacology (Berl) 2006;187:133–137. doi: 10.1007/s00213-006-0422-3. [DOI] [PubMed] [Google Scholar]
- Parker LA, Burton P, Sorge RE, Yakiwchuk C, Mechoulam R. Effect of low doses of delta9-tetrahydrocannabinol and cannabidiol on the extinction of cocaine-induced and amphetamine-induced conditioned place preference learning in rats. Psychopharmacology (Berl) 2004;175:360–366. doi: 10.1007/s00213-004-1825-7. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford, U.K: Oxford University Press; 1927. [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J Neurosci. 2008a;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology (Berl) 2008b;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- Pfeiffer UJ, Fendt M. Prefrontal dopamine D4 receptors are involved in encoding fear extinction. Neuroreport. 2006;17:847–850. doi: 10.1097/01.wnr.0000220142.29413.6f. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fertig J, Baker L, Cooney N. Reactivity to alcohol cues in alcoholics and non-alcoholics: implications for a stimulus control analysis of drinking. Addict Behav. 1983;8:1–10. doi: 10.1016/0306-4603(83)90048-5. [DOI] [PubMed] [Google Scholar]
- Powell J. Conditioned responses to drug-related stimuli: is context crucial? Addiction. 1995;90:1089–1095. doi: 10.1046/j.1360-0443.1995.90810897.x. [DOI] [PubMed] [Google Scholar]
- Powell J, Gray J, Bradley B. Subjective craving for opiates: evaluation of a cue exposure protocol for use with detoxified opiate addicts. Br J Clin Psychol. 1993;32 ( Pt 1):39–53. doi: 10.1111/j.2044-8260.1993.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Inhibitory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:999–1009. doi: 10.1152/jn.1991.66.3.999. [DOI] [PubMed] [Google Scholar]
- Raw M, Russell MA. Rapid smoking, cue exposure and support in the modification of smoking. Behav Res Ther. 1980;18:363–372. doi: 10.1016/0005-7967(80)90001-7. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Extinction of facilitation. J Exp Psychol Anim Behav Proc. 1986;12:16–24. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Reti IM, Crombag HS, Takamiya K, Sutton JM, Guo N, Dinenna ML, Huganir RL, Holland PC, Baraban JM. Narp regulates long-term aversive effects of morphine withdrawal. Behav Neurosci. 2008;122:760–768. doi: 10.1037/a0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O’Brien CP. Relationships among physiological and self-report responses produced by cocaine-related cues. Addict Behav. 1997;22:157–167. doi: 10.1016/s0306-4603(96)00007-x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roche M, O’Connor E, Diskin C, Finn DP. The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci. 2007;26:2643–2653. doi: 10.1111/j.1460-9568.2007.05861.x. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. 0896–6273. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking behavior in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohsenow DJ, Monti PM, Rubonis AV, Gulliver SB, Colby SM, Binkoff JA, Abrams DB. Cue exposure with coping skills training and communication skills training for alcohol dependence: 6- and 12-month outcomes. Addiction. 2001;96:1161–1174. doi: 10.1046/j.1360-0443.2001.96811619.x. [DOI] [PubMed] [Google Scholar]
- Room P, Russchen FT, Groenewegen HJ, Lohman AH. Efferent connections of the prelimbic (area 32) and the infralimbic (area 25) cortices: an anterograde tracing study in the cat. J Comp Neurol. 1985;242:40–55. doi: 10.1002/cne.902420104. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Ann N Y Acad Sci. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Pare D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Graap K, Rothbaum BO. A preliminary report on the use of virtual reality technology to elicit craving and cue reactivity in cocaine dependent individuals. Addict Behav. 2006;31:1881–1894. doi: 10.1016/j.addbeh.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Santa Ana EJ, Rounsaville BJ, Frankforter TL, Nich C, Babuscio T, Poling J, Gonsai K, Hill KP, Carroll KM. D-cycloserine attenuates reactivity to smoking cues in nicotine dependent smokers: a pilot investigation. Drug Alcohol Depend. 2009;104:220–227. doi: 10.1016/j.drugalcdep.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA- independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt ED, Voorn P, Binnekade R, Schoffelmeer AN, De Vries TJ. Differential involvement of the prelimbic cortex and striatum in conditioned heroin and sucrose seeking following long-term extinction. Eur J Neurosci. 2005;22:2347–2356. doi: 10.1111/j.1460-9568.2005.04435.x. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Kelley AE. Conditioned Fos expression following morphine-paired contextual cue exposure is environment specific. Behav Neurosci. 2002;116:727–732. doi: 10.1037//0735-7044.116.4.727. [DOI] [PubMed] [Google Scholar]
- Schroeder BE, Holahan MR, Landry CF, Kelley AE. Morphine-associated environmental cues elicit conditioned gene expression. Synapse. 2000;37:146–158. doi: 10.1002/1098-2396(200008)37:2<146::AID-SYN8>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Systemic or intra-amygdala injections of glucose facilitate memory consolidation for extinction of drug-induced conditioned reward. Eur J Neurosci. 2003;17:1482–1488. doi: 10.1046/j.1460-9568.2003.02578.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Packard MG. Facilitation of memory for extinction of drug-induced conditioned reward: role of amygdala and acetylcholine. Learn Mem. 2004;11:641–647. doi: 10.1101/lm.78504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Stinus L, Risbrough VB, Koob GF. Clonidine blocks acquisition but not expression of conditioned opiate withdrawal in rats. Neuropsychopharmacology. 1998;19:406–416. doi: 10.1016/S0893-133X(98)00036-0. [DOI] [PubMed] [Google Scholar]
- See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris J, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ. Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend. 2000;60:207–216. doi: 10.1016/s0376-8716(99)00158-1. [DOI] [PubMed] [Google Scholar]
- Sheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology. 2001;41:151–158. doi: 10.1016/s0028-3908(01)00073-9. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sideroff SI, Jarvik ME. Conditioned responses to a videotape showing heroin-related stimuli. Int J Addict. 1980;15:529–536. doi: 10.3109/10826088009040035. [DOI] [PubMed] [Google Scholar]
- Siegel S. Evidence from rats that morphine tolerance is a learned response. J Comp Physiol Psychol. 1975;89:498–506. doi: 10.1037/h0077058. [DOI] [PubMed] [Google Scholar]
- Siegel S. Morphine analgesic tolerance: its situation specificity supports a Pavlovian conditioning model. Science. 1976;193:323–325. doi: 10.1126/science.935870. [DOI] [PubMed] [Google Scholar]
- Siegel S, Ramos BM. Applying laboratory research: drug anticipation and the treatment of drug addiction. Exp Clin Psychopharmacol. 2002;10:162–183. doi: 10.1037//1064-1297.10.3.162. [DOI] [PubMed] [Google Scholar]
- Siegel S, Hinson RE, Krank MD, McCully J. Heroin “overdose” death: contribution of drug-associated environmental cues. Science. 1982;216:436–437. doi: 10.1126/science.7200260. [DOI] [PubMed] [Google Scholar]
- Siegel S, Baptista MA, Kim JA, McDonald RV, Weise-Kelly L. Pavlovian psychopharmacology: the associative basis of tolerance. Exp Clin Psychopharmacol. 2000;8:276–293. doi: 10.1037//1064-1297.8.3.276. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Sitharthan T, Sitharthan G, Hough MJ, Kavanagh DJ. Cue exposure in moderation drinking: a comparison with cognitive-behavior therapy. J Consult Clin Psychol. 1997;65:878–882. doi: 10.1037//0022-006x.65.5.878. [DOI] [PubMed] [Google Scholar]
- Smolka MN, Buhler M, Klein S, Zimmermann U, Mann K, Heinz A, Braus DF. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl) 2006;184:577–588. doi: 10.1007/s00213-005-0080-x. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, Ledoux JE. Dissociable Roles for the Ventromedial Prefrontal Cortex and Amygdala in Fear Extinction: NR2B Contribution. Cereb Cortex. 2009;19:474–482. doi: 10.1093/cercor/bhn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiewicz PR, Brandon TH, Bradizza CM. Effects of extinction context and retrieval cues on renewal of alcohol-cue reactivity among alcohol-dependent outpatients. Psychol Addict Behav. 2007;21:244–248. doi: 10.1037/0893-164X.21.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinus L, Caille S, Koob GF. Opiate withdrawal-induced place aversion lasts for up to 16 weeks. Psychopharmacology (Berl) 2000;149:115–120. doi: 10.1007/s002139900358. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Thewissen R, van den Hout M, Havermans RC, Jansen A. Context-dependency of cue-elicited urge to smoke. Addiction. 2005;100:387–396. doi: 10.1111/j.1360-0443.2005.00996.x. [DOI] [PubMed] [Google Scholar]
- Thewissen R, Snijders SJ, Havermans RC, van den Hout M, Jansen A. Renewal of cue-elicited urge to smoke: implications for cue exposure treatment. Behav Res Ther. 2006;44:1441–1449. doi: 10.1016/j.brat.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Loh HH, Wei ET. Effects of clonidine on morphine withdrawal signs in the rat. Eur J Pharmacol. 1975;30:93–99. doi: 10.1016/0014-2999(75)90208-3. [DOI] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer ANM, Mansvelder HD, Smit AB, Spijker S, De Vries TJ. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin seeking. Nat Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Kiefer F, Spanagel R. D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol. 2008;43:626–629. doi: 10.1093/alcalc/agn067. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology (Berl) 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Twining RC, Jones JL, Slater JM, Grigson PS, Carelli RM. Behavioral and electrophysiological indices of negative affect predict cocaine self-administration. Neuron. 2008;57:774–785. doi: 10.1016/j.neuron.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- Wikler A. Recent progress in research on the neurophysiologic basis of morphine addiction. Am J Psychiatry. 1948;105:329–338. doi: 10.1176/ajp.105.5.329. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine Tob Res. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AM, Bouton ME. D-Cycloserine Facilitates Extinction but Does Not Eliminate Renewal of the Conditioned Emotional Response. Behav Neurosci. 2006;120:1159–1162. doi: 10.1037/0735-7044.120.5.1159. [DOI] [PubMed] [Google Scholar]
- Yang YL, Lu KT. Facilitation of conditioned fear extinction by d-cycloserine is mediated by mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades and requires de novo protein synthesis in basolateral nucleus of amygdala. Neuroscience. 2005;134:247–260. doi: 10.1016/j.neuroscience.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharmacology. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- Yang Z, Xie J, Shao YC, Xie CM, Fu LP, Li DJ, Fan M, Ma L, Li SJ. Dynamic neural responses to cue-reactivity paradigms in heroin-dependent users: an fMRI study. Hum Brain Mapp. 2009;30:766–775. doi: 10.1002/hbm.20542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Lubman DI. Neurocognitive and neuroimaging evidence of behavioural dysregulation in human drug addiction: implications for diagnosis, treatment and prevention. Drug Alcohol Rev. 2007;26:33–39. doi: 10.1080/09595230601036978. [DOI] [PubMed] [Google Scholar]
- Yucel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Wood SJ, Forman SD, Pantelis C. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12:611, 691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Biswas S, Harlan RE, Neisewander JL. Fos and glutamate AMPA receptor subunit coexpression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience. 2007;145:438–452. doi: 10.1016/j.neuroscience.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Osredkar T, Joyce JN, Neisewander JL. Upregulation of Arc mRNA expression in the prefrontal cortex following cue-induced reinstatement of extinguished cocaine-seeking behavior. Synapse. 2008;62:421–431. doi: 10.1002/syn.20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala AR, Weber SM, Rice HJ, Alleweireldt AT, Neisewander JL. Role of the prelimbic subregion of the medial prefrontal cortex in acquisition, extinction, and reinstatement of cocaine-conditioned place preference. Brain Res. 2003;990:157–164. doi: 10.1016/s0006-8993(03)03452-8. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Jiao H, Zhang Q, Zhang D, Lou D, Katz JL, Xu M. c-Fos facilitates the acquisition and extinction of cocaine-induced persistent changes. J Neurosci. 2006;26:13287–13296. doi: 10.1523/JNEUROSCI.3795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, Kalivas PW. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144:1209–1218. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zushida K, Sakurai M, Wada K, Sekiguchi M. Facilitation of extinction learning for contextual fear memory by PEPA: a potentiator of AMPA receptors. J Neurosci. 2007;27:158–166. doi: 10.1523/JNEUROSCI.3842-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]