Abstract
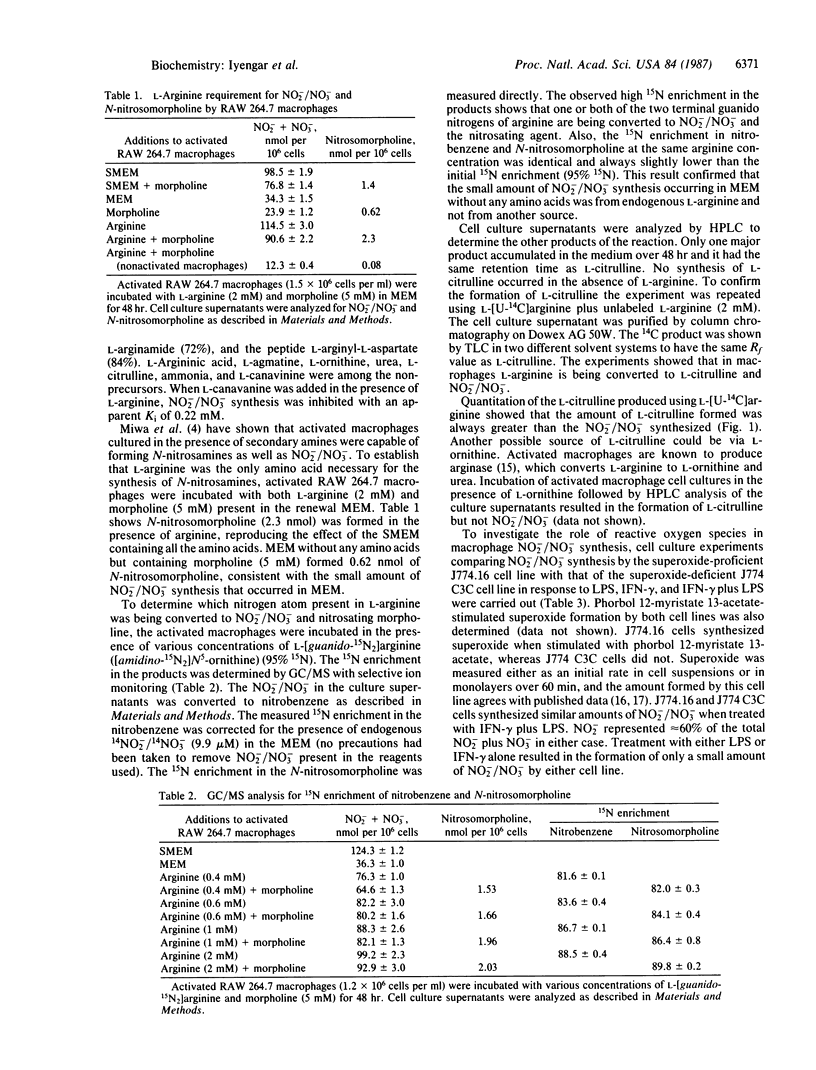
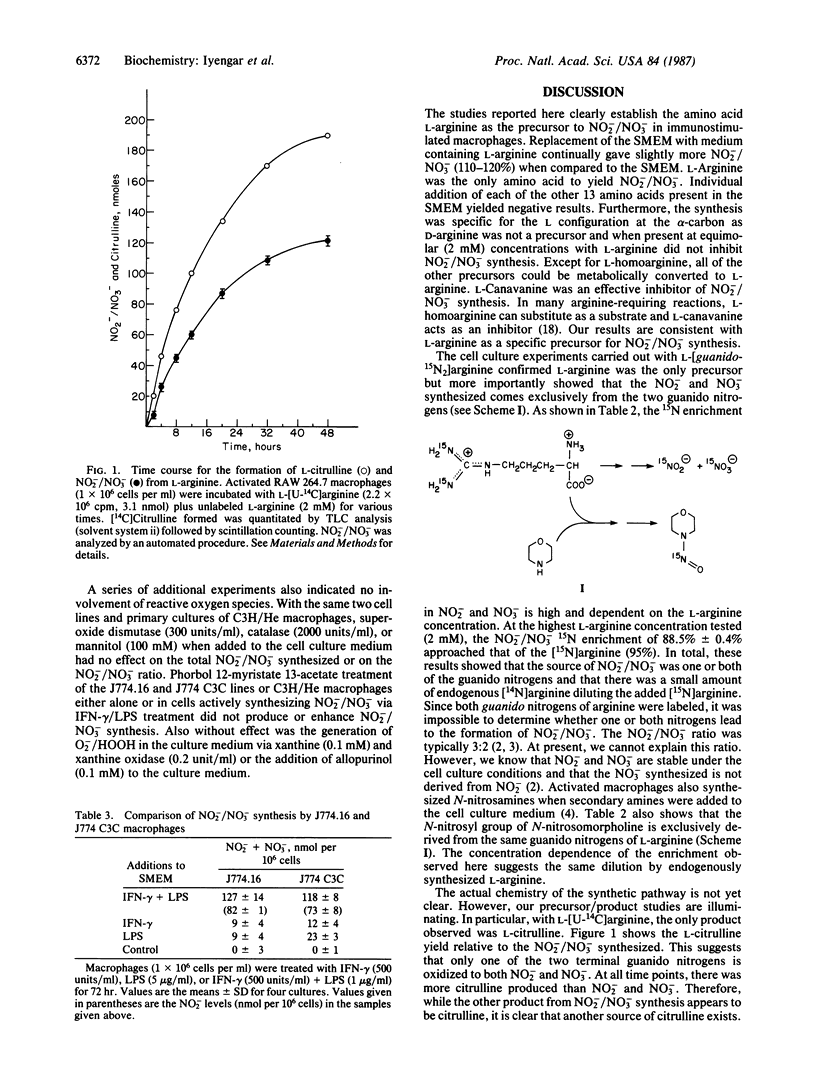
The macrophage cell line RAW 264.7 when activated with Escherichia coli lipopolysaccharide and interferon-gamma synthesized nitrite (NO3-) and nitrate (NO3-). Medium change after the activation showed that L-arginine was the only amino acid essential for this synthesis. D-Arginine would not substitute for L-arginine. Other analogues that could replace L-arginine were L-homoarginine, L-arginine methyl ester, L-arginamide, and the peptide L-arginyl-L-aspartate. L-Argininic acid, L-agmatine, L-ornithine, urea, L-citrulline, and ammonia were among the nonprecursors, while L-canavanine inhibited this L-arginine-derived NO2-/NO3- synthesis. When morpholine was added to the culture medium of the activated RAW 264.7 macrophages, N-nitrosation took place, generating N-nitrosomorpholine. GC/MS experiments using L-[guanido-15N2]arginine established that the NO2-/NO3- and the nitrosyl group of N-nitrosomorpholine were derived exclusively from one or both of the terminal guanido nitrogens of arginine. Chromatographic analysis showed that the other product of the L-arginine synthesis of NO2-/NO3- was L-citrulline. The role of the respiratory burst in NO2-/NO3- synthesis was examined using the macrophage cell lines J774.16 and J774 C3C. Both cell lines synthesized similar amounts of NO2-/NO3-. However, J774 C3C cells do not produce superoxide and hence do not exhibit the respiratory burst. Additional experiments also ruled out the involvement of the respiratory burst in NO2-/NO3- synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTELLANI A. G., NIVEN C. F., Jr Factors affecting the bacteriostatic action of sodium nitrite. Appl Microbiol. 1955 May;3(3):154–159. doi: 10.1128/am.3.3.154-159.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G. A. Activated macrophages kill tumour cells by releasing arginase. Nature. 1978 Jun 29;273(5665):758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Damiani G., Kiyotaki C., Soeller W., Sasada M., Peisach J., Bloom B. R. Macrophage variants in oxygen metabolism. J Exp Med. 1980 Oct 1;152(4):808–822. doi: 10.1084/jem.152.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom M. H., Fernstrom J. D. Rapid measurement of free amino acids in serum and CSF using high-performance liquid chromatography. Life Sci. 1981 Nov 16;29(20):2119–2130. doi: 10.1016/0024-3205(81)90669-x. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna R., Nagarajan B. Separation and estimation of arginine-related metabolites in tissues. Anal Biochem. 1980 Sep 15;107(2):318–323. doi: 10.1016/0003-2697(80)90390-5. [DOI] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Kiyotaki C., Peisach J., Bloom B. R. Oxygen metabolism in cloned macrophage cell lines: glucose dependence of superoxide production, metabolic and spectral analysis. J Immunol. 1984 Feb;132(2):857–866. [PubMed] [Google Scholar]
- Miwa M., Stuehr D. J., Marletta M. A., Wishnok J. S., Tannenbaum S. R. Nitrosation of amines by stimulated macrophages. Carcinogenesis. 1987 Jul;8(7):955–958. doi: 10.1093/carcin/8.7.955. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai A. W., Lien E. J., Lai M. M., Khwaja T. A. Novel N-hydroxyguanidine derivatives as anticancer and antiviral agents. J Med Chem. 1984 Feb;27(2):236–238. doi: 10.1021/jm00368a024. [DOI] [PubMed] [Google Scholar]
- Tesch J. W., Rehg W. R., Sievers R. E. Microdetermination of nitrates and nitrites in saliva, blood, water, and suspended particulates in air by gas chromatography. J Chromatogr. 1976 Nov 3;126:743–755. doi: 10.1016/s0021-9673(01)84117-0. [DOI] [PubMed] [Google Scholar]
- Young C. W., Schochetman G., Hodas S., Balis M. E. Inhibition of DNA synthesis by hydroxyurea: structure-activity relationships. Cancer Res. 1967 Mar;27(3):535–540. [PubMed] [Google Scholar]



