Abstract
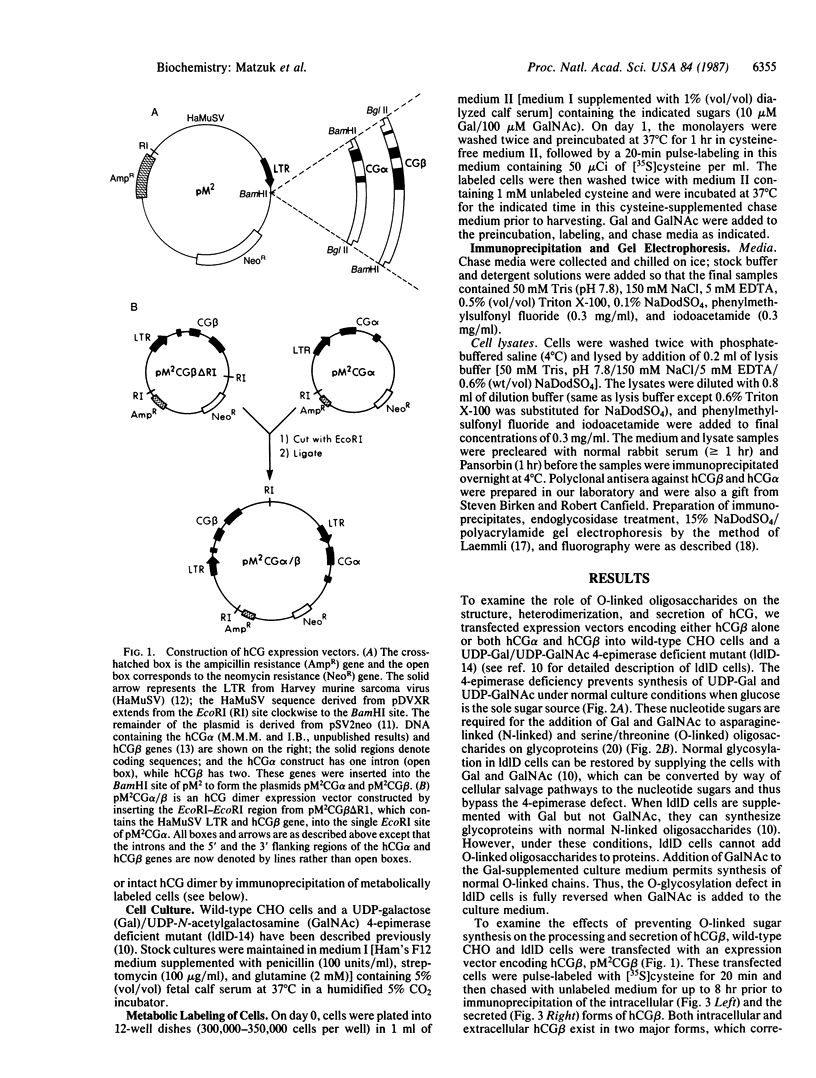
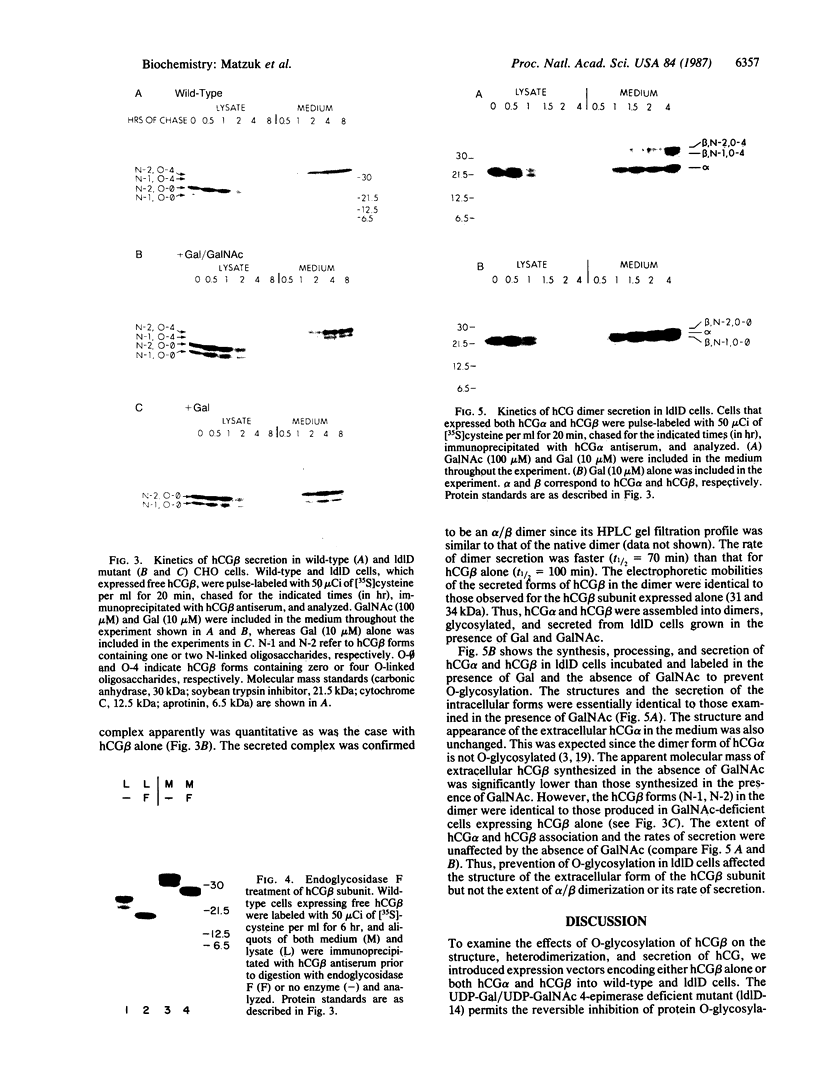
Human chorionic gonadotropin (hCG) is a member of a family of heterodimeric glycoprotein hormones that have a common alpha subunit but differ in their hormone-specific beta subunits. The beta subunit of hCG (hCG beta) is unique among the beta subunits in that it contains four mucin-like O-linked oligosaccharides attached to a carboxyl-terminal extension. To study the effects of O-glycosylation on the secretion and assembly of hCG, expression vectors containing either the hCG beta gene alone or together with the hCG alpha gene were transfected into a mutant Chinese hamster ovary cell line, IdID, which exhibits a reversible defect in O-glycosylation. Our results reveal that hCG beta can be secreted normally in the absence of its O-linked oligosaccharides. hCG beta devoid of O-linked carbohydrate can also combine efficiently with hCG alpha and be secreted as an intact dimer. We conclude that in Chinese hamster ovary cells, the hCG beta O-linked chains play no role in the assembly and secretion of hCG. The normal and O-linked oligosaccharide-deficient forms of hCG secreted by these cells should prove useful in examining the role of O-linked chains on the biological function of hCG.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Subcellular site of synthesis of the N-acetylgalactosamine (alpha 1-0) serine (or threonine) linkage in rat liver. J Biol Chem. 1987 Mar 25;262(9):4153–4159. [PubMed] [Google Scholar]
- Birken S., Canfield R. E. Isolation and amino acid sequence of COOH-terminal fragments from the beta subunit of human choriogonadotropin. J Biol Chem. 1977 Aug 10;252(15):5386–5392. [PubMed] [Google Scholar]
- Carlsen R. B., Bahl O. P., Swaminathan N. Human chorionic gonadotropin. Linear amino acid sequence of the beta subunit. J Biol Chem. 1973 Oct 10;248(19):6810–6827. [PubMed] [Google Scholar]
- Corless C. L., Matzuk M. M., Ramabhadran T. V., Krichevsky A., Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. J Cell Biol. 1987 May;104(5):1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The cDNA for the beta-subunit of human chorionic gonadotropin suggests evolution of a gene by readthrough into the 3'-untranslated region. Nature. 1980 Aug 14;286(5774):684–687. doi: 10.1038/286684a0. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hoshina H., Boime I. Combination of rat lutropin subunits occurs early in the secretory pathway. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7649–7653. doi: 10.1073/pnas.79.24.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Spear P. G. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell. 1983 Mar;32(3):987–997. doi: 10.1016/0092-8674(83)90083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. J., Mise T., Ghai R. D., Bahl O. P. Structure and location of the O-glycosidic carbohydrate units of human chorionic gonadotropin. J Biol Chem. 1979 Aug 25;254(16):7909–7914. [PubMed] [Google Scholar]
- Keutmann H. T., Williams R. M. Human chorionic gonadotropin. Amino acid sequence of the hormone-specific COOH-terminal region. J Biol Chem. 1977 Aug 10;252(15):5393–5397. [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Hobbie L., Krieger M. Reversible defects in O-linked glycosylation and LDL receptor expression in a UDP-Gal/UDP-GalNAc 4-epimerase deficient mutant. Cell. 1986 Mar 14;44(5):749–759. doi: 10.1016/0092-8674(86)90841-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Niemann H., Boschek B., Evans D., Rosing M., Tamura T., Klenk H. D. Post-translational glycosylation of coronavirus glycoprotein E1: inhibition by monensin. EMBO J. 1982;1(12):1499–1504. doi: 10.1002/j.1460-2075.1982.tb01346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. P., Krzesicki R. F., Hartle R. J., Perini F., Ruddon R. W. A kinetic comparison of the processing and secretion of the alpha beta dimer and the uncombined alpha and beta subunits of chorionic gonadotropin synthesized by human choriocarcinoma cells. J Biol Chem. 1984 Dec 25;259(24):15123–15130. [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Policastro P., Ovitt C. E., Hoshina M., Fukuoka H., Boothby M. R., Boime I. The beta subunit of human chorionic gonadotropin is encoded by multiple genes. J Biol Chem. 1983 Oct 10;258(19):11492–11499. [PubMed] [Google Scholar]
- Sege R. D., Kozarsky K., Nelson D. L., Krieger M. Expression and regulation of human low-density lipoprotein receptors in Chinese hamster ovary cells. Nature. 1984 Feb 23;307(5953):742–745. doi: 10.1038/307742a0. [DOI] [PubMed] [Google Scholar]
- Shome B., Parlow A. F. The primary structure of the hormone-specific, beta subunit of human pituitary luteinizing hormone (hLH). J Clin Endocrinol Metab. 1973 Mar;36(3):618–621. doi: 10.1210/jcem-36-3-618. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]









