Abstract
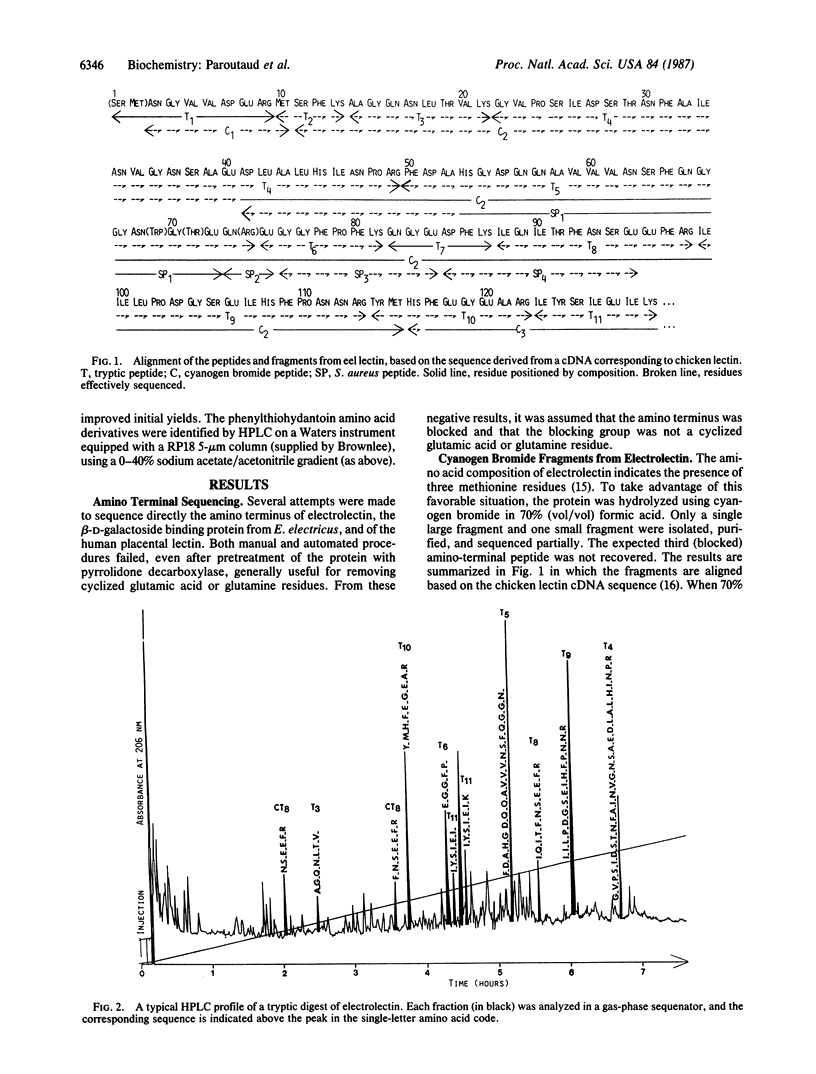
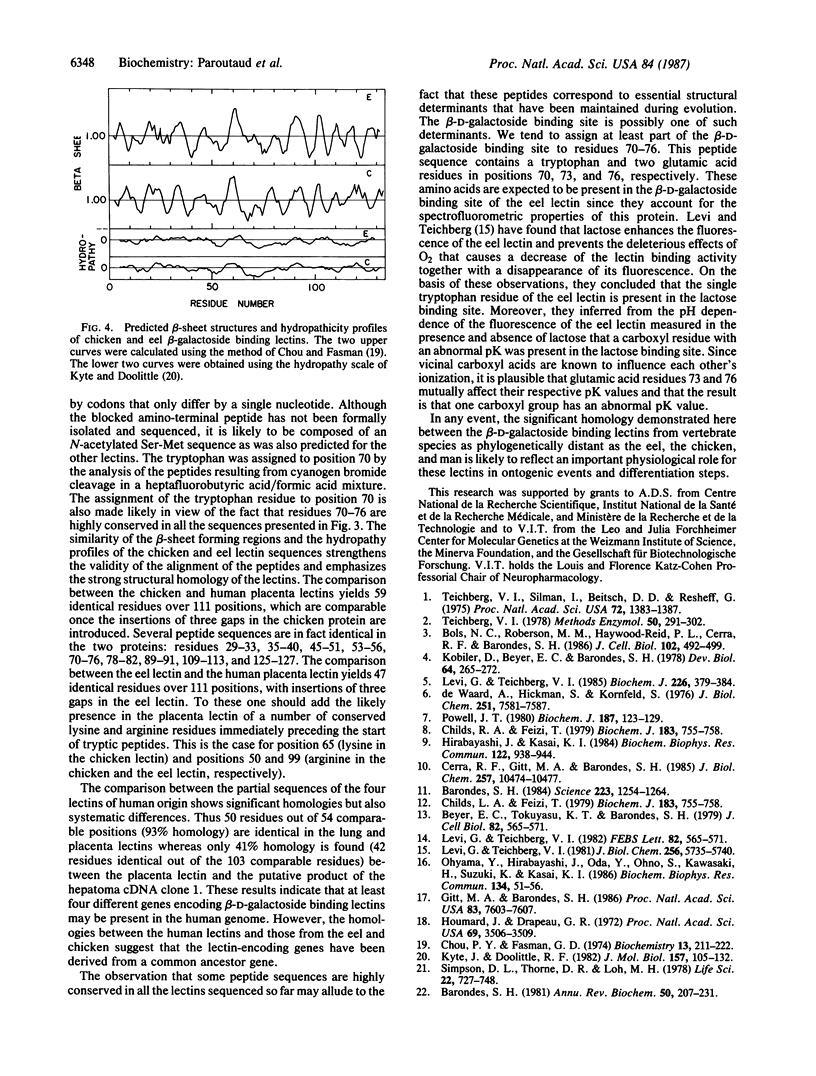
We have established the amino acid sequence of the beta-D-galactoside binding lectin from the electric eel and the sequences of several peptides from a similar lectin isolated from human placenta. These sequences were compared with the published sequences of peptides derived from the beta-D-galactoside binding lectin from human lung and with sequences deduced from cDNAs assigned to the beta-D-galactoside binding lectins from chicken embryo skin and human hepatomas. Significant homologies were observed. One of the highly conserved regions that contains a tryptophan residue and two glutamic acid residues is probably part of the beta-D-galactoside binding site, which, on the basis of spectroscopic studies of the electric eel lectin, is expected to contain such residues. The similarity of the hydropathy profiles and the predicted secondary structure of the lectins from chicken skin and electric eel, in spite of differences in their amino acid sequences, strongly suggests that these proteins have maintained structural homologies during evolution and together with the other beta-D-galactoside binding lectins were derived from a common ancestor gene.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H. Lectins: their multiple endogenous cellular functions. Annu Rev Biochem. 1981;50:207–231. doi: 10.1146/annurev.bi.50.070181.001231. [DOI] [PubMed] [Google Scholar]
- Barondes S. H. Soluble lectins: a new class of extracellular proteins. Science. 1984 Mar 23;223(4642):1259–1264. doi: 10.1126/science.6367039. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Tokuyasu K. T., Barondes S. H. Localization of an endogenous lectin in chicken liver, intestine, and pancreas. J Cell Biol. 1979 Aug;82(2):565–571. doi: 10.1083/jcb.82.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bols N. C., Roberson M. M., Haywood-Reid P. L., Cerra R. F., Barondes S. H. Secretion of a cytoplasmic lectin from Xenopus laevis skin. J Cell Biol. 1986 Feb;102(2):492–499. doi: 10.1083/jcb.102.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerra R. F., Gitt M. A., Barondes S. H. Three soluble rat beta-galactoside-binding lectins. J Biol Chem. 1985 Sep 5;260(19):10474–10477. [PubMed] [Google Scholar]
- Childs R. A., Feizi T. beta-Galactoside-binding muscle lectins of man and monkey show antigenic cross-reactions with those of bovine origin. Biochem J. 1979 Dec 1;183(3):755–758. doi: 10.1042/bj1830755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs R. A., Feizi T. beta-Galactoside-binding muscle lectins of man and monkey show antigenic cross-reactions with those of bovine origin. Biochem J. 1979 Dec 1;183(3):755–758. doi: 10.1042/bj1830755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Conformational parameters for amino acids in helical, beta-sheet, and random coil regions calculated from proteins. Biochemistry. 1974 Jan 15;13(2):211–222. doi: 10.1021/bi00699a001. [DOI] [PubMed] [Google Scholar]
- Gitt M. A., Barondes S. H. Evidence that a human soluble beta-galactoside-binding lectin is encoded by a family of genes. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7603–7607. doi: 10.1073/pnas.83.20.7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi J., Kasai K. Human placenta beta-galactoside-binding lectin. Purification and some properties. Biochem Biophys Res Commun. 1984 Aug 16;122(3):938–944. doi: 10.1016/0006-291x(84)91181-1. [DOI] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobiler D., Beyer E. C., Barondes S. H. Developmentally regulated lectins from chick muscle, brain, and liver have similar chemical and immunological properties. Dev Biol. 1978 Jun;64(2):265–272. doi: 10.1016/0012-1606(78)90077-5. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and characterization of chicken thymic electrolectin. Biochem J. 1985 Mar 1;226(2):379–384. doi: 10.1042/bj2260379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi G., Teichberg V. I. Isolation and physicochemical characterization of electrolectin, a beta-D-galactoside binding lectin from the electric organ of Electrophorus electricus. J Biol Chem. 1981 Jun 10;256(11):5735–5740. [PubMed] [Google Scholar]
- Ohyama Y., Hirabayashi J., Oda Y., Ohno S., Kawasaki H., Suzuki K., Kasai K. Nucleotide sequence of chick 14K beta-galactoside-binding lectin mRNA. Biochem Biophys Res Commun. 1986 Jan 14;134(1):51–56. doi: 10.1016/0006-291x(86)90525-5. [DOI] [PubMed] [Google Scholar]
- Powell J. T. Purification and properties of lung lectin. Rat lung and human lung beta-galactoside-binding proteins. Biochem J. 1980 Apr 1;187(1):123–129. doi: 10.1042/bj1870123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Thorne D. R., Loh H. H. Lectins: endogenous carbohydrate-binding proteins from vertebrate tissues: functional role in recognition processes? Life Sci. 1978 Mar;22(9):727–748. doi: 10.1016/0024-3205(78)90242-4. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I. Electrolectins: beta-D-galactoside-binding proteins. Methods Enzymol. 1978;50:291–302. doi: 10.1016/0076-6879(78)50031-1. [DOI] [PubMed] [Google Scholar]
- Teichberg V. I., Silman I., Beitsch D. D., Resheff G. A beta-D-galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1383–1387. doi: 10.1073/pnas.72.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waard A., Hickman S., Kornfeld S. Isolation and properties of beta-galactoside binding lectins of calf heart and lung. J Biol Chem. 1976 Dec 10;251(23):7581–7587. [PubMed] [Google Scholar]



