Dyskerin is a highly conserved and essential nucleolar protein that is required for the assembly and maturation of a specific subtype of ribonucleoproteins (RNPs).1 These RNPs incorporate a class of non-coding RNA characterized by the consensus H/ACA secondary structure. There are over 100 human H/ACA RNA, including telomerase RNA and several small nucleolar (snoRNA) and Cajal body RNA. The specific function of each RNP is dependent on its component RNA and each RNP incorporates a single H/ACA RNA. Through binding to H/ACA snoRNA and Cajal body RNA, dyskerin catalyzes the site-specific conversion of uridine residues to pseudouridines in nascent ribosomal RNA and spliceosomal RNA, respectively.1–3 Through interaction with telomerase RNA, dyskerin is also required for normal telomerase activity and telomere maintenance.4 However, emerging evidence suggests that dyskerin may regulate other important cellular processes.5,6 To that end, recent in silico analyses indicate that a subset of microRNAs (miRNAs) are encoded within and processed from either known or predicted H/ACA snoRNA.6–8 MiRNAs are a class of small non-coding RNAs that directly regulate post-transcriptional gene expression.6 MiRNAs typically exert their regulatory effects through binding to complementary sequences found in 3’-untranslated regions of their cognate mRNA.8 Dyskerin was recently shown to directly bind to several miRNA sequences embedded within known and predicted H/ACA snoRNA.6 Loss of dyskerin function reduces H/ACA RNA accumulation resulting in telomere dysfunction, impaired rRNA maturation and decreased cell proliferation.1–4 In this study, we tested the effects of dyskerin depletion on the accumulation of H/ACA snoRNA-encoded miRNAs. We treated telomerase-positive UM-SCC1 human oral cancer and telomerase-negative U2OS human osteosarcoma cells with siGENOME SMARTPool siRNA duplexes targeting dyskerin, GAPDH, or a non-specific sequence (Dharmacon, Lafayette, CO). Forty-eight hours later, total RNA was harvested using the miRNeasy kit (Qiagen, Valencia, CA) and then enriched for small RNA using the RNeasy MinElute Cleanup kit (Qiagen). The miScript PCR system (Qiagen) was used for quantitative RT-PCR analysis.
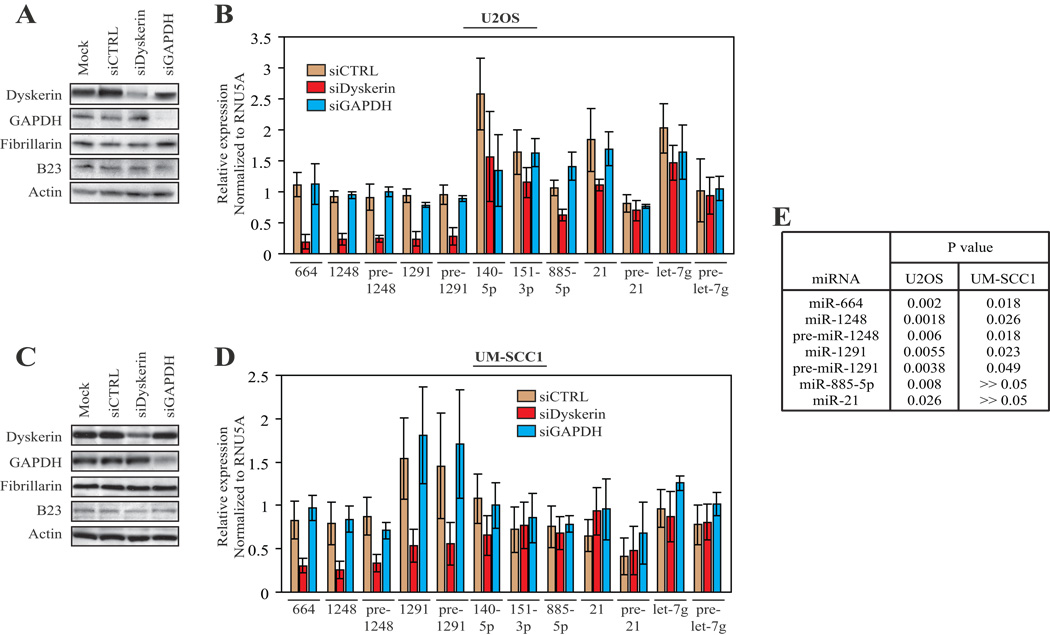
MiR-664 is encoded within H/ACA snoRNA ACA36B, miRNA-1248 is embedded within ACA81 and miRNA-1291 is found within ACA34.6 Irrespective of cell type, dyskerin depletion significantly reduced the levels of miR-664, miR-1248, miR-1291, and their respective immediate precursors pre-miR-1248 and pre-miR-1291, by 2- to 4-fold relative to the siRNA controls (Figure 1). We were unable to reliably measure pre-miR-664 expression. Similar results were obtained using a distinct, custom-designed, anti-dyskerin SMARTPool reagent (not shown). Dyskerin was shown to directly bind to H/ACA snoRNA sequences that overlap miR-664, miR-140 and miR-151.6 Yet, unlike miR-664, the levels of miR-140-5p and miR-151-3p were unaffected by dyskerin depletion. As recently suggested, there may be differences not only in the mechanisms by which telomerase RNA and H/ACA snoRNA are processed, but also in the way cells process prototypical H/ACA snoRNP and miRNA-containing H/ACA snoRNP.9 However, our intriguing findings suggest the possibility of an additional layer of complexity in the regulatory mechanisms that underlie the assembly and processing of miRNA-containing H/ACA snoRNP. Nonetheless, we cannot discount the possibility that miR-140-5p and miR-151-3p may be stable and that loss of dyskerin may not significantly affect their steady-state levels. Similarly, levels of miR-885-5p, which is also encoded within a predicted H/ACA snoRNA, remained unchanged in the dyskerin-depleted UM-SCC1 cells. In contrast, loss of dyskerin significantly decreased miR-885 levels in U2OS cells, albeit less than 2-fold relative to the controls. We were unable to measure pre-miR-885-5p levels. Thus, the possibility that there may be cell-type specific effects of dyskerin on regulation of miRNA-containing H/ACA snoRNA cannot be excluded.
Figure 1. Dyskerin depletion reduces the accumulation of a subset of miRNA.
Using the small RNA enriched samples obtained from the siRNA-treated cells, a panel of miRNA and, where indicated, their corresponding precursors were assessed by quantitative RT-PCR. A, Dyskerin expression was reduced by 80% in U2OS cells, with no effect on levels of other rRNA processing factors, including B23 and fibrillarin. As a control for transfection, GAPDH expression is also shown. Actin was used as a control for protein loading. B, In U2OS cells, dyskerin depletion (siDyskerin) resulted in a greater than 4-fold decrease in the levels of miR-664, -1248 and -1291 and pre-miR-1248 and pre-miR1291 relative to the non-target (siCTRL) and GAPDH (siGAPDH) controls. In contrast, miR-885-5p and miR-21 expression was reduced less than 2-fold. There was no change in the levels of the other miRNA or precursors tested. Precursors of miR-140, -151, -664 and -885 were beyond the threshold of detection. C, Dyskerin expression was reduced by almost 70% in UM-SCC1 cells. D, In these cells, the effect of siDyskerin on miRNA levels was not as pronounced. Nonetheless, loss of dyskerin reduced the levels of miR-664, miR-1248, miR-1291 and pre-mir-1248 and pre-mir-1291 by at least 2-fold relative to controls. There was no effect on the other miRNA tested. The levels of each transcript was measured relative to that of RNU5A, and then normalized to the ratio of the respective transcripts in the wild-type, untreated cells. Under our experimental conditions, levels of RNU5A remained essentially unchanged irrespective of the siRNA used. The solid bars represent the mean values derived from qRT-PCR analyses of triplicate transfections conducted in one representative experiment; error bars denote standard deviations. The experiment was repeated twice with similar results. E, P values for each of the miRNA that exhibited reproducible decreases in their expression. miR-885-5p and miR-21 were significantly reduced in U2OS but not in UM-SCC1 cells. For statistical analysis, levels of the miRNA from siDyskerin cells were compared to their corresponding levels in whichever of the siGAPDH or siCTRL controls exhibited the lower mean expression. Statistical significance was assessed using Student’s t-tests and set at 0.05.
As controls, we tested the effects on miRNA that do not bind dyskerin and do not derive from H/ACA snoRNA. Loss of dyskerin did not affect the levels of pre-miR-let-7g or mature miR-let-7g in either cell type. Similarly, miR-21 and pre-miR-21 were unaffected in UM-SCC1 cells. In U2OS cells, mature miR-21 was reduced less than 2-fold but this achieved statistical significance. However, since levels of its corresponding precursor were unchanged, we reason that the effect on miR-21 may be non-specific. This will require more detailed investigation.
It is currently unknown if dyskerin contributes to enzymatic processing of miRNA. Alternatively, it is possible that the observed reduction in miRNA levels results from a decrease in accumulation of the parent H/ACA snoRNA. Irrespective of the molecular mechanism and cell of origin, we show for the first time that loss of dyskerin function disrupts the accumulation of a subset of H/ACA snoRNA-derived miRNAs. MiR-664, miR-1248 and miR-1291 have a wide array of possible target mRNA, including many overlapping candidates. To our knowledge, miR-664, miR-1248 and miR-1291 have not yet been functionally validated. However, miRNA sequences (not yet annotated) derived from the H/ACA Cajal body RNA ACA45 can directly regulate post-transcriptional expression of the transcription regulatory factor CDK8 / CDC2L6.8 Interestingly, CDK8 also has a site in its 3’-untranslated region that is complementary to miR-664. Dyskerin is overexpressed in sporadically-occurring cancers and high tumor levels are associated with poor prognosis.10 Conversely, germline mutations in the dyskerin gene are associated with cancer susceptibility in individuals with X-linked dyskeratosis congenita.11 To that end, targeted inhibition of ACA45 results in upregulation of CDK88 and CDK8 is an oncoprotein12 whose overexpression can drive tumorigenesis. Thus, it is highly intriguing to speculate that dyskerin may potentially contribute to post-transcriptional gene expression, and, by extension, regulation of other important cellular processes in addition to rRNA processing and telomere maintenance that may be important for sporadic tumorigenesis and in the pathogenesis and phenotype of X-linked dyskeratosis congenita.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants DE018416 and the American Cancer Society #IRG-78-002-30.
REFERENCES
- 1.Meier UT. Chromosoma. 2005;114:1–14. doi: 10.1007/s00412-005-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggero D, et al. Science. 2003;299:259–262. doi: 10.1126/science.1079447. [DOI] [PubMed] [Google Scholar]
- 3.Ge J, et al. Mol Cell Biol. 2010;30:413–422. doi: 10.1128/MCB.01128-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell JR, et al. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 5.Yoon A, et al. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- 6.Scott MS, et al. PLoS Comput Biol. 2009;5:e1000507. doi: 10.1371/journal.pcbi.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taft RJ, et al. RNA. 2009;15:1233–1240. doi: 10.1261/rna.1528909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ender C, et al. Mol Cell. 2008;32:519–528. doi: 10.1016/j.molcel.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Trahan C, et al. Hum Mol Genet. 2010;19:825–836. doi: 10.1093/hmg/ddp551. [DOI] [PubMed] [Google Scholar]
- 10.Carter SL, et al. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 11.Heiss NS, et al. Nat Genet. 1998;19:32–38. doi: 10.1038/ng0598-32. [DOI] [PubMed] [Google Scholar]
- 12.Firestein R, et al. Nature. 2008;455:547–551. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]