Abstract
Background
Some patients with Zollinger-Ellison syndrome(ZES) postcurative gastrinoma resection continue to show gastric acid hypersecretion, however the mechanism is unknown.
Aim
Prospectively study acid secretion following curative gastrinoma resection and analyze factors contributing in patients with ZES.
Methods
Fifty patients cured post gastrinoma resection were studied with serial assessments of acid-secretory status, cure status and ECL-cell status/activity (with serial biopsies, CgA, urinary N-MIAA). Correlative analysis was performed to determine predictive factors.
Results
Hypersecretion occurred in 31 patients(62%) and 14 had extreme-hypersecretion. There was an initial decline(3–6 mos) in BAO/MAO and then remained stable for the 8 years. Preoperative-BAO correlated with the postoperative secretion, but not other clinical, tumoral, laboratory variables, the degree of postoperative acid suppression or type of antisecretory drug needed. Hypersecretors had greater postoperative ECL changes (P= 0.005), serum CGA (P= 0.009) and 24-hr urinary N-MIAA (P=0.0038)
Conclusions
Postcurative-resection, gastric hypersecretion persists long-term (mean-8 yrs) in 62% of patients and in 28% it is extreme, despite normogastrinemia. No preoperative variable except BAO correlates with postresection hypersecretion. The persistent increased ECL-cell extent postcurative resection suggests prolonged hypergastrinemia can lead to changes in ECL-cells that are either irreversible in humans or sustained by unknown mechanisms not involving hypergastrinemia and which can result in hypersecretion, in a proportion of which it can be extreme. Whether similar findings may occur in patients with idiopathic GERD treated for prolonged periods (>10 years) with PPIs, at present is unknown.
Keywords: Zollinger-Ellison syndrome, gastrinoma, acid secretion, proton pump inhibitors, ECL cells
Introduction
Zollinger-Ellison syndrome (ZES) is caused by gastric acid hypersecretion due to the ectopic release of gastrin from a neuroendocrine tumor (i.e. gastrinoma)[1,2]. Because the diagnosis is often delayed, patients have hypergastrinemia for >4–8 years prior to surgical exploration for cure and many, postoperatively for life, because <40% are cured postresection long-term [3]. This prolonged hypergastrinemia not only results in marked increases in acid secretion, it also has profound trophic effects on the gastric mucosal cells causing an increase in parietal cell mass, gastric mucosal thickness and gastric enterochromaffin-like cells [ECL cells] [2,4–8]. Therefore, ZES has been proposed to be an excellent natural model to study the chronic effects of hypergastrinemia in man, as well as a possible natural model to investigate the effects of the rapid reversal of chronic hypergastrinemia in man, by studying those ZES patients who are successfully, surgically cured [8,9].
Both of the above areas are receiving increasing attention because of the increasing number of patients with gastro-esophageal reflux disease (GERD) chronically taking proton pump inhibitors (PPI), with 80–100% developing hypergastrinemia, including a significant percent in a range seen in ZES patients [9–13]. Whereas numerous studies have shown that such treatment is safe and effective short term (<5 years) [8,14,15], the long-term consequences of such treatment are still largely unknown. The importance of the changes is that ECL-cell hyperplasia can progress to gastric carcinoids [11,16,17], which can occasionally be malignant [18]. The ECL and parietal cell hyperplasia can lead to gastric hypersecretion after stopping antisecretory treatment (rebound hypersecretion) which can make it difficult to wean patients with GERD off of PPIs [11,19–22]. A recent study reports even healthy, asymptomatic individuals treated for only an 8-week period with PPIs, can develop dyspeptic symptoms when the PPIs are stopped [23]. Whereas the possible development of gastric carcinoid tumors in both animals and humans [4,5,8,11,24] with chronic long-term gastric antisecretory treatment has been extensively studied; in humans much less information is available on rebound hypersecretion and few long term studies on the effects of rapid reversal of chronic hypergastrinemia, such as after stopping chronic PPI treatment [11,19,20,22].
Previous studies show approximately 30–40% of ZES patients are cured long-term [3,25] and in proportion; mild gastric acid hypersecretion may persist [26]. However, neither the possible mechanisms leading to this latter finding are known or have been studied, nor is it known if similar results might be seen in GERD patients treated for very prolonged periods >10 years). Studying the gastric acid secretion following curative resection of the gastrinoma could serve as a natural model for acid secretory changes that might occur after withdrawal of prolonged treatment with PPIs and may provide an insight into the mechanisms that are involved. To address these questions we studied gastric acid secretion in 50 ZES patients who underwent curative gastrinoma resection and attempted to define factors that may be associated with gastric acid hypersecretion postcurative resection in these patients.
METHODS
All patients with ZES referred to the National Institutes of Health were considered for the current study. These patients are included in the prospective study of ZES approved by the Clinical Research Committee of the National Institute of Diabetes and Digestive and Kidney Diseases. To be eligible for participation in the present study, patients needed to fulfill the following criteria: 1) have an established diagnosis of ZES as previously defined [27]; 2) agree to undergo exploratory laparotomy for possible cure; 3) be eligible for curative surgery as previously defined [3,25]; 4) have no medical contraindications to surgery; 5) have no medical illness limiting life expectancy; and 6) agree to long term follow-up including an assessment of disease-free status, gastric acid secretory status and ECL-cell activity.
The diagnosis of ZES was established by a combination of the following criteria as specified previously [2,25,27] including: 1) an elevated fasting serum gastrin concentration [13]; 2) gastric acid hypersecretion with a basal acid output (BAO) >15 mEq/h in patients without previous gastric acid-reducing surgery and >5 mEq/h in patients with previous gastric acid-reducing surgery [2]; 3) basal gastric fluid pH of ≤2 [2]; 4) positive secretin or calcium provocative test result [28] and/or; 5) positive histological confirmation of gastrinoma.
Serum gastrin concentrations were measured by Bioscience Laboratories (New York, NY) until 1994 and subsequently by Mayo Clinic Laboratories (Rochester, MN). Secretin provocation testing was performed as described previously [28] with a positive secretin provocation test result was defined as a ≥120-pg/ml increase over the pre-injection fasting gastrin concentration [28]. The calcium provocative test was performed as described previously [28] using calcium gluconate (10%) [54 mg/kg per hour (5 mg calcium/kg per hour)] given by intravenous infusion for 3 hours. An increase of ≥395 pg/ml over the average of the pre-infusion values was considered a positive test [28].
The extent of ECL-cell hyperplasia was determined from endoscopic biopsies [5,24,29] and also assessed quantitatively by determination of fasting chromogranin A (CgA) levels [30–32] and 24-hour urinary N-methylimidazoleacetic acid (N-MIAA) excretion rate [33,34]. The extent of ECL-cell changes was determined as outlined in the specific protocol section. Serum CgA levels were determined by immunochemilluminometric assay using an amino and carboxyl directed antibody [30,34] (Quest Diagnostics Nichols Institutes, San Juan Capistrano, CA). The normal range is 1.6–5.6 ng/ml. The intra-assay variation was 11.6% for a normal control (i.e., 4.1 ng/ml) and 8% for a high control (i.e., 12.8 ng/ml). The inter-assay variation was 5.9% for a normal control, 6.7% for the high control, and 6.6% for a very high control (i.e., 100 ng/ml). A 24-hour urine was collected while patients were on a histamine-restricted diet and was analyzed for N-MIAA as described previously [34]. The N-MIAA determination was done using quantitative mass spectrometry (Vanderbilt Pathology and Laboratories Services, Nashville, TN). The normal range is 50–230 mcg of N-MIAA/gm of creatinine.
To be evaluated for surgery, all patients underwent tumor localization studies and studies to determine the presence or absence of MEN1. Preoperative tumor localization included conventional imaging studies were performed including transabdominal ultrasonography (US), computerized tomography (CT scan), magnetic resonance imaging (MRI) and selective abdominal angiography in patients in whom the tumor location or extent was unclear after these other conventional imaging studies [3,25,35,36]. Since June 1994 all patients underwent somatostatin receptor scintigraphy (SRS) using 6 mCi of [111 In-DTPA-DPhe1] octreotide with spot views, whole body views and with single photon emission CT imaging at 4 hours and 24 hours postinjection [25,35,37]. Distant metastasis to the spine was evaluated using 99M-technetium bone scanning after the injection of 25 mCi of methylene diphosphonate, SRS and spinal MRI [38]. Functional localization of the gastrinoma was also done with patients undergoing either selective transhepatic portal venous gastrin sampling (January 1980-April 1992) or later selective intra-arterial injection of secretin with hepatic venous gastrin sampling as described previously [35,39].
Patients were considered for attempted curative surgery according to previously defined criteria [25,35,40]. Surgical exploration was performed on all patients with ZES without MEN1 who did not have diffuse liver metastases or an illness limiting life expectancy. Patients with ZES with MEN1 also underwent exploratory laparotomy if they had an imagable lesion ≥2 cm and did not have diffuse liver metastases or an associated illness limiting life expectancy, as described previously [35,41,41–43].
The surgical protocol employed was described previously [25,35,40,42]. Between 1980 and 1987 an extensive search for the gastrinoma was done using palpation, intraoperative ultrasonography with a 10 MHz real time transducer, and an extended Kocher maneuver of the duodenum to examine the pancreatic head area. Intraoperative endoscopic transillumination of the duodenum and a routine duodenotomy has been performed as well since 1987 [25,35,44].
Diagnostic criteria for ZES with Multiple Endocrine Neoplasia-type 1 (MEN1) were as previously described [41,43,45].
Specific Protocol
All eligible surgical patients who met the following criteria were included in this prospective study: 1) their highest postoperative BAO was measured >6 months postresection; 2) the patient underwent yearly follow-up evaluation for disease activity, including acid secretory studies and assessment of tumor location and extent; and 3) the patient had at least two years disease-free follow-up from the last postoperative acid secretory studies.
After surgery and prior to discharge from the hospital when the patient was eating on maintained on their standard dose of antisecretory drug, fasting serum gastrin concentrations were determined on at least 3 separate days. If resection was thought complete, a secretin test was performed [25–27,35]. Each patient was discharged on the same dose of oral antisecretory drug that he or she was taking before surgery [25,35]. Patients were evaluated at 3–6 months after surgery and then yearly as described above with fasting gastrins and detailed imaging studies. If fasting gastrin were normal, then gastric secretory studies and secretin testing were done after withdrawing any antisecretory drug [25–27,35]. Disease-free was defined as a patient having all three of the following at each postoperative follow-up visit: 1) normal fasting serum gastrin concentrations [13]; 2) negative gastrin provocation testing with secretin [28]; and 3) no evidence of tumor on imaging studies [25,27,35].
Upper gastrointestinal endoscopy was performed on all patients at each visit. Any abnormality was biopsied. On at least one occasion eight biopsy specimens (4 - greater curvature and 4 -lesser curvature) from the gastric body mucosa were taken as described previously [5,24,29]. Serial 5 μm thick sections of gastric body mucosa perpendicular to the mucosal surface were stained with H&E for evaluation of the type of mucosa and immunostained for endocrine cells using a monoclonal antibody against CgA (clone LK2H10; working dilution-1: 250; Biogenex Laboratories, San Ramon, CA). Detection was done using biotinylated anti-mouse immunoglobulin as a secondary antibody and an avidin-biotin-complex-peroxidase technique with diaminobenzidine tetrahydrochloride as a chromogen substrate [5,24,29]. Gastric endocrine cell distribution was determined as described previously [5,24,29]. The ECL-cell changes were evaluated qualitatively using the histopathologic classification proposed by Solcia et al. [16] according to the criteria previously published [5,24,29]. Using these criteria [5,24,29], ECL-cell changes were classified as diffuse hyperplasia (DH), linear hyperplasia (LH), micronodular hyperplasia (MH), adenomatoid hyperplasia (AH), or dysplasia. DH, LH and MH were further subdivided into 3 grades - mild, moderate, or severe [5,24,29]. Carcinoid tumors were diagnosed when the ECL-cell lesions exceeded 0.5 mm in diameter [16]. Patients were classified both according to the highest grade of endocrine cell hyperplasia and by the magnitude of the ECL-cell index [5,24,29] which was the average value for all biopsies using a scoring system as described previously [5,24,29,46]. Presence of H. pylori was assessed on routine biopsies obtained for histology [5,24,29].
Postoperative BAO and MAO were measured in patients that were possibly cured as described previously [2,26,47]. At each follow-up visit an attempt was made to reduce the antisecretory drug requirement as described previously [26,48]. Specifically, for patients taking PPIs the dose was first reduced to the equivalent of omeprazole 20 mg/day, then an H2 receptor antagonist started, which was then attempted to be withdrawn on subsequent visits.
The highest acid secretory rate post resection was used to stratify patients into three groups: normosecretors had highest recorded BAO <15 mEq/h; moderate hypersecretors, BAO 15–24.9 mEq/h; and extreme hypersecretors, BAO ≥25 mEq/h. These hypersecretory stratifications were used because >95% of patients without ZES with idiopathic hypersecretion have a BAO<25mEq/hr [49]. With prior acid-reducing surgery (vagotomy and/or partial gastrectomy) the corresponding values were <5 mEq/h, >5–14.9 mEq/h and ≥15 mEq/h. Patients were also stratified according to the level of acid control postoperatively based on the acid secretory rates as described previously [50]. Specifically, patients were classified as showing drug induced achlorhydria if all antisecretory drug measurements showed no acid, and as showing drug induced sustained hypochlorhydria if the gastric acid secretion for the hour prior to the next dose of antisecretory drug was <0.2 mEq/Hr for >50% of the assessment periods [50].
Statistical Analysis
Statistical analysis was performed using the Student’s t test, the Fisher Exact test and ANOVA. For a post hoc test the Bonferroni/Dunn test was used. Values of P <0.05 were considered significant. All values are expressed as mean ± SEM.
RESULTS
This study included 181 consecutive patients with ZES undergoing surgery for gastrinoma resection at the National Institutes of Health (Figure 1). To be eligible for this study patients had to be disease-free post-resection, have gastric secretory studies at least 6 months postresection, and have at least 2 years of follow-up from their last evaluation to insure disease-free status. Following surgery and at 3–6 months postoperatively, 53 patients were disease-free. Three of these 53 patients were not included in the present study because their follow-up time was less than the required 2 years. Of the 50 patients who fulfilled all the criteria for the present study, 44 were disease-free following the first surgery while six were disease-free after the second surgery.
Figure 1. Algorithm of patients studied.
Numbers in parentheses refer to the numbers of patients in each category. To be eligible for the study patients had to be cured postresection and have at least a two-year follow-up from their last postoperative BAO. Patients were stratified into three groups based on their highest postoperative acid secretory level. Patients with or without a previous acid-reducing surgery were classified as postoperative normosecretors if the BAO was <5 or 15 mEq/h, respectively; moderate hypersecretors if BAO was 5–14.9 or 15–24.9, respectively; and extreme hypersecretors if BAO was ≥15 or ≥25 mEq/h, respectively.
In the 50 patients in the present study, there were 19 patients (38%) who became and remained normosecretors on all of their postoperative follow-up evaluations and 31 patients (62%) who had hypersecretion on at least one postoperative follow-up visit (Figure 1). For these 31 hypersecretors, 17 patients (34%) (with or without previous acid-reducing surgery) were moderate hypersecretors (BAO 5–14.9 or 15–24.9 mEq/h, respectively), and 14 patients (28%) were extreme hypersecretors (BAO ≥15 or ≥25 mEq/h, respectively). The mean postoperative BAO differed significantly (P <0.0001) between these three secretory categories (Figure 2). In the 41 patients without gastric acid-reducing surgery, the highest postoperative BAO for the 17 patients who were normosecretors varied from 0 to 13 with a mean of 8 ± 1 mEq/h (Figure 2, left panel). In the 13 patients with moderate hypersecretion without acid-reducing surgery, the highest postoperative BAO varied from 15 to 24 with a mean of 21 ± 1 mEq/h, which was significantly greater (P = 0.0012) than the BAO in normosecretors (Figure 2, middle panel). In the extreme hypersecretors, the highest postoperative BAO varied from 26 to 67 with a mean of 37 ± 4 mEq/h, which was significantly greater (P <0.0001) than the BAO of the moderate hypersecretors (Figure 2, right panel). The comparable postoperative mean BAOs [ranges] for patients with previous acid-reducing surgery were 1 ± 1 (0.3–1.3 mEq/h, n=2), 7 ± 1 (5–9 mEq/h, n=4), and 17 ± 2 (15–20 mEq/h, n=3) for the normosecretors, moderate, and extreme hypersecretors, respectively.
Figure 2. Highest postoperative BAO after curative resection of the gastrinoma.
Each symbol represents data from one patient. Based on the level of the highest postoperative BAO, patients were stratified into three acid secretory categories defined in Figure 1 legend. Nine patients had previous gastric acid-reducing surgery while 41 patients did not. Data from patients without acid-reducing surgery are shown by the solid symbols and with previous acid-reducing surgery by the half-filled symbols. The horizontal line represents the mean while the vertical bar represents the SEM for each acid secretory category.
A comparison of the demographic data and other clinical variables between the patients in the three postoperative secretory groups did not reveal any significant difference in age at surgery, gender, age at ZES onset or diagnosis, duration of disease at surgery, duration from surgery to highest postoperative BAO, duration of disease-free follow-up from diagnosis or frequency of gastric acid-reducing surgery in each secretory group (Table 1). Diarrhea (82% overall) was the most frequent symptom at diagnosis in all three groups and was present in 15 (79%) normosecretors, 14 (82%) moderate hypersecretors, and 12 (86%) extreme hypersecretors. Other presenting symptoms did not differ in frequency between the three groups (Table 1). The duration from surgery to the highest postoperative BAO varied from 0.5 to 14 with a mean of 4 ± 0.5 years overall and did not differ significantly between normosecretors, moderate hypersecretors and extreme hypersecretors (Figure 3).
Table 1.
Clinical characteristics of gastric acid normosecretors and hypersecretors postcurative resection(1)
| Clinical Characteristic |
Number (percent)(8) |
||
|---|---|---|---|
| Normosecretors |
Hypersecretors |
||
| (n=19) |
Moderate (n=17) |
Extreme (n=14) |
|
| Age at surgery (yrs) | 51 ± 3 | 54 ± 2 | 49 ± 2 |
| Males | 12 (63%) | 11 (65%) | 11 (79%) |
| Age at ZES onset (yrs)(2) | 41 ± 2 | 44 ± 3 | 42 ± 3 |
| Age at ZES diagnosis (yrs) | 49 ± 3 | 49 ± 3 | 47 ± 2 |
| Duration of symptoms at surgery (yrs) | 10 ± 2 | 10 ± 2 | 6 ± 2 |
| Duration from diagnosis to surgery (yrs) | 2 ± 1 | 4 ± 1 | 2 ± 1 |
| Duration from ZES onset to postop highest BAO (yrs)(3) | 14 ± 2 | 14 ± 2 | 10 ± 2 |
| Duration from diagnosis to postop highest BAO (yrs) | 7 ± 1 | 8 ± 1 | 6 ± 1 |
| Duration from surgery to postop highest BAO (yrs) | 5 ± 1 | 4 ± 1 | 4 ± 1 |
| Disease-free follow-up from diagnosis (yrs)(4) | 11 ± 1 | 12 ± 1 | 10 ± 1 |
| Symptoms and signs at diagnosis (preoperatively)(5) | |||
| Diarrhea | 15 (79%) | 14 (82%) | 12(86%) |
| Heartburn | 9 (47%) | 9 (53%) | 7 (50%) |
| Bleeding | 4 (21%) | 4 (24%) | 6 (43%) |
| Ulcers(6) | 13 (68%) | 12 (71%) | 8 (57%) |
| Gastric acid-reducing surgery(7) | 2 (11%) | 4 (24%) | 3 (21%) |
Secretory status is based on highest basal acid output (BAO) measured postoperatively (with or without previous gastric surgery): normosecretors BAO <5 or <15; moderate 5–14.9 or 15–24.9; extreme hypersecretors ≥15 or ≥25 mEq/h, respectively.
ZES onset is the time of onset of continuous symptoms compatible with ZES [27].
Highest BAO is the highest basal acid output measured at anytime postoperatively, from beyond 1 year post-resection and prior to at least 2 years before the last disease-free visit.
This is the time interval from the diagnosis of ZES to the last disease-free visit at the NIH following curative resection.
Initial symptoms and signs present prior to the diagnosis of ZES.
Diagnosed at endoscopy or by upper gastrointestinal series.
Includes patients with parietal cell vagotomy (n=5), vagotomy and drainage procedure or with a partial gastrectomy (Billroth I - 2 patients, Billroth II - 2 patients) with or without vagotomy.
Number refers to the number of patients with the indicated clinical characteristic. The percent is the proportion of the total number of patients in the indicated secretory group with the indicated clinical characteristic.
Abbreviations: ZES - Zollinger-Ellison syndrome, BAO - basal acid output
Figure 3. Duration from surgery to highest postoperative BAO.
Each symbol represents duration for a single patient. Each of the patients postoperatively was stratified into one of three acid secretory categories as defined in Figure 1 legend. Duration is the time in years to the highest postoperative BAO. The mean ± SEM of the time from surgery to highest postoperative BAO was 5 ± 1, 4 ± 1, and 4 ± 1 years in the normosecretors, moderate hypersecretors, and severe hypersecretors, respectively.
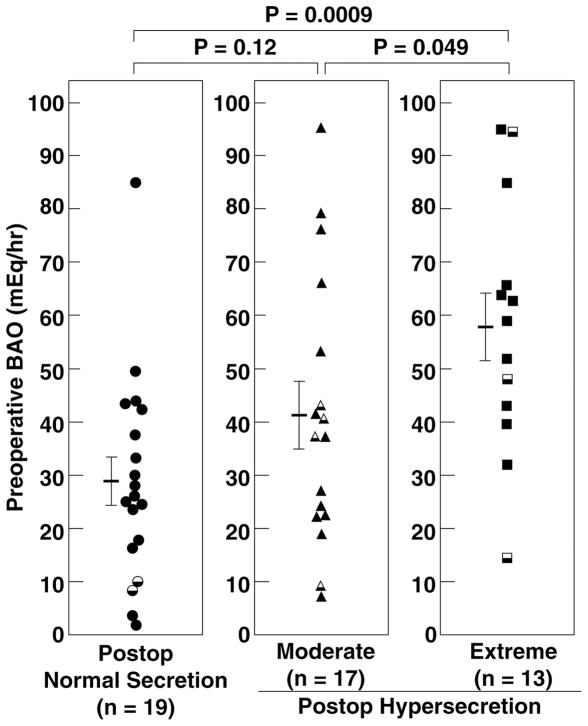
To determine whether the magnitude of preoperative basal acid secretion correlated with postoperative acid secretory status, the preoperative BAO was compared between the three postoperative acid secretory groups (Table 2, PFigure 4). The mean preoperative BAO in the extreme hypersecretors varied from 15 to 95 with a mean of 58 ± 7 mEq/h (Figure 4, right panel) and was significantly higher ( <0.05) than in moderate hypersecretors with a mean of 41 ± 6 (range 7–95) mEq/h (Figure 4, middle panel), and in normosecretors (P <0.001) whose values varied between 2 and 85 with a mean of 29 ± 5 mEq/h (Table 2, PFigure 4, right panel). Following curative resection, the mean BAO declined in all three secretory groups (Figure 5, left panel) with a 75 ± 8, 54 ± 10, and 53 ± 10 percent decrease at the first postoperative evaluation in normosecretors, moderate hypersecretors, and extreme hypersecretors, respectively. The mean BAO at the first postoperative evaluation was higher ( <0.001) in extreme hypersecretors 21 ± 3 (range 6–47) mEq/h and moderate hypersecretors 14 ± 2 (range 3–36) mEq/h (P <0.01) than in normosecretors 6 ± 1 (range 0–19) mEq/h (Table 3, Figure 5). However, after this initial decrease in BAO postcurative resection, the mean postoperative BAO remained fairly stable for each secretory group for the next 8 years (Figure 5, left panel).
Table 2.
Preoperative laboratory characteristics and antisecretory drug requirement of gastric acid normosecretors and hypersecretors postcurative resection(1)
| Preoperative Characteristic |
Number (percent)(6) |
||
|---|---|---|---|
| Normosecretors |
Hypersecretors |
||
| (n=19) |
Moderate (n=17) |
Extreme (n=14) |
|
| Basal acid output (mEq/h) | 28.9 ± 4.5 | 41.0 ± 6.1 | 57.9 ± 6.6(a, c) |
| Maximal acid output (mEq/h) | 43.9 ± 6.2 | 67.2 ± 8.8(b) | 87.6 ± 7.5(a) |
| Fasting serum gastrin (pg/ml) | 1284 ± 450 | 1200 ± 422 | 1435 ± 616 |
| Delta secretin (pg/ml)(2) | 2143 ± 612 | 1550 ± 589 | 2417 ± 778 |
| Preoperative Antisecretory treatment | |||
| Drug(3) | |||
| Histamine H2-inhibitors | 9 (47%) | 7 (41%) | 3 (21%) |
| Omeprazole | 10 (53%) | 10 (59%) | 11 (79%) |
| Duration (yrs)(4) | |||
| Total | 4 ± 1 | 5 ± 1 | 3 ± 1 |
| Histamine H2-inhibitors | 3 ± 1 | 2 ± 1 | 2 ± 1 |
| Omeprazole | 2 ± 1 | 3 ± 1 | 2 ± 1 |
| Dosage (mg/d)(5) | |||
| Histamine H2-inhibitors | 1284 ± 454 | 2203 ± 318 | 1426 ± 122 |
| Omeprazole | 52 ± 8 | 66 ± 13 | 65 ± 18 |
Compared to normosecretors:
P <0.001,
P <0.03: Compared to moderate hypersecretors:
P <0.05.
Secretory groups are as defined in Table 1 legend.
Increase in serum gastrin level following secretin injection [28].
This is the last gastric acid antisecretory drug used prior to surgery.
Total duration is the total time the patient was treated with any gastric acid antisecretory drug prior to curative resection.
Dose of antisecretory drug patient was taking at the time of curative resection. All histamine H2-inhibitors were converted to ranitidine equivalents as previously described [26,80].
Number and percent are as defined in Table 1 legend.
Figure 4. Comparison of Preoperative BAO with postoperative acid secretory category in patients disease-free after resection of gastrinoma.
Each symbol represents data from one patient. Closed symbols are from patients without previous gastric acid-reducing surgery (n=9) and half symbols are from patients with previous acid-reducing surgery (n=41). Patients were stratified into 3 different groups based on their highest postoperative acid secretory rate as defined in Figure legend 1. The horizontal line represents the mean while the vertical bars on either side represent the SEM for each acid secretory category.
Figure 5. Change in the BAO (left panel) and MAO (right panel) postcurative gastrinoma resection.
The mean BAO and MAO preoperatively and, at the indicated times, postoperatively are shown for each of the three postoperative acid secretory categories as defined in Figure legend 1. There were 19 patients who were postoperative normosecretors, 17 patients with moderate hypersecretion, and 14 patients with extreme hypersecretion.
Table 3.
Gastric acid output, fasting serum gastrin, and Helicobacter pylori status following curative resection
| Postoperative Parameter |
Number (percent)(1) |
||
|---|---|---|---|
| Normosecretors |
Hypersecretors |
||
| (n=19) |
Moderate (n=17) |
Extreme (n=14) |
|
| BAO at 3–6 mos (mEq/h)(2) | 5.7 ± 1.3 | 13.9 ± 2.1(a) | 21.3 ± 3.0(b) |
| BAO at 3–6 mos (% of pre-op value)(2) | 25.0 ± 7.7 | 46.4 ± 10.0(a) | 46.5 ± 10.0(a) |
| MAO at 3–6 mos (mEq/h) | 31.6 ± 5.8 | 44.8 ± 5.4 | 63.5 ± 9.1(a) |
| MAO at 3–6 mos (% of pre-op value)(2) | 65.1 ± 8.7 | 80.9 ± 9.2 | 70.9 ± 10.4 |
| Highest BAO (mEq/h)(3) | 7.1 ± 1.1 | 17.7 ± 1.7(b) | 33.1 ± 4.0(c, d) |
| Highest BAO (% of pre-op value)(4) | 32.6 ± 8.4 | 68.4 ± 9.2 | 67.2 ± 9.6 |
| Highest MAO (mEq/h)(3) | 34.9 ± 6.6 | 52.8 ± 6.8 | 82.7 ± 9.4(c) |
| Highest MAO (% of pre-op value)(4) | 71.5 ± 12.4 | 90.5 ± 9.2 | 98.2 ± 9.9 |
| Fasting serum gastrin immediate post-op (pg/ml) | 69.0 ± 6.4 | 102.5 ± 26.8 | 72.1 ± 5.5 |
| Delta secretin immediate post-op (pg/ml)(5) | 37.3 ± 18.1 | 22.3 ± 5.5 | 31.2 ± 6.2 |
| Fasting serum gastrin at time of highest BAO (pg/ml) | 58.6 ± 8.1 | 53.6 ± 6.3 | 85.1 ± 12.9 |
| Delta secretin at time of highest BAO (pg/ml)(5) | 28.3 ± 17.6 | 28.8 ± 5.6 | 52.5 ± 15.1 |
| Helicobacter pylori status (% present)(6) | 3 (16%) | 2 (12%) | 0 |
Compared to normosecretors:
P <0.01,
P <0.001,
P <0.0001; compared to moderate hypersecretors:
P <0.0001.
Number and percent are as defined in Table 1 legend.
Basal acid output (BAO) or maximal acid output (MAO) at 3 to 6 months post-curative resection are expressed as a percentage of the pre-operative value.
Highest BAO is as defined in Table 1 legend. Highest MAO is similarly defined.
Highest BAO or MAO expressed as a percentage of the pre-operative value.
Immediate post-op means within two weeks of curative resection. Delta secretin is the increase in serum gastrin following secretin injection [28].
H. pylori status refers to the results of evaluation for the presence of H. pylori as defined in METHODS post-curative resection and prior to or at time of highest BAO.
The preoperative MAO was also compared between the three secretory groups. The mean preoperative MAO in the extreme hypersecretors varied from 42 to 136 mEq/h with a mean of 88 ± 8 mEq/h and was higher (P = 0.0003) than in normosecretors with mean of 44 ± 6 (range 4–121) mEq/h (Table 2, PFigure 6). The preoperative MAO in moderate hypersecretors varied from 22 to 127 with a mean of 67 ± 9 mEq/h and was also significantly higher ( = 0.03) than in nomosecretors (Table 2, PFigure 6). Similar to the BAO following curative resection, the MAO also declined in all three acid secretory groups postcurative resection (Figure 5, right panel), with a 35 ± 9, 19 ± 9 and 29 ± 10 percent decrease at the first postoperative evaluation in normosecretors, moderate hypersecretors, and extreme hypersecretors, respectively. The mean MAO at the first postoperative evaluation was higher ( <0.01) in extreme hypersecretors 64 ± 9 (19–143) mEq/h than in normosecretors 32 ± 6 (0–97) mEq/h (Table 3). The rate of change in the MAO postcurative resection was also similar to the postoperative BAO-time curve (Figure 5). Specifically, after the initial decrease in the MAO at the first postoperative evaluation following curative resection, the mean MAO remained fairly constant at this level for the next 8 years (Figure 5, right panel).
Figure 6. Preoperative MAO in patients disease-free after resection of gastrinoma.
Each symbol represents data from one patient. Closed symbols are from patients without previous gastric acid-reducing surgery (n=9) and half symbols are from patients with previous acid-reducing surgery (n=41). Patients were stratified into 3 different groups based on their highest postoperative acid secretory rate as defined in Figure legend 1. The horizontal line represents the mean while the vertical bars on either side represent the SEM for each acid secretory category.
To determine if preoperative fasting gastrin levels and changes in these levels correlated with the magnitude of the postoperative acid secretion, we compared the three acid secretory groups, their preoperative serum gastrin levels, preoperative change in serum gastrin with secretin stimulation (delta secretin), immediate postoperative fasting serum gastrin level, immediate postoperative delta secretin, fasting serum gastrin at the time of the highest BAO and delta secretin at the time of the highest BAO (Tables 2 and 3). There were no significant differences in any of these gastrin levels between the three postoperative acid secretory categories (Tables 2 and 3). The presence or absence of H. pylori and previous antisecretory drug treatment can affect acid secretory rates [51–53]. Therefore, we compared the effect of both of these variables on postoperative acid secretory rate (Tables 2 and 3). All patients were treated preoperatively with either a histamine H2 antagonist or omeprazole (Table 2). Overall, 19 patients (38%) were on treatment with a histamine H2 antagonist while 31 patients (62%) were on treatment with omeprazole at the time of surgery. There were no significant differences between the three acid secretory groups in the type of antisecretory drug (omeprazole or histamine H2 antagonist), its dosage, or duration of their treatment prior to surgery (Table 2). Only 10% of patients had H. pylori present and there was no significant difference in its frequency in the three postcurative resection acid secretory groups (Table 3).
To provide insight into whether the postoperative long-term use of PPIs could be contributing to the presence of the hypersecretion postcurative resection, the type of maintenance antisecretory drug, if any at the time of the highest postoperative BAO was compared in patients with or without hypersecretion. Long-term 9/50 patients need no antisecretory drug, 15 a low dose of H2 blocker, and 26 required omeprazole. There was no significant difference in the percentage of patients maintained or not maintained on PPIs that showed acid hypersecretion during long-term follow-up (65% vs. % 59%).
To determine if any tumoral characteristics correlated with the magnitude of postoperative acid secretion, we compared the primary tumor location and size as well as the extent of the tumor at surgery between the 3 postoperative acid secretory groups (Table 4). The duodenum was the most common primary site with 17 patients (34%) having a gastrinoma in this location. Overall, the gastrinoma primary was ≤1 cm in 22 patients (44%), between 1.1 and 2.9 cm in 17 patients (34%), and >2.9 cm in 11 patients (22%). Thirty-three patients (66%) had a primary tumor only resected and the remainder had a primary tumor with a lymph node found at surgery (Table 4). There was no significant difference in the location of the primary, size of the primary, or extent of the tumor at surgery between the three acid secretory groups (Table 4).
Table 4.
Tumor characteristics in normosecretors and hypersecretors postcurative resection(1)
| Tumor Characteristic |
Number (percent)(6) |
||
|---|---|---|---|
| Normosecretors |
Hypersecretors |
||
| (n=19) |
Moderate (n=17) |
Extreme (n=14) |
|
| Location of primary(2) | |||
| Pancreas | 3 (16%) | 2 (12%) | 2 (14%) |
| Duodenum | 4 (21%) | 9 (53%) | 4 (29%) |
| Other(3) | 12 (63%) | 6 (35%) | 8 (57%) |
| Size of primary(4) | |||
| ≤1 cm | 7 (37%) | 8 (47%) | 7 (50%) |
| 1.1–2.9 cm | 8 (42%) | 4 (24%) | 5 (36%) |
| >2.9 cm | 4 (21%) | 5 (29%) | 2 (14%) |
| Extent of tumor(5) | |||
| Primary only | 13 (68%) | 11 (65%) | 9 (64%) |
| Primary and lymph node | 6 (32%) | 6 (35%) | 5 (36%) |
Secretory groups are as defined in Table 1 legend.
Location, size and extent of tumor are based on operative findings.
Other primary locations include lymph node (n=22), ovary (n=1), liver (n=2), and omentum (n=1).
Diameter of largest tumor seen at surgery.
Extent is defined as >primary only= if only a primary tumor is removed and the patient was cured.
Number and percent are as defined in Table 1 legend.
To assess whether the need or extent of control of acid secretion postoperatively might correlate with the degree of postcurative acid secretion, these variables were correlated (Table 5). Postcurative resection the antisecretory drug dose needed to control acid secretion could be reduced in all patients, with 18/50 (36%) able to stop all drugs for at least 6 months and with 23 (46%) either requiring no drug or only a low dose of H2 receptor antagonist for at least a 6-month period. At last follow-up, 30 patients were taking low doses of omeprazole (median dose 20 QD). Between postcurative normosecretors or moderate/extreme hypersecretors, there was no difference in the median postoperative dose of omeprazole used for those who required PPIs, the percentage who had dose reductions or the percentage in which BAO was assessed after at least 6 months of no treatment or a low dose of H2 receptor antagonist (Table 5). Furthermore, the average level of control of acid secretion for the hour prior to the next dose of antisecretory drug was similar for each of the three postcurative acid secretory groups. Lastly, the percentage of patients showing marked drug induced acid suppression (complete achlorhydria, or sustained hypochlorhydria) did not differ between the three groups (Table 5). These results support the conclusion that the postoperative drug dosage, level of acid control or drug used were not contributing to the magnitude of the postoperative acid secretion. .
Table 5.
Postoperative antisecretory drug requirements and control of gastric acid in normosecretors and hypersecretors postcurative resection(1)
| Number (percentage) |
|||
|---|---|---|---|
| Normosecretors |
Hypersecretors(1) |
||
| (N=19) |
Moderate (n=17) |
Extreme (n=14) |
|
| Postoperative acid secretion control | |||
| Control (mEq/hr)(2) | |||
| 6 mos postop | 2.5 ± 0.6 | 2.5 ± 0.5 | 3.1 ± 1.1 |
| 1 year postop | 2.1 ± 0.6 | 2.8 ± 0.5 | 3.2 ± 1.1 |
| 1 year postop | 2.1 ± 0.6 | 2.8 ± 0.4 | 3.1 ± 1.0 |
| No. with achlorhydria(3) | 3 (15%) | 1 (6%) | 0(0%) |
| No. with sustained hypochlorhydria (3) | 3 (15%) | 1 (6%) | 0(0%) |
| Postop antisecretory drug/dose | |||
| No. patients taking no drug/H2R antag ≥6mos(4) | 9 (47%) | 10(53%) | 5 (36%) |
| No. patients with drug dosage reduced postop (4) | 19(100%) | 17(100%) | 14(100%) |
| Median postoperative dosage of omeprazole in patients requiring PPI (mg/day) | 20 | 20 | 20 |
Secretory groups are as defined in Table 1 legend.
Acid secretion one hour prior to next dose of antisecretory drug for patients taking antisecretory drugs (n=40)
Definitions as outlined in [50] with drug induced achlorhydria requiring no acid detected at any follow-up on drug and sustained hypochlorhydria requiring acid secretion reduced to <0.2mEq/hr in >50% of follow-ups.
Antisecretory drug dosage was attempted to be reduced in all patients postoperatively as described in methods with the aim if possible to remove all antisecretory drug.
Since the magnitude of the mean preoperative BAO correlated with the postoperative acid secretory status and prediction of postoperative extreme hypersecretion would be clinically important, we attempted to define the likelihood of a given patient having postoperative extreme acid hypersecretion by calculating the sensitivity, specificity, positive predictive and negative predictive value of various preoperative BAO levels in the occurrence of extreme hypersecretion (Table 6). A preoperative BAO of >30 or >35 mEq/h had excellent sensitivity (i.e., 90–100%) and negative predictive value, but low specificity or positive predictive value (42–53%). A preoperative BAO >45 mEq/h still retained acceptable sensitivity and negative predictive value (i.e., 75–89%), whereas it also had good specificity (78%) but had a low positive predictive value (i.e., 56%) (Table 6).
Table 6.
Sensitivity, specificity, positive and negative predictive values of various preoperative basal acid output values for predicting extreme hypersecretors postcurative resection(1)
| Preoperative BAO |
Percent |
|||
|---|---|---|---|---|
| Sensitivity |
Specificity |
Positive Predictive Value |
Negative Predictive Value |
|
| >30 mEq/hr | 100 | 50 | 43 | 100 |
| >35 mEq/hr | 92 | 53 | 42 | 94 |
| >40 mEq/hr | 83 | 62 | 44 | 90 |
| >45 mEq/hr | 75 | 78 | 56 | 89 |
| >50 mEq/hr | 67 | 81 | 57 | 87 |
Results are from the 44 patients without previous acid-reducing surgery.
To determine if differences in the postoperative ECL-cell changes correlated with postoperative acid secretory status, we compared the qualitative degree of ECL-cell hyperplasia in patients with or without acid hypersecretion expressed either as the most advanced ECL-cell change seen [5,6,24,29,54] or the ECL-cell index, a mean numerical value of hyperplasia for all biopsies [5,24,46]. We also compared the urinary N-methylimidazole acetic acid (N-MIAA) excretion rate and serum CgA levels in the different groups, both measures of the degree of ECL-cell hyperplasia [30–32]. Sixteen of the 19 normosecretors had >2 gastric biopsies (median=8), which allowed reliable assessment of ECL-cell changes. The most advanced ECL-cell change was a normal pattern (n=1), mild (n=7) or moderate (n=5) DH, mild LH (n=1), and mild MH (n=1). The mean ECL-cell index was 1.16 ± 0.24 with a range from 0 to 3.6. In contrast, for the 29 patients with postoperative hypersecretion with >2 gastric biopsies allowing assessment of postoperative ECL-cell change, the most advanced ECL-cell change was mild (n=2), moderate (n=9) or severe (n=12) DH; mild (n=3) or moderate (n=2) LH, and dysplasia (n=1). The mean ECL-cell index overall for the hypersecretors was 1.90 ± 0.23 with a value of 1.51 ± 0.13 for the moderate hypersecretors and 2.30 ± 0.43 for the extreme hypersecretors. Only 23% (3/16) of the 19 normosecretors had severe DH or a more advanced ECL-cell change which was significantly less (P = 0.005) than the 63% (18/29) seen in hypersecretors. Representative results from 4 patients are shown in Figure 7. The mean ECL-cell index was significantly higher (P = 0.0124) in all hypersecretors than normosecretors (1.90 ± 0.23) vs. (1.16 ± 0.24). The gastric biopsies assessing ECL-cell changes were obtained 7.8 ± 0.91, 7.4± 1.1 and 6.3 ± 1.2 years postresection for normosecretors, moderate, and extreme hypersecretors, respectively, which were not significantly different. The mean 24-hour urinary N-MIAA excretion varied from 35 to 226 with a mean of 109 ± 11.5 mcg/g creatinine in normosecretors and from 52 to 390 with a mean of 157 ± 17.2 mcg/g creatinine in hypersecretors. There were significantly (P = 0.038) more hypersecretors (11 of 28 patients) with 24-hour urinary N-MIAA greater >150mcg/g creatinine compared to normosecretors (2 of 18 patients). The mean CGA levels in the hypersecretors varied from 3.4 to 93 with a mean of 18.8 ± 3.8 ng/ml and were significantly higher (P = 0.009) than in normosecretors with a mean of 5.5 ± 0.9 (range 2–15) ng/ml. The mean CGA levels were also significantly higher (P = 0.04) in extreme hypersecretors, 17.5 ± 6.6 (range 3.5–93) ng/ml compared to normosecretors 5.5 ± 0.9 (range 2–15) ng/ml.
Figure 7. ECL-cell changes in patients with or without postcurative acid hypersecretion.
The top two panels are the ECL-cell pattern in two patients with postoperative normosecretion with one patient (top left) showing a normal ECL-cell pattern and the other (top right) showing mild diffuse hyperplasia. The bottom two panels show severe diffuse hyperplasia in a patient (bottom left) with moderate hypersecretion and moderate linear hyperplasia in a patient with extreme hyperplasia. Shown are CgA immunoperoxidase stains with hematoxylin counterstaining. These results are representative of those for all patients with patients having hypersecretion showing significantly (P = 0.005) more advanced ECL-cell changes than normosecretors.
DISCUSSION
This study was undertaken to attempt to provide insights into the mechanism(s) that contribute to some patients with ZES continuing to show various degrees of prolonged gastric acid hypersecretion after rapid reversal of the chronic hypergastrinemia by curative gastrinoma resection [26]. This study not only is important in the management of patients with ZES, but has a potentially wider importance, in that in could serve as a natural model of the effects of the rapid reversal of chronic hypergastrinemia in man, an area that is currently receiving increased attention. This area is receiving increased attention because of the widespread use of PPIs in the general population for GERD which has been shown to result in 80–100% developing hypergastrinemia, including a significant percent in a range seen in ZES patients [9–13]. Because GERD symptoms recur in 80% of patients with advanced GERD within 6 months when PPIs are stopped, long-term continuous treatment is being increasingly used [10,12,55]. The possible consequences of very long-term treatment with PPIs are still uncertain, although those possibly due to the PPI-induced hypochlorhydria/achlorhydria are receiving increased attention [4,7,11,56,57]. One of the main areas of potential concern is the development with chronic PPI treatment of achlorhydria-induced hypergastrinemia, which can cause trophic effects in the gastric mucosa [4,7,11,56,57]. The ability of chronic hypergastrinemia to cause gastric ECL-cell proliferation which can result in gastric carcinoids, of which some are malignant, has been extensively studied both in animals and humans [4,11,56,57]. A chronic hypergastrinemic effect that has been less well studied, but is receiving increasing attention, is its ability to lead to the development of rebound acid hypersecretion when the antisecretory drug is stopped [11,19–22]. This is receiving increased attention because it can result in the recurrence or worsening of symptoms after withdrawal of gastric antisecretory drug therapy [19,23,55,58]. Studies in animals demonstrate that marked acid inhibition by antisecretory agents can result in up to a 400% increase in BAO and 100% increase in MAO post-treatment [59,60]. That the drug-induced hypergastrinemia resulting in ECL and parietal cell hyperplasia/hypertrophy is responsible for the rebound hypersecretion following PPI treatment and withdrawal is suggested by the fact that it is abolished by antrectomy and by gastrin receptor blockade [61,62].
Rebound hypersecretion is generally short lasting and mild, if present at all after prolonged treatment with H2 blockers [11,21]. Recent studies have now demonstrated acid rebound does occur after PPI treatment and that it is not infrequent, occurring in 62–90% of patients [11,19–22]. There is, in general, little known about rebound acid hypersecretion after prolonged gastric antisecretory treatment in humans. It is unknown how severe it might be with chronic treatment for years with such potent acid suppressants such as PPI. It is unknown the duration of rebound acid hypersecretion after such prolonged treatment. Also unknown are prognostic factors that might predict either the severity or duration of rebound hypersecretion. There are several reasons why the results from animal studies may not be applicable to man. First, in rats, the most commonly used laboratory animal, a more profound hypergastrinemia develops with omeprazole treatment compared to other species including humans [56,63]. Second, the time period of development of these changes in animals may not be applicable to humans. Third, the levels of acid suppressants utilized in animal studies may not be similar to that seen in long-term treatment in humans. Since the induction of hypergastrinemia has been demonstrated to be the underlying mechanism by which PPIs or other potent gastric antisecretory drugs induce ECL-cell hyperplasia and increased parietal cell density [7,22,61,64]a potential good natural model to study to provide insights in humans to the effect of suddenly ending chronic hypergastrinemia, as occurs when a potent gastric acid anti-suppressant is stopped, is to study patients with Zollinger-Ellison syndrome post-curative gastrinoma resection.
Only one prospectively evaluated gastric acid secretion following gastrinoma resection [26]. This study did not investigate the possible mechanisms involved and contained insufficient numbers of patients to allow detailed correlative analysis to provide possible insights into contributing factors. The present study has none of these limitations. It includes a large number of patients assessed postcurative resection (i.e., 50 patients), a sufficient number of patients in this group had postcurative resection hypersecretion, allowing enough patients for correlative analysis (i.e., 30 patients) and well-defined criteria were used to exclude the possibility of early recurrence at the time of the gastric acid secretory analysis [2,27].
In the present study we found that 62% of our patients continued to have acid hypersecretion postcurative resection, of which 28% exceeded levels of hypersecretion seen in 95% of patients with idiopathic hypersecretion [49] [i.e. were extreme hypersecretors (BAO ≥25 mEq/h)]. In a previous study involving 20 patients with gastrinoma postcurative resection [26] 67% of the patients had continued hypersecretion. However, no patient had extreme hypersecretion. These studies show acid hypersecretion is frequent in patients with gastrinoma postcurative resection and that high levels of hypersecretion are not infrequent, occurring in over a quarter of the patients cured. The high frequency of gastric acid hypersecretion and significant proportion with high levels of hypersecretion was not due to the fact patients were examined too soon after curative resection before postresection gastric adaptive changes occurred or because the patients were not cured. The latter possibility was excluded because all patients remained cured for at least two years after the highest BAO and cure was assessed by both gastrin studies (fasting and secretin provocative testing) and imaging studies, which detect all relapses [13,27,28]. The former possibility was excluded because in all patients the highest BAO was at least 1 year after curative resection except in one patient where it was 6 months after curative resection. In our study the mean time from surgery to the highest BAO was 4 years in all postoperative secretory groups and in all but one patient was ≥1 year, so the patients were assessed well beyond the time when postoperative gastric secretory changes have reversed due to the decrease in fasting gastrin levels to normal levels postcurative resection. In a previous study [26] in patients with gastrinoma postcurative resection, the BAO and MAO were reported to remain unchanged from 6 months to 4 years postoperatively after an initial decrease during the first 3–6 months. In the present study the BAO declined from preoperative levels in all three secretory groups postoperatively with a mean decrease of 75%, 54%, and 53% in normosecretors, moderate hypersecretors, and extreme hypersecretors, respectively, at the 3–6 months follow-up. The MAO also declined postoperatively in all three groups with a 29%, 19%, and 35% decrease at the first postoperative evaluation in extreme hypersecretors, moderate hypersecretors, and normosecretors, respectively. After this initial decline, the mean postoperative BAO (and MAO) remained stable for each secretory group for the next 8 yrs. These results show that the gastric acid hypersecretion seen in many cured patients following reversal of prolonged hypergastrinemia can be long-term, persistent, and generally does not diminish for up to almost a decade after curative resection. Except for the study noted above [26], there are currently no human studies addressing the acid secretory changes that occur following reversal of prolonged hypergastrinemia of >5 years duration induced by any cause. Weinstein, et al. [65] studied the gastric endoscopic and biopsy changes after 2 years of omeprazole treatment in patients with Barrett’s esophagus. They found that gastric hypersecretion and parietal cell hyperplasia which occurred during the treatment reverted to normal within 3 months of discontinuation of omeprazole. Fossmark [22] demonstrated in 7 patients with GERD who had taken PPIs for >1 year and were studied post anti-reflux surgery after stopping the PPI, the MAO decreased over a 26 week period as well as the serum CGA, serum gastrin and number of gastric HDC positive cells on biopsy. In one animal study [59] in rats treated with omeprazole for 30 days, ECL and parietal cell hyperplasia developed. Withdrawal of omeprazole was followed by a rebound increase in MAO with a gradual return toward control levels with a high-dose omeprazole group having an MAO significantly above the control level 70 days after omeprazole cessation. In another study [66] following 10 weeks of omeprazole treatment in rats ECL density was completely reversed 140 days after stopping treatment, whereas in a third study the ECL-cell density returned to control levels between 20 and 100 days following withdrawal of omeprazole treatment of 16 days [67]. The studies reviewed demonstrate that except for the present study there is no data in humans or from animal studies on the time-course of changes in gastric secretory function after prolonged hypergastrinemia (i.e., >3–5 years).
At present the mechanism(s) responsible for the persistent hypersecretion postcurative gastrinoma resection in our patients are unknown, however our study provides some possible insight. There are a number of different possible explanations for our findings including the following: the patients were not cured following resection of gastrinoma or were developing relapses; hypersecretion of a non-gastrin secretagogue persisted after gastrinoma resection; rebound hypersecretion secondary to postoperative PPI use occurred; exaggerated idiopathic hypersecretion, persistent ECL and/or parietal cell hyperplasia occurred postcurative resection or very long-term hypergastrinemia may activate some unknown, irreversible process(es) that results in persistent hypersecretion.
First, Lack of cure of the gastrinoma is unlikely, as discussed above, because our patients underwent rigorous assessment of cure to insure disease-free status including serial gastrin studies (fasting and secretin provocation tests) and imaging studies (including somatostatin scintigraphy) performed at each visit. Early relapse was excluded by requiring all patients to be followed at least 2 years after the last observed BAO and MAO.
The second possible explanation that an unknown non-gastrin secretagogue may be released by an additional unrelated pancreatic endocrine tumor after removal of the gastrinoma is also unlikely. Although pancreatic endocrine tumors producing acid hypersecretion without hypergastrinemia are described [68–70]and it has been suggested that the peptide secreted by these tumors can potentiate the effect of gastrin on acid secretion [71], our patients had no evidence of any primary tumor or metastases on imaging studies despite follow-up of up to 14 years in some cases. Almost all non-gastrinoma islet tumors have high densities of somatostatin receptors [72] and since 1994; somatostatin receptor scintigraphy (SRS) has been used in our patients [37]. However, no recurrent PETs were seen on SRS.
A third possible explanation for the gastric acid hypersecretion postcurative resection, is that many of these patients remained on gastric antisecretory drugs and that the gastric acid hypersecretion noted could be due to an exaggerated rebound hypersecretion following their withdrawal. However, there are several reasons why drug-induced rebound acid hypersecretion is unlikely to explain our findings. First, the mechanism of rebound hypersecretion is due to drug-induced hypergastrinemia secondary to profound acid inhibition [11,20–22,58]. This did not occur in our patient’s postcurative resection, because both while taking the antisecretory drugs, as well as when not taking them, fasting gastrin levels remained normal. This is consistent with the fact that none of the hypersecretory patients had profound acid suppression, due to the careful reduction of drug dosage, and in fact, their acid control levels was indistinguishable from the normosecretors. Second, one half of the hypersecretors were evaluated after a 6 month period of taking either no antisecretory drug or only a low dose of H2 antagonist, which, in the literature are reported to either no rebound or at most, mild and transient rebound [11,21,73]; and this had no effect on the presence of the hypersecretion in our patients. Third, the magnitude of the acid hypersecretion in our study is much higher than that reported in the published studies of post-antisecretory drug rebound acid hypersecretion. In two human studies after short term PPI treatment (<90 days) [51,74], when the PPI was stopped the rebound acid secretion showed the BAOs that only increased <4mEq/h with only two patients reaching levels up to 17 mEq/h. In contrast, in our patients the median BAO in our study was 20.7 mEq/h in moderate hypersecretors and 30.1 mEq/h in extreme hypersecretors.
A fourth possible explanation for the gastric acid hypersecretion postcurative resection is suggested by findings in a previous study [51] showing in H. pylori-negative healthy volunteers, the greater the drug-induced acid suppression, the greater the acid rebound level. This was not the case for our patients, because those with or without postcurative gastrinoma resection, acid hypersecretion showed the same level of acid control while on antisecretory drugs. Furthermore, in our study >95% hypersecretory patients had their acid levels reduced by antisecretory agents only to normal levels of acid secretion and profound acid inhibition, which occurs in most studies reporting acid rebound, was not present. Lastly, <10% of our hypersecretors has H. pylori present and this percentage was similar to that in normosecretors.
A fifth possible explanation for the gastric acid hypersecretion postcurative resection could be proposed that it involves similar mechanisms to that causing idiopathic hypersecretion in patients without ZES. It has been proposed that increased vagal tone is an important contributory factor in patients with idiopathic hypersecretion [75]. Results in our patients support the conclusion that maintenance of vagal tone is an important contributory factor to the magnitude of postoperative acid secretion. This conclusion is supported by our finding that cured patients who had undergone a vagotomy had levels of hypersecretion of about 40% that seen in patients without vagotomy. However, postcurative resection hypersecretion was still seen in 80% (7/9) of patients with vagotomy postcurative resection showing other factors besides vagal tone were contributory to this acid hypersecretion. Furthermore, in almost one-half of the patients with postoperative hypersecretion (i.e., the patients (the 14/50 with extreme hypersecretion), the magnitude of the hypersecretion was greater than that found in >95% of patients with idiopathic hypersecretion [49].
A final possible explanation for the gastric acid hypersecretion postcurative resection could be proposed which involved proposing that persistent ECL and/or parietal cell hyperplasia could be present, which could be contributory to the continuing gastric acid hypersecretion. As noted above in short-term studies in animals and humans, results show that the drug-induced hypergastrinemia causes ECL and parietal cell hyperplasia/hypertrophy, which can cause rebound hypersecretion following antisecretory treatment and withdrawal. Furthermore, Fossmark [22] demonstrated in 7 patients with GERD who had taken PPIs for >1 year and were studied post anti-reflux surgery after stopping the PPI, with the decreased in MAO over a 26 week period there was also a decrease in serum CGA, serum gastrin and number of gastric HDC positive cells on biopsy. They proposed increased ECL mass and/or activity, in addition to increased parietal cell mass [22], likely contributed to the rebound hypersecretion in these patients treated with PPIS for at least 1 year. In patients with the Zollinger-Ellison syndrome the long-standing hypergastrinemia results in marked ECL-cell hyperplasia [5,6,11,24] and parietal cell hyperplasia/hypertrophy [76]. Numerous studies show a close positive correlation between the MAO and parietal cell mass [59,77] and thus, the increased parietal cell mass in ZES could lead to increased MAO seen in these patients. Studies also suggest the ECL mass can also have an important effect on MAO [22]. A recent study suggests that the BAO is positively correlated with the ECL-cell density in hypersecretory patients in various gastric acid hypersecretory states [78]. Since the MAO is correlated with parietal cell mass and possibly ECL cell mass and the BAO correlated with ECL-cell density, a possible explanation for the persisting hypersecretion is that contrary to what the short term studies show, after prolonged hypergastrinemia of several years duration, these ECL and/or parietal cell changes may not be completely reversible. Alternatively, there may be a functional alteration in these cells such that despite a normal ECL and parietal cell mass, acid hypersecretion persists. Our results provide some support for the former possibility. We found that the mean highest postoperative MAO was significantly higher (P <0.0001) in extreme hypersecretors than in normosecretors. This suggests that increased parietal cell mass and/or ECL-cell mass was being maintained in the extreme hypersecretors postcurative resection. The possibility that increased ECL-cell hyperplasia could be contributing to the acid hypersecretion postresection in our patients is supported by the finding of more advanced ECL-cell changes by three different methods of assessment in hypersecretors compared to normosecretors. There was a significantly higher frequency of advanced ECL-cell change determined on gastric mucosal biopsy, whether assessed by the most advanced qualitative ECL-cell change [5,24,29,54] or the ECL-cell index [5,24,46] in hypersecretors; a significantly higher serum CGA level in extreme hypersecretors, and a significantly higher proportion of hypersecretors with 24 h urinary N-MIAA excretion of >150 mcg/g creatinine. Both of the latter measurements have been shown to reflect the extent of ECL-cell hyperplasia in various studies [30–32,34]. Numerous in vitro and in vivo studies have shown that histamine released from ECL-cells is critical to stimulated acid secretion [79]. What remains unclear from our study is the mechanism causing the continued ECL/parietal cell hyperplasia postcurative gastrinoma resection in our patients for a mean of 7 years after reversal of the hypergastrinemia. In all short-term animal and human studies of persistent hypergastrinemia [65–67], these parameters return to normal in a few months. To explain our results one has to postulate either very prolonged hypergastrinemia can trigger mechanisms that cause irreversible changes in ECL/parietal cell hyperplasia, or changes that can be maintained subsequently by normal levels of gastrin. These mechanisms are at present completely unknown. Whether such changes will occur in patients with idiopathic GERD or peptic ulcer disease treated for prolonged periods of time with PPIs, at present are unknown.
Acknowledgments
Declaration of funding interests. This study was partially supported by intramural funds of NIDDK, NIH and by grants from the Italian Association for Cancer Research (AIRC), Milan; the Italian Ministry for University, Scientific and Technological Research (MURST); the Italian Ministry of Health (grant number ICS060.2/RF00-57).
Abbreviations
- ZES
Zollinger-Ellison syndrome
- BAO
basal acid output
- MAO
maximal acid output
- ECL
enterochromaffin-like cell
- CgA
chromogranin A
- N-MIAA
N-methyl imidazole acetic acid
- PPI
proton pump inhibitor
- GERD
gastroesophageal reflux disease
- MEN1
multiple endocrine neoplasia-type 1
- SRS
somatostatin receptor scintigraphy
- PET
pancreatic endocrine tumor
- DH
diffuse ECL-cell hyperplasia
- LH
linear ECL-cell hyperplasia
- MH
micronodular ECL-cell hyperplasia
Footnotes
Author Financial disclosures: none
Contributor Information
Jeremiah V. Ojeaburu, Email: ojeaburu@hotmail.com.
Tetsuhide Ito, Email: itopapa@intmed3.med.kyushu-u.ac.jp.
Pellegrino Crafa, Email: rcrafa@ao.pr.it.
Cesare Bordi, Email: cesare.bordi@unipr.it.
Robert T. Jensen, Email: RobertJ@bdg10.niddk.nih.gov.
References
- 1.Jensen RT, Niederle B, Mitry E, Ramage JK, et al. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84:173–182. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 2.Roy P, Venzon DJ, Feigenbaum KM, Koviack PD, et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore) 2001;80:189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Annals of Surgery. 2004;240:757–773. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maton PN, Dayal Y. Clinical implications of hypergastrinemia. In: Zakim D, Dannenberg AJ, editors. Peptic ulcer disease and other acid-related disorders. Armonk, NY: Academic Research Associates; 1991. pp. 213–246. [Google Scholar]
- 5.Peghini PL, Annibale B, Azzoni C, Milione M, et al. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- 6.Maton PN, Lack EE, Collen MJ, Cornelius MJ, et al. The effect of Zollinger-Ellison syndrome and omeprazole therapy on gastric oxyntic endocrine cells. Gastroenterology. 1990;99:943–950. doi: 10.1016/0016-5085(90)90611-4. [DOI] [PubMed] [Google Scholar]
- 7.Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39:61–79. doi: 10.1159/000199609. [DOI] [PubMed] [Google Scholar]
- 8.Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacology and Toxicology. 2002;91:333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- 9.Jensen RT. Gastrinoma as a model for prolonged hypergastrinemia in man. In: Walsh JH, editor. Gastrin. New York, NY: Raven Press Publishing Co; 1993. pp. 373–393. [Google Scholar]
- 10.Richter JE. Gastroesophageal reflux disease. In: Yamada T, Alpers DH, Kalloo AN, Kaplowitz N, Owyang C, Powell DW, editors. Textbook of Gastroentorology. Oxford, UK: Blackwell; 2009. pp. 772–801. [Google Scholar]
- 11.Jensen RT. Consequences of long-term proton pump blockade: Highlighting insights from studies of patients with gastrinomas. Basic ClinPharmacol Toxicol. 2006;98:4–19. doi: 10.1111/j.1742-7843.2006.pto_378.x. [DOI] [PubMed] [Google Scholar]
- 12.Jansen JB, Klinkenberg-Knol EC, Meuwissen SG, De Bruijne JW, et al. Effect of long-term treatment with omeprazole on serum gastrin and serum group A and C pepsinogens in patients with reflux esophagitis. Gastroenterology. 1990;99:621–628. doi: 10.1016/0016-5085(90)90946-x. [DOI] [PubMed] [Google Scholar]
- 13.Berna MJ, Hoffmann KM, Serrano J, Gibril F, Jensen RT. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295–330. doi: 10.1097/01.md.0000236956.74128.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klinkenberg-Knol EC, Festen HPM, Jansen JBMJ, Lamers CBHW, et al. Long-term treatment wih omeprazole for refractory reflux esophagitis: efficacy and safety. Annals of Internal Medicine. 1994;121:161–167. doi: 10.7326/0003-4819-121-3-199408010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Klinkenberg-Knol EC, Nelis F, Dent J, Snel P, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety and influence on gastric mucosa. Gastroenterology. 2000;118:661–669. doi: 10.1016/s0016-5085(00)70135-1. [DOI] [PubMed] [Google Scholar]
- 16.Solcia E, Bordi C, Creutzfeldt W, Dayal Y, et al. Histopathological classification of nonantral gastric endocrine growths in man. Digestion. 1988;41:185–200. doi: 10.1159/000199786. [DOI] [PubMed] [Google Scholar]
- 17.Delle Fave G, Capurso G, Milione M, Panzuto F. Endocrine tumours of the stomach. Best PractResClin Gastroenterol. 2005;19:659–673. doi: 10.1016/j.bpg.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Wangberg B, Nilsson O, Theodorsson E, Modlin IM, et al. Are enterochromaffinlike cell tumours reversible? An experimental study on gastric carcinoids induced in Mastomys by histamine2-receptor blockade. Regul Pept. 1995;56:19–33. doi: 10.1016/0167-0115(95)00123-s. [DOI] [PubMed] [Google Scholar]
- 19.Gillen D, McColl KE. Problems related to acid rebound and tachyphylaxis. Best PractResClin Gastroenterol. 2001;15:487–495. doi: 10.1053/bega.2001.0190. [DOI] [PubMed] [Google Scholar]
- 20.Hunfeld NG, Geus WP, Kuipers EJ. Systematic review: Rebound acid hypersecretion after therapy with proton pump inhibitors. Aliment Pharmacol Ther. 2007;25:39–46. doi: 10.1111/j.1365-2036.2006.03171.x. [DOI] [PubMed] [Google Scholar]
- 21.Waldum HL, Qvigstad G, Fossmark R, Kleveland PM, Sandvik AK. Rebound acid hypersecretion from a physiological, pathophysiological and clinical viewpoint. ScandJ Gastroenterol. 2009 doi: 10.3109/00365520903477348. [DOI] [PubMed] [Google Scholar]
- 22.Fossmark R, Johnsen G, Johanessen E, Waldum HL. Rebound acid hypersecretion after long-term inhibition of gastric acid secretion. Alimentary Pharmacology and Therapeutics. 2005;21:149–154. doi: 10.1111/j.1365-2036.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- 23.Reimer C, Sondergaard B, Hilsted L, Bytzer P. Proton-pump inhibitor therapy induces acid-related symptoms in healthy volunteers after withdrawal of therapy. Gastroenterology. 2009;137:80–7. 87. doi: 10.1053/j.gastro.2009.03.058. [DOI] [PubMed] [Google Scholar]
- 24.Berna MJ, Annibale B, Marignani M, Luong TV, et al. A prospective study of gastric carcinoids and enterochromaffin-like cells changes in Multple Endocrine Neoplaisa Type 1 and Zollinger-Ellison syndrome: Identification of risk factors. Journal of Clinical Endocrinology and Metabolism. 2008;93:1582–1591. doi: 10.1210/jc.2007-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Annals of Surgery. 2004;239:617–626. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pisegna JR, Norton JA, Slimak GG, Metz DC, et al. Effects of curative resection on gastric secretory function and antisecretory drug requirement in the Zollinger-Ellison syndrome. Gastroenterology. 1992;102:767–778. doi: 10.1016/0016-5085(92)90157-t. [DOI] [PubMed] [Google Scholar]
- 27.Fishbeyn VA, Norton JA, Benya RV, Pisegna JR, et al. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Annals of Internal Medicine. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berna MJ, Hoffmann KM, Long SH, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: II. Prospective study of gastrin provocative testing in 293 patients from the National Institutes of Health and comparison with 537 cases from the literature. evaluation of diagnostic criteria, proposal of new criteria, and correlations with clinical and tumoral features. Medicine (Baltimore) 2006;85:331–364. doi: 10.1097/MD.0b013e31802b518c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bordi C, Azzoni C, Ferraro G, Corleto VD, et al. Sampling strategies for analysis of enterochromaffin-like cell changes in Zollinger-Ellison syndrome. American Journal of Clinical Pathology. 2000;114:419–425. doi: 10.1093/ajcp/114.3.419. [DOI] [PubMed] [Google Scholar]
- 30.Goebel SU, Serrano J, Yu F, Gibril F, et al. Prospective study of the value of serum chromogranin A or serum gastrin levels in assessment of the presence, extent, or growth of gastrinomas. Cancer. 1999;85:1470–1483. [PubMed] [Google Scholar]
- 31.Borch K, Stridsberg M, Burman P, Rehfeld JF. Basal chromogranin A and gastrin concentrations in circulation correlate to endocrine cell proliferation in type-A gastritis. Scandinavian Journal of Gastroenterology. 1997;32:198–202. doi: 10.3109/00365529709000194. [DOI] [PubMed] [Google Scholar]
- 32.Sanduleanu S, Stridsberg M, Jonkers D, Hameeteman W, et al. Serum gastrin and chromogranin A during medium- and long-term acid suppressive therapy: a case-control study. Alimentary Pharmacology and Therapeutics. 1999;13:145–153. doi: 10.1046/j.1365-2036.1999.00466.x. [DOI] [PubMed] [Google Scholar]
- 33.Granerus G. Effects of oral histamine, histidine and diet on urinary excretion of histamine, methylhistamine and 1-methyl-4-imidazoleacetic acid in man. Scandinavian Journal of Clinical and Laboratory Investigation. 1968;104:49–58. [PubMed] [Google Scholar]
- 34.Bashir S, Gibril F, Ojeaburu J, Asgharian B, et al. Prospective study of the ability of histamine, serotonin or chromogranin A levels to identify gastric carcinoids in patients with gastrinomas. Alimentary Pharmacology and Therapeutics. 2002;16:1367–1382. doi: 10.1046/j.1365-2036.2002.01249.x. [DOI] [PubMed] [Google Scholar]
- 35.Norton JA, Fraker DL, Alexander HR, Venzon DJ, et al. Surgery to cure the Zollinger-Ellison syndrome. New England Journal of Medicine. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 36.Krudy AG, Doppman JL, Jensen RT, Norton JA, et al. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. American Journal of Roentgenology. 1984;143:585–589. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 37.Gibril F, Reynolds JC, Chen CC, Yu F, et al. Specificity of somatostatin receptor scintigraphy: a prospective study and the effects of false positive localizations on management in patients with gastrinomas. Journal of Nuclear Medicine. 1999;40:539–553. [PubMed] [Google Scholar]
- 38.Gibril F, Doppman JL, Reynolds JC, Chen CC, et al. Bone metastases in patients with gastrinomas: a prospective study of bone scanning, somatostatin receptor scanning, and MRI in their detection, their frequency, location and effect of their detection on management. Journal of Clinical Oncology. 1998;16:1040–1053. doi: 10.1200/JCO.1998.16.3.1040. [DOI] [PubMed] [Google Scholar]
- 39.Doppman JL, Miller DL, Chang R, Maton PN, et al. Gastrinomas: localization by means of selective intraarterial injection of secretin. Radiology. 1990;174:25–29. doi: 10.1148/radiology.174.1.2294556. [DOI] [PubMed] [Google Scholar]
- 40.Alexander HR, Fraker DL, Norton JA, Barlett DL, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Annals of Surgery. 1998;228:228–238. doi: 10.1097/00000658-199808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. Journal of Internal Medicine. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 42.Norton JA, Alexander HR, Fraker DL, Venzon DJ, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Annals of Surgery. 2001;234:495–506. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine. 2004;83:43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 44.Frucht H, Norton JA, London JF, Vinayek R, et al. Detection of duodenal gastrinomas by operative endoscopic transillumination: a prospective study. Gastroenterology. 1990;99:1622–1627. doi: 10.1016/0016-5085(90)90466-e. [DOI] [PubMed] [Google Scholar]
- 45.Benya RV, Metz DC, Venzon DJ, Fishbeyn VA, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. American Journal of Medicine. 1994;97:436–444. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 46.Aprile MR, Azzoni C, Gibril F, Jensen RT, Bordi C. Intramucosal cysts in the gastric body of patients with Zollinger-Ellison syndrome. Human Pathol. 2000;31:140–148. doi: 10.1016/s0046-8177(00)80213-0. [DOI] [PubMed] [Google Scholar]
- 47.Roy P, Venzon DJ, Shojamanesh H, Abou-Saif A, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Metz DC, Pisegna JR, Fishbeyn VA, Benya RV, et al. Currently used doses of omeprazole in Zollinger-Ellison syndrome are too high. Gastroenterology. 1992;103:1498–1508. doi: 10.1016/0016-5085(92)91170-9. [DOI] [PubMed] [Google Scholar]
- 49.Collen MJ, Jensen RT. Idiopathic gastric acid hypersecretion: comparison with Zollinger-Ellison syndrome. Digestive Diseases and Sciences. 1994;39:1434–1440. doi: 10.1007/BF02088045. [DOI] [PubMed] [Google Scholar]
- 50.Termanini B, Gibril F, Sutliff VE, III, Yu F, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. American Journal of Medicine. 1998;104:422–430. doi: 10.1016/s0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 51.Gillen D, Wirz AA, Ardill JE, McColl KE. Rebound hypersecretion after omeprazole and its relation to on-treatment acid suppression and Helicobacter pylori status. Gastroenterology. 1999;116:239–247. doi: 10.1016/s0016-5085(99)70118-6. [DOI] [PubMed] [Google Scholar]
- 52.Metz DC, Weber HC, Orbuch M, Strader DB, et al. Helicobacter pylori infection: a reversible cause of hypergastrinemia and hyperchlorhydria which can mimic Zollinger-Ellison syndrome. Digestive Diseases and Sciences. 1995;40:153–159. doi: 10.1007/BF02063959. [DOI] [PubMed] [Google Scholar]
- 53.Weber HC, Venzon DJ, Jensen RT, Metz DC. Studies on the interrelation between Zollinger-Ellison syndrome, Helicobacter pylori and proton pump inhibitor therapy. Gastroenterology. 1997;112:84–91. doi: 10.1016/s0016-5085(97)70222-1. [DOI] [PubMed] [Google Scholar]
- 54.Lamberts R, Creutzfeldt W, Struber HG, Brunner G, Solcia E. Long-term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth, and gastritis. Gastroenterology. 1993;104:1356–1370. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- 55.McColl KE, Gillen D. Evidence that proton-pump inhibitor therapy induces the symptoms it is used to treat. Gastroenterology. 2009;137:20–22. doi: 10.1053/j.gastro.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Ekman L, Hansson E, Havu N, Carlsson E, Lundberg C. Toxicological studies on omeprazole. Scandinavian Journal of Gastroenterology. 1985;20(Suppl 108):53–69. [PubMed] [Google Scholar]
- 57.Waldum HL, Brenna E, Sandvik AK. Long-term safety of proton pump inhibitors: risks of gastric neoplasia and infections. Expert Opin Drug Saf. 2002;1:29–38. doi: 10.1517/14740338.1.1.29. [DOI] [PubMed] [Google Scholar]
- 58.Sandvik AK, Brenna E, Waldum HL. Review article: the pharmacological inhibition of gastric acid secretion--tolerance and rebound. Alimentary Pharmacology and Therapeutics. 1997;11:1013–1018. doi: 10.1046/j.1365-2036.1997.00257.x. [DOI] [PubMed] [Google Scholar]
- 59.Larsson H, Carlsson E, Ryberg B, Fryklund J, Wallmark B. Rat parietal cell function after prolonged inhibition of gastric acid secretion. AmJ Physiol. 1988;254:G33–G39. doi: 10.1152/ajpgi.1988.254.1.G33. [DOI] [PubMed] [Google Scholar]
- 60.LePard KJ, Mohammed JR, Stephens RL., Jr Gastric ECL-cell hyperplasia produces enhanced basal and stimulated gastric acid output but not gastric erosion formation in the rat. General Pharmacology. 1997;28:415–420. doi: 10.1016/s0306-3623(96)00296-0. [DOI] [PubMed] [Google Scholar]
- 61.Larsson H, Carlsson E, Mattsson H, Lundell L, et al. Plasma gastrin and gastric enterochromaffin-like cell activation and proliferation. Studies with omeprazole and ranitidine in intact and antrectomized rats. Gastroenterology. 1986;90:391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- 62.Nishida A, Kobayashi-Uchida A, Akuzawa S, Takinami Y, et al. Gastrin receptor antagonist YM022 prevents hypersecretion after long-term acid suppression. AmJ Physiol. 1995;269:G699–G705. doi: 10.1152/ajpgi.1995.269.5.G699. [DOI] [PubMed] [Google Scholar]
- 63.Havu N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion. 1986;35(Suppl 1):42–55. doi: 10.1159/000199381. [DOI] [PubMed] [Google Scholar]
- 64.Carlsson E, Larsson H, Mattsson H, Ryberg B, Sundell G. Pharmacology and toxicology of omeprazole--with special reference to the effects on the gastric mucosa. ScandJ Gastroenterol(Suppl) 1986;118:31–38. doi: 10.3109/00365528609090884. [DOI] [PubMed] [Google Scholar]
- 65.Weinstein WM, Ippoliti AF, Lee SW, Weber LJ, Lieberman DA. Acid hypersecretion, parietal cell hyperplasia, and endoscopic changes after withdrawal of long term high dose omeprazole therapy: a prospective study. Gastroenterology. 1996;110(4):A294. [Google Scholar]
- 66.Larsson H, Carlsson E, Hakanson R, Mattsson H, et al. Time-course of development and reversal of gastric endocrine cell hyperplasia after inhibition of acid secretion. Gastroenterology. 1988;95:1477–1486. doi: 10.1016/s0016-5085(88)80066-0. [DOI] [PubMed] [Google Scholar]
- 67.Tielemans Y, Chen D, Sundler F, Hakanson R, Willems G. Reversibility of the cell kinetic changes induced by omeprazole in the rat oxyntic mucosa. An autoradiographic study using tritiated thymidine. Scandinavian Journal of Gastroenterology. 1992;27:155–160. doi: 10.3109/00365529209165437. [DOI] [PubMed] [Google Scholar]
- 68.Chey WY, Chang TM, Park HJ, Lee KY, et al. Non-gastrin secretagogue in ulcerogenic tumors of the pancreas. Annals of Internal Medicine. 1984;101:7–13. doi: 10.7326/0003-4819-101-1-7. [DOI] [PubMed] [Google Scholar]
- 69.Chey WY, Chang TM, Lee KY, Sun G, et al. Ulcerogenic tumor syndrome of the pancreas associated with a nongastric acid secretogogue. Annals of Surgery. 1989;210:139–149. doi: 10.1097/00000658-198908000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jensen RT. Treatment of pancreatic Zollinger-Ellison syndrome and other gastric hypersecretory states. In: Wolfe MM, editor. Therapy of Digestive Disorders. A companion to Sleisenger and Fordtran’s Gastrointestinal and Liver Diseases. Philadelphia: W.B. Saunders Co; 2000. pp. 169–187. [Google Scholar]
- 71.Song Y, Chey WY, Chang TM, Lee KY. Mechanism of gastric acid hypersecretion in patients with islet cell tumor without hypergastrinemia: studies in rats. Gastroenterology. 1997;113:1129–1135. doi: 10.1053/gast.1997.v113.pm9322507. [DOI] [PubMed] [Google Scholar]
- 72.Krenning EP, Kwekkeboom DJ, Bakker WH, Breeman WAP, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. European Journal of Nuclear Medicine. 1993;20:716–731. doi: 10.1007/BF00181765. [DOI] [PubMed] [Google Scholar]
- 73.Jensen RT, Berna MJ, Bingham MD, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management and controversies. Cancer. 2008;113(7 suppl):1807–1843. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Waldum HL, Arnestad JS, Brenna E, Eide I, et al. Marked increase in gastric acid secretory capacity after omeprazole treatment. Gut. 1996;39:649–653. doi: 10.1136/gut.39.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kirkpatrick PM, Jr, Hirschowitz BI. Duodenal ulcer with unexplained marked basal gastric acid hypersecretion. Gastroenterology. 1980;79:4–10. [PubMed] [Google Scholar]
- 76.Jensen RT, Gardner JD. Gastrinoma. In: Go VLW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The Pancreas: Biology, Pathobiology and Disease. New York: Raven Press Publishing Co; 1993. pp. 931–978. [Google Scholar]
- 77.Crean GP, Marshall MW, Ramsey RD. Parietal cell hyperplasia induced by the administration of pentagastrin (ICI - 50,123) to rats. Gastroenterology. 1969;57:147–155. [PubMed] [Google Scholar]
- 78.Annibale B, Aprile MR, Ferraro G, Marignani M, et al. Relationship between fundic endocrine cells and gastric acid secretion in hypersecretory duodenal ulcer diseases. Alimentary Pharmacology and Therapeutics. 1998;12:779–788. doi: 10.1046/j.1365-2036.1998.00365.x. [DOI] [PubMed] [Google Scholar]
- 79.Hakanson R, Chen D, Lindstrom E, Norlen P, et al. Physiology of the ECL cells. Yale Journal of Biology and Medicine. 1998;71:163–171. [PMC free article] [PubMed] [Google Scholar]
- 80.Howard JM, Chremos AN, Collen MJ, McArthur KE, et al. Famotidine, a new, potent, long-acting histamine H2-receptor antagonist: comparison with cimetidine and ranitidine in the treatment of Zollinger-Ellison syndrome. Gastroenterology. 1985;88:1026–1033. doi: 10.1016/s0016-5085(85)80024-x. [DOI] [PubMed] [Google Scholar]