Abstract
The development of a functional microvasculature is critical to the long-term survival of implanted tissue-engineered constructs. Dynamic culture conditions have shown to significantly modulate phenotypic characteristics and stimulate proliferation of cells within hydrogel-based tissue engineered blood vessels. Although prior work has described the effects uniaxial or equibiaxial mechanical stimulation has on endothelial cells, no work has outlined effects of three-dimensional mechanical stimulation on endothelial cells within tubular vessel analogs. We demonstrate here that 7 days of 10% cyclic volumetric distension has a deleterious effect on the average length and density of angiogenic sprouts derived from pellets of bovine aortic endothelial cells. Although both groups demonstrated lumen formation, the sprouts grown under dynamic culture conditions typically had wider, less-branching sprout patterns. These results suggest that prolonged mechanical stimulation could represent a cue for angiogenic sprouts to preferentially develop larger lumens over cellular migration and subsequent sprout length.
Keywords: Tissue Engineering, Angiogenesis, Vasa Vasorum, Bioreactor, Fibrin, BAEC
1. Introduction
Tissue engineering is a multidisciplinary field that applies engineering principles to create devices for the study, restoration, modification, and assembly of functional tissues from native or synthetic sources. For survival, thicker engineered or native tissues require either a microvascular network during development or the ability to rapidly foster neovascular ingrowth from pre-existing vasculature after implantation. (Black et al., 1998) This requirement is based on the observation that metabolically active cells cannot survive >200μm from their nutrient source, (Folkman and Hochberg 1973) and it is a phenomenon that can be clearly demonstrated within vascular tissue. After the removal of adventitia supplying vasa vasorum within vessels with a tunica media thicker than 29 smooth muscles cell (SMC) layers, (Wolinsky and Glagov 1967) arterial segments undergo medial necrosis.(Heistad et al., 1978)
Several approaches have been adopted to engineer vascular tissue (i.e. cellular self assembly, synthetic graft, hydrogel, etc.). (L’Heureux et al., 1998), (Schmidt et al., 2006), (Grassl et al., 2002) Of these, biologic hydrogels offer advantages of cell signaling and direct attachment. However these constructs are inherently weak and prone to dilation or mechanical failure. Early work with these tubular hydrogels required reinforcement by synthetic tubular grafts. (Weinberg and Bell 1986) More recently, dynamic culture conditions including cyclic distension with resultant strain have been shown to augment the mechanical characteristics of hydrogel-based tissue engineered blood vessels (TEBV). (Seliktar et al., 2003), (Syedain et al., 2008) Specifically, hydrogels comprised of fibrin or collagen and containing SMC’s cultured in vitro within bioreactors generating 10% cyclic strain have demonstrated significant cellular alignment, cellular proliferation, and extracellular matrix (ECM) deposition. (Grassl et al., 2002), (Stegemann and Nerem 2003), (Butcher et al., 2006)
Besides stimulating SMC’s, the effect of cyclic strain has also been studied for dynamic stimulation for endothelial cells (EC’s). These observations have been made in relation to EC’s subjected to uniaxial or equibiaxial strain. Joung et al. demonstrated that a monolayer of EC’s seeded on top of collagen hydrogels exposed to uniaxial cyclic strain demonstrate increased sprout width. (Joung et al., 2006) Von Offenberg Sweeney et al. noted that a monolayer of EC’s exposed to 5% equibiaxial cyclic strain for 24 hr and subsequently harvested and placed as a monolayer on top of collagen gels demonstrated subsequent increased tubulogenesis. (Von Offenberg Sweeney et al., 2005) Matsumoto et al. demonstrated that EC’s on cytodex beads in a three dimensional culture system composed of fibrin hydrogels subjected to 10% equibiaxial strain for 48 hours have less angiogenic sprout branching and increased “sprout thickness”.(Matsumoto et al., 2007)
The intent of the current work was to decipher what effect 10% cyclic strain has on angiogenic sprout formation from pelleted endothelial cells within the wall of a fibrin hydrogel-based TEBV. This pursuit differs from the aforementioned work by employing a bioreactor culture system modified from one previously described to engineer TEBVs. (Seliktar et al., 2003) This approach allows for tubular hydrogels to be cyclically strained in concert with the volumetric distension of inner silicone sleeves. Using this system we demonstrate that volumetric cyclic distension significantly alters the behavior of angiogenic sprouts within the wall of a tubular hydrogel. The information gained from this in vitro work could be relevant to the development of vasa vasorum within thicker vascular tissue constructs or model and assess what role local mechanical stimulation has on angiogenesis within the fibrin coagulum surrounding an implanted, pulsating construct. Additionally, this work suggests that spheroids of endothelial cells may be used to populate a hydrogel-based tissue construct to create a microvascular network.
2. Methods
2.1. Cell Culture
Bovine aortic endothelial cells (BAECs), were enzymatically harvested with collagenase from adult bovine aortas procured from a local abattoir within minutes of the animals’ demise (Booyse et al., 1975), (Schwartz 1978) After isolation and identification of >95% purity by staining with anti-vWF, the cells were seeded at 5.0×105 cells/flask in fibronectin coated (2.5mg/cm2) t75 flasks. The cells (passages 2–5) were grown with media changes every 3 days in complete media composed of M199 supplemented with L-Glutamine, Hepes salts, Earle’s salts, 10% fetal bovine serum, streptomycin/penicillin (100 μg/mL), and fungisone (250 μg/mL) in a 37°C environment supplemented with 5% CO2. At 90% confluence the cells were transferred from culture flasks using a short digestion (≤10 minute) with trypsin-EDTA.
2.2. Pellet Formation
Cell pellets were formed in a similar fashion to the protocol of EC spheroid fabrication described by Korff et al.(Korff et al., 2001) Briefly, pellets of 2,000 BAECs were created by combining 1% sterile methylcellulose in M199 and an EC suspension at a (1:3) ratio and then incubated at 37°C with 5% CO2 in a 96-well round-bottom culture plate for 24 hours. The pellets were transferred to a centrifugation tube, spun at 250 RPM for two minutes, and the methylcellulose supernatant solution was removed.
2.3. Tubular Construct Formation
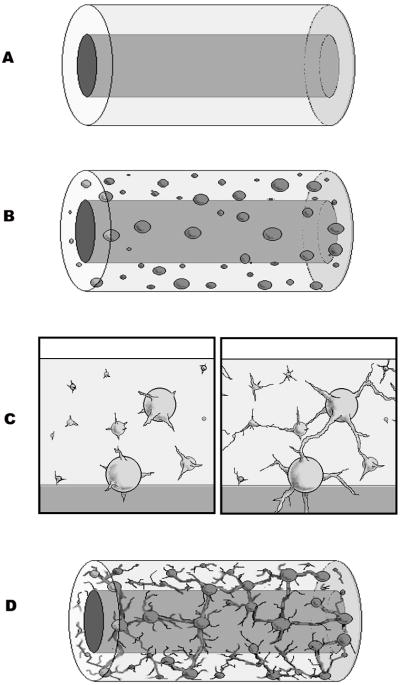
Endothelial cell pellets were re-suspended in a solution of M199 medium (supplemented with L-Glutamine, Hepes salts, and Earle’s salts), fibrinogen, heparin sulfate, vascular endothelial growth factor (VEGF), fibroblast growth factor-1 (FGF-1), and aprotinin. Thrombin dissolved in an equal volume of the same M199 was then added to the suspension yielding a final concentration of EC pellets (20 pellets/mL), fibrinogen (2.5 mg/mL), thrombin (0.32 U/mL), heparin sulfate (5 U/mL), VEGF (1 ng/mL), FGF-1 (10 ng/mL), and aprotinin (100KIU/mL). Immediately after mixing, the solution was added to a tubular mold comprised of five parts: 1) an outer glass cylinder (3 cm long × 8 mm I.D.), 2) an inner glass mandrel (3 cm long × 2 mm O.D.), 3) a sterile silicon sleeve (2 cm × 3.15 mm I.D.× 3.55 mm − 50 durometer) covering the mandrel, 4) two sterile silicone stoppers enclosing both open ends of the outer cylinder, and 5) an 18 G needle. The needle evacuated air from the mold as stoppers closed each end. To ensure proper hydrogel adhesion, prior to the experiment, the silicone sleeves were etched by overnight submersion in 10N HCl, rinsed with sterile water, and then adjusted to a pH of 8 with sequential rinses with M199 of the same pH. During crosslinking, the mold was gently rolled for 4 minutes to ensure equal distribution of EC pellets. After 8 minutes at 37°C, the hydrogel was careful removed from the mold and placed within assay media composed of the same reagents and concentrations as the hydrogel except lacking fibrinogen and thrombin for another 30 minutes to allow final equilibration. Manipulated by the sleeve alone, the tubular hydrogels’ inner silicone sleeves were attached to the inlet and outlet mounting pins within the previously described bioreactor chamber and sutured into place.(Seliktar et al., 2003) Special care was taken to fix each end of the hydrogel with a stay suture. Constructs were then covered with more assay media, which was replaced on the 3rd day of culture. Both dynamic and static controls were mounted in the same fashion. The bioreactor, sealed with exception of a 0.22μ filter vent port, was placed within a 37°C incubator with 100% humidity and 5% CO2.
2.4. Dynamic culture
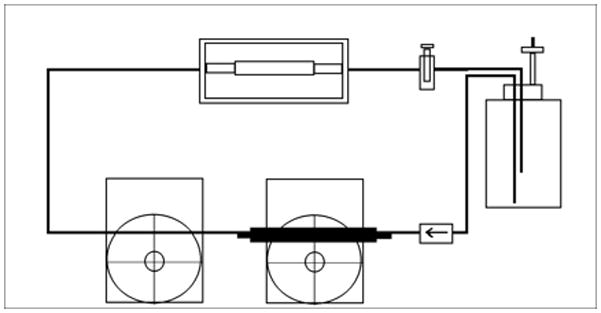
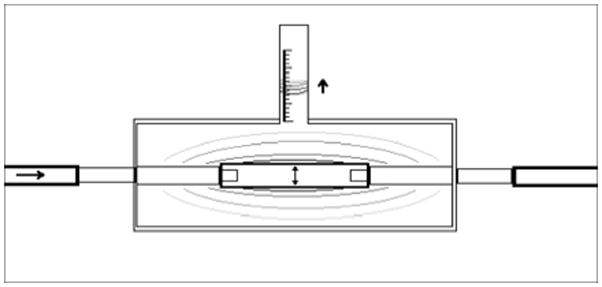
A Windkessel effect pump system,(Dumont et al., 2002), (Hahn et al., 2007) comprised of two peristaltic pumps arranged in series was used to generate a fluid pulse wave capable of distending the dynamic culture groups’ silicone sleeves by 10% (Figure 1). Briefly, a computer controlled L/S brushless pump system with check valve (Cole-Parmer, Vernon Hills, IL USA) continuously delivered perfusate composed of PBS 1x supplemented with streptomycin/penicillin (100 μg/mL), and fungisone (250 μg/mL) into large caliber high-pressure, platinum-cured, LS 35 silicone tubing (Cole-Parmer). This fluid became pressurized as the flow was limited by another occlusive peristaltic pump (Cole-Parmer) that sequentially released fluid pulsations at 1 Hz. The released pressure wave propagated through minimally distensible Tygon tubing (0.125″ ID × 0.250″ OD) (Cole-Parmer) until it reached the bioreactor chambers where it was able to volumetrically displace 10% of the silicon sleeve’s outer volume. An afterload resistance valve was placed in the circuit after the bioreactor chambers and before the perfusate reservoir. This device ensured positive circuit pressures during pulsatile flow. Briefly, the volume of displacement was determined by filling an identical bioreactor chamber, termed the calibration chamber, with water and pulsing the system with the same Windkessel-effect pumps used in the experimental trial. After a brief modification to the calibration chamber’s vent port, the volume displaced by silicon sleeve distension was determined by measuring the distance the fluid within the vent tubing moved with each pulsation (Figure: 1a). As water is essentially non-compressible at atmospheric pressure and 37°C, the volume of fluid that moved within the vent port corresponds directly with the external volume change of the silicone sleeves within the calibration chamber. The Windkessel-effect pump system was adjusted until the volume within the vent port changed by 10% of the resting, external volume of silicon sleeves present between fixation sutures in the calibration chamber. Of note, pressure tracings were also taken throughout the system at every bifurcation point, inlet, and outlet and ANOVA analysis of these “systolic” and “diastolic” pressures documented that there was no significant difference in pressure profiles, which would have indicated discrepancies in pressure transduction throughout the system (data not shown). Additionally, a calibration chamber was used intermittently to ensure 10% volumetric distension was maintained throughout the experimental trial.
Figure 1.
2.5. Sprout Quantification
To maintain their delicate angiogenic sprouts, each tubular construct was carefully removed from the bioreactor in its original shape with its inner silicone sleeve in place. The constructs were fixed in 4% paraformaldehyde (30 min. at 25°C), permeabilized with 0.5% Triton-X (1 hour at 25°C), blocked with 2% bovine serum albumin (BSA) (1 hour at 37°C), labeled with Alexafluor 488 labeled phalloidin (1:40 concentration for 1 hour at 37°C), and washed several times with 1x PBS before and after each step. Angiogenesis was then assessed with a Carl Zeiss Axiovert 200 inverted fluorescence microscope and a 5MP 36-bit RGF Axiocam MRc5 color digital camera. To avoid the obscuring silicone sleeve, fluorescently labeled EC pellets and sprouts were visualized tangentially through the construct, and images through all focal planes involving the sprouts were taken.(Joung et al., 2006) The acquired images through all focal planes involving sprout lengths were combined for each pellet using Adobe® Photoshop® v 5.0 software. Average sprout length and sprout number were quantified as described by Vernon et al. (Vernon and Sage 1999) Briefly, a 36 spoke radial grid (one every 10°) was overlaid over each merged image. The number of radians crossed and furthest distance from the center for each radian cross was recorded and tabulated.
2.6. Ultrastructure Assessment: Confocal microscopy
The sprout ultrastructure was analyzed with confocal microscopy. Briefly, the stained pellets were optically sectioned by exciting their flurophore at 488 nm through a 10x Plan-Neofluor air objective on a laser-scanning microscope (LSM) (Carl Zeiss Microimaging, Thornwood, NY USA). Stacks of images (every 10 μm), acquired by the Pascal confocal program (Carl Zeiss), were reconstructed into 3D images to view sprout growth. Optical sections of individual sprouts were taken at 20x magnification every 5 μm.
2.7. Ultrastructure Assessment: Transmission electron microscopy
At the end of the assay, angiogenic networks and pellets along with 1 mm hydrogel margins were carefully excised from the fixed tubular constructs and prepared for morphologic analysis via light and transmission electron microscopy. For light microscopy, the pellets were dehydrated, embedded in paraffin, sectioned perpendicularly at 5 μm thickness, and stained with hematoxylin and eosin. For transmission electron microscopy, the pellets were fixed were subsequently fixed again with 1% glutaraldehyde solution, postfixed in 1% osmium tetroxide, dehydrated in a graded series of acetone washes, and embedded in resin. Ultrathin sections were cut, collected on copper grids, and stained with uranyl acetate and lead citrate. The sections were viewed on a transmission Hitachi H-600 electron microscope at 50 kV.
2.8. Statistics
The length of sprouts and number of sprouts per pellet were calculated for each pellet and averaged for each construct. Averages and standard deviations for static and dynamically cultured pellet groups were compared using a student t-test assuming equal variance. P-values < 0.05 were considered significant.
3. Results
3.1. 10% volumetric strain reduces BAEC average sprout length
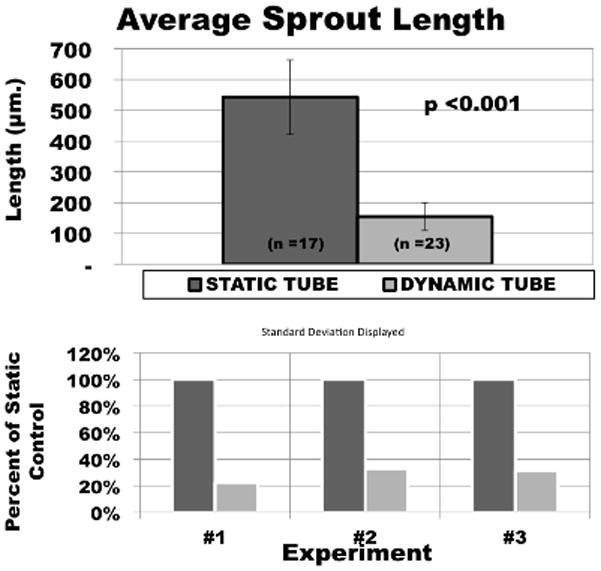
Volumetric cyclic strain (10%, 1 Hz, for 7 days) limited the length of BAEC pellet’s angiogenic sprout invasion within the wall of tubular fibrin hydrogels by 63.3–81.6% as compared to static controls (Figure 2). Similar trends were found in each of the three experimental trials (Figure 3). The first demonstrated an average sprout length from static ycontrol pellets of 710 ± 104 μm versus 130 ± 71 μm for the volumetric strain group (p<0.001). The second and third trials demonstrated 484 ± 134 μm vs. 177 ± 21 μm (p <0.001) and 576 ± 99 μm vs. 110 ± 76 μm (p<0.05), respectively. Additionally, to assess potential effects of variability between experimental trials, average sprout lengths for both statically and dynamically cultured pellets in all experiments were pooled and compared with a student T-test. Average length of sprout for statically cultured pellets for all experiments was 545 ± 105 μm versus 155 ± 42 μm for all volumetric strained pellets (p<0.001).
Figure 2.
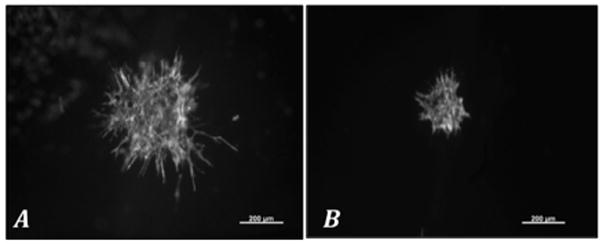
Figure 3.
3.2. 10% volumetric strain reduces BAEC average sprout density
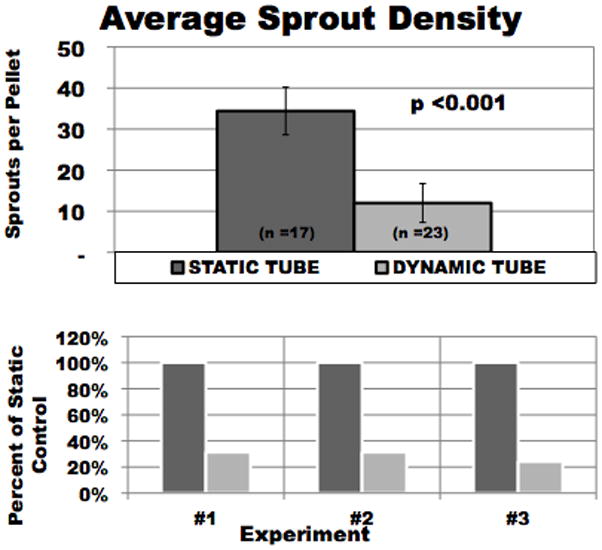
Sprout number was defined as the number of radial grid lines crossed within Vernon and Sage’s radial grid system. (Vernon and Sage 1999) In all experimental trials, the dynamic culture conditions had fewer sprouts per pellet than their statically cultured counterparts (Figure 4). Volumetric cyclic strain reduced the average number of sprouts from each cell pellet by 50 to 77% as compared to static controls. Sprout number for static vs. volumetric strain groups for the first, second, and third trials demonstrated 23 ± 13 vs. 11 ± 10, 36 ± 0 vs. 8 ± 3, and 36 ± 0 vs. 9 ± 1 sprouts, respectively. Statistical significance (p< 0.001) was reached for the 2nd and 3rd trials. Additionally, when all experimental trials were averaged a statically significant difference was found between statically and dynamically cultured pellets (p<0.001).
Figure 4.
3.3. Lumen Formation
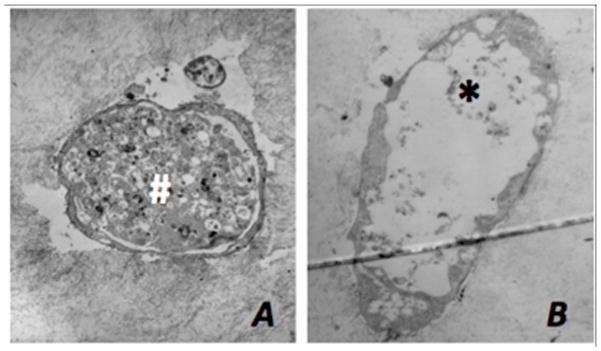
Transmission electron microscopy demonstrated that endothelial cell pellets both dynamically and statically cultured for 7 days in the presence of FGF-1 and VEGF form angiogenic sprouts with lumens. The lumens commonly involved a central cavity within the cell pellet and extended out peripherally through the angiogenic sprout (Figure 5). Sections were taken at varying levels through each pellet’s sprout network. Cross sections of the angiogenic sprouts derived from the dynamically cultured pellets typically displayed lumens extending the length of the sprout while their statically grown counterparts typically displayed lumens that terminated closer to the pellet origin (Figure 6). However, these differences could not be quantified.
Figure 5.
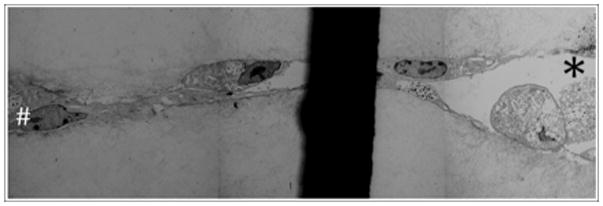
Figure 6.
3.4. Sprout Morphology
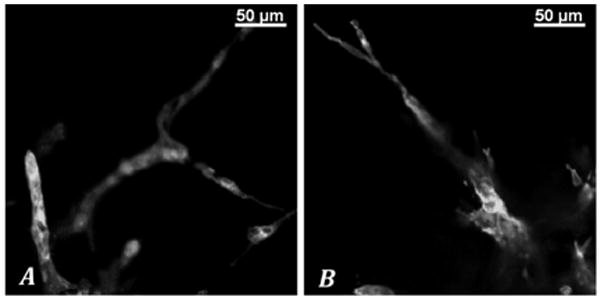
Images from static control pellets acquired at 10x magnification demonstrated a dense network of thin highly branching angiogenic sprouts. The pellets subjected to dynamic stimulation however typically displayed sprouts with less branching but greater diameters. At higher magnification (20x) and under confocal microscopy, pellets subjected to dynamic stimulation were clearly seen to generate sprouts with diameters commonly in the range of 30 μm as compared to the static controls, which were often on the order of 10 μm (Figure 7). No sprouts within the static control group were > 15μm in diameter. Less sprout branching, once again, was noted (Figure 8). We did not perform precise measurements for statistical analysis because one cannot specifically rule out artifact in cross-sectional area that may result from in tangential views. However this limitation faces all assessments made by this common method of morphologic assessment and is not inherent to our conditions. The differences we appreciated were subjectively obvious.
Figure 7.

Figure 8.
4. Discussion
The formation of lumens within angiogenic sprouts, or tubulogenesis, is a hallmark of angiogenesis. It is the capacity of ECs to create luminal compartments connected to multicellular chains, which allows for the flow of blood from the pre-existing vasculature to the neovasculature. At the outset of this work we developed a model to investigate the EC response to cyclic three-dimensional deformation within tubular fibrin hydrogels. It was our hope to describe the role cyclic strain has on EC behavior either during the in vitro development of TEBV’s or within the perivascular region after the implantation of a pulsating engineered construct. Prior work noted that uniaxial or equibiaxial cyclic strain augments endothelial migration and tubulogenesis. (Joung et al., 2006), (Matsumoto et al., 2007), (Von Offenberg Sweeney et al., 2005) We hypothesized that tubular constructs subjected to 10% volumetric distension would similarly demonstrate increased angiogenic sprout length and sprout density. However, our results suggest that 10% cyclic volumetric distension in a three-dimensional fibrin hydrogel limits the depth of sprout penetration. Our results also suggest that mechanical stimulation augments tubulogenesis while inhibiting sprout branching
Bovine aortic endothelial cells were selected for this work because of both their robust sprout formation and extensive description of their behavior in relation to mechanical stimulation. (Joung et al., 2006), (Vouyouka et al., 1998), (Von Offenberg Sweeney et al. 2005), (Zhao et al., 1995), (Sumpio et al., 1987), (Azuma et al., 2000), (Ito et al., 2007) Previously in our lab, the combination of VEGF and FGF-1 was shown to synergistically augment sprout formation from pelleted BAEC’s within fibrin hydrogels. (Xue and Greisler 2002) Taken together, we continued this approach to increase the EC’s sprouting potential within an environment of volumetric cyclic strain.
The premise of this work is that cells in vivo rarely survive beyond 200μm distance from a nutrient and waste exchange interface. However, we demonstrated angiogenic sprout formation in hydrogels that are > 1.0 mm in thickness. Griffith et al. also described that endothelial cell populations may sprout in fibrin hydrogels > 7.2mm from the surface of their nutrient and waste exchange. (Griffith et al., 2005) The substrates that comprise the tissue are the key distinction. For example, the aforementioned diffusion distance limitation of 200μm typically describes dense tissues heavily populated by metabolically active cells. In both Griffith’s work and the data presented here, the in vitro assays were carried out in fibrin hydrogels of similar density (2.5 mg/mL). These loose matrices, sparsely populated by endothelial cell spheroids, likely allow for both nutrient and waste exchange despite having a thickness > 1.0 mm.
For this study, 20 pellets per mL at 2,000 cells/pellet was found to be a reasonable density/size ratio to limit pellet encroachment which subsequently was found to limit facility of optical sectioning and sprout identification. For the development of a functional vasa vasorum within a hydrogel-based tissue construct using the aforementioned technique, the optimal exact pellet size and density relationship have yet to be determined. The selection listed here was made pragmatically based on tangential visualization.
Prior work may offer some insight into the decrease in average angiogenic sprout length and perhaps an increase in tubulogenesis. Carosi et al. demonstrated that BAEC’s subjected to 10% cyclical strain demonstrate increased prostacyclin and endothelin secretion.(Carosi et al., 1992) Prostacyclin has been described to augment both endothelial cell proliferation and tube formation, while endothelin is a potent mitogenfor EC’s, promoting cord formation on Matrigel.(Salani et al., 2000; He et al., 2008) Collins et al. reported that BAEC’s subjected to 5% equibiaxial cyclic strain demonstrated an increase in tight junctional protein expression (i.e. Occludin and ZO-1), protein localization to cell borders, and intracellular tight junction formation.(Collins et al., 2006) EC tight junction assembly represents an augmentation of barrier integrity. Such cellular behaviors are necessary for subsequent vessel integrity and perhaps represent another step in differentiation. Within a three-dimensional culture environment, volumetric cyclic strain may represent a cue for EC’s to differentiate and undergo tubulogenesis over further migration or substrate invasion.
The model we described above differs from prior work by adding another dimension to in vitro mechanical stimulation. By casting a hydrogel around and suturing it to a distensible silicone sleeve we have three axes of mechanical stimulation. When the silicone sleeve distends, it does so circumferentially. Locally this type of distension causes distortion of the hydrogel in two dimensions. However, given that the silicone sleeves were etched to ensure tight contact and that the hydrogels were immobilized at either end, the hydrogels also experience a lengthening component. We described this form of stimulation as volumetric distension for two reasons. First, it has three unique axes of stimulation. Second, to assess this type of stimulation, measurement of volumetric displacement of the central silicone sleeve is imperative. Although prior work provides great insight into the local activity of ECs within planar shaped tissues, these models do not accurately describe the local mechanical stimulation within either mural or directly contiguous perivascular tissue. We believe that this form of stimulation more closely reflects the mechanical environment within vessels that are subjected to pulsatile flow in vivo. Native vessels not only distend circumferentially, they also experience a lengthening element as a pulse wave propagates through both the lumen and wall. Unless transected, each vessel segment is attached to both proximal and distal elements that serve as counter-traction to pulse wave lengthening.
Volumetric stimulation may represent another discreet form of mechanical stimulation. Dynamic culture conditions have different excitatory effects depending on the type of stimulation. For example, Azuma et al. (Azuma et al., 2000) described that cyclic strain and flow shear stimulate BAEC’s to express differing levels of intracellular signaling molecules (i.e. ERK and p38).
The time spent mechanically stimulating tissue may be another key feature in the differences displayed between the prior work and our results. Most studies describing EC responses to cyclic strain have been done in experiments that last 48 hours or less. (Joung et al., 2006), (Von Offenberg Sweeney et al., 2005), (Collins et al., 2006), (Rosales and Sumpio 1992), (Sumpio et al., 1998), (Upchurch et al., 1998) Although data from early time points is critical to an understanding of the initial influence of mechanical stimulation has on engineered tissue, these early time points may not reflect what these cells experience in long term culture. For example, tPA has been demonstrated to play a critical role in EC invasion of fibrin hydrogels. Tsukurov et al. noted that EC tPA mRNA levels decrease the longer EC’s are subject to cyclic strain, with no mRNA detectible at 48 hours. (Tsukurov et al., 2000)
Endothelial cells have been shown to align themselves in relation to cyclic strain. (Joung et al., 2006), (Matsumoto et al., 2007), (Moretti et al., 2004) We cannot currently comment on the role volumetric strain played on angiogenic sprout orientation. Due to the fragile nature of these hydrogels, sectioning or removal from their inner silicone sleeves was impossible. All attempts to do so resulted in deformation or destruction of angiogenic sprouts regardless of fixation technique. As such, angiogenic sprout lengths were documented by tangential optical sectioning through hydrogels that were not obstructed by silicone sleeves. We found the bright label and deep tissue penetration of the Alexafluor 488 labeled Phalloidin critical to visualization. Although it is possible that tangential image acquisition could miss accelerated sprout growth in the transaxial direction, we noted multi dimensional sprout growth. Although not impossible, it is not likely that missed sprout orientation due to angle of view could account for the 63.3–81.6% reduction in average sprout length we found in this study.
Nutrient and gas exchange is both a requirement and a challenge in developing a tissue engineered construct. General approaches to vascularization include either the engineering of a microvasculature in vitro, prior to implantation, or the generation of a microvasculature early in the post-implantation period. Our understanding of the regulation of angiogenic processes is steadily improving, but remains far from complete. This work suggests that suspended endothelial cell pellets under the correct conditions can generate an interconnected network of endothelial-lined lumens that resemble a capillary network (Figure 9). Within a blood vessel wall, this network could serve as a vasa vasorum after the recipient’s vascular network either inosculates with surface openings or with trans-adventitial neovascularization into this engineered network. However, it is important to note that this is in vitro work and that in vivo endothelial cells may behave quite differently. Additionally, until such networks are implanted in vivo, We have no way of knowing how stabile these neovessel will be in terms of their long-term spatial organization and patency during the in vivo remodeling processes.
Figure 9.
5. Conclusions
We created lumen-forming angiogenic sprouts of bovine aortic endothelial cells within tubular fibrin hydrogels under both static and dynamic culture conditions. The presence of 10% volumetric strain appears to limit the length of sprout formation at one week when compared to static controls. However, other findings (i.e. sprout diameters and lumen extension) seem to suggest that mechanical stimulation may be contributing to other cellular behaviors required for neovascularization other than matrix invasion, including tubulogenesis. As the development of perfused capillary networks requires both cellular invasion and tubulogenesis, prolonged mechanical stimulation may aid the development of microvasculature microvascular system capable of sustaining blood flow and supporting the metabolic activities of thick, engineered tissues.
Acknowledgments
Sponsorship: This work was supported by grants from the National Institutes of Health (NIH R01-HL41272), the Department of Veterans’ Affairs, the American Heart Association (AHA 0815674G), the National Science Foundation (NSF 0731201a0) and the Falk Medical Research Trust.
Acknowledgements/Funding: This work was supported by grants from the National Institutes of Health (NIH R01-HL41272), the Department of Veterans’ Affairs, the American Heart Association (AHA 0815674G), the National Science Foundation (Grant number 0731201a0) and the Falk Medical Research Trust.
References
- Azuma N, Duzgun SA, et al. Endothelial cell response to different mechanical forces. Journal of vascular surgery: official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2000;32(4):789. doi: 10.1067/mva.2000.107989. [DOI] [PubMed] [Google Scholar]
- Black AF, Berthod F, et al. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12(13):1331–40. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- Booyse FM, Sedlak BJ, et al. Culture of arterial endothelial cells: characterization and growth of bovine aortic cells. Thromb Diath Haemorrh. 1975;34(3):825–39. [PubMed] [Google Scholar]
- Butcher JT, Barrett BC, et al. Equibiaxial strain stimulates fibroblastic phenotype shift in smooth muscle cells in an engineered tissue model of the aortic wall. Biomaterials. 2006;27(30):5252. doi: 10.1016/j.biomaterials.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Carosi JA, Eskin SG, et al. Cyclical strain effects on production of vasoactive materials in cultured endothelial cells. Journal of cellular physiology. 1992;151(1):29. doi: 10.1002/jcp.1041510106. [DOI] [PubMed] [Google Scholar]
- Collins NT, Cummins PM, et al. Cyclic strain-mediated regulation of vascular endothelial occludin and ZO-1: influence on intercellular tight junction assembly and function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(1):62. doi: 10.1161/01.ATV.0000194097.92824.b3. [DOI] [PubMed] [Google Scholar]
- Dumont K, Yperman J, et al. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif Organs. 2002;26(8):710–4. doi: 10.1046/j.1525-1594.2002.06931_3.x. [DOI] [PubMed] [Google Scholar]
- Folkman J, Hochberg M. Self-regulation of growth in three dimensions. J Exp Med. 1973;138(4):745–53. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassl ED, Oegema TR, et al. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. Journal of Biomedical Materials Research. 2002;60(4):607. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- Grassl ED, Oegema TR, et al. Fibrin as an alternative biopolymer to type-I collagen for the fabrication of a media equivalent. J Biomed Mater Res. 2002;60(4):607–12. doi: 10.1002/jbm.10107. [DOI] [PubMed] [Google Scholar]
- Griffith CK, Miller C, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11(1–2):257–66. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- Hahn MS, McHale MK, et al. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Annals of Biomedical Engineering. 2007;35(2):190. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]
- He T, Lu T, et al. Angiogenic function of prostacyclin biosynthesis in human endothelial progenitor cells. Circ Res. 2008;103(1):80–8. doi: 10.1161/CIRCRESAHA.108.176057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heistad DD, Marcus ML, et al. Regulation of blood flow to the aortic media in dogs. J Clin Invest. 1978;62(1):133–40. doi: 10.1172/JCI109097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Sakamoto N, et al. Effects of frequency of pulsatile flow on morphology and integrin expression of vascular endothelial cells. Technology and health care: official journal of the European Society for Engineering and Medicine. 2007;15(2):91. [PubMed] [Google Scholar]
- Joung IS, Iwamoto MN, et al. Cyclic strain modulates tubulogenesis of endothelial cells in a 3D tissue culture model. Microvascular research. 2006;71(1):1. doi: 10.1016/j.mvr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Korff T, Kimmina S, et al. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15(2):447–57. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- L’Heureux N, Paquet S, et al. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Yung YC, et al. Mechanical strain regulates endothelial cell patterning in vitro. Tissue engineering. 2007;13(1):207. doi: 10.1089/ten.2006.0058. [DOI] [PubMed] [Google Scholar]
- Moretti M, Prina-Mello A, et al. Endothelial cell alignment on cyclically-stretched silicone surfaces. Journal of materials science Materials in medicine. 2004;15(10):1159. doi: 10.1023/B:JMSM.0000046400.18607.72. [DOI] [PubMed] [Google Scholar]
- Rosales OR, Sumpio BE. Protein kinase C is a mediator of the adaptation of vascular endothelial cells to cyclic strain in vitro. Surgery. 1992;112(2):459. [PubMed] [Google Scholar]
- Salani D, Taraboletti G, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157(5):1703–11. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Asmis LM, et al. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82(4):1465–71. doi: 10.1016/j.athoracsur.2006.05.066. discussion 1471. [DOI] [PubMed] [Google Scholar]
- Schwartz SM. Selection and characterization of bovine aortic endothelial cells. In Vitro. 1978;14(12):966–80. doi: 10.1007/BF02616210. [DOI] [PubMed] [Google Scholar]
- Seliktar D, Nerem RM, et al. Mechanical strain-stimulated remodeling of tissue-engineered blood vessel constructs. Tissue engineering. 2003;9(4):657. doi: 10.1089/107632703768247359. [DOI] [PubMed] [Google Scholar]
- Stegemann JP, Nerem RM. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Annals of Biomedical Engineering. 2003;31(4):391. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- Sumpio BE, Banes AJ, et al. Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg. 1987;6(3):252–6. [PubMed] [Google Scholar]
- Sumpio BE, Du W, et al. Regulation of PDGF-B in endothelial cells exposed to cyclic strain. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18(3):349. doi: 10.1161/01.atv.18.3.349. [DOI] [PubMed] [Google Scholar]
- Syedain ZH, Weinberg JS, et al. Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A. 2008;105(18):6537–42. doi: 10.1073/pnas.0711217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukurov OI, Kwolek CJ, et al. The response of adult human saphenous vein endothelial cells to combined pressurized pulsatile flow and cyclic strain, in vitro. Annals of Vascular Surgery. 2000;14(3):260. doi: 10.1007/s100169910044. [DOI] [PubMed] [Google Scholar]
- Upchurch GR, Jr, Leopold JA, et al. Nitric Oxide Alters Human Microvascular Endothelial Cell Response to Cyclic Strain. Journal of cardiovascular pharmacology and therapeutics. 1998;3(2):135. doi: 10.1177/107424849800300206. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Sage EH. A novel, quantitative model for study of endothelial cell migration and sprout formation within three-dimensional collagen matrices. Microvascular research. 1999;57(2):118. doi: 10.1006/mvre.1998.2122. [DOI] [PubMed] [Google Scholar]
- Von Offenberg Sweeney N, Cummins PM, et al. Cyclic strain-mediated regulation of vascular endothelial cell migration and tube formation. Biochemical and biophysical research communications. 2005;329(2):573. doi: 10.1016/j.bbrc.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Vouyouka AG, Powell RJ, et al. Ambient pulsatile pressure modulates endothelial cell proliferation. Journal of Molecular and Cellular Cardiology. 1998;30(3):609. doi: 10.1006/jmcc.1997.0625. [DOI] [PubMed] [Google Scholar]
- Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science (New York, NY) 1986;231(4736):397. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20(4):409–21. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- Xue L, Greisler HP. Angiogenic effect of fibroblast growth factor-1 and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin-based 3-dimensional angiogenesis system. Surgery. 2002;132(2):259–67. doi: 10.1067/msy.2002.125720. [DOI] [PubMed] [Google Scholar]
- Zhao S, Suciu A, et al. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arteriosclerosis, Thrombosis, and Vascular Biology. 1995;15(10):1781. doi: 10.1161/01.atv.15.10.1781. [DOI] [PubMed] [Google Scholar]