Abstract
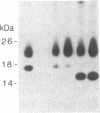
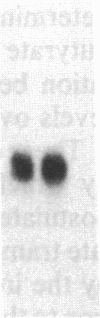
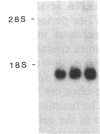
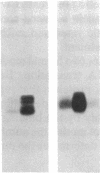
In this paper, we examine the response of a translational regulatory mechanism when changes in mRNA levels are induced. The gene that encodes the human ferritin heavy chain has been transfected into mouse fibroblasts. Stable transformants that express the human ferritin heavy chain have been isolated. This protein assembles into ferritin polymers and can co-assemble with host mouse ferritin. Biosynthetic rates of the expressed human ferritin varied over a wide range in response to perturbations in iron supply, but total and cytoplasmic messenger RNA levels remained unchanged. When changes in ferritin mRNA levels were induced by treatment with sodium butyrate, proportional changes in the biosynthetic rates of ferritin were observed, but the capacity for modulating biosynthesis in response to alterations in iron availability was preserved. These findings suggest that the final protein biosynthetic rate of a translationally regulated gene depends on both translational regulatory signals and underlying transcription rates.
Full text
PDF
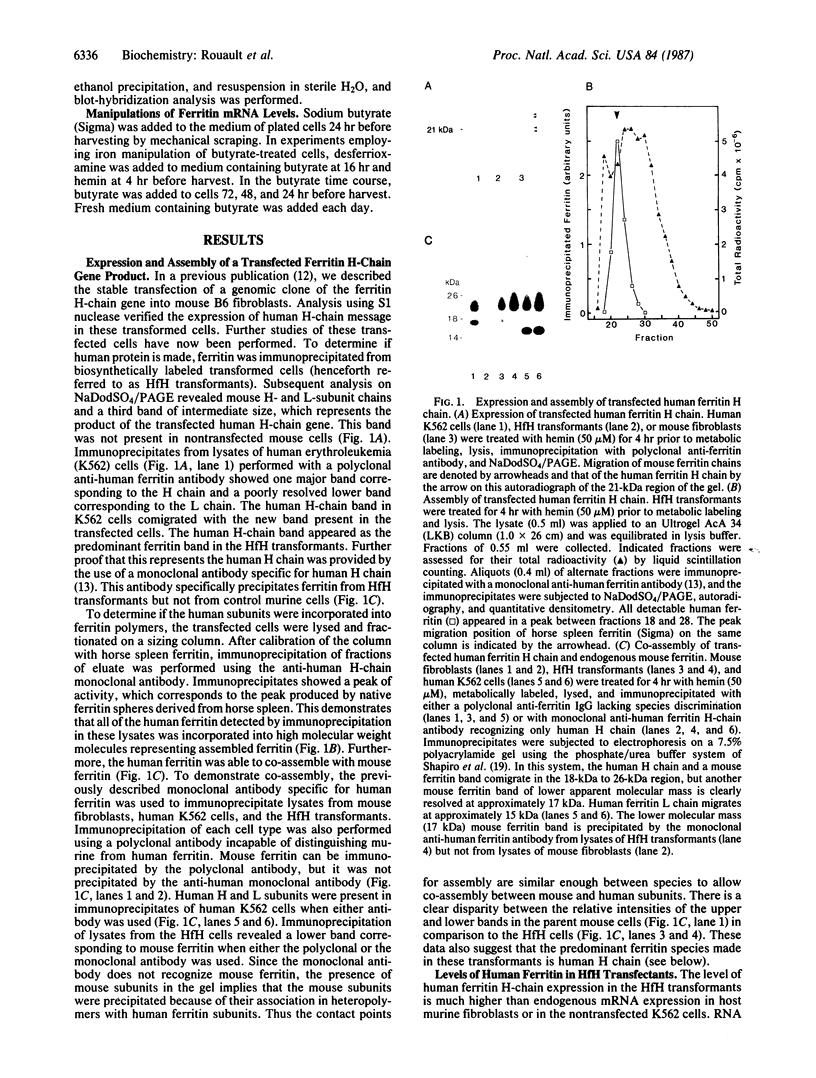

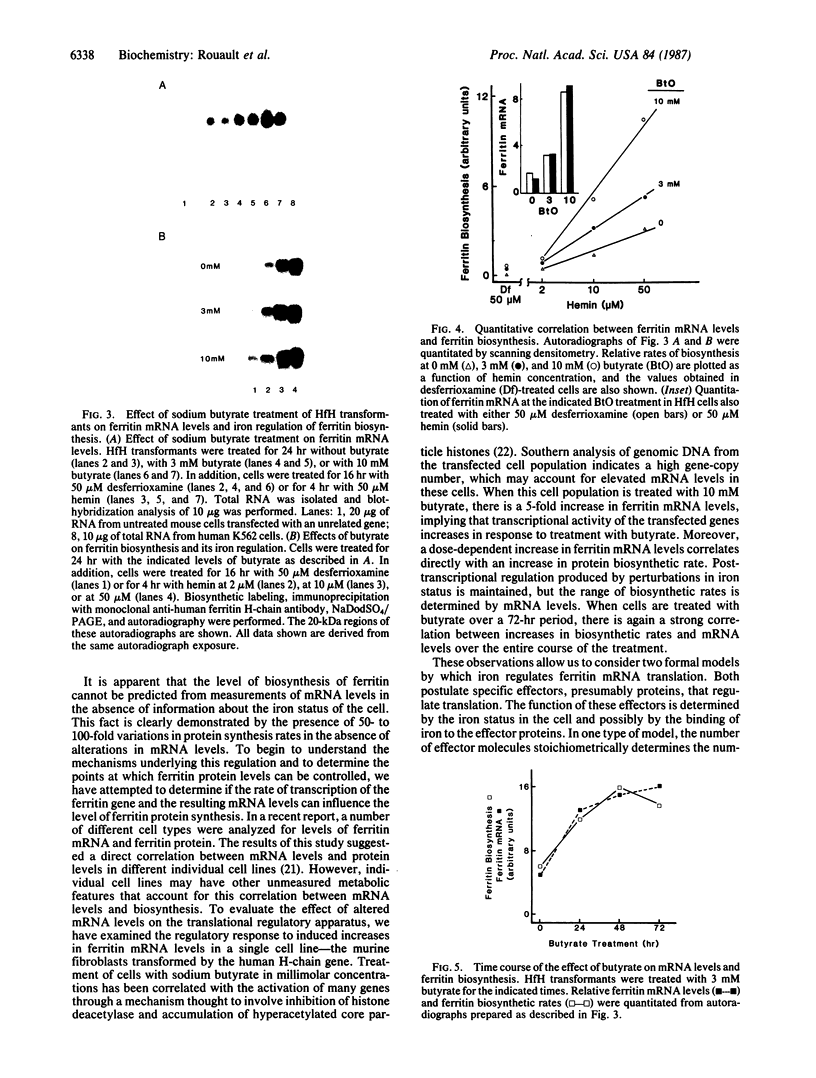

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Both subunits of rat liver ferritin are regulated at a translational level by iron induction. Nucleic Acids Res. 1986 Jan 24;14(2):915–927. doi: 10.1093/nar/14.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Jain S. K., Crampton J., Barrett K. J., Drysdale J. Isolation and characterization of a cDNA clone for human ferritin heavy chain. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4751–4755. doi: 10.1073/pnas.81.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd D., Vecoli C., Belcher D. M., Jain S. K., Drysdale J. W. Structural and functional relationships of human ferritin H and L chains deduced from cDNA clones. J Biol Chem. 1985 Sep 25;260(21):11755–11761. [PubMed] [Google Scholar]
- Cairo G., Vezzoni P., Bardella L., Schiaffonati L., Rappocciolo E., Levi S., Arosio P., Bernelli-Zazzera A. Regulation of ferritin synthesis in malignant and non-malignant lymphoid cells. Biochem Biophys Res Commun. 1986 Sep 14;139(2):652–657. doi: 10.1016/s0006-291x(86)80040-7. [DOI] [PubMed] [Google Scholar]
- Chou C. C., Gatti R. A., Fuller M. L., Concannon P., Wong A., Chada S., Davis R. C., Salser W. A. Structure and expression of ferritin genes in a human promyelocytic cell line that differentiates in vitro. Mol Cell Biol. 1986 Feb;6(2):566–573. doi: 10.1128/mcb.6.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo F., Colombo M., Staempfli S., Santoro C., Marone M., Frank R., Delius H., Cortese R. Structure of gene and pseudogenes of human apoferritin H. Nucleic Acids Res. 1986 Jan 24;14(2):721–736. doi: 10.1093/nar/14.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Keim S., Papadopoulos P., O'Brien S., Modi W., Drysdale J., Leonard W. J., Harford J. B., Klausner R. D. Cloning, characterization, expression, and chromosomal localization of a human ferritin heavy-chain gene. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7226–7230. doi: 10.1073/pnas.83.19.7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Barrett K. J., Boyd D., Favreau M. F., Crampton J., Drysdale J. W. Ferritin H and L chains are derived from different multigene families. J Biol Chem. 1985 Sep 25;260(21):11762–11768. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luzzago A., Arosio P., Iacobello C., Ruggeri G., Capucci L., Brocchi E., De Simone F., Gamba D., Gabri E., Levi S. Immunochemical characterization of human liver and heart ferritins with monoclonal antibodies. Biochim Biophys Acta. 1986 Jul 25;872(1-2):61–71. doi: 10.1016/0167-4838(86)90147-0. [DOI] [PubMed] [Google Scholar]
- Mattia E., Josic D., Ashwell G., Klausner R., van Renswoude J. Regulation of intracellular iron distribution in K562 human erythroleukemia cells. J Biol Chem. 1986 Apr 5;261(10):4587–4593. [PubMed] [Google Scholar]
- Mattia E., Rao K., Shapiro D. S., Sussman H. H., Klausner R. D. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J Biol Chem. 1984 Mar 10;259(5):2689–2692. [PubMed] [Google Scholar]
- McCue P. A., Gubler M. L., Sherman M. I., Cohen B. N. Sodium butyrate induces histone hyperacetylation and differentiation of murine embryonal carcinoma cells. J Cell Biol. 1984 Feb;98(2):602–608. doi: 10.1083/jcb.98.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa S., de Haro C. Regulation of protein synthesis in eukaryotes. Annu Rev Biochem. 1979;48:549–580. doi: 10.1146/annurev.bi.48.070179.003001. [DOI] [PubMed] [Google Scholar]
- Rouault T., Rao K., Harford J., Mattia E., Klausner R. D. Hemin, chelatable iron, and the regulation of transferrin receptor biosynthesis. J Biol Chem. 1985 Nov 25;260(27):14862–14866. [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Regulation of ferritin mRNA: a possible gene-sparing phenomenon. Induction of ferritin synthesis by iron in liver as well as red cells combines high translational efficiency with increased utilization of preformed ferritin mRNA. J Biol Chem. 1983 Jul 10;258(13):7921–7923. [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]