Abstract
The literature suggests that nondeclarative, or nonconscious, learning might be impaired among HIV+ individuals compared with HIV− matched control groups, but these studies have included relatively few women. We administered measures of motor skill and probabilistic learning, tasks with a nondeclarative or procedural learning component that are dependent on integrity of prefrontal-striatal systems, to well-matched groups of 148 men and 65 women with a history of substance dependence that included 45 men and 30 women seropositive for HIV. All participants were abstinent at testing. Compared to HIV− women, HIV+ women performed significantly more poorly on both tasks, but HIV+ men’s performance did not differ significantly compared to HIV− men on either task. These different patterns of performance indicate that features of HIV-associated neurocognitive disorder (HAND) can not always be generalized from men to women. Additional studies are needed to address directly the possibility of sex differences in HIV-associated neurocognitive disorder (HAND) and the possibility that women might be more vulnerable to the effects of HIV and substance dependence on some neurocognitive functions.
Keywords: HIV, neurocognition, substance use disorders, addiction, sex differences, procedural learning
Converging evidence has shown that a positive HIV serostatus or history of substance dependence is associated with impaired executive functions critically dependent on the integrity of prefrontal-striatal circuitry, such as response inhibition and decision-making (Bechara et al., 2001; Goldstein & Volkow, 2002). Given the regional distribution of HIV-associated neuropathology, which includes prominent atrophy of caudate and putamen and neuron loss in prefrontal cortex, it is not surprising that HIV-seropositive substance dependent individuals (SDIs) have shown consistent impairment in these functions compared with matched HIV− SDIs. More recently, the question has been raised whether HIV+ SDIs show impaired performance compared with HIV− SDIs on non-executive tasks that are critically dependent on striatal integrity.
“Nondeclarative” memory refers to acquisition and retention processes with a nonconscious component that are critically (although not solely) dependent on the integrity of prefrontal-striatal neural systems. ”Procedural” learning (PL) refers to a type of nondeclarative memory for motor and cognitive abilities that is often disrupted among patients with basal ganglia disorders, such as Parkinson disease. PL integrity has thus been of particular interest to investigators of HIV-associated neurocognitive disorder (HAND). Several studies have reported that HIV+ subjects performed more poorly on motor skill learning tasks such as the Rotary Pursuit compared with HIV− controls (e.g., Gonzalez et al., 2008; A. Martin, Heyes, Salazar, Law, & Williams, 1993). In a recent study by Gonzalez et al. (2008), HIV+ SDIs performed poorly overall on two measures of motor skill learning, the rotary pursuit task (RPT) and the Star Mirror Tracing (SMT). Overall SMT performance was significantly impaired among the HIV+ compared with the HIV− subjects while group comparison of overall RPT performance showed a nonsignificant trend (p=.06) toward impaired performance among the HIV+ participants; however, the groups showed no evidence of different learning rates, as indexed by patterns of performance across trial blocks. The investigators concluded that the HIV+ participants’ impaired performance on these tasks was more consistent with a general deficit in complex motor skills rather than a selective impairment of procedural learning. This conclusion received support from their finding of no significant differences in performance among HIV+ and HIV− participants on the Weather Prediction Task (WPT), a non-declarative measure of probabilistic learning without a significant motor component (Knowlton, Mangels, & Squire 1996).
It is critical to note that these studies tested all- or predominantly male samples of HIV+ individuals. Relatively few studies of HIV-associated neurocognitive disorder (HAND) have included sufficient numbers of HIV+ women to explore gender differences (e.g., Fox-Tierney, Ickovics, Cerreta, & Ethier, 1999). Multiple lines of evidence suggest that patterns of neurocognitive performance observed among HIV+ compared with HIV− men might not be readily generalizable to women, although the current state of knowledge is insufficiently detailed to support more specific hypotheses regarding sex differences in HAND. For example, male performance advantage, sex differences in underlying regional brain activity, and sex differences in patterns of deficit following lateralized damage to the ventromedial prefrontal cortex have all been reported for a specific measure with known sensitivity to HIV, the Iowa Gambling Task, (Bolla, Eldreth, Matochik, & Cadet,, 2004; Hardy, Hinkin, Levine, Castellon, & Lam, (2006). Martin et al., 2004; Tranel, Damasio, Denburg & Bechara, 2005). Additionally, there are high concentrations of estrogen receptors in multiple brain regions affected by HIV, including striatum (Donahue et al., 2000); prefrontal cortex (MacLusky, Naftolin, & Goldman-Rakic, 1986) and hippocampus (Lu, Zeng, Swaab, Ravid, & Zhou, 2004; Ishunina, Fischer & Swaab, 2007), raising the question of increased detrimental effects on some aspects of neurocognitive function among HIV+ women since fluctuations in ovarian hormone activity can influence both declarative and non-declarative memory performance (e.g., Maki, Rich, & Rosenbaum, 2002); conversely, estradiol is protective of striatal dopamine neurons (see Dluzen, 2000 for a review), suggesting that HIV+ women might be paradoxically less vulnerable to impairment of some aspects of neurocognitive function.
In this study we administered the rotary pursuit task (RPT) and the Weather Prediction Task (WPT) to an independent sample of HIV+ and HIV− SDIs that included a larger group of women compared with previous studies of PL and HIV, in order to explore the possibility that patterns of PL might be different for HIV+ men and women compared with their respective HIV-controls. Different patterns of neurocognitive performance (and specifically in learning) associated with gender and HIV serostatus would have significant translational implications for development of targeted interventions such as cognitive stimulation therapies or behavioral components of addiction treatment.
Method
Participants
Study participants were recruited from infectious disease and substance dependence treatment clinics at the University of Illinois-Chicago and the VAMC-Jesse Brown, community substance dependence treatment programs, and by word of mouth. We studied 148 men and 65 women with a mean age of 41.3 years (IQR = 37, 47) and mean education of 11.9 years (IQR 11, 13) enrolled in a larger ongoing study of cognitive effects of HIV and drug abuse. The participant sample was approximately 87% African American and 13% Euro-American or Hispanic; there were no differences across groups in ethnic composition, χ2 < 1. All subjects met DSM-IV criteria for past cocaine or heroin dependence. Exclusion criteria for both HIV+ and HIV− groups included a positive rapid urine toxicology screen or Breathalyzer result at testing, history of AIDS-defining or other CNS disease, a diagnosis of current substance use disorder or major depression by DSM-IV criteria, positive hepatitis C serostatus, or history of schizophrenia, learning disability, open head injury of any type, seizure disorder or closed head injury with more than 30 minutes’ loss of consciousness. The majority of subjects abused multiple substances: 70% of subjects were diagnosed with previous alcohol dependence; 60% with cannabis dependence; 50% with opioid dependence; and 82% with previous cocaine dependence. No subject had participated in the previous procedural learning study conducted by our group (Gonzalez et al., 2008).
The HIV-seropositive group included 30 women and 45 men. All HIV+ subjects were ambulatory and capable of outpatient testing; median CD4 count was 315 and 30% of participants were diagnosed with immunologic AIDS (CD4 < 200) at the time of testing. HIV RNA (viral load) levels were undetectable among 64% of the sample, and 89% were currently prescribed highly active antiretroviral therapy (HAART). There were no significant sex differences on any of these disease characteristics at testing (Current CD4: Mann Whitney Z = −.97, p = .33; nadir CD4; Mann-Whitney Z = −1.61, p = .11; undetectable viral load: χ2 < 1; current AIDS diagnosis: χ2(1) = 1.87, p = .17; and current HAART: χ2 < 1 There was a nonsignificant trend toward a greater lifetime prevalence (76%) of an immunologic AIDS diagnosis [i.e. a nadir CD4 count < 200] among men compared with women (76% vs. 54%; χ2(1) = 3.3, p = .07). The HIV− SDI group included 35 women and 103 men verified HIV− by ELISA at testing.
Abstinence from cocaine, opioids, and methamphetamine was verified by rapid urine toxicology screening and breath tests for alcohol were negative for all subjects. Approximately 6% of subjects tested positive for tetrahydrocannabinol (THC), consistent with relatively recent marijuana use; following accepted practice in the current literature (e.g., Rippeth et al., 2004) we did not exclude these participants, since THC can be detected on urine toxicology screens up to a month following cessation of use and no participant was acutely intoxicated.
Rotary Pursuit
The RPT (Lafayette Instruments) has been widely used in the literature to measure procedural learning of motor skills among healthy participants and multiple clinical groups. Patients with diseases that primarily affect the basal ganglia (e.g., Huntington's and Parkinson's disease) tend to show impaired performance on the RPT relative to controls and relative to patients with neurodegenerative diseases that show relative sparing of basal ganglia, such as Alzheimer disease or midtemporal amnestic disorder (Salmon & Butters, 1995). The testing apparatus consists of a turntable with a disk, which can be set to rotate at varying speeds. The RPT requires participants to hold a plastic stylus steadily over a target (patch of light) on a rotating disk as it spins (van Gorp et al., 1999). Participants complete 10 20-second testing trials with a 10s rest following each trial1. For the present study the revolutions per minute (RPMs) of the turntable was set to 55 for all participants; this value represents the average RPMs reported from previous studies using the pursuit rotor among healthy controls and patient groups without dementia (Weickert et al., 2002). We chose to employ a single RPM in order to minimize potential procedural learning likely to occur with the "training-to-criterion" method sometimes employed to establish individual RPM for each subjects. Current experience in our laboratory (i.e., Gonzalez et al., 2008) with this task suggests that this speed is adequate for our patient population in that it appears to be of appropriate difficulty to minimize floor effects, but challenging enough to prevent participants from rapidly reaching an asymptote in their performance. Time on target is the outcome measure calculated for each trial.
Weather Prediction Task
The WPT is a computerized task has been used successfully as a probe of probabilistic classification learning in healthy adults (Poldrack, Prabhakaran, Seger, & Gabrieli, 1999; Poldrack et al., 2001) and in patient groups with basal ganglia pathology or midtemporal amnesia (Knowlton et al.,, 1996; Shohamy, Myers, Onlaor, & Gluck, 2004). Test stimuli consist of a set of four cards with varying geometric patterns. On each trial, a combination of up to three different cards is displayed on the computer monitor. The subjects are told that some card combinations predict sunny weather and others predict rain. They are instructed to press a key labeled "sun" or a key labeled "rain" to register their prediction as each card display appears. Participants are told that they may need to guess at first. No other instructions are provided. After each key-press, the computer provides visual and auditory feedback to indicate if the choice is correct. Participants are administered 200 consecutive trials. For 50% of trials the correct outcome is "sun" and for 50% the correct outcome is "rain." Each of the four display cards is associated with a fixed probability of predicting "rain" or "sun." The test employs a total of 14 different card patterns, each with a specific probability of occurring across the 200 trials. "Optimal" responses are those when a participant chooses the most likely outcome associated with a particular combination of cards, regardless of the feedback provided by the computer on that given trial (i.e., optimal responses are based on probabilities). For example, if 80% is the fixed probability of a specific card combination, on 80% of the trials this combination appears “rain” might be the correct response; in this instance on the additional 20% of trials the “rain” response would be considered incorrect. Task performance is measured by the percent of optimal responses, overall and for each of four 50-trial blocks. These procedures (Knowlton et al., 1996) and the probability structure employed (Gluck, Shohamy, and Myers, 2002, Experiment 2) have been previously described in detail. As with Gonzalez et al (2008) we modified the WPT parameters to minimize any impact that processing speed problems may have on task performance by allowing participants 10 seconds to provide a response, rather than 5 seconds.
Results
Group characteristics
We conducted a preliminary series of analyses in order to determine the comparability of the participant groups (i.e., HIV− men, HIV+ men, HIV− women and HIV+ women) on a series of demographic, substance abuse and comorbid characteristics using one-way analyses of variance (ANOVA) for continuous data and chi-square analyses for categorical data, employing a significance level of .01 to minimize the possibility of Type I error. Tables 1 to 3 show the results of these comparisons. Results of this series of analyses indicated satisfactory matching of groups, sexes, and sex within groups on demographic and substance use characteristics and on comorbid conditions associated with substance use disorders (SUDs).
Table 1.
Demographic Characteristics for All Participants
| Men | Women | |||||
|---|---|---|---|---|---|---|
| n |
HIV− 103 M (SD) |
HIV+ 45 M (SD) |
HIV− 35 M (SD) |
HIV+ 30 M (SD) |
F | P |
| Age, yrs | 42.4 (8.4) | 41.0 (7.0) | 41.7 (8.2) | 39.9 (7.6) | .89 | .45 |
| Education, yrs | 11.7 (1.5) | 12.4 (2.1) | 12.0 (2.0) | 11.8 (1.8) | 1.53 | .21 |
| Estimated IQ | 87.2 (9.4) | 85.5 (8.3) | 89.8 (11.6) | 86.7 (8.9) | 1.32 | .27 |
| % African American | 84 | 87 | 82 | 100 | χ2=11.23 | .26 |
Note: All values are means unless otherwise indicated. Full Scale IQ (FSIQ) was estimated using the Wechsler Test of Adult Reading (WTAR).
Table 3.
Comorbid Characteristics for All Groups
| Men | Women | |||||
|---|---|---|---|---|---|---|
|
HIV− M (SD) |
HIV+ M (SD) |
HIV− M (SD) |
HIV+ M (SD) |
F | p | |
| SSS-V | 14.9 (5.8) | 13.5 (5.0) | 14.2 (6.6) | 14.8 (5.5) | 0.70 | .55 |
| PCLC | 35.8 (12.5) | 37.7 (14.3) | 34.8 (17.0) | 36.3 (13.1) | 0.30 | .82 |
| Wender | 32.5 (18.5) | 33.5(20.1) | 27.4 (19.3) | 33.0 (19.0) | 0.80 | .49 |
| SRPS | 52.2 (10.5) | 52.9 (10.9) | 47.7 (8.8) | 54.8 (9.6) | 2.8 | .04 |
| BDI-II | 10.4 (7.8) | 15.2 (13.7) | 13.2 (10.6) | 11.3 (8.8) | 2.8 | .04 |
Note. All descriptive statistics are means unless otherwise indicated; SSS-V = Sensation Seeking Scale v. 5; PCLC = PTSD Checklist: Civilian version; Wender = Wender Utah Rating Scale; SRPS = Self-Report Psychopathy Scale; BDI-II = Beck Depression Inventory-II.
Rotary Pursuit
Figure 1 shows the Rotary Pursuit task performance. Following Gonzalez et al. (2008), we reduced data from 10-trial to 5-trial blocks by averaging trials 1 and 2, trials 3 and 4, and so forth, in order to decrease variability. The RPT data were analyzed by mixed factor analysis of variance (ANOVA) with HIV Serostatus and Sex as between-subject factors and Trial Block as the within-subjects factor. Analysis of the RPT data showed the expected significant main effect for Trial Block, F (4,824) = 97.0, p < .00001, verifying that task performance improved (e.g., time on target increased) significantly over trial blocks across Serostatus and Sex. There was a marginally significant trend toward poorer overall RPT performance by the HIV+ compared to the HIV− group, F(1,206) = 3.54, p = .06, and no significant main effect for Sex, F < 1. There was a significant Sex x Serostatus interaction, F (1,206) = 4.42, p < .05, ε2 = .02; follow up ANOVA indicated that HIV− women outperformed the HIV+ women, showing significantly more time on target across Trial Blocks, F (1, 59) = 6.80, p = .01 ε2 = .09, but there were no significant differences in overall RPT performance between HIV+ and HIV− men, F < 1. There were no statistically significant Serostatus x Trial Block, Sex x Trial Block, or Sex x Serostatus x Trial Block interactions, F < 1 for each comparison.
Figure 1.
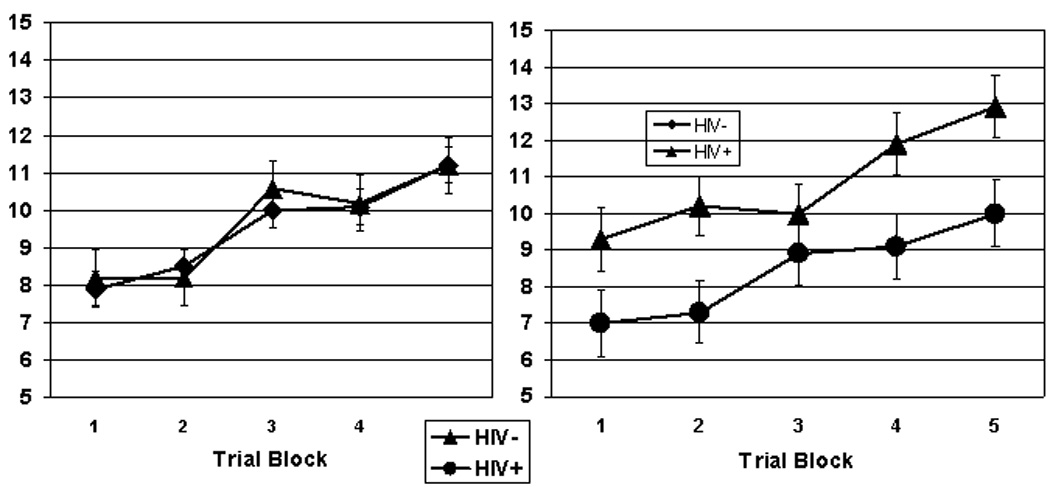
Rotary Pursuit Task performance for HIV+ vs. HIV− men (left) and HIV+ vs. HIV− women (right)
Weather Prediction Task
Figure 2 shows WPT performance. WPT data were also analyzed by mixed factor ANOVA with Sex and Serostatus as between factors and Trial Block as within factor. Subjects’ performance of the WPT showed the expected significant main effect for trial block, F(3,634) = 11.7, p < .00001, verifying that overall performance improved significantly (i.e., total number of optimal choices increased) across trial blocks, serostatus, and sex. Review of main effects for Serostatus and Sex indicated that the HIV+ group performed the WPT significantly more poorly overall compared with HIV− controls, F(1,208) = 4.99, p < .05, ε2 = .02,. The main effect for Sex and the Sex x Serostatus, Block x Sex and Block x Serostatus interactions did not reach statistical significance, F < 1 for each test. However, we found a significant Block x Sex x Serostatus interaction, F(3,600) = 3.12, p = .02, ε2 = .02, indicating that the Block x Serostatus interaction differed significantly for men and women; this suggested that the learning rate for HIV+ compared to HIV-participants was different for men and women. Thus, we analyzed the linear trend components of the Block x Serostatus interaction separately for men and women. The linear component of the Block x Serostatus interaction differed significantly for the women, F(1,62)=4.12, p = .05, ε2 = .06, but not for the men, F(1,146) = 2.71, p = .10. Inspection of the means (Figures 3 and 4) indicated that HIV− women’s WPT performance steadily increased while the HIV+ women’s performance showed minimal improvement, F < 1.
Figure 2.
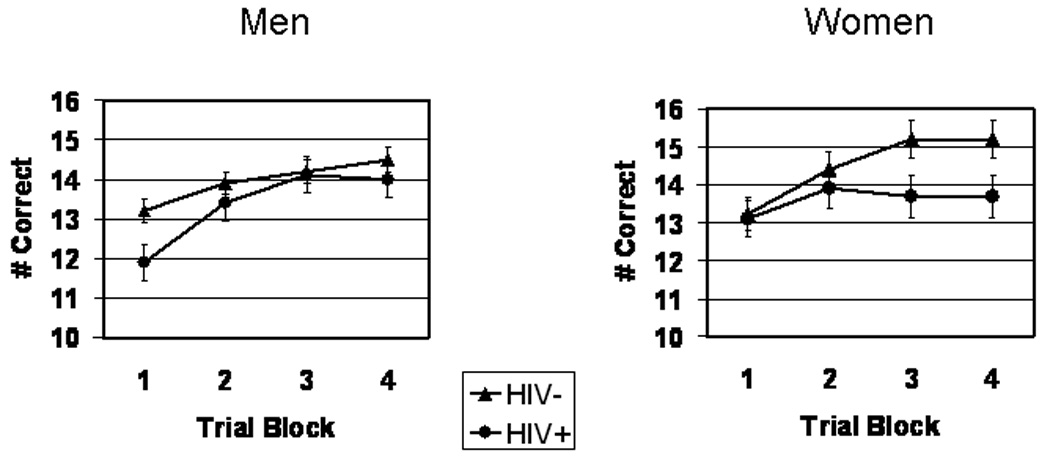
Weather Prediction Task performance for HIV+ vs. HIV− men (left) and HIV+ vs. HIV− women (right)
We then repeated both PL analyses controlling for BDI and SRPS scores and results were unchanged.
HIV-associated variables and PL task performance
We then conducted a series of exploratory analyses of data for the HIV+ groups in order to evaluate potential relationships between task performance and indicators of disease status for men and women. We employed a probability level of .01 for these exploratory analyses to reduce the chance of Type I error. For the HIV+ women, current CD4 count correlated significantly with overall RPT performance, r = .50, p = .005, but not with WPT, r = .30, p =.12. There were no significant performance differences for HIV+ women with undetectable versus detectable HIV RNA (RPT: t < 1; WPT: t(26) = −1.1, p =.32). CD4 counts did not correlate significantly with either task for the HIV+ men, (RPT: r = .06, p = .68; WPT: r = −.14, p = .37), and both RPT and WPT performance did not differ significantly for men with detectable versus undetectable viral load, F < 1 for both comparisons. Almost all subjects were prescribed HAART at testing so no comparisons were made between treated versus untreated subjects.
Discussion
Performance on tasks with a procedural learning component depends primarily on the integrity of prefrontal-striatal brain systems, regions prominently affected by HIV, with critical involvement of striatum. There is mixed evidence in the literature of HIV-associated deficits in PL, but available studies have tested relatively few women (e.g., Martin et al., 1993; Gonzalez et al., 2008). In the current study we administered tasks of motor skill and probabilistic classification learning to well-matched groups of substance dependent men and women to explore patterns of PL task performance among HIV+ men and women compared with their respective HIV− peers. We found that the HIV+ women performed both the motor (Rotary Pursuit Task (RPT) and cognitive (Weather Prediction Task (WPT) learning tasks significantly more poorly compared with HIV− women, but HIV+ compared to HIV− men’s performance did not differ significantly on either of these tasks. Although all participants showed significant improvements in their performance across Trial Blocks of the RPT, suggesting adequate PL, this was not the case for the WPT. HIV+ women did not show significant improvements in their performance across Trial Blocks of the WPT, whereas HIV− women, HIV+ men, and HIV− men did improve significantly.
Gonzalez et al (2008) recently reported that compared to HIV− controls, HIV+ participants performed the Star Mirror Tracing (SMT) significantly more poorly and showed a marginally significant trend toward impaired performance of the RPT but showed no evidence of impairment on the Weather Prediction Task; however, their study sample was primarily male. In the current study, we did not employ the SMT, but did find that the HIV+ women showed overall poorer performance on the RPT compared with HIV− women but showed no evidence of a specific PL impairment, which would have been manifested by a significant interaction involving the Trial Block factor; these RPT findings support Gonzalez et al.’s hypothesis that impaired complex motor function, rather than PL deficits, might underlie the HIV+ subjects’ poorer RPT performance. Our findings reinforce Gonzalez et al.’s conclusion that more specific measures of simple and complex motor performance are needed to investigate the underlying mechanisms of impaired RPT performance among HIV+ compared with HIV− SDIs. Additionally, our finding of a sex-specific deficit in RPT performance suggests that the trend toward significantly poorer overall RPT performance among HIV+ compared with HIV− groups observed by Gonzalez et al might have been driven primarily by deficits among the small number of HIV+ women in their sample. Finally, RPT performance for the HIV+ women correlated significantly and positively with current CD4 count, suggesting that RPT scores might index current level of immune function; however, this finding awaits replication, since RPT performance did not correlate significantly with CD4 among the HIV+ men.
The HIV+ women in our study also performed more poorly compared to HIV− women on the Weather Prediction Task, a measure of probabilistic classification learning with essentially no motor component. Unlike the RPT findings, the HIV+ women’s pattern of WPT performance was consistent with a specific impairment in PL; the linear increase in their task performance over trial blocks was significantly lower than the HIV− women’s performance. One might speculate that impairments in different component functions or underlying neural systems might contribute to the HIV+ women’s deficits in WPT compared with RPT performance. This possibility is compatible with reports from the cognitive neuroscience literature emphasizing activity of both striatal and medial-temporal-hippocampal systems during probabilistic learning (e.g., Poldrack et al., 1999; Poldrack & Packard, 2003), in relative contrast with primary involvement of striatal and cerebellar involvement during motor skill learning (Doyon & Benali, 2005; Doyon, Penhune, & Ungerleider, 2003). However, these studies also caution against overly simplistic models of PL or dichotomous classification of declarative/nondeclarative learning systems: for example, multiple learning strategies can be employed for successful WPT performance (Gluck et al., 2002) and WPT task parameters can be manipulated to increase demands on declarative memory with corresponding changes in patterns of brain activity (Poldrack et al., 2001). Thus, questions of differences in underlying component functions or patterns of brain activity contributing to HIV+ women’s performance on PL tasks are appealing but entirely speculative at present and await more detailed study.
Direct investigation of possible sex differences in PL task performance among HIV+ drug users and their potential neural mechanisms is the logical next step in this program of studies. Compared to men, women show less dopamine release from the caudate following amphetamine administration (Munro et al., 2006) and show higher presynaptic dopamine synthesis capacity (Laakso et al., 2002); so the specificity of the impairment among only the HIV+ women may reflect sex differences in the extent to which these two non-declarative tasks engage striatal mechanisms. Alternatively, it is possible that the HIV+ women were less able than men to compensate for prefrontal-striatal dysfunction by using midtemporal-hippocampal learning strategies to perform the WPT successfully. The hippocampus is noteworthy for high concentrations of both virus and estrogen receptors (Maki & Martin, 2009) and hippocampal activity is significantly lower among HIV+ individuals compared with HIV− controls in functional brain imaging studies (Maki et al., 2009; Castelo, Sherman, Courtney, Melrose, & Stern, 2006).
The question of generalizability of our findings beyond nondeclarative learning will require future studies with measures of additional neurocognitive functions such as declarative learning. Interpretation of our results is also necessarily limited because all participants had a history of substance use disorder (SUD). There is strong evidence in the literature that HIV can interact with certain substances of abuse resulting in additive or synergistic effects on neurocognition (Gonzalez & Cherner, 2008; Rippeth et al., 2004; Sassoon et al., 2007). One might speculate that any sex differences in neurocognition among HIV+ drug users might reflect a greater vulnerability to these interactive effects among HIV+ women. In this regard we note that significantly more HIV+ men in the current study had a lifetime diagnosis of immunologic AIDS compared to HIV+ women, suggesting that the HIV+ women’s impairment on PL tasks might appear earlier in the course of disease progression
Findings from the addiction neuroscience literature also support the possibility that combined effects of a positive HIV serostatus and substance dependence could influence neurocognitive performance differently for men and women, though considerable work is needed to disentangle these effects. Numerous studies have documented sex differences in components of the addictive process (Cahill, 2006; Wetherington, 2007); for example, women escalate their drug use more rapidly than men and are more responsive to drug-related cues (Becker & Hu, 2008). Additionally, underlying neural correlates of addictive behavior observed among women are in some cases distinct or even opposite of effects observed in males. For example, a PET study by Kilts, Gross, Ely & Drexler (2004) demonstrated that cue-induced craving was associated with increased amygdala activation in males but decreased activity in women. Finally, Adinoff et al (2006) have reported evidence of sex differences in the effects of cocaine use on OFC activation. In the absence of a non-SDI control group we cannot rule out the possibility that our results can be explained partly or entirely by sex differences in neurocognitive aspects of addiction rather than HIV serostatus. Inclusion of non drug using control groups will be enable us to address this question more directly by comparing neurocognitive performance between men and women with single (i.e., HIV or substance dependence), multiple or no risk factors, and these studies are currently in progress in our laboratory. Additionally, our observations were limited to behavioral performance of experimental cognitive tasks without functional neuroimaging or standard clinical neuropsychological tasks; collaborative studies incorporating these measures are currently in progress (e.g., Maki et al., 2009).
Current recommendations for the neurocognitive assessment of HIV disease are essentially gender neutral; however, Maki and Martin (2009) note that possibly female-specific patterns of HAND have not been investigated despite their potential significance. The results of this descriptive preliminary study suggest neurocognitive test performance by HIV+ men is not automatically generalizable to HIV+ women. Further investigation of sex differences in learning and memory function in this population could have critical translational implications such as emphasis on different learning strategies for men and women in substance abuse treatment as well as management of HIV disease (Cahill, 2006).
Table 2.
Substance Use Characteristics – All Subjects
| Men | Women | |||||
|---|---|---|---|---|---|---|
| HIV− M(SD) |
HIV+ M (SD) |
HIV− M (SD) |
HIV+ M (SD) |
Test Statistic |
p | |
| Addiction Severity Index (means) | ||||||
| Alcohol | .15 (.12) | .14 (.09) | .14 (.11) | .15 (.13) | F = 0.07 | .98 |
| Drug | .08 (.05) | .07 (.04) | .07 (.04) | .06 (.04) | F = 1.16 | .32 |
| Years used (means): | ||||||
| Alcohol | 19.77 (10.91) | 20.88 (10.73) | 16.88 (9.84) | 20.39 (10.77) | F = 0.98 | .40 |
| Cocaine | 14.0 (8.67) | 13.1 (8.7) | 12.4 (8.0) | 14.3 (7.94) | F = 0.36 | .78 |
| Opioids | 14.3 (10.51) | 16.4 (12.0) | 16.1 (9.44) | 15.5 (11.25) | F = 0.25 | .86 |
| Days since use (median, IQR) | ||||||
| Alcohol | 251 (661) | 2920 (3763) | 335 (3590) | 608 (1611) | χ2 = 1.51 | .68 |
| Cocaine | 251 (1377) | 306 (2739) | 260 (1755) | 191 (744) | χ2 = 1.64 | .65 |
| Opioids | 183 (986) | 547 (3237) | 308 (2199) | 456 (1493) | χ2 = 1.43 | .70 |
| % Past Dependence | ||||||
| Alcohol | 65 | 82 | 68 | 67 | χ2 = 4.55 | .21 |
| Cannabis | 63 | 56 | 63 | 53 | χ2 = 1.46 | .69 |
| Cocaine | 79 | 82 | 86 | 83 | χ2 = 0.70 | .87 |
| Opioid | 55 | 44 | 41 | 47 | χ2 = 2.90 | .41 |
| % IDU | 10 | 5 | 7 | 7 | χ2= 1.13 | .77 |
Table 4.
Disease characteristics for HIV+ Participants
| Men | Women | Test Statistic* | p | |
|---|---|---|---|---|
| % HAART | 91 | 86 | χ2 = .40 | .53 |
| % Current AIDS | 36 | 21 | χ2 = 1.87 | .11 |
| % Lifetime AIDS | 76 | 54 | χ2 = 3.3 | .07 |
| Current CD4 count (median) | 292 | 370 | Mann Whitney Z = −.97 | .33 |
| Nadir CD4 count (median) | 90 | 186 | Mann-Whitney Z = −1.61 | .11 |
| % undetectable HIV RNA | 62 | 66 | χ2 = .08 | .77 |
Acknowledgements
We thank Leslie Ladd, Sarah Wicks, Erin Scheidemantel and Brian Wolf for subject testing.
Supported by grants from the National Institute on Drug Abuse to EM (R01DA12828) and RG (K23DA023560)
References
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr. Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gender Medicine. 2006;3:206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Frontiers in Neuroendocrinology. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cerebral Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bouwman FH, Skolasky RL, Hes D, Selnes OA, Glass JD, Nance-Sproson TE, Royal W, Dal Pan GJ, McArthur JC. Variable progression of HIV-associated dementia. Neurology. 1998;50:1814–1820. doi: 10.1212/wnl.50.6.1814. [DOI] [PubMed] [Google Scholar]
- Cahill L. Why sex matters in neuroscience. Nature Reviews Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Castelo JMB, Sherman SJ, Courtney MG, Melrose RA, Stern CE. Altered hippocampal-prefrontal activation in HIV patients during episodic memory encoding. Neurology. 2006;66:1688–1695. doi: 10.1212/01.wnl.0000218305.09183.70. [DOI] [PubMed] [Google Scholar]
- Chiesi A, Vella S, Dally G, Pedersen C, Danner S, Johnson AM, Schwander S, Goebel FD, Glauser M, Antunes F. Epidemiology of AIDS Dementia Complex in Europe. AIDS in Europe Study Group. Journal of Acquired Immune Deficiency Syndromes. 1996;11:39–44. doi: 10.1097/00042560-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Dluzen DE. Neuroprotective effects of estrogen on the nigrostriatal dopamine system. Journal of Neurocytology. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- Donahue JE, Stopa EG, Chorsky RL, King JC, Schipper HM, Tobet SA, Blaustein JD, Reichlin S. Cells containing immunoreactive estrogen receptor-alpha in the human basal forebrain. Brain Research. 2000;8:142–151. doi: 10.1016/s0006-8993(99)02413-0. [DOI] [PubMed] [Google Scholar]
- Doyon J, Benali H. Reorganization and plasticity in the adult brain during learning of motor skills. Current Opinion in Neurobiology. 2005;15:161–167. doi: 10.1016/j.conb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia. 2003;41:252–262. doi: 10.1016/s0028-3932(02)00158-6. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Fox-Tierney RA, Ickovics JR, Cerreta CL, Ethier KA. Potential sex differences remain understudied: A case study of the inclusion of women in HIV/AIDS-related neuropsychological research. Review of General Psychology. 1999;3:44–54. [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the weather prediction task? Individual variability in strategies for probabilistic category learning. Learning and Memory. 2002;9:408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Cherner M. Co-factors in HIV neurobehavioural disturbances: substance abuse, hepatitis C and aging. International Review of Psychiatry. 2008;20:49–60. doi: 10.1080/09540260701872028. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Jacobus J, Vassileva J, Quartana P, Amatya A, Martin E. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence. Neuropsychology. 2008:240–251. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. Journal of Neuroscience. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishunina TA, Fischer DF, Swaab DF. Estrogen receptor alpha and its splice variants in the hippocampus in aging and Alzheimer's disease. Neurobiology and Aging. 2007;28:1670–1681. doi: 10.1016/j.neurobiolaging.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Hinkin CH, van Gorp WG, Castellon SA, Satz P. Depression predicts procedural but not episodic memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 1998;20:529–535. doi: 10.1076/jcen.20.4.529.1473. [DOI] [PubMed] [Google Scholar]
- Keane TM, Wolfe J, Taylor KL. Post-traumatic stress disorder: Evidence for diagnostic validity and methods of psychological measurement. Journal of Clinical Psychology. 1987;43:32–43. doi: 10.1002/1097-4679(198701)43:1<32::aid-jclp2270430106>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kilts CD, Gross R, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. American Journal of Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Laakso A, Vilmane H, Bergmana J, Haaparantab M, Solina O, Syvälahtie E, Salokangasc RKR, Hietala J. Sex differences in striatal presynaptic dopamine synthesis capacity in healthy subjects. Biological Psychiatry. 2002;52:759–763. doi: 10.1016/s0006-3223(02)01369-0. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a non institutionalized population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Lu Y-P, Zeng M, Swaab DF, Ravid R, Jiang-Ning Zhou. Colocalization and alteration of estrogen receptor-alpha and -beta in the hippocampus in Alzheimer's disease. Human Pathology. 2004;35:275–280. doi: 10.1016/j.humpath.2003.11.004. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proceedings of the National Academy of Sciences U.S.A. 1986;83:513–516. doi: 10.1073/pnas.83.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer EM. HIV, cognition and women. Neuropsychology Review. 2009;19:204–214. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs. HIV-negative women. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- Martin A, Heyes MP, Salazar AM, Law WA, Williams J. Impaired motor-skill learning, slowed reaction time, and elevated cerebrospinal fluid quinolinic acid in a subgroup of HIV-infected individuals. Neuropsychology. 1993;7:149–157. [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. Journal of the International Neuropsychological Society. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- Martin-Thormeyer E, Paul RH. Drug abuse and Hepatitis C infection as comorbid features of HIV Associated Neurocognitive Disorder: Neurocognitive and neuroimaging features. Neuropsychology Review. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, O'Brien CP. New data from the Addiction Severity Index: Reliability and validity in three centers. Journal of Nervous and Mental Disease. 1985;173:12–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- Munro C, McCaul M, Wong D, Oswald L, Zhou Y, Brasic J, Kuwabara H, Kumar A, Alexander M, Ye W. Sex differences in striatal dopamine release in healthy adults. Biological Psychiatry. 2006;59:966–974. doi: 10.1016/j.biopsych.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso MJ, Myers C. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/s0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I the HNRC Group. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10:1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinions in Neurobiology. 1995;5:184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Sassoon SA, Fama R, Rosenbloom MJ, O’Reilly A, Pfefferbaum A, Sullivan EV. Component cognitive and motor processes of the Digit Symbol Test: Differential deficits in alcoholism, HIV infection, and their comorbidity. Alcoholism: Clinical and Experimental Research. 2007;31:1315–1324. doi: 10.1111/j.1530-0277.2007.00426.x. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Onlaor S, Gluck MA. Role of the basal ganglia in category learning: How do patients with Parkinson’s disease learn? Behavioral Neuroscience. 2004;118:676–686. doi: 10.1037/0735-7044.118.4.676. [DOI] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, Klein Z. Psychometric characteristics of the Wender Utah Rating Scale (WURS): Reliability and factor structures for men and women. Psychopharmacology Bulletin. 1995;31:425–433. [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Wilkins JN, Hinkin CH, Moore LH, Hull J, Horner MD, et al. Declarative and procedural memory functioning in abstinent cocaine abusers. Archives of General Psychiatry. 1999;56:85–89. doi: 10.1001/archpsyc.56.1.85. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, Weinberger DR, Goldberg TE. Habit and skill learning in schizophrenia: Evidence of normal striatal processing with abnormal cortical input. Learning and Memory. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington CL. Sex-gender differences in drug abuse: A shift in the burden of proof? Experimental and Clinical Psychopharmacology. 2007;15:411–417. doi: 10.1037/1064-1297.15.5.411. [DOI] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation seeking: A comparative approach. Neuropsychobiology. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]


