Abstract
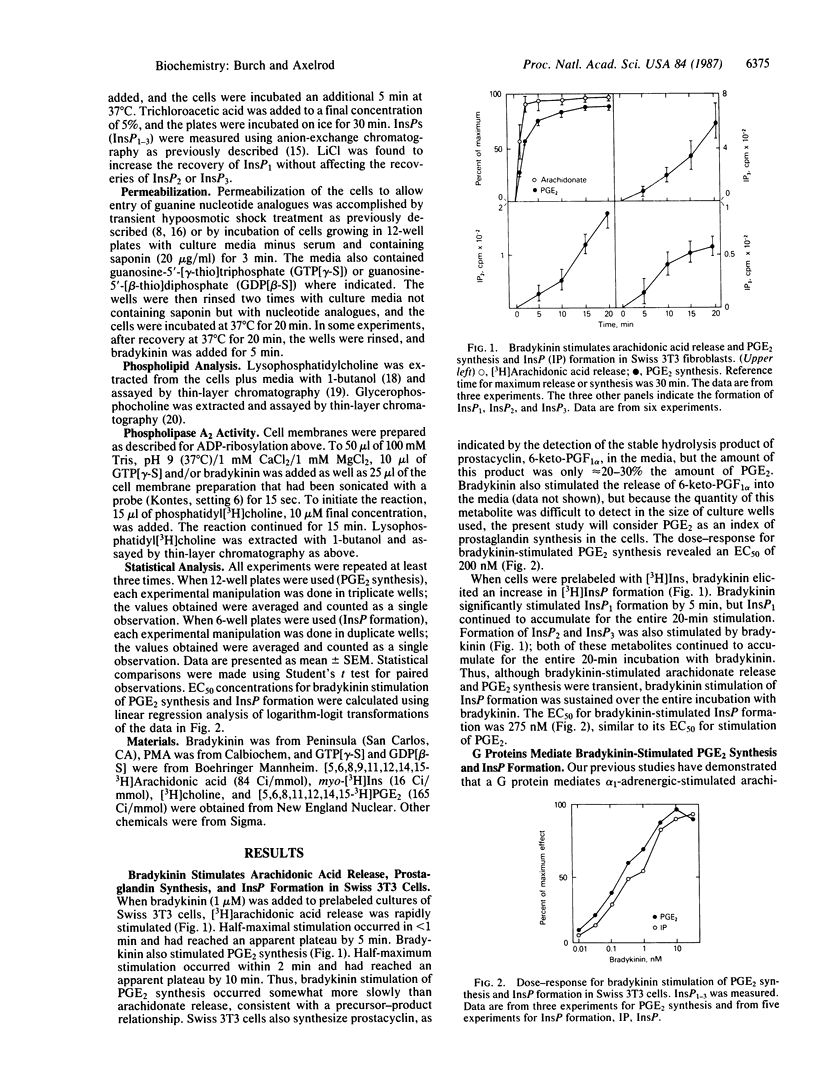
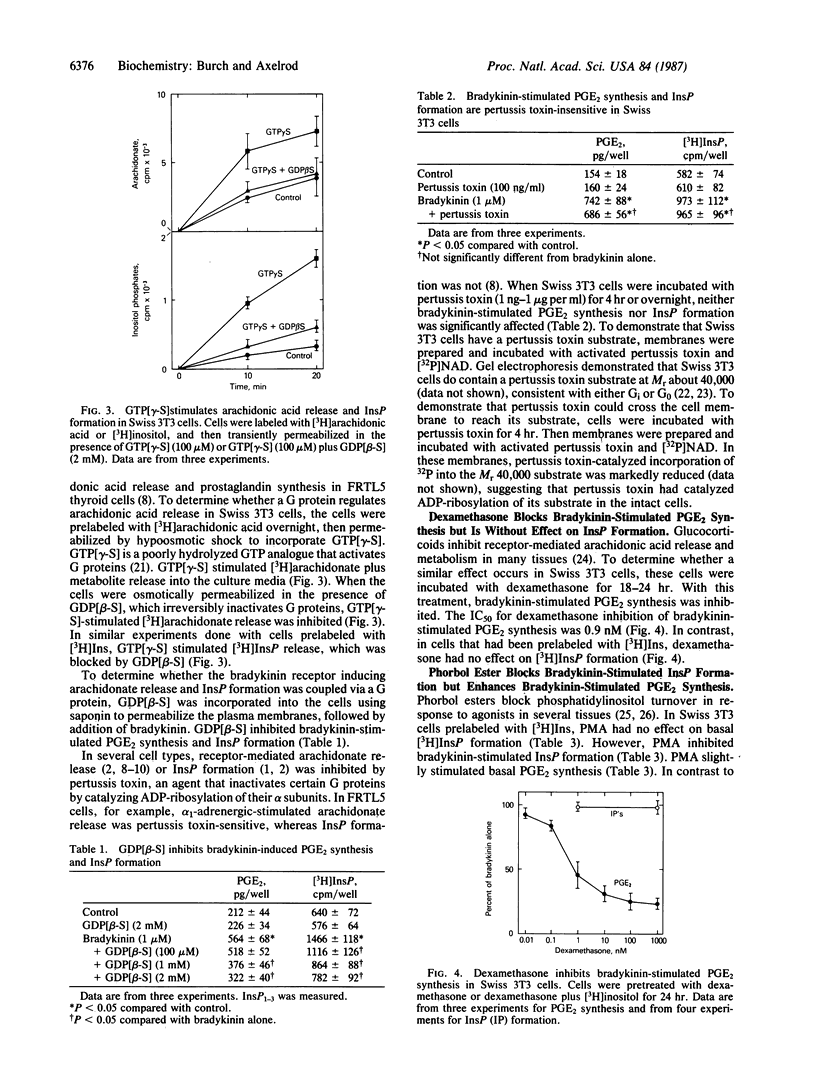
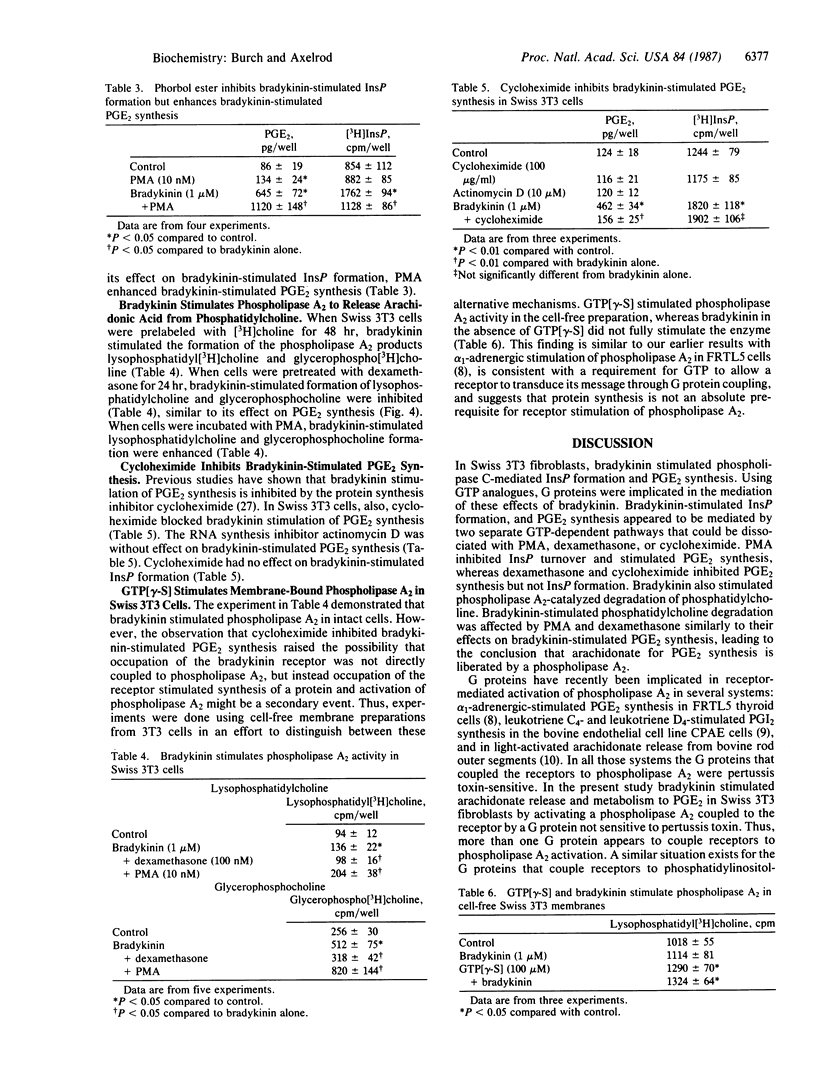
In Swiss 3T3 fibroblasts bradykinin stimulated inositol phosphate (InsP) formation and prostaglandin E2 (PGE2) synthesis. The EC50 values for stimulation of PGE2 synthesis and InsP formation by bradykinin were similar, 200 pM and 275 pM, respectively. Guanosine-5'-[gamma-thio]triphosphate stimulated PGE2 synthesis and InsP formation, and guanosine-5'-[beta-thio]diphosphate inhibited both PGE2 synthesis and InsP formation stimulated by bradykinin. Neither bradykinin-stimulated PGE2 synthesis nor InsP formation was sensitive to pertussis toxin. Phorbol ester, dexamethasone, and cycloheximide distinguished between bradykinin-stimulated PGE2 synthesis and InsP formation. Phorbol 12-myristate 13-acetate enhanced bradykinin-stimulated PGE2 synthesis but inhibited bradykinin-stimulated InsP formation. Pretreatment of cells with dexamethasone for 24 hr inhibited bradykinin-stimulated PGE2 synthesis but was without effect on bradykinin-stimulated InsP formation. Cycloheximide inhibited bradykinin-stimulated PGE2 synthesis but was without effect on bradykinin-stimulated InsP formation. When bradykinin was added to cells prelabeled with [3H]choline, the phospholipase A2 products lysophosphatidylcholine and glycerophosphocholine were generated. In cells pretreated with dexamethasone, lysophosphatidylcholine and glycerophosphocholine formation induced by bradykinin were inhibited. Treatment of cells with phorbol ester enhanced bradykinin-induced formation of these metabolites. The data suggest that bradykinin receptors are coupled by GTP-binding proteins to both phospholipase C and phospholipase A2 and that phospholipase A2 is the enzyme that catalyzes release of arachidonate for prostaglandin synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell R. L., Kennerly D. A., Stanford N., Majerus P. W. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J. 1983 Jun 15;212(3):849–858. doi: 10.1042/bj2120849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. The selective loss of lysophospholipids in some commonly used lipid-extraction procedures. Anal Biochem. 1974 Mar;58(1):238–245. doi: 10.1016/0003-2697(74)90463-1. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Snowdowne K. W. Measurement of intracellular free calcium in monkey kidney cells with aequorin. Science. 1982 Jul 16;217(4556):252–254. doi: 10.1126/science.6806904. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Conway T. M., Bennett C. F., Crooke S. T., Stadel J. M. Islet-activating protein inhibits leukotriene D4- and leukotriene C4- but not bradykinin- or calcium ionophore-induced prostacyclin synthesis in bovine endothelial cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7320–7324. doi: 10.1073/pnas.83.19.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Conway T. M., Shorr R. G., Crooke S. T. Identification and isolation of a mammalian protein which is antigenically and functionally related to the phospholipase A2 stimulatory peptide melittin. J Biol Chem. 1987 Mar 25;262(9):4402–4406. [PubMed] [Google Scholar]
- Clark M. A., Littlejohn D., Mong S., Crooke S. T. Effect of leukotrienes, bradykinin and calcium ionophore (A 23187) on bovine endothelial cells: release of prostacyclin. Prostaglandins. 1986 Jan;31(1):157–166. doi: 10.1016/0090-6980(86)90233-9. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hirata F., Corcoran B. A., Venkatasubramanian K., Schiffmann E., Axelrod J. Chemoattractants stimulate degradation of methylated phospholipids and release of arachidonic acid in rabbit leukocytes. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2640–2643. doi: 10.1073/pnas.76.6.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- Korte K., Casey M. L. Phospholipid and neutral lipid separation by one-dimensional thin-layer chromatography. J Chromatogr. 1982 Oct 8;232(1):47–53. doi: 10.1016/s0378-4347(00)86006-5. [DOI] [PubMed] [Google Scholar]
- Kunze H., Vogt W. Significance of phospholipase A for prostaglandin formation. Ann N Y Acad Sci. 1971 Apr 30;180:123–125. doi: 10.1111/j.1749-6632.1971.tb53191.x. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molski T. F., Naccache P. H., Marsh M. L., Kermode J., Becker E. L., Sha'afi R. I. Pertussis toxin inhibits the rise in the intracellular concentration of free calcium that is induced by chemotactic factors in rabbit neutrophils: possible role of the "G proteins" in calcium mobilization. Biochem Biophys Res Commun. 1984 Oct 30;124(2):644–650. doi: 10.1016/0006-291x(84)91603-6. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Lok J. M., Wolf L. G. Purification and properties of the inhibitory guanine nucleotide regulatory unit of brain adenylate cyclase. J Biol Chem. 1984 Nov 25;259(22):14222–14229. [PubMed] [Google Scholar]
- Okajima F., Ui M. ADP-ribosylation of the specific membrane protein by islet-activating protein, pertussis toxin, associated with inhibition of a chemotactic peptide-induced arachidonate release in neutrophils. A possible role of the toxin substrate in Ca2+-mobilizing biosignaling. J Biol Chem. 1984 Nov 25;259(22):13863–13871. [PubMed] [Google Scholar]
- Paris S., Pouysségur J. Further evidence for a phospholipase C-coupled G protein in hamster fibroblasts. Induction of inositol phosphate formation by fluoroaluminate and vanadate and inhibition by pertussis toxin. J Biol Chem. 1987 Feb 15;262(5):1970–1976. [PubMed] [Google Scholar]
- Pong S. S., Hong S. L., Levine L. Prostaglandin production by methylcholanthrene-transformed mouse BALB/3T3. Requirement for protein synthesis. J Biol Chem. 1977 Feb 25;252(4):1408–1413. [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Smith C. D., Uhing R. J., Snyderman R. Nucleotide regulatory protein-mediated activation of phospholipase C in human polymorphonuclear leukocytes is disrupted by phorbol esters. J Biol Chem. 1987 May 5;262(13):6121–6127. [PubMed] [Google Scholar]
- Sternweis P. C., Robishaw J. D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J Biol Chem. 1984 Nov 25;259(22):13806–13813. [PubMed] [Google Scholar]
- Sweatt J. D., Connolly T. M., Cragoe E. J., Limbird L. E. Evidence that Na+/H+ exchange regulates receptor-mediated phospholipase A2 activation in human platelets. J Biol Chem. 1986 Jul 5;261(19):8667–8673. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Wallace M. A., Fain J. N. Guanosine 5'-O-thiotriphosphate stimulates phospholipase C activity in plasma membranes of rat hepatocytes. J Biol Chem. 1985 Aug 15;260(17):9527–9530. [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]



