Abstract
Researchers are interested in respiratory sinus arrhythmia (RSA) as an index of cardiac vagal activity. Yet, debate exists about how to account for respiratory influences on quantitative indices of RSA. Ritz and colleagues (2001) developed a within-individual correction procedure by which the effects of respiration on RSA may be estimated using regression models. We replicated their procedure substituting a spectral high-frequency measure of RSA for a time-domain statistic and a respiratory belt’s relative measure of tidal volume for the direct assessment provided by a pneumotachograph. The standardized slopes from the respiratory belt and pneumotachography-derived regression equations (estimated across a 6 mins paced breathing protocol) were positively correlated (r = 0.93, p < 0.001); correlations were similar across 2 and 4-min time courses parsed from the 6-min protocol. Our results offer methodological alternatives to the research community.
Respiratory sinus arrhythmia (RSA) is the rhythmic fluctuation in heart rate at the respiratory frequency. The psychophysiological research community has primarily become interested in RSA as an index of cardiac vagal activity. Commonly-used quantitative RSA measurement techniques derived from electrocardiogram recordings have been previously described in detail (Berntson et al., 1997; Camm et al., 1996). In this report, we focus on a frequency-domain measure of RSA, high frequency spectral power. High frequency spectral power, a common measure of heart rate variability, can be derived using spectral methods such as fast Fourier transform or autoregressive modeling. These methods decompose the total variation in a heart rate or inter-beat interval time series into frequency components. The high frequency band is considered to be between 0.15 and 0.4 Hz in adult participants; since, younger participants breathe at faster rates, the frequency range may need to be reconsidered for such samples (Berntson et al., 1997; Camm et al., 1996). An alternative measure is the peak-to-valley statistic, a time-domain measure of RSA. This statistic produces a breath-by-breath index of heart rate fluctuations that reflects the difference between the longest and the shortest heart rates within a given respiratory cycle. While these methods of RSA quantification are generally accepted, questions remain regarding the optimal manner in which to account for respiratory influences on such indices of RSA.
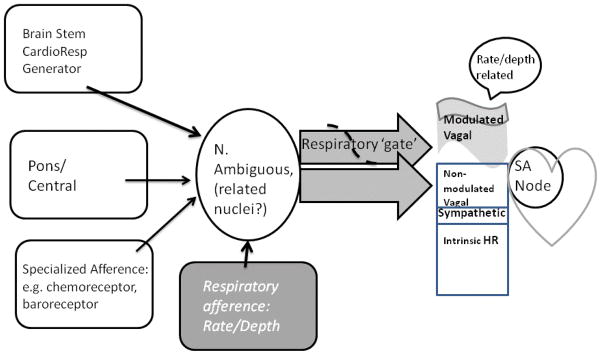
The relationship of respiratory rate and depth to respiratory sinus arrhythmia (RSA) has been a topic of substantial discussion (e.g., Ritz and Dahme, 2006; Grossman & Taylor, 2007; Denver, Reed, & Porges, 2007). Of particular concern, some have advocated that measures of RSA be ‘corrected’ or ‘unconfounded’ from respiratory effects (e.g., Grossman & Taylor, 2008; Ritz, 2009). Respiratory phase and duration (rate), though, essentially defines RSA. RSA is the result of cardiac-respiratory interaction. An understanding of this interaction permits the investigator to define clearly a measurement strategy that defines the dependent measure(s) appropriate to the question being posed. The nature of the cardiac-respiratory interaction is relatively well understood and has been described elsewhere in some detail (Grossman & Taylor, 2007, Berntson, Cacioppo, & Quigley, 1993; Berntson et al., 1997; Grossman & Kollai, 1993; Eckberg, 2003). Figure 1 summarizes the understanding and is closely related to the description presented earlier by Berntson, Cacioppo, & Quigley (1993). In the absence of any neural input, an intrinsic heart rate is present that represents a characteristic of that person’s cardiac sinus node pacemaker (e.g., Noble,1979). Heart rate level is typically set by a combination of vagal inhibition and sympathetic activation acting on the sinus node pacemaker. Vagal control of heart rate is accepted as predominant; particularly at rest, although some, relatively minor sympathetic nervous system control is also present. Variation over time in heart rate at respiratory frequencies is attributed to a vagal gating phenomenon, located in medullary nuclei known to channel vagal efference, i.e. the nucleus ambiguous and possibly the dorsal motor nucleus (Gilbey, Jordan, Richter, & Spyer, 1984). Vagal gating results in a modulation of heart rate such that heart rate decelerates as vagal firing occurs during expiration; while during inspiration, gating of such vagal activation results in cardiac acceleration. Importantly as illustrated in Figure 1, the gating typically modulates vagal input to the sinus node pacemaker of the heart rather than the gate completely inhibiting vagal influence during inspiration. Thus, if the aim of the investigator is to assess vagal influences on the heart, then two components are present—one component that modulated by respiration and another not altered by this modulation (See, e.g., Grossman, Karemaker, & Weiling, 1991, for experimental manipulation of these components). Finally, respiratory rate and depth are illustrated in Figure 1 as providing afferent information that can moderate the cyclic output from neural centers and thus contribute variance to the modulatory component of heart rate.
Figure 1.
Diagrammatic view of likely sources of vagal influence on the heart, omitting central influences and emphasizing areas related to respiratory function.
Despite some disagreement (Denver, Reed, & Porges, 2007), respiratory frequency and depth typically vary the degree of vagal modulation (review in Eckberg, 2003). Fast, shallow breathing is related to less vagal modulation than slow, deep breathing. This variation may be in part intrinsic to medullary cardiac-respiratory control and in part due to peripheral factors, e.g., slower respiratory rate permits greater time for vagal/cholinergic effects on the sinus node to be expressed (cf., Cooper, Parkes, & Clutton-Brock, 2003; Strauss-Blasche, Moser, Voica, McLeod, Klammer, & Markti, 2000). Measures of RSA, e.g. power in the respiratory band or peak to trough measures, assess the amplitude of the modulation—similar to the variance due to respiratory gating. As measures of modulation, they do not measure the non-modulated component of vagal influence. The modulatory portion is, however, subject to moderation by respiratory rate and depth. This moderation can be assessed and is the focus of the current article. Degree of moderation becomes a separate measure of modulations as RSA typically cannot be completely predicted by respiratory rate and depth (Ritz, Thons, & Dahme, 2001). The measurement of the non-modulated component is not straightforward, but use of average heart rate less a measure of the modulated component has been suggested (Grossman, Karemaker & Weiling, 1991; Ritz, Thons, & Dahme, 2001). Interpretation is somewhat clouded, however, by the known possibility of sympathetic influences on average heart rate as well as individual differences in intrinsic heart rate.
Note that both the modulated and non-modulated components represent vagal influences on the heart. Given this, ‘correcting’ or ‘unconfounding’ does not discard non-vagal influences. Depending on the aims of an investigator, the modulated, non-modulated or combined vagal effects may be of interest. Acceptance of the use of respiratory measures in conjunction with heart rate variability measures, has been slowed both by arguments over ‘unconfounding’ as well as cost in time and money of adding respiratory measurement. The current experiment was directed at testing a simple, inexpensive respiratory assessment that would ease the latter concern. We view the technique as permitting a reasonably easy means of assessing the modulatory components of vagal control and as such under some circumstances, to further permit assessment of non-modulatory and combined vagal control. Note that we have avoided the physiologically outdated term ‘vagal tone’. It remains unclear whether ‘vagal tone’ refers specifically to vagal effects on the sinoatrial node of the heart, and even if so, whether it refers to the modulated, non-modulated, or combined vagal influence on the heart. This vagueness of the ‘vagal tone’ terminology seems to contribute to the confusion over respiratory-cardiac interactions. Given this, we prefer to avoid the term (and suggest that others do so as well).
Based on earlier work in the field, (e.g., Grossman & Kollai, 1993), Ritz, Thons, and Dahme (2001) have proposed a within-individual procedure by which respiratory rate and depth’s influence on RSA may be estimated. The procedure calls for a baseline paced breathing protocol in which measures of respiratory rate, tidal volume, and an electrocardiogram-derived index of RSA are obtained. From these measures, the index of RSA can be apportioned for respiratory influences by regressing RSA divided by tidal volume on a measure of respiratory cycle length (Ritz & Dahme, 2006; Ritz, Thons, & Dahme, 2001). Following this baseline period, participants breathe freely during the experimental session. The predicted value of the index of RSA is obtained for the experimental data using the regression equation derived during the paced breathing period. The predicted value from the equation provides a measure of the modulatory component that is not linearly related to breathing frequency and depth; the predicted less observed measure assesses variance due to breathing frequency and depth.
This method has been tested by Ritz, Thons, and Dahme (2001). Their protocol employed the peak-to-valley statistic as an index of RSA and utilized pneumotachography to measure respiratory rate and tidal volume. The current study aimed to determine whether this correction procedure could be approximated using HF-HRV to assess RSA and a respiratory belt to assess thoracic contraction and expansion. HF-HRV was substituted because spectral measures of heart rate variability are used widely. The equivalency of the peak-to-valley and HF-HRV methods of RSA analysis has been discussed elsewhere (e.g. Grossman, van Beek, & Weintjes, 1990). Indeed, the multiple time- and frequency-domain measurements of RSA, of which the peak-to-valley and HF-HRV methods are only two, are highly correlated (between- and within-subjects correlations range from 0.58- 0.98 (e.g. Allen, Chambers, & Towers, 2007). Similarly, while the initial purchase cost and participant burden (i.e. breathing through a tube while nasal passages are blocked) associated with pneumotachography is somewhat prohibitive, the respiratory belt offers cost-effective, minimally invasive assessment of respiration. In typical measurement using respiratory belts, a combination of 2 respiratory belts spaced across the thorax is used to assess respiratory rate. Such 2-belt systems can provide measures of tidal volume when pre-calibrated using a device that measures tidal volume directly (e.g. a pneumotachograph) (Konno & Mead, 1967; Sackner, 1996). Instead, we tested the ability of a simply-designed, single uncalibrated respiratory belt to provide a relative, not quantitative, measure of tidal volume comparable to that obtained with pneumotachography. The belt tested was commercially available, relatively inexpensive, and wider than those previously used and possibly responsive to both chest and diaphragmatic breathing. Finally, while Ritz and colleagues’ protocol called for 3-min blocks of paced breathing, we examined the accuracy of our methodology during 6, 4, and 2-min blocks. These 4 and 2-min blocks represent the first 4 and 2 mins of the full 6-min block. If the correction procedure is validated using various time-scales, the flexible, relatively inexpensive technique would encourage routine implementation of respiratory assessment during evaluation of heart rate variability.
Method
Participants
Participants were 20 college students (10 male, 10 female; mean age=20.8 years old, SD=1.7). Seventy-five percent of the sample was Caucasian. Participants abstained from drinking caffeine as well as from consuming nicotine for at least 2 hours prior to their participation in the study. All received monetary compensation for their participation. The University of Pittsburgh Institutional Review Board approved all procedures and all participants provided their informed consent.
Materials
The respiratory flow curve was measured with a pneumotachograph (47304 A Flow Transducer, Hewlett Packard, Palo Alto, CA). Participants breathed through an oral tube connected to the transducer; participants’ noses occluded to ensure airflow solely through the tube. The calibration of the pneumotachograph was checked using a standard 3-liter syringe approximately twice per week throughout the study. The signal from the pneumotachograph, as well as the respiratory belt and electrocardiogram signal, was digitized at 512 Hz using the software and 12 bit analog to digital converter supplied with the respiratory belt (Vernier, Beaverton, OR, USA). The pneumotach data were visualized to check for artifacts after being digitized using a locally-developed Matlab program. Artifacts were rare and when present did not interfere with the determination of respiratory peak and trough. A Visual BASIC program was developed to calculate tidal volume from the pneumotach/flow-transducer. For each 2-min segment, all flow values >0 were summed to indicate volume and this was volume was divided by the number of breaths to form a tidal volume measure. Respiratory rate was calculated directly from the pneumograph readings and also verified by the rate estimate from the Mindware (Ghana, OH, USA) Heart Rate Variability Scoring Module, which separately analyzed the respiratory signal obtained from the respiratory belt (see below).
Respiratory belt
A single respiratory belt (Respiration Monitor Belt, Vernier, Beaverton, OR, USA) measured the changes in pressure associated with the thoracic expansion and contraction accompanying participants’ breathing. The belt was situated such that it covered the area between and including the 5th and 8th ribs and it was wrapped tightly enough so that only the experimenter’s index and middle finger could fit underneath. The air bladder of the belt was pumped to approximately 100 kPa before each participant began the protocol. A Visual BASIC program was created to calculate peak-to-trough values from the respiratory belt data. Zero-crossings in the pneumotach data (derived as described in the previous section using the Visual BASIC program) were used to identify the peaks and troughs within the pressure data. Peak-to-trough differences in the pressure data were summed and divided by the number of breaths to provide an indicator of tidal volume.
Electrocardiogram
HF-HRV was obtained via electrocardiogram (ECG). A modified lead II electrode placement with 3 Ag-AgCl electrodes was used. The ECG signal was digitized (12 bit), sampled (again with the Vernier software at 512 Hz), and stored for offline processing. R wave markers in the ECG signal, indications of each heartbeat, were assessed for artifacts by visual inspection and by an artifact detection algorithm in a commercial software package (Mindware Heart Rate Variability Scoring Module, version 2.16; Mindware Technologies Ltd., Columbus, OH, USA). After the correction of artifacts, 2-min estimates of heart rate and HF-HRV were established. Specifically, for each 2-min period, a time series was created with an appropriately weighted conversion of the interbeat interval (the time in ms between sequential ECG R spikes) creating a value for each 250 ms period within the 2-min period. This time series was linearly detrended, mean-centered, and tapered using a Hamming window. Then spectral power values were calculated (in ms2/Hz) via fast Fourier transformations, and the power values in the 0.15- to 0.40-Hz spectral bandwidth were identified (ms2). Both raw and natural log-transformed HF-HRV values were examined. Using the Shapiro-Wilk test, 35% of participants’ had raw HF-HRV values that were skewed whereas 25% of participants had skewed HF-HRV values after natural log transformation (raw HF-HRV W-statistic range = 0.69–0.96; lnHF-HRV W-statistic range = 0.82–0.97).
Procedure
Upon arrival, participants were given an overview of the experiment. ECG electrodes and the respiratory belt were fitted to participants, who remained seated for the duration of the protocol. Data from the pneuomotachograph, respiratory belt, and ECG were collected simultaneously throughout the session. To provide a pacing mechanism for respiration, a 300Hz tone was amplitude modulated at each of the necessary respiration rates. The tones were copied to audio CD for playback via speakers in the subject chamber. The respiration rates were 8, 10.5, 13, and 18 breaths/min for 6 mins each. Participants first engaged in a practice trial of 1–2 mins to ensure that they breathed accurately in synchrony with the frequency variation of the pacing tone. Following the practice trial, participants breathed for 6 mins at each of the rates presented in random order. Breaks of approximately 3 mins were given in between each rate.
Data Reduction and Statistical Analyses
A measure of tidal volume (VT; in liters) was calculated based on the calibration of the pneumotachography signal. Raw and natural log-transformed HF-HRV values were divided by VT (HF-HRV/VT Pneumo in ms per liter). Raw and natural log-transformed HF-HRV values were also divided by the output from the respiratory belt (HF-HRV/VT Belt in ms per kPa). Average respiratory cycle length in seconds (TTOT) was also calculated by dividing respiration rate for 2 mins into 120s. Separate within-individual regression equations were calculated regressing HF-HRV/VT Pneumo upon TTOT and regressing HF-HRV/VT Belt upon TTOT. These equations included all breathing rates from the paced breathing protocol. The resultant standardized slopes (β values) from the pneuomotachography and respiratory belt regressions were correlated to determine whether both respiration monitoring devices similarly estimated the effects of respiration on HF-HRV. The regression equations and standardized slope correlations were calculated using data from the entire 6 mins of each auditory activity from the paced breathing protocol, from the first 4 mins of each activity, and from the first 2 mins of each activity.
Results
Effects of Paced Breathing Protocol
Participants followed the paced breathing signal correctly, resulting in four distinct respiratory frequencies (e.g. 8 breaths/min should result in 60/8 = 7.5 cycles/second; experimental mean value = 7.54 cycles/second; Table 1). One participant did not follow the paced breathing protocol as directed; however, this individual did breathe reliably at twice the prescribed frequency. Thus, this individual was still included in the final sample. HF-HRV and lnHF-HRV varied across the respiratory rates such that HF-HRV and lnHF-HRV decreased as respiratory frequency increased, F(1, 19)=13.19, p=0.000001, η2=0.41 and F(1,19)=36.27, p=0.00, η2=0.66, respectively. The heart period (HP) did not vary significantly across the different breathing frequencies, F(1, 19)=1.17, p=0.33, η2=0.06. VT decreased as the frequency of respiration increased, showing the usual physiologic relationship between tidal volume and HF-HRV for both pneumotachography measures of VT, F(1, 19)=8.20, p=0.00013, η2=0.30, and respiratory belt measures of VT, F(1, 19)=4.26, p=0.0088, η2=0.18. All of these values are shown in Table 1. Finally, a correlation matrix including values for raw and lnHF-HRV, heart period, pneumotachography and respiratory belt measures of VT, and TTOT is shown in Table 2.
Table 1.
Means (SD) of Cardiac and Respiratory Parameters During Paced Breathing
| Parameter | 8 cycles/min | 10.5 cycles/min | 13 cycles/min | 18 cycles/min |
|---|---|---|---|---|
| TTOT (s) | 7.54 (0.09) | 5.71 (0.04) | 4.72 (0.50) | 3.34 (0.03) |
| VT Pneumo (ms/l) | 0.59 (0.28) | 0.49 (0.23) | 0.44 (0.21) | 0.36 (0.15) |
| VT Belt (ms/kPa) | 0.73 (0.47) | 0.67 (0.35) | 0.60 (0.34) | 0.56 (0.24) |
| HP (ms) | 901(115.76) | 887 (123.63) | 893 (117.04) | 887 (119.24) |
| HF-HRV (ms) | 6140 (3441) | 4058 (3678) | 2622 (2034) | 1991 (3045) |
| lnHF-HRV (ms) | 8.50 (0.71) | 7.95 (0.89) | 7.53 (0.96) | 6.98 (0.97) |
| HF-HRV/VT Pneumo (ms/l) | 14272 (15735) | 10793 (11621) | 8012 (7896) | 5645 (6729) |
| lnHF-HRV/VT Pneumo (ms/l) | 19.40 (11.23) | 20.30 (9.37) | 20.66 (8.44) | 22.42 (9.12) |
| HF-HRV/VT Belt (ms/kPa) | 12008 (9434) | 8015 (7027) | 5880 (4913) | 3531 (3444) |
| lnHF-HRV/VT Belt (ms/kPa) | 17.97 (13.50) | 15.98 (8.16) | 16.31 (8.18) | 15.33 (8.12) |
Note. Values for TTOT do not include those for the participant who breathed at an aberrant frequency. Including this participant, the TTOT values for each tone are as follows: 7.29 (1.70), 6.00 (1.31), 5.17 (1.45), 3.51 (0.75).
Table 2.
Correlation Matrix of Key Variables
| HF-HRV | lnHF-HRV | Heart Period | TTOT | VTPneumo | VTBelt | |
|---|---|---|---|---|---|---|
| HF-HRV | 0.55* | 0.53* | 0.20 | −0.39 | −0.08 | |
| lnHF-HRV | 0.37 | −0.24 | −0.95** | −0.23 | ||
| Heart Period | 0.16 | −0.37 | −0.41 | |||
| Ttot | 0.30 | 0.13 | ||||
| VTPneumo | 0.31 |
p<0.05
p<0.001
Within-Individual Regressions
Separate within-individual regressions were calculated regressing each HF-HRV, lnHF-HRV, HF-HRV/VT Pneumo, and HF-HRV/VT Belt (dependent variables) upon TTOT (the independent variable) (see Table 3). Ritz, Thons, and Dahme (2001) found that the standardized slope for the regression of the peak-to-valley statistic upon TTOT was notably larger than the standardized slope that resulted from the regression of the peak-to-valley statistic as corrected for pneumotachography-derived tidal volume upon TTOT. Comparing the standardized slopes for the regressions of HF-HRV upon TTOT and of HF-HRV/VT Pneumo upon TTOT for 6, 4, and 2 mins per activity of the paced breathing protocol, we also found that our standardized slope for HF-HRV was larger than the standardized slopes obtained using the respiratory-corrected measures.
Table 3.
Mean Slopes and Intercepts of the Regressions of HF-HRV, lnHF-HRV, HF-HRV/VT Pneumo, lnHF-HRV/VT Pneumo, HF-HRV/VT Belt, and lnHF-HRV/VT Belt upon TTOT
| Mean | SD | |
|---|---|---|
| HF-HRV | ||
| Slope | 0.69 | 0.44 |
| Intercept | −1478 | 5340 |
| lnHF-HRV | ||
| Slope | 0.34 | 0.18 |
| Intercept | 5.96 | 1.39 |
| HF-HRV/VT Pneumo (6 min) | ||
| Slope | 0.59 | 0.48 |
| Intercept | −1667 | 11205 |
| lnHF-HRV/VT Pneumo (6 min) | ||
| Slope | −0.76 | 1.71 |
| Intercept | 25.05 | 10.88 |
| HF-HRV/VT Pneumo (4 min) | ||
| Slope | 0.61 | 0.48 |
| Intercept | −1702 | 10896 |
| lnHF-HRV/VT Pneumo (4 min) | ||
| Slope | −0.78 | 1.57 |
| Intercept | 25.31 | 10.65 |
| HF-HRV/VT Pneumo (2 min) | ||
| Slope | 0.66 | 0.48 |
| Intercept | −2955 | 11922 |
| lnHF-HRV/VT Pneumo (2 min) | ||
| Slope | −0.68 | 1.48 |
| Intercept | 25.60 | 10.38 |
| HF-HRV/VT Belt (6 min) | ||
| Slope | 0.63 | 0.42 |
| Intercept | −3354 | 9704 |
| lnHF-HRV/VT Belt (6 min) | ||
| Slope | 0.51 | 2.99 |
| Intercept | 13.68 | 15.42 |
| HF-HRV/VT Belt (4 min) | ||
| Slope | 0.62 | 0.44 |
| Intercept | −3406 | 10344 |
| lnHF-HRV/VT Belt (4 min) | ||
| Slope | 0.42 | 2.86 |
| Intercept | 14.21 | 14.42 |
| HF-HRV/VT Belt (2 min) | ||
| Slope | 0.65 | 0.45 |
| Intercept | −3372 | 10373 |
| lnHF-HRV/VT Belt (2 min) | ||
| Slope | 0.20 | 2.58 |
| Intercept | 15.74 | 13.27 |
Moreover, the respiratory belt (in combination with HF-HRV) yielded results essentially equivalent to those found using the pneumotachograph. Note that, because of the large difference in measurement units between the peak-to-valley statistic and HF-HRV and between the pneumotachtograph and respiratory belt output, our standardized slope and intercept values cannot be expected to estimate directly the values obtained by Ritz, Thons, and Dahme. However, the relative values of tidal volume from the respiratory belt show a relationship with HF-HRV that is similar to that derived by Ritz and colleagues. Finally, there were no significant differences between standardized slopes resulting from 6 and 2-min-per-auditory activity estimations of the influence of respiration on HF-HRV as measured by either the pneumotachograph (t = 0.50, df = 19, p = 0.62) or respiratory belt (t = 0.20, df = 19, p = 0.85).
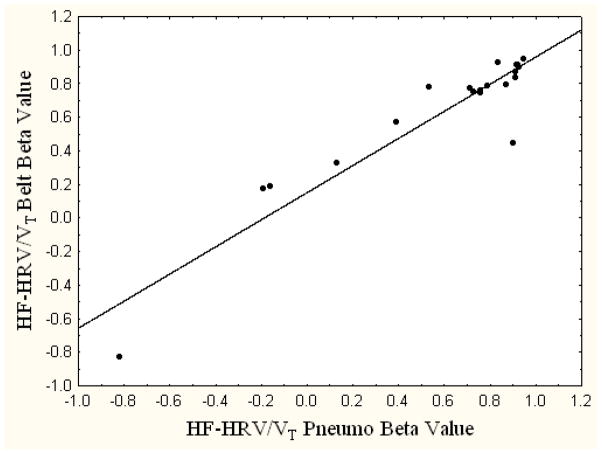
The observed standardized slopes from the pneumotachography and respiratory belt regression equations estimated across 6 mins of the paced breathing protocol were positively correlated (r = 0.93, p=0.00; see Figure 2). The correlations between the pneumotachography and respiratory belt standardized slopes derived from regression equations examining the first 4 or 2 mins of pacing were also positively correlated (4-min estimate: r = 0.91, p=0.00; 2-min estimate: r = 0.95, p=0.00).
Figure 2.
Correlation between the slopes from the regressions of HF-HRV/VT Pneumo and HF-HRV/VT Belt upon TTOT.
Note. r = 0.93, p < 0.01.
When conducting duplicate analyses using the natural log-transformed HF-HRV values, the relationship between belt and pneumotachograph β values was reduced, as can be seen in Table 3. Additionally, the 6-min correlation of 0.93 between belt and pneumotachograph was reduced to 0.36 when the natural log values were employed. Examination of the distributions and summary statistics showed that the variance relative to the mean (the coefficient of variation) was increased substantially with the natural log transformation. This factor rather than any change in seeming outliers appeared to account for the reduction in the correlation. Thus, for this particular belt measure, raw values performed better than natural log-transformed values. It also is important to note that a) our raw HF-HRV values were not extremely skewed and b) the skew of those raw values was only improved minimally by natural log transformation.
Discussion
We found that the standardized slopes of the equations regressing HF-HRV/VT Pneumo upon TTOT and HF-HRV/VT Belt upon TTOT were highly correlated. This result indicates that the procedure for correcting respiratory influences on quantitative indices of RSA presented by Ritz, Thons, and Dahme (2001) can be closely approximated when HF-HRV is substituted for the peak-to-valley statistic and when a respiratory belt replaces a pneumotachography device. Additionally, we demonstrated that our methods can be used effectively over 6, 4, and 2-min time courses. As such, the correction procedure appears useful with as little as 3 pacing periods of 2 mins each, rendering it an efficient addition to psychophysiological research designs assessing RSA. It is notable that these results were strongest when raw HF-HRV values, not those that were natural log-transformed, were used in the analyses. Thus, this correction procedure may be better suited for use with raw HF-HRV values that are not extremely skewed.
Regarding the respiratory belt itself, we emphasize that our results do not establish that the belt provided a quantitative measure of tidal volume. Rather, they demonstrate that data from the respiratory belt can be used in the within-individual (not between-subjects) respiratory correction procedure comparably to direct measures of tidal volume. While this study does support the use of a respiratory belt in the measurement of relative tidal volume, it remains unclear what effects, if any, body habitus may have on the accuracy of its measurement. Although measures of body habitus such as body mass index or waist circumference were not taken, such factors may influence the position and fit of the belt, altering its measurement of thoracic expansion and contraction. Future research should address how such variations in participants’ physical characteristics may influence respiratory belt assessment.
We also note that additional research is needed to validate our HF-HRV and respiratory belt substitution method against Ritz and colleagues’ original peak-to-valley statistic and pneumotachograph method. We acknowledge that the ratio of HF-HRV/VT Belt to HF-HRV/VT Pneumo varies somewhat as a function of breathing rate. To further assess their comparability, both methodologies should be conducted using the same participant sample so that the resulting within-individual regression models can be directly compared. Finally, such a study might incorporate the use of multi-level regression analyses, another technique often used to assess individual differences in within-subjects intercepts and standardized slopes.
Despite these limitations, our findings are intriguing. They are particularly notable as there are certain advantages to the use of HF-HRV and the respiratory belt over the peak-to-valley statistic and pneumotachography, respectively. For example, several commercial software packages for automated spectral analysis have been validated and are in common use, standardizing the popular frequency-domain measurement. Correspondingly, the use of a respiratory belt can be seen as more cost-effective and less invasive than pneumotachography. Generally, our demonstration that this respiratory correction procedure can be executed using HF-HRV and a respiratory belt provides methodological alternatives to the research community, increasing the ease with which it can be implemented. Naturally, different applications such as ambulatory versus laboratory studies may require different instrumentation. We cannot guarantee that our results will generalize to all situations, but we hope they are reasonably representative of non-invasive respiration and typical heart rate variability measures.
We recognize that there is some debate concerning whether ‘correcting or ‘unconfounding’ quantitative measures of RSA for respiratory influences is appropriate or necessary. Both centrally-mediated cardiac vagal activity and respiration appear to contribute to the generation of RSA (Berntson et al., 1997; Berntson, Cacioppo, & Quigley, 1993; Denver, Reed, & Porges, 2007; Grossman, Karemaker, & Wieling, 1991; Grossman & Kollai, 1993; Grossman, Stemmler, & Meinhardt, 1990; Grossman & Taylor, 2007; Martinmaki, Rusko, Kooistra, Kettunen, & Saalasti, 2006; Medigue et al., 2001; Penttila et al., 2001; Pyetan, Toledo, Zoran, & Akselrod, 2003). And, given one’s research question, the respiratory-modulated, non-respiratory modulated or combined vagal effects may be of interest. The current experiment examined a simple, inexpensive respiratory assessment technique which we view as permitting a relatively easy means of assessing the modulatory components of vagal control. Given the developing nature of this field, researchers interested in using quantitative measures of RSA may initially find it appropriate to reflect on how respiratory changes might relate to their research question. For example, if it can be assumed that changes in respiration are related to the psychological concept being studied, they may be valid experimental effects. However, if changes in respiration do not follow from the psychological concept being investigated, they may warrant statistical removal of this portion of the vagal modulation. In such cases, it might be prudent to compare RSA values produced using the correction procedure described in this manuscript to raw values. Attention should also be paid as to whether there are systematic changes in respiratory rate and/or tidal volume as a function of experimental manipulations.
This said, uncorrected RSA can also be conceptualized as an index of cardiorespiratory control with empirical relationships to dimensions of interest such as depression or physical fitness. This conceptualization does not attempt to infer vagal function, but considers HRV a meaningful index of the central and peripheral control of both cardiac and respiratory function. In short, any ‘confounding’ is viewed as intrinsic to this index and separation of respiratory and vagal influences may be inappropriate, compromising predictive validity (See elaboration of the general point in (Jennings & Gianaros, 2007). Separation of these influences may, of course, be of conceptual interest despite the predictivity of the ‘confounded’ index.
In conclusion, we report results demonstrating that the procedure for correcting respiratory influences on quantitative indices of RSA developed by Ritz, Thons, and Dahme (2001) can be modified for use with a spectrally-derived measure of RSA and with a respiratory belt used to provide a relative measure of tidal volume. This methodology can be implemented over 6, 4, and 2-min time courses. Given these findings, we present an efficient and minimally invasive technique for respiratory correction.
Acknowledgments
Research support was provided by the Pittsburgh Mind-Body Center (National Institutes of Health grant HL 076852/076858) and by National Institutes of Health grant T32HL007560. We thank Charles Atwood, M.D. and the University of Pittsburgh Department of Pulmonary Medicine for their assistance and loan of the pneumotachograph. For reprints, please contact Victoria Egizio at the Western Psychiatric Institute and Clinic, 3811 O’Hara St. Room E1329, Pittsburgh, PA 15213.
References
- Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Camm AJ, Malik M, Bigger JT, Breithardt G, Cerutti S, Cohen RJ, et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biol Psychol. 2007;74(2):286–294. doi: 10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28(2):201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrhythmia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30(5):486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Stemmler G, Meinhardt E. Paced respiratory sinus arrhythmia as an index of cardiac parasympathetic tone during varying behavioral tasks. Psychophysiology. 1990;27(4):404–416. doi: 10.1111/j.1469-8986.1990.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol. 2007;74(2):263–285. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Gianaros PJ. Methodology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. New York: Cambridge University Press; 2007. [Google Scholar]
- Martinmaki K, Rusko H, Kooistra L, Kettunen J, Saalasti S. Intraindividual validation of heart rate variability indexes to measure vagal effects on hearts. Am J Physiol Heart Circ Physiol. 2006;290(2):H640–647. doi: 10.1152/ajpheart.00054.2005. [DOI] [PubMed] [Google Scholar]
- Medigue C, Girard A, Laude D, Monti A, Wargon M, Elghozi JL. Relationship between pulse interval and respiratory sinus arrhythmia: a time- and frequency-domain analysis of the effects of atropine. Pflugers Arch. 2001;441(5):650–655. doi: 10.1007/s004240000486. [DOI] [PubMed] [Google Scholar]
- Penttila J, Helminen A, Jartti T, Kuusela T, Huikuri HV, Tulppo MP, et al. Time domain, geometrical and frequency domain analysis of cardiac vagal outflow: effects of various respiratory patterns. Clin Physiol. 2001;21(3):365–376. doi: 10.1046/j.1365-2281.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- Pyetan E, Toledo E, Zoran O, Akselrod S. Parametric description of cardiac vagal control. Auton Neurosci. 2003;109(1–2):42–52. doi: 10.1016/j.autneu.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Ritz T, Dahme B. Implementation and interpretation of respiratory sinus arrhythmia measures in psychosomatic medicine: practice against better evidence? Psychosom Med. 2006;68(4):617–627. doi: 10.1097/01.psy.0000228010.96408.ed. [DOI] [PubMed] [Google Scholar]
- Ritz T, Thons M, Dahme B. Modulation of respiratory sinus arrhythmia by respiration rate and volume: stability across posture and volume variations. Psychophysiology. 2001;38(5):858–862. [PubMed] [Google Scholar]