Abstract
Hypocalcemia and hyperphosphatemia with low/normal parathyroid hormone (PTH) levels can be observed in hypoparathyroidism (HP), a disorder that may follow an autosomal dominant (AD) or autosomal recessive (AR) mode of inheritance. Similar biochemical changes are also observed in pseudohypoparathyroidism (PHP) type Ia and Ib, but affected patients usually show elevated PTH levels indicative of hormonal resistance. Features of Albright’s hereditary osteodystrophy (AHO) are typically not observed in patients affected by familial forms of PHP-Ib, which are most frequently caused by maternally inherited, heterozygous microdeletions within STX16 and are associated with isolated loss of methylation at GNAS exon A/B.
We established the molecular defect in two children of consanguineous Turkish parents, who presented with hypocalcemia, hyperphosphatemia, and low 25-OH vitamin D levels, but initially normal or only mildly elevated PTH levels, i.e. findings that do not readily exclude HP. After normalizing serum magnesium levels, hypocalcemia and hyperphosphatemia persisted, and PTH levels increased, suggesting PTH-resistance rather than PTH-deficiency. Because of the absence of AHO and parental consanguinity, an AR form of PHP-Ib appeared plausible, which had previously been suggested for sporadic cases. However, loss of GNAS methylation was restricted to exon A/B, which led to the identification of the 3-kb STX16 microdeletion. The same mutation was also detected in the healthy mother, who did not show any GNAS methylation abnormality, indicating that her deletion resides on the paternal allele.
Our findings emphasize the importance of considering a parentally imprinted, autosomal dominant disorder even if consanguinity suggests an autosomal recessive mode of inheritance.
Keywords: Pseudohypoparathyroidism type Ib, syntaxin 16, GNAS, autosomal dominant, parental imprinting, consanguinity
Hypocalcemia and hyperphosphatemia with inappropriately low/normal or only slightly elevated parathyroid hormone (PTH) levels are typically observed in patients affected by isolated hypoparathyroidism (HP), a rare disorder that can follow an autosomal recessive (AR) or an autosomal dominant (AD) mode of inheritance.1–6 HP can be caused by impaired synthesis or secretion of PTH as a result of mutations in the PTH gene itself,2, 3 due to activating mutations in the calcium-sensing receptor,4 or due to homozygous or heterozygous mutations in glial cells missing B (GCMB).5, 6
Abnormalities in serum calcium and phosphorous similar to those encountered in HP are also observed in patients with pseudohypoparathyroidism type Ia (PHP-Ia), a disorder that is caused by heterozygous, inactivating mutations in those GNAS exons encoding the alpha-subunit of the stimulatory G protein (Gsα). Affected individuals show, in addition to PTH-resistance, resistance towards few other hormones that mediate their actions through Gsα-coupled receptors, and they usually exhibit features of Albright’s Hereditary Osteodystrophy (AHO) such as round face, short stature, obesity, brachydactyly, heterotopic ossifications, and mental retardation, i.e. clinical findings not present in HP.7, 8
In contrast, patients affected by PHP type Ib (PHP-Ib) typically show PTH and, in some cases, TSH resistance without any features of AHO, although few reports have revealed subtle evidence for AHO.9, 10, 11 At least two distinct types of epigenetic/genetic defects have been described in autosomal dominant PHP-Ib (AD-PHP-Ib), which lead to indistinguishable clinical and laboratory phenotypes. These familial forms of PHP-Ib are caused by maternally inherited, heterozygous deletions within or up-stream of the GNAS locus, which are associated either with a loss of all maternal GNAS methylation imprints or with a loss of exon A/B methylation alone;12–15 paternal inheritance of these deletions does not lead to laboratory or clinically obvious abnormalities. Similar to AD-PHP-Ib caused by deletions within GNAS, sporadic PHP-Ib cases display broad GNAS imprinting defects, but no molecular defect has yet been identified. Haplotype sharing with an unaffected sibling has been demonstrated in some sporadic PHP-Ib cases,16 suggesting either de novo mutations, small paternal uniparental iso- or heterodisomy within the chromosome 20q13.3 region, or an autosomal recessive (AR) form of PHP-Ib that could be caused by homozygous or compound heterozygous mutations in a different gene.
Here, we describe clinical and laboratory findings of a patient, who presented with hypocalcemia, hypomagnesemia, and hyperphosphatemia, and inappropriately normal PTH levels, but no evidence for developmental abnormalities. After correction of serum magnesium levels, PTH levels increased suggesting PHP-Ib rather than HP, which was subsequently confirmed through epigenetic and genetic investigations.
Case Report
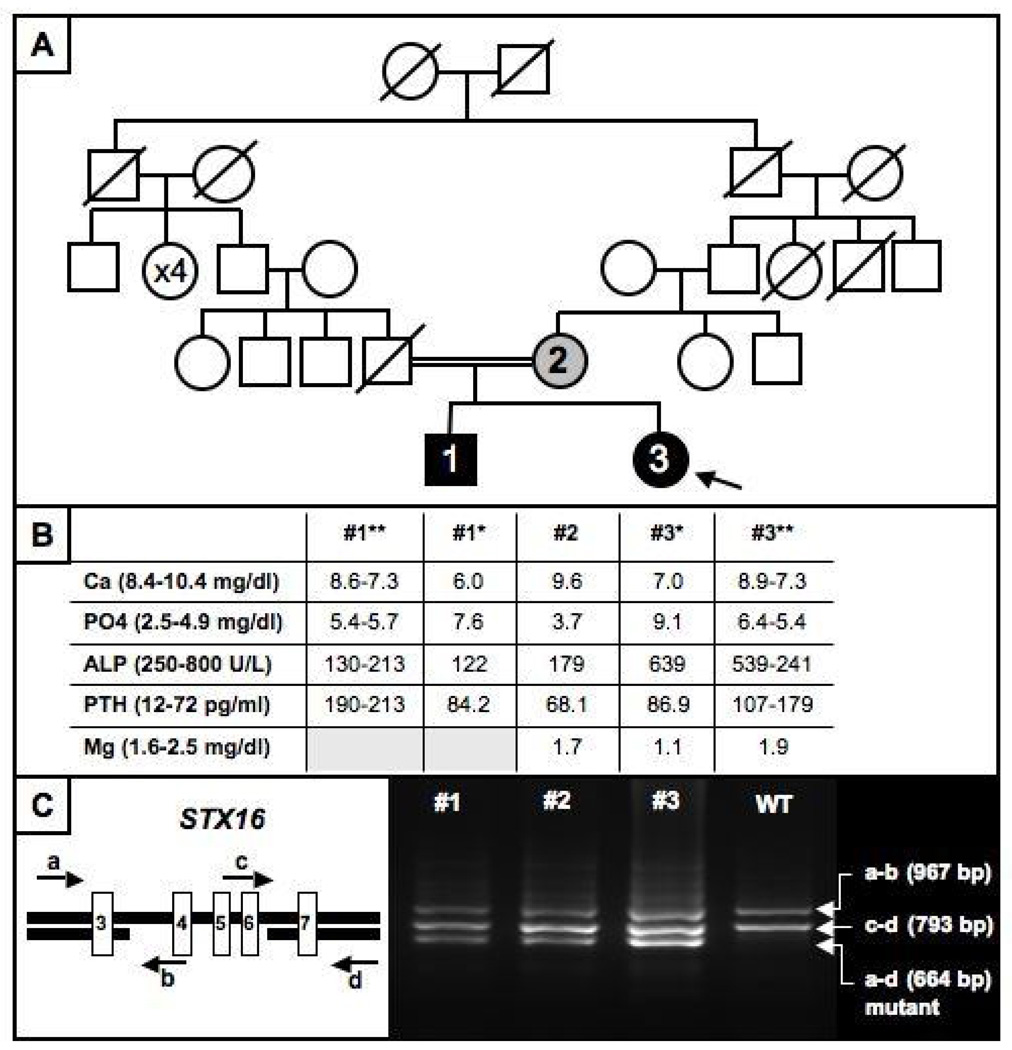
An 11 year old Turkish female (Fig. 1A; patient #3) presented to clinic with tetany, and laboratory testing revealed hypocalcemia, hypomagnesemia, and hyperphosphatemia [Fig. 1B: 7.0 mg/dl (normal: 8.4–10.4); 1.1 mg/dl (normal: 1.6–2.5); and 9.1 mg/dl (normal: 2.5–4.5), respectively]. Alkaline phosphatase was 639 U/L (normal: 300–1100) and PTH was 44 pg/ml (normal: 9–55); the urinary calcium-to-creatinine ratio low at 0.01–0.05 (normal: <0.2). Her height was 147 cm (0.3 SDS above the mean of age-matched Turkish controls), her weight was 51 kg (1.4 SDS above the mean of age-matched Turkish controls), and her BMI was 23.6 kg/m2 (1.6 SDS above the mean of age-matched Turkish controls). She revealed no evidence for AHO. The physical examination was within normal limits and the past medical history was unremarkable; birth weight had been 3000 g, and she had shown normal growth and development. At the time of presentation, her pubertal development was consistent with Tanner stage III.
Figure 1.
Panel A: Pedigree of the AD-PHP-Ib family showing a mode of inheritance that is consistent with an autosomal recessive disorder.
Panel B: Laboratory findings. * at presentation; ** range after normalization of serum magnesium level in the index case. For conversion of metric unit to SI unit; multiple by 0.25 for Ca, 0.3229 for phosphorus and 0.18 for Mg to mmol/L, 0.102 for PTH to pmol/L.
Panel C: Analysis of the STX-16 region by multiplex PCR using primers a, b, c, and d (arrows) leading to the identification of the previously described heterozygous 3-kb microdeletion comprising exons 4–6. The shortest PCR product, which was present in the two affected family members, #1 and #3, and in unaffected carrier #2, is derived from the mutant allele and amplified by primers a and d. The 967 bp product, representing the mutant product, is amplified by primers a and b, while the 793 bp product by primers c and d represents the wild-type allele.
Because of the low serum calcium and magnesium levels, she was first treated with intravenous calcium gluconate (500 mg/kg/day) and MgSO4 (100 mg/kg/day) for 3 days. She furthermore received oral vitamin D (300,000 IU) because of a low 25-OH vitamin D level (5.3 ng/ml; normal: 6–46). Because PTH was initially normal despite hypocalcemia, it was re-measured after correction of the hypomagnesemia, i.e. 10 days after the first measurement, and at that time it was shown to be mildly elevated at 86.9 pg/ml (normal: 12–72) when the serum levels of Ca, Pi, and Mg were 7.4 mg/dl, 7.8 mg/dl, and 1.8 mg/dl respectively. Based on the laboratory findings at presentation, a tentative diagnosis of hypoparathyroidism in combination with vitamin D deficiency was made. Treatment with oral calcium carbonate and calcitriol was therefore initiated. However, the doses of both medications were probably too low since PTH levels increased further (107–203 pg/ml) and hyperphosphatemia persisted (5.3 and 5.7 mg/dl) (Fig. 1B), indicating PTH resistance rather than hypoparathyroidism. It was therefore suspected that she might be affected by pseudohypoparathyroidism, e.g. PHP-Ib because of the absence of AHO. T4 and TSH were within normal limits.
The older brother of the patient (Fig. 1A; patient #1) presented at the age of 18 years with pain in his arms and legs that had started about 5 years ago. Laboratory evaluation revealed hypocalcemia and hyperphosphatemia (6.0 mg/dl and 7.6 mg/dl, respectively), with mildly elevated PTH (84.2 pg/ml) (Fig. 1B). T4 and TSH were within normal limits; there was no evidence for AHO. Because of the findings in his younger sister, the index case #3, therapy with oral calcium carbonate and 1,25(OH)2 vitamin D was initiated, which resulted initially in a further increase in PTH levels (190–213 pg/ml).
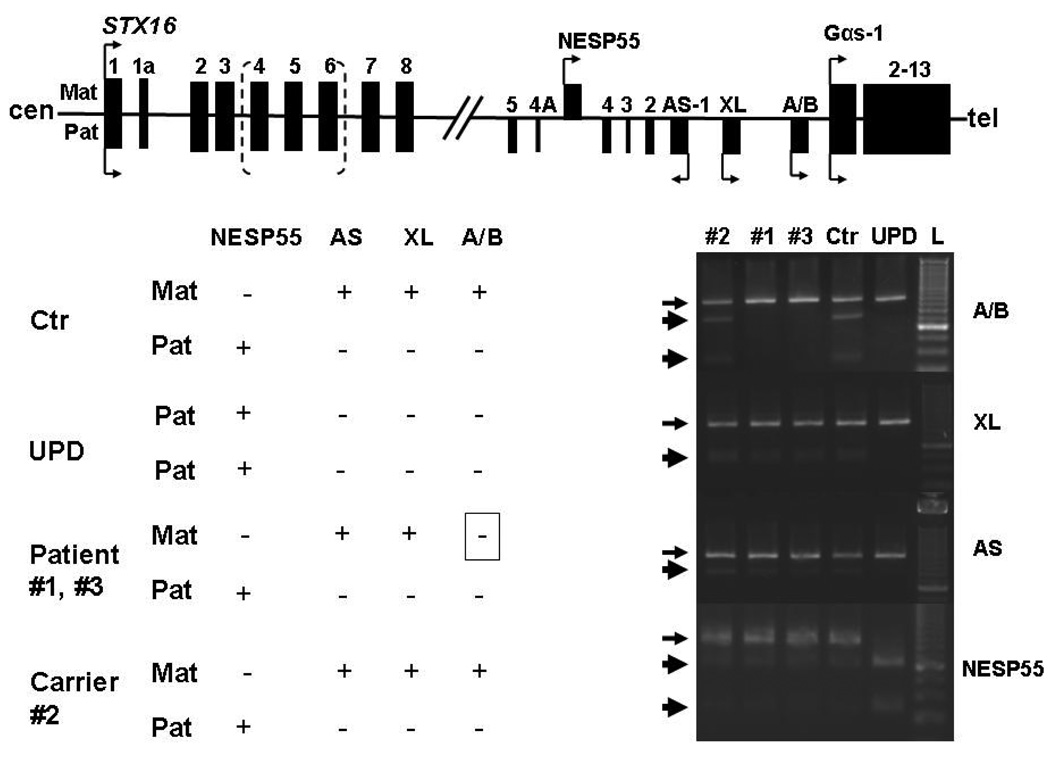
Lymphocyte DNA was extracted from both patients and the mother using standard methods after obtaining informed consents;12 the study was approved by Massachusetts General Hospital Institutional Review Board. The father had been healthy until he died in a traffic accident, and laboratory and genetic analyses could therefore not be performed. GNAS methylation analysis was carried out as described12 using bisulfite-modified genomic DNA sequence analysis, which established normal methylation at three of the four differentially methylated GNAS regions, NESP55, AS, and XL. However, only exon A/B revealed a loss of methylation in both patients; GNAS methylation analysis of the mother showed no abnormality (Figure 2). Because of consanguinity of the healthy parents, a recessive form of PHP-Ib had been initially considered. However, because of the loss of A/B methylation alone, we first searched for one of the two known microdeletions within the gene encoding syntaxin 16 (STX16), using multiplex PCR analysis as described.11 These studies revealed the previously reported 3-kb deletion within STX16, which was also identified in the unaffected mother (Fig. 1C), thereby establishing that both patients are affected by AD-PHP-Ib.
Figure 2.
Schematic representation of the human STX16 gene and the GNAS locus. Exons are shown as black boxes; arrows indicate the transcriptional direction (sense, antisense) and allelic origin (Maternal, Mat: above the line; Paternal, Pat: below the line). Hatched bracket shows deleted region within STX16.
Lower left panel: Methylation changes observed in the differentially methylated regions (DMR) for several individuals; ‘+’, methylated DMR; ‘-‘, non-methylated DMR: Ctr, healthy individual; UPD, patient with paternal uniparental isodisomy of chromosome 20q; patients #1 and #3 with 3-kb STX16 deletion; carrier #2, healthy mother of #1 and #3.
Lower right panel: Bisulfite-treated genomic DNA was amplified by PCR and the digested with the endonuclease FauI for exon A/B, BstUI for exons XL and AS, with AciI for exon NESP55 to assess the methylation status. Only the products is derived from the methylated allele were digested (thick arrows); absence of digestion thus indicates that genomic DNA was unmethylated before bisulfite treatment (thin arrows).
Discussion
In this report, we describe laboratory, epigenetic, and genetic findings in a patient, who presented with hypocalcemia, hypomagnesemia, and hyperphosphatemia. Because the parents of the patient are related and because serum PTH levels were initially normal, an autosomal recessive form of HP was considered. After correction of serum magnesium levels, however, PTH concentrations increased while calcium levels remained low, making a form of pseudohypoparathyroidism without AHO more likely. Because the patient’s brother showed similar biochemical abnormalities as the index case and because of the parental consanguinity, an autosomal recessive form of PHP-Ib appeared plausible. However, subsequent epigenetic and molecular characterization revealed only a loss of GNAS exon A/B methylation, and the previously described heterozygous 3-kb STX16 deletion was identified in the affected siblings and their mother, thereby establishing AD-PHP-Ib. Both patients (#1 and #3) carry the mutation on the maternal allele, and both show an associated loss of exon A/B methylation and PTH resistance. In contrast, their healthy mother presumably carries the mutation on the paternal allele and thus does not show any loss of exon A/B methylation.
Only approximately 100 genes in the human genome undergo parent-of-origin specific methylation, thereby limiting expression specifically to a single parental allele.17, 18 However, only less than 10 imprinted genes have thus far been implicated in human diseases, including Prader-Willi syndrome, Angelman syndrome, Beckwith-Wiedeman syndrome, Silver-Russell Syndrome, and transient neonatal diabetes,17, 19 as well as disorders that are caused by mutations within the GNAS locus. These include PHP-Ia, in which affected individuals carry maternally inherited, inactivating mutations located in those GNAS exons that encode Gsα.20–23 When inherited paternally, the same mutations lead to pseudo-PHP (PPHP) or progressive osseous heteroplasia (POH), i.e. related disorders characterized by the presence of AHO features without hormonal resistance.20–23 AD-PHP-Ib can be caused by one of two different, maternally inherited microdeletions within STX16, a gene about 220-kb up-stream of the GNAS locus; these 3-kb and 4.4-kb deletions, which are overlapping, are usually not associated with AHO-like abnormalities. PTH-resistance, which can be quite variable, is observed only with maternally inherited STX16 deletions.12, 13, 16, 24, 25
Because the consanguineous parents are healthy and did not show any laboratory abnormalities, the siblings described herein were initially thought to be affected by an autosomal recessive disorder. However, identification of the 3-kb STX16 deletion and knowledge about the paternally imprinted mode of inheritance for AD-PHP-Ib led to the conclusion that consanguinity had been misleading. Since the rates of consanguineous marriages in the Turkish population are reported to be as high as 20–25%, our findings raise the possibility that additional cases of AD-PHP-Ib will be identified in Turkey or other countries with high frequency of marriages between closely related relatives.26
In conclusion, we identified the 3-kb STX16 deletion, the most frequent cause of AD-PHP-Ib,12 in two siblings in whom an autosomal recessive form of PHP-Ib was initially suspected because of the parental consanguinity. Our findings indicate that children from related parents are not necessarily affected by an autosomal recessive disorder and that a parentally imprinted disorder should be considered. For patients affected by PHP-Ib, whose parents are related, it is thus important to first exclude the known genetic defects before searching for a novel genetic locus even though parental consanguinity suggests the possibility of an autosomal recessive disorder.
Acknowledgment
ST was recipient of a grant from Fulbright Scholarship Program. This study was supported by research grants from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK073911 to MB and R37 DK46718 to HJ). We thank the members of the investigated AD-PHP-Ib kindred for participating in this research study.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
3Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in European Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the journal accepts no responsibility for any errors of omissions it may contain. The definitive version is now freely available at [10.1530/EJE-10-0348]. © [September 2010] European Society of Endocrinology.2
References
- 1.Bringhurst FR, Demay MB, Kronenberg HM. Hormones and disorder of mineral metabolism. ch. 27. In: Kronenberg HM, Shlomo M, Polonsky KS, Larsen PR, editors. Williams Textbook of Endocrinology. edn 11. Philadelphia: Elsevier; 2008. pp. 1203–1268. [Google Scholar]
- 2.Arnold A, Horst SA, Gardella TJ, Baba H, Levine MA, Kronenberg HM. Mutation of the signal peptide-encoding region of the preproparathyroid hormone gene in familial isolated hypoparathyroidism. J Clin Invest. 1990;86:1084–1087. doi: 10.1172/JCI114811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunthornthepvarakul T, Churesigaew S, Ngowngarmratana S. A novel mutation of the signal peptide of the preproparathyroid hormone gene associated with autosomal recessive familial isolated hypoparathyroidism. J Clin Endocrinol Metab. 1999;84:3792–3796. doi: 10.1210/jcem.84.10.6070. [DOI] [PubMed] [Google Scholar]
- 4.Baron J, Winer KK, Yanovski JA, Cunningham AW, Laue L, Zimmerman D, Cutler GB., Jr Mutations in the Ca(2+)-sensing receptor gene cause autosomal dominant and sporadic hypoparathyroidism. Hum Mol Genet. 1996;5:601–606. doi: 10.1093/hmg/5.5.601. [DOI] [PubMed] [Google Scholar]
- 5.Ding C, Buckingham B, Levine MA. Familial isolated hypoparathyroidism caused by a mutation in the gene for the transcription factor GCMB. J Clin Invest. 2001;108:1215–1220. doi: 10.1172/JCI13180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mannstadt M, Bertrand G, Muresan M, Weryha G, Leheup B, Pulusani SR, Grandchamp B, Jüppner H, Silve C. Dominant-negative GCMB mutations cause an autosomal dominant form of hypoparathyroidism. J Clin Endocrinol Metab. 2008;93:3568–3576. doi: 10.1210/jc.2007-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine MA. Pseudohypoparathyroidism. In: Bilezikian JP, Raisz LG, Rodan GA, editors. Principles of Bone Biology. edn 2. New York: Academic Press; 2002. pp. 1137–1163. [Google Scholar]
- 8.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory G protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 9.de Nanclares GP, Fernández-Rebollo E, Santin I, García-Cuartero B, Gaztambide S, Menéndez E, Morales MJ, Pombo M, Bilbao JR, Barros F, Zazo N, Ahrens W, Jüppner H, Hiort O, Castaño L, Bastepe M. Epigenetic defects of GNAS in patients with pseudohypoparathyroidism and mild features of Albright's hereditary osteodystrophy. J Clin Endocrinol Metabol. 2007;92:2370–2373. doi: 10.1210/jc.2006-2287. [DOI] [PubMed] [Google Scholar]
- 10.Mariot V, Maupetit-Méhouas S, Sinding C, Kottler ML, Linglart A. A maternal epimutation of GNAS leads to Albright osteodystrophy and parathyroid hormone resistance. J Clin Endocrinol Metabol. 2008;93:661–665. doi: 10.1210/jc.2007-0927. [DOI] [PubMed] [Google Scholar]
- 11.Unluturk U, Harmanci A, Babaoglu M, Yasar U, Varli K, Bastepe M, Bayraktar M. Molecular diagnosis and clinical characterization of pseudohypoparathyroidism type-Ib in a patient with mild Albright's hereditary osteodystrophy-like features, epileptic seizures, and defective renal handling of uric acid. Am J Med Sci. 2008;336:84–90. doi: 10.1097/MAJ.0b013e31815b218f. [DOI] [PubMed] [Google Scholar]
- 12.Bastepe M, Fröhlich LF, Hendy GN, Indridason OS, Josse RG, Koshiyama H, Körkkö J, Nakamoto JM, Rosenbloom AL, Slyper AH, Sugimoto T, Tsatsoulis A, Crawford JD, Jüppner H. Autosomal dominant pseudohypoparathyroidism type Ib is associated with a heterozygous microdeletion that likely disrupts a putative imprinting control element of GNAS. J Clin Invest. 2003;112:1255–1263. doi: 10.1172/JCI19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linglart A, Gensure RC, Olney RC, Jüppner H, Bastepe M. A novel STX16 deletion in autosomal dominant pseudohypoparathyroidism type Ib redefines the boundaries of a cis-acting imprinting control element of GNAS. Am J Hum Gen. 2005;76:804–814. doi: 10.1086/429932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bastepe M, Fröhlich LF, Linglart A, Abu-Zahra HS, Tojo K, Ward LM, Jüppner H. Deletion of the NESP55 differentially methylated region causes loss of maternal GNAS imprints and pseudohypoparathyroidism type Ib. Nat Genet. 2005;37:25–27. doi: 10.1038/ng1487. [DOI] [PubMed] [Google Scholar]
- 15.Chillambhi S, Turan S, Hwang DY, Chen HC, Jüppner H, Bastepe M. Deletion of the Noncoding GNAS Antisense Transcript Causes Pseudohypoparathyroidism Type Ib and Biparental Defects of GNAS Methylation in cis. J Clin Endocrinol Metab. 2010 May 5; doi: 10.1210/jc.2009-2205. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linglart A, Bastepe M, Jüppner H. Similar clinical and laboratory findings in patients with symptomatic autosomal dominant and sporadic pseudohypoparathyroidism type Ib despite different epigenetic changes at the GNAS locus. Clin Endocrinol (Oxf) 2007;67:822–831. doi: 10.1111/j.1365-2265.2007.02969.x. [DOI] [PubMed] [Google Scholar]
- 17.Morison IM, Ramsay JP, Spencer HG. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. doi: 10.1016/j.tig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Renfree MB, Hore TA, Shaw G, Graves JA, Pask AJ. Evolution of genomic imprinting: insights from marsupials and monotremes. Annu Rev Genomics Hum Genet. 2009;10:241–262. doi: 10.1146/annurev-genom-082908-150026. [DOI] [PubMed] [Google Scholar]
- 19.Polychronakos C, Kukuvitis A. Parental genomic imprinting in endocrinopathies. Eur J Endocrinol. 2002;147:561–569. doi: 10.1530/eje.0.1470561. [DOI] [PubMed] [Google Scholar]
- 20.Bastepe M. The GNAS locus and pseudohypoparathyroidism. Adv Exp Med Biol. 2008;626:27–40. doi: 10.1007/978-0-387-77576-0_3. [DOI] [PubMed] [Google Scholar]
- 21.Jüppner H, Bastepe M. Different mutations within or upstream of the GNAS locus cause distinct forms of pseudohypoparathyroidism. J Pediatr Endocrinol Metab. 2006;19 Suppl 2:641–646. doi: 10.1515/jpem.2006.19.s2.641. [DOI] [PubMed] [Google Scholar]
- 22.Germain-Lee EL. Short stature, obesity, and growth hormone deficiency in pseudohypoparathyroidism type 1a. Pediatr Endocrinol Rev. 2006;3 Suppl 2:318–327. [PubMed] [Google Scholar]
- 23.Mantovani G, Spada A. Mutations in the Gs alpha gene causing hormone resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:501–513. doi: 10.1016/j.beem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Jüppner H, Schipani E, Bastepe M, Cole DE, Lawson ML, Mannstadt M, Hendy GN, Plotkin H, Koshiyama The gene responsible for pseudohypoparathyroidism type Ib is paternally imprinted and maps in four unrelated kindreds to chromosome 20q13.3. Proc Natl Acad Sci U S A. 1998;95:11798–11803. doi: 10.1073/pnas.95.20.11798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laspa E, Bastepe M, Jüppner H, Tsatsoulis A. Phenotypic and molecular genetic aspects of pseudohypoparathyroidism type Ib in a Greek kindred: evidence for enhanced uric acid excretion due to parathyroid hormone resistance. J Clin Endocrinol Metab. 2004;89:5942–5947. doi: 10.1210/jc.2004-0249. [DOI] [PubMed] [Google Scholar]
- 26.Tuncbilek E. Clinical outcomes of consanguineous marriages in Turkey. Turk J Pediatr. 2001;43:277–279. [PubMed] [Google Scholar]