Abstract
The recent identification of genes implicated in hereditary recurrent fevers has allowed their specific diagnosis. So far however, only punctual mutations have been identified and a significant number of patients remain with no genetic confirmation of their disease after routine molecular approaches such as sequencing. The possible involvement of sequence rearrangements in these patients has only been examined in familial Mediterranean fever and was found to be unlikely. To assess the existence of larger genetic alterations in 3 other concerned genes, MVK (Mevalonate kinase), NLRP3 (Nod like receptor family, pyrin domain containing 3) and TNFRSF1A (TNF receptor superfamily 1A), we adapted the qPCR-HRM method to study possible intragenic deletions and duplications. This single-tube approach, combining both qualitative (mutations) and quantitative (rearrangement) screening, has proven effective in Lynch syndrome diagnosis. Using this approach, we studied 113 unselected (prospective group) and 88 selected (retrospective group) patients and identified no intragenic rearrangements in the 3 genes. Only qualitative alterations were found with a sensitivity similar to that obtained using classical molecular techniques for screening punctual mutations. Our results support that deleterious copy number alterations in MVK, NLRP3 and TNFRSF1A are rare or absent from the mutational spectrum of hereditary recurrent fevers, and demonstrate that a routine combined method such as qPCR-HRM provides no further help in genetic diagnosis. However, quantitative approaches such as qPCR or SQF-PCR did prove to be quick and effective and could still be useful after non contributory punctual mutation screening in the presence of clinically evocative signs.
Introduction
The hereditary recurrent fever syndromes are a group of auto-inflammatory disorders characterized by self-limited episodes of fever accompanied by inflammation of the serosal membranes, without apparent infectious etiology [1]. Included in this group are familial Mediterranean fever (FMF) and mevalonate kinase deficiencies (MKD; MIM#610377 for mevalonic aciduria and 260920 for hyper-IgD syndrome), all of which are recessively inherited. Dominantly inherited syndromes have also been described, such as TNF receptor associated periodic syndrome (TRAPS; MIM# 142680) and cryopyrin associated periodic syndromes (CAPS; MIM#120100 for familial cold autoinflammatory syndrome 1, 191900 for Muckle-Wells syndrome and 607115 for CINCA syndrome) [2].
The vast majority (FMF 97%; CAPS 96%; TRAPS 95%; MKD 81%) of the mutations implicated in the hereditary recurrent fever syndromes are punctual substitutions, and have been recorded in the dedicated online database INFEVERS (http://fmf.igh.cnrs.fr/ISSAID/infevers/). However, most laboratories use molecular techniques such as sequence analysis or exon screening (DGGE, Denaturing Gradient Gel Electrophoresis, dHLPC, denaturing high performance chromatography, HRM, High Resolution Melting analysis) that only allow for the identification of micro-rearrangements. On the other hand, a significant number of apparently mutation-free patients show evocative symptoms of hereditary recurrent fevers. The possible involvement of sequence rearrangements in these diseases has only been examined in FMF and was found to be unlikely [3]. Several methodological approaches have been have been described to search for such gene alterations: SQF-PCR, semi-quantitative fluorescent PCR [4]; MLPA®, multiplex ligation-dependent probe amplification [5]; MAPH, multiplex amplification probe hybridization [5]; qPCR, quantitative PCR [6]. More recently, innovative approaches have been developed that allow the simultaneous, rapid and accurate detection of both micro and macro-rearrangements in genes responsible for hereditary cancers. This strategy, called qPCR-HRM, is based on a real time PCR assay (gene dosage quantitative analysis) combined with HRM curves (qualitative analysis of punctual mutations) in a single tube [7].
To search for sequence rearrangements in our patients, we adapted the qPCR-HRM method for the study of intragenic deletions and duplications in the MVK (Mevalonate kinase, MIM#251170; NM_000431.1), NLRP3 (Nod like receptor family, pyrin domain containing 3, MIM#606416; NM_004895.3) and TNFRSF1A (TNF receptor superfamily 1A, MIM#191190; NM_001065.2) genes, responsible for MKD, CAPS and TRAPS respectively. We used SQF-PCR as a confirmatory approach.
Materials and Methods
Study group
The study includes a total of 201 patients referred for the genetic diagnosis of hereditary recurrent fevers in our reference laboratory specialized in auto-inflammatory disorders (Montpellier, France). Informed consent was obtained from all participating subjects.
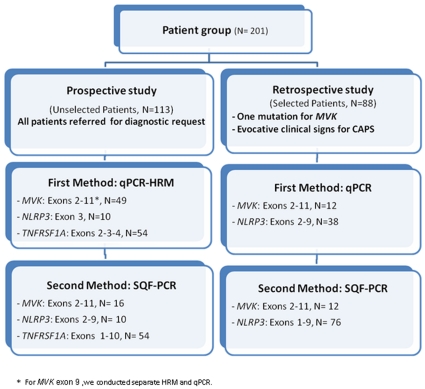
The study design consisted in the delineation of two groups of patients (summarized in Figure 1). The prospective study included patients unselectively recruited over a period of 6 months in 2009 (N = 113). The retrospective study included 88 selected patients previously genotyped using our routine diagnosis strategy (screening by sequencing and/or DGGE of hot spot mutations, i.e. MVK, all exons; NLRP3: exon 3). The selection criteria for MKD (N = 12) was the detection of only a single mutation and for CAPS (N = 76), it was evocative signs, i.e. recurrent fever, urticaria, arthralgia and sometimes neurosensorial deficiency. No TRAPS patients were retrospectively selected since the clinical spectrum for this disease is too unspecific.
Figure 1. Flow chart for the screening of mutations in the genes MVK, NLRP3 and TNFRSF1A.
In our patient cohort, we performed a simultaneous search for punctual mutations and gene rearrangements using qPCR-HRM in the prospective group and qPCR alone in the retrospective group (as these patients had already been assessed for qualitative alterations), then SQF-PCR in both groups as a confirmatory quantitative approach.
DNA extraction
Human genomic DNA was prepared from whole-blood samples using the QIAmp DNA blood kit (Qiagen, Courtaboeuf, France) according to the manufacturer's standard protocol. The quality and concentration of the DNA was assessed using a Nanodrop® spectrophotometer (Coleman Technologies, Orlando, FL). DNA working solutions were prepared at a concentration of 5ng/ul for qPCR-HRM and qPCR, and 50ng/ul for SQF-PCR.
qPCR-HRM and qPCR
Primer design
Common primers were used for qPCR-HRM and qPCR for the screening of exons containing mutation hot spots. Specific primers were designed for the other exons (retrospective study). Since effective primer design is an important component of HRM analysis, all amplicons were chosen to contain a single melting domain as assessed using the Poland program (http://www.biophys.uni-duesseldorf.de/local/POLAND/poland.html [8]. The PrimerBlast software (NCBI, http://www.ncbi.nlm.nih.gov/tools/primer-blast/, date of access 03/20/2010) was used to design primers with the same melting temperature (60°C), and to result in amplicons ranging between 100 and 400 bp in size. All primer sequences used in this work are listed in Table 1. The primer pairs were validated if they matched the following conditions: PCR efficiency range between 80–100% as determined using a DNA concentration gradient (40ng-20ng-10ng-5ng-2.5ng), Cycle threshold (Ct) lower than 30 cycles, and all pathological genotypes detected in the validation set.
Table 1. Primers used in this study.
| Gene/Exon | Forward Primer | Reverse Primer | Amplicon size (bp) | Multiplex set | |
| qPCR-HRM1 | |||||
| B2M/2 | cactgaaaaagatgagtatgcc | Aacattccctgacaatccc | 231 | ||
| DMD/16 | tctatgcaaatgagcaaatacacgc | ggtatcactaacctgtgctgtactc | 290 | ||
| MVK/2 | tcttcctgctggttctgaca | gcatcagggagaaggggta | 203 | ||
| MVK/3 | tcaccctcaggcttattgct | tggtttctcctccttgcact | 210 | ||
| MVK/4 | ccctctcacccacttgtgtt | aggctgaatctggactccttc | 226 | ||
| MVK/5 | cgggagagtcacgtttcac | gacactggccaggtaaggac | 221 | ||
| MVK/6 | ccactcctcactgccacag | ctcttgggcacctaccattg | 221 | ||
| MVK/7 | cctctctcccaagtagcacag | ccctgcacacatcaatgc | 170 | ||
| MVK/8 | tgagttcagtgtggacctgc | taattgtgtcctggccttcc | 181 | ||
| MVK/92 | gaacacctcctccctccac | ttctgagcacagccagattg | 295 | ||
| MVK/10 | aagtgggaacagatggaacct | ccaatgaggaagcaaagacc | 346 | ||
| MVK/11 | gtcaagggtgacctgccttc | gcctctccagcagtgtcag | 300 | ||
| NLRP3/3a | gatgtgtgtatactttccccctaa | aaacctgtcttggtagagtgtcc | 399 | ||
| NLRP3/3c | agcctcatcagaaagaagctg | tgaggtcggactcctcaaac | 469 | ||
| NLRP3/3d | tctttggctgcagatggaat | ttgctgagagatcttgcaactta | 399 | ||
| NLRP3/3e | tttcctctttggcctggtaa | agaagaagctggcgaggaag | 477 | ||
| NLRP3/3c3 | gcctctctgctcatcacgac | gctcttgccactctccatct | 264 | ||
| TNFRSF1A/2 | ttgatggtgtctcctctatctga | aagaagcagcaccccagac | 240 | ||
| TNFRSF1A/3 | gggctccttccttgtgttct | cacatagacaggcacccaca | 247 | ||
| TNFRSF1A/4 | agaaatgggtcaggtggagat | gccagagaggagttggttgt | 280 | ||
| qPCR | |||||
| MVK/92 | accagccgttccttcttttt | ttctgagcacagccagattg | 230 | ||
| NLRP3/2 | ggggtctcctctctcatgc | ctagaagcaccaccccagtc | 242 | ||
| NLRP3/4 | tcgaggctgatttcttttctg | gcactcacacagatcacatgc | 248 | ||
| NLRP3/5 | tctgatgctttctgcctctgt | cgctggcagaacttccttag | 244 | ||
| NLRP3/6 | tgactgacattctgccatctct | tctggtaagacacccatgaaga | 227 | ||
| NLRP3/7 | ggaacagctgggtactgagg | cttgtggccactctgccta | 295 | ||
| NLRP3/8 | aatcaccccctttttgcag | agagccatcctggattttga | 274 | ||
| NLRP3/9a | agtgcaacccaggctttcta | tcacagagctgtggtcttgg | 240 | ||
| SQF-PCR4 | |||||
| GFAP/3 | gaggaaaggattgatggcca | gaggaggagatccggttctt | 249 | Control (A to H) | |
| DMD/28 | tgcattttgaattacctgctaca | agtaccaaatagaagacaaatccaaag | 361 | Control (A to H) | |
| MVK/2 | tattatgatgggcttgaactagg | tgcctcagggtgtcctttta | 292 | A | |
| MVK/3 | cttcttagcacgtgggtcct | ctctctgtaggctctt | 286 | A | |
| MVK/4 | gtcgattttctgtgttctgttgtt | agagcatgtgcattctccag | 337 | B | |
| MVK/5 | ctggaccagatgcttggagt | aagccacgtccctgtcctg | 336 | A | |
| MVK/6 | gagtggacttgttctttctgagc | agactcttgggcacctacca | 328 | B | |
| MVK/7 | ttcctgaatggggcaaaat | ctgcctcctatggtacttccc | 266 | C | |
| MVK/8 | gtatcagggtgggcggcttcc | gagggagacctcgaaaatcc | 279 | A | |
| MVK/9 | ggctgtgtgaacacctcctc | cacagccagattgcagagcca | 314 | B | |
| MVK/10 | tctccagccaacaactgtca | tcaagggaattctccaggtg | 348 | C | |
| MVK/11 | ctgggcttttgccttgaat | aataatccagaaaggggcatc | 304 | C | |
| TNFRSF1A/1 | accaggccgtgatctctatg | cactcttccctttgtccctg | 224 | H | |
| TNFRSF1A/2 | aggacttgagccagggaagt | tttccttggggacacacact | 198 | D | |
| TNFRSF1A/3 | ctggctgttgtccctagcat | cacccacacaccactcaaga | 256 | E | |
| TNFRSF1A/4 | agaaatgggtcaggtggagat | ttggttgtcagacccacaga | 268 | D | |
| TNFRSF1A/5 | acaaccaacttcctctctggc | atctgttgcccagctaatgg | 277 | I | |
| TNFRSF1A/6 | caccagtgccgtctcttctt | atagatggatgggtgggatg | 186 | I | |
| TNFRSF1A/7 | aacacctgctttgtctgcag | accttctgcccagagtccc | 268 | H | |
| TNFRSF1A/8–9 | gggaaatcgacacctgaaaa | aagctccccctgaaagagag | 233 | H | |
| TNFRSF1A/9–10 | cccttcagaagtgggaggac | gatcgatctcgtggtcgct | 305 | I | |
| NLRP3/1 | ccagagccttcagtttggag | aggagtgtgtcctgagccat | 269 | G | |
| NLRP3/2 | ccactgtgatatgccaggaa | gcattccaaagagcaggaac | 208 | F | |
| NLRP3/3 | gcatctcaggtggatgtgtg | caggctcagaatgctcatca | 307 | G | |
| NLRP3/4 | cctcacttccagtttttgcc | gcactcacacagatcacatgc | 189 | G | |
| NLRP3/5 | tctgtgtgtgggactgaagc | ctttccccacgacaaacact | 228 | F | |
| NLRP3/6 | tcagtattgagcaccagcca | tgaccaaagtaacccccatc | 213 | G | |
| NLRP3/7 | gagtcaaagcagctgcacaa | ccaccatgtgttctcattgc | 283 | F | |
| NLRP3/8 | gggatggttaaggggacatt | caggcccaacctaatcttga | 339 | G | |
| NLRP3/9a | tgcaacccaggctttctatt | tgctgtcattgtcctggtgt | 348 | F |
All primers (except HRM-qPCR MVK/9 primer) showed a PCR efficiency range between 80 and 100% (IC+/−0.2%).
qPCR and HRM of MVK exon 9 could not be performed in the same run due to low PCR efficiency. We used therefore two different primer pairs.
p.T348M allele specific primer.
Forward primers were 6FAM labeled. Nine distinct PCR sets (Multiplex A to I) were designed, each yielding a pattern of 3 to 6 peaks including the 2 control fragments (DMD and GFAP).
Assay conditions
qPCR-HRM and qPCR were performed in a single run using a LightCycler 480 instrument (Roche diagnostics, Meylan, France). Reaction mixtures contained 10 ng genomic DNA, 0.25µM of each primer, 3mM MgCl2 and 1× LightCycler 480 High Resolution Melting Master Mix (Roche Diagnostics, Meylan, France) containing Taq polymerase, nucleotides and the dye Resolight in a total volume of 10µl. We used the same cycling conditions for all reactions: an activation step at 95°C for 10 min followed by 50 cycles of 95°C for 15s, a touchdown from 65 to 55°C for 15s (0.5°C/cycle) and 72°C for 20s. Following amplification, products were heated to 95°C for 1 min and cooled to 40°C for 1 min to favor heteroduplex formation. For the melting step, the temperature was raised from 75°C to 97°C with a ramp rate of 0.02°C/s and 25 acquisitions/°C. All reactions were performed at least twice. Each experiment included male or female wild-type DNA (for quantitative calibration and as a qualitative negative control), and positive control DNA with a known point mutation (as a qualitative positive control). All controls are summarized in Table 2.
Table 2. Controls used in this work.
| qPCR-HRM | SQF-PCR | ||||||||
| HRM (Qualitative) | qPCR (Quantitative) | (Semi-Quantitative) | |||||||
| Validation Set | |||||||||
| Negative Controls | N = 48 | Wild-type DNAs | N = 10 | Asymptomatic individual | N = 10 | Asymptomatic individual | |||
| Positive Controls | N = 49 | DNAs with known punctual mutations: | N = 3 | DNAs with or mimicking a CNV†: | N = 1 | DNA with a deletion | |||
| MVK (N = 19) | p.S52N(1)(2),c.371+8C>T(1)(2), p.S135L(1), p.S135S(1), p.D170D(1)(2),c.632−18A>G (1)(2), c.769-7_769-6dupT(1) *,c.885+24G>A(1)(2), p.G309S(1), p.S329N(1), p.V377I(1)(2), p.R388X(1), p.E296GfsX14(1) | MVK (N = 1) | Chr12q24.1del | MVK (N = 1) | Chr12q24.1del | ||||
| NLRP3 (N = 23) | c.278−45T>C(1), p.Y141Y(1) *, p.R168Q(3), p.V198M(1), p.T219T(1), p.A242A(1)(2) *, p.P340P(1)(3), p.L344L(1)(3), p.A439P(1), p.H463H(1), p.T587I(1), p.Q703K(1)(2), p.Y859C(1),c.3005+25C>T(1), p.T348M(1), p.L411L(3), p.S434S(1)(2), p.T436P(3) | NLRP3 (N = 1) | p.T348M artificial deletion (allele specific primer) | ||||||
| TNFRSF1A (N = 7) | p.C30R(1), p.C30F(1), p.C73W(1), p.H69fs(1), p.E54E(1), p.P46L(1), p.R92Q(1) | ITGB5 (N = 1) | Chr3q21.2dup | ||||||
| Study group | |||||||||
| Negative Controls | N = 1 | Wild-type DNA | N = 1 | Asymptomatic individual | N = 1 | Asymptomatic individual | |||
| Positive Controls | N = 1 | DNA with a known variation per exon tested where available | N = 1 | DMD (Internal control) | N = 1 | DMD (Internal control) | |||
(1) heterozogote, (2) homozygote, (3) compound heterozygote.
*These polymorphisms were not detected by HRM.
Copy number variation.
Qualitative analysis for punctual mutation screening
Upon completion of the run of the qPCR-HRM (approximately 2 hours), HRM curve analysis was performed using the LightCycler 480 Software version 1.5 supplied with the device. The melting curves were normalized and temperature shifted to allow the direct comparison of samples. Difference plots were generated by selecting a negative control, converting the melting profile to a horizontal line and normalizing the melting profiles of the other samples against the test sample. Significant differences in fluorescence from the horizontal baseline were indicative of mutations. Differences were judged as significant if the replicates fell outside the range of variation seen in the wild type samples.
Quantitative analysis for sequence rearrangement screening
Standard curves were generated for all exons investigated (Figure 1) and for two reference genes (exon 16 of the X-linked gene DMD, and exon 2 of B2M) to derive sample and calibrator (negative control) relative DNA amounts. The ratio (R) representing the relative copy number of the target exon in the patient was automatically calculated by the LightCycler 480 data analysis software (LC480; Roche) according to the formula:
The gene DMD was analyzed in each experiment as a control of correct gene dosage: the ratio DMD/B2M should be equal to two for female samples, and one for male samples.
SQF-PCR
Primer design
The method of SQF-PCR is based on the comparison of fluorescent profiles of multiplex PCR fragments obtained from different samples (patients and control)4. Primers labeled with 6FAM fluorochrome (Table 1) for exons 2–10 of MVK, exons 1–10 of TNFRSFA1, exons 1–9 of NLRP3, and 2 internal control genes (exon 28 of DMD and exon 3 of GFAP) were designed using the PrimerBlast software (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). We chose allele pairs with the same annealing temperature to perform multiplex PCR resulting in fragments of sizes differing by at least 20bp (186–361 bp). We checked that all exons were adequately co-amplified.
Assay conditions
The PCR reactions were performed using the QIAGEN Multiplex kit (Qiagen, Courtaboeuf, France) in a total volume of 20 ul containing 100 ng of genomic DNA and 5 to 20 pMol of each primer. The amplification was stopped at the exponential phase (22 cycles). The PCR cycling conditions used were those suggested in the manufacturer's instructions. The PCR products were then separated on an ABI3130xL DNA Sequencer (Applied Biosystems, Foster city, CA, USA).
Semi-quantitative analysis
Electrophoregrams were analyzed using the GeneMapper software v4.0 (Applied Biosystems, Foster City, CA, USA). In each experiment, the genes GFAP and DMD were analyzed to determine the relative gene dosage for MVK, NLRP3 and TNFRSFA1. To calculate the relative copy number (R) of each target region, each peak value including height and area from the patient samples was compared with those from the normal control after normalization with the reference gene GFAP. The data (area and height of peak) were exported to Microsoft Excel and inserted into the equation:
The DMD gene was used as a control of correct gene dosage: the ratio (R) DMD/B2M should be equal to two for female samples and one for male samples.
Results
We first implemented qPCR-HRM as a valid method for screening hereditary recurrent fever genes in our laboratory. Then in our patient cohort, we conducted a simultaneous search for punctual mutations and gene rearrangements using qPCR-HRM in the prospective group and qPCR alone in the retrospective group (as these patients had already been assessed for qualitative alterations), then SQF-PCR in both groups as a confirmatory quantitative approach (Figure 1).
Validation of qPCR-HRM
To implement a strategy that could be used on a routine basis, we set the method up to focus on mutation hot spots (MVK, exons 2–11; NLRP3, exon 3; TNFRSF1A, exons 2–4). The qPCR-HRM method could be performed for all regions except MVK exon 9 for which the Ct could not be obtained below 30 cycles. For this exon, we therefore conducted separate HRM and qPCR. The validation process included a number of DNA controls to assess the successful detection of a variety of gene alterations.
To validate the mutation analysis (HRM), we used 49 DNA samples carrying known mutations and 48 wild-type controls (Table 2). The sensitivity of the technique in our conditions (93.8%) was comparable to other mutation screening methods [9]. Indeed, all pathological mutations (homozygous or heterozygous) were detected, and only 3 polymorphisms (c.769-7_769-6dupT in MVK and p.A242A, p.Y141Y in NLRP3) went undetected (not shown).
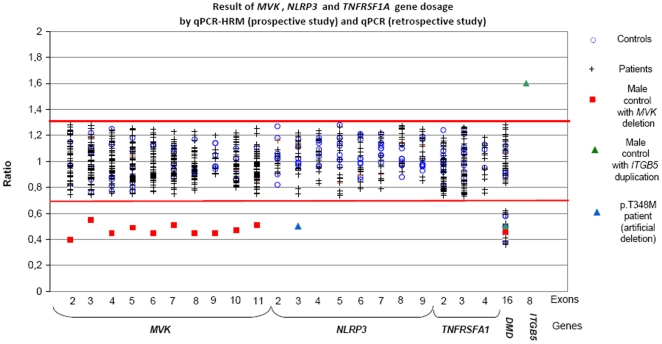
To validate the gene dosage (qPCR), we used DNA samples from 3 individuals (Table 2): 1) One patient with no recurrent fever in whom was found a large deletion (2,13Mb, Chr12:107813096..109948089, version: NCBI36/hg18) including the MVK gene in an unrelated study (micro-array screening for mental retardation); 2) One patient with a p.T348M (c.1043C>T) mutation in NLRP3 in whom we performed allele specific PCR using a wild-type primer to mimic a deletion; 3) One patient with 3 copies of the gene ITGB5 (154.9kb, Chr3:125946672..126101558, version NCBI36/hg18), since we had no available sample with a duplication in our genes of interest. As normal controls, 10 DNA samples from asymptomatic individuals were screened. Expected copy numbers were obtained (Figure 2).
Figure 2. Results of MVK, NLRP3 and TNFRSF1A gene dosage analysis by qPCR-HRM (prospective study) and qPCR (retrospective study).
Prospective study: qPCR-HRM has little relevance for routine genetic diagnosis of hereditary recurrent fevers
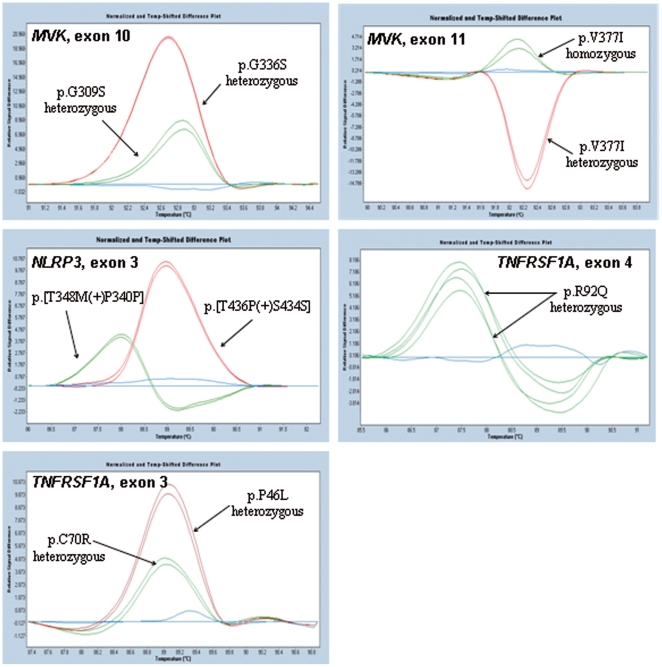
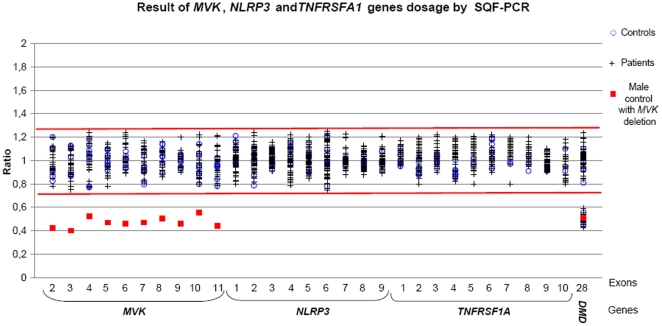
To assess the usefulness of this approach for routine genetic diagnosis, we applied it to our prospective study group consisting of 113 unselected patients referred for genetic diagnosis. All amplicons were sequenced in parallel. We confirmed diagnosis in 6 patients, all of whom bore already known mutations: three associated with MKD, p.[V377I]+[V377I], p.[V377I]+[G226S], and p.[T237S]+[T237S]; one with CAPS, p.T348M; and two with TRAPS, p.C70R and p.R92Q. Representative HRM difference plots are shown in Figure 3. The qualitative step (melting curves) detected all the mutations except the T237S homozygous genotype which was only detected after equimolar mixing with a wild type sample using the HRM method alone (not shown). The quantitative step detected no gene dosage abnormalities (Figure 2). A second method (SQF-PCR) was performed on a subset of these patients and confirmed the absence of copy number variants in this cohort (Figure 4).
Figure 3. Pathological genotypes detected by qPCR-HRM in the prospective study.
Qualitative changes were assessed using the gene scanning module of the LightCycler 480 Software version 1.5. Wild type sequences were used to define baselines. Difference plots revealed disease associated variants.
Figure 4. Results of MVK, NLRP3 and TNFRSF1A gene dosage analysis by SQF-PCR.
Retrospective study: sequence rearrangements are likely absent in hereditary recurrent fever genes
To confirm this absence of copy number alterations in our genes of interest and enhance the probability of finding new mutations, we extended our series to a panel of selected patients with recurrent fever (N = 88, Figure 1) with either one MVK punctual mutation for MKD, or urticaria and/or neurosensorial symptoms for CAPS. Indeed, FMF had previously been addressed3 and TRAPS does not show a clear cut phenotype. In this retrospective series, punctual mutations in all coding exons and intron boundaries had already been investigated using classical one-step routine analysis (HRM and/or sequencing). No genetic rearrangements were identified (Figure 2). We also performed a second SQF-PCR method in this retrospective panel which confirmed the absence of intragenic duplications or deletions (Figure 4).
Discussion
The existence of a significant number of patients with convincing symptoms though not carrying point mutations suggests that other genes and/or mutational mechanisms, such as gene dosage alteration, could be involved in the etiology of hereditary recurrent fever syndromes. The aim of this study was therefore to explore the possible existence of copy number variants in hereditary recurrent fever genes and estimate the usefulness of qPCR-HRM in routine diagnosis. Duplications and deletions of the genes involved in MKD, a recessive enzymopathy [10], and TRAPS, thought to be a protein misfolding disorder could theoretically lead to these two diseases [11]. CAPS is due to gain-of-function mutations in NLRP3, a major protein of the inflammasome platform that activates pro-caspase-1 into its active form caspase 1, which in turn promotes the processing of the potent pro-inflammatory cytokine interleukin-1β [12]. Classical gain-of-function rearrangements (e.g. duplications) have never been recorded in CAPS. However, a nonsense mutation (p.Arg554X) was identified in NLRP3 putatively resulting in a truncated protein lacking all leucine-rich repeats [13]. A partially deleted protein could therefore result in a gain-of-function.
To address the issue of possible pathological copy number variations in hereditary recurrent fevers, we took advantage of the fact that the HRM mutation screening technique is already routinely used in our laboratories, and implemented a recently described strategy that combines point mutation and gene rearrangement detection in a single assay (qPCR-HRM). This approach proved useful for the genetic analysis of MLH1 in Lynch syndrome [7]. The main advantage of qPCR-HRM is the gain in terms of cost and time. Another major benefit is the validation of amplification of the two alleles through the quantitative measure, which avoids the misinterpretation of primer mismatch of one allele as a deletion, and validates homozygous genotypes. We found the sensitivity of qPCR-HRM for qualitative analysis as compared to sequencing (93.8%) to be similar to that of classical qualitative methods such as DGGE or dHPLC [9]. The sensitivity of qPCR-HRM and SQF-PCR for quantitative analysis was 100% for the positive controls. The known limit of qPCR-HRM is the difficulty in designing primers efficient for both qualitative detection and quantitative dosage. Among the 21 amplicons we designed, only exon 9 of MVK could not be assessed by qPCR-HRM.
Having validated this new method being in our laboratories, we then used it to assess our patients in a prospective and retrospective study. We detected no gene dosage alteration in a total of 159 patients suspected of MKD, CAPS or TRAPS. A previous study in patients with FMF using MLPA also found no genetic rearrangements. A recent review demonstrated that copy number variants are more frequent in large genes [14]. The hereditary recurrent genes are in contrast rather small (MVK: 23434bp, NLRP3: 31054bp, TNFRSF1A: 13231bp). To investigate whether the chromosomal regions containing these genes could contain fragile sites, we consulted the Database of Genomics Variants (http://projects.tcag.ca/variation) and the Copy Number Variation project at the Children's Hospital of Philadelphia (htpp://cnv.chop.edu). We retrieved no copy variant within the regions of the MVK and TNFRSF1A genes. However, 3 variants have been reported in apparently healthy individuals in the 1q44 region of NLRP3, the largest of the 3 genes: 1) an insertion of 147bp in intron 3 of the NLRP3 gene (position 245657612 on chromosome 1q, version NCBI36/hg18) in one Caucasian individual [15]; 2) a deletion spanning intron 4 to the 5′extremity of exon 5 (chr1:245663794..245664071) in one Asian individual [16]; and 3) complex rearrangements upstream of the NLRP3 gene in 50 French individuals [17]. This may indicate the localization of NLRP3 in or close to a minor fragile site, although we identified no pathological rearrangements in our series.
In conclusion, our previous and present results support that deleterious intragenic rearrangements in MEFV, MVK, NLRP3 and TNFRSF1A are rare or absent from the mutational spectrum of hereditary recurrent fevers, suggesting that other mutations in seemingly affected patients should be searched for either in other gene regions (e.g. promoter and introns) or in other candidate genes. The use of the combined qPCR-HRM as a first line approach for genetic diagnosis is not relevant for these diseases since only qualitative alterations were identified. However, rare pathological gene dosage alterations could still be found in future series and the method we have developed could be used punctually as a second approach since it proved effective and quick.
Acknowledgments
We thank the micro-array platform team for the positive samples with a deletion in Chr12q24.1 and duplication in Chr3q21.2. We are grateful to Dr S. Grandemange for her helpful reviewing of the manuscript, and to Angloscribe for editing the English language.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the French Ministry of Health, the University Hospital of Montpellier, and the Coordination Theme 1 (Health) of the European Community's FP7, Grant Agreement Number HEALTH-F2-2008-200923. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Simon A, van der Meer JW. Pathogenesis of familial periodic fever syndromes or hereditary autoinflammatory syndromes. Am J Physiol Regul Integr Comp Physiol. 2007;292:R86–98. doi: 10.1152/ajpregu.00504.2006. [DOI] [PubMed] [Google Scholar]
- 2.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Gijn ME, Soler S, de la Chapelle C, Mulder M, Ritorre C, et al. Search for copy number alterations in the MEFV gene using multiplex ligation probe amplification, experience from three diagnostic centres. Eur J Hum Genet. 2008;16:1404–1406. doi: 10.1038/ejhg.2008.135. [DOI] [PubMed] [Google Scholar]
- 4.Giansily-Blaizot M, Thorel D, Khau Van Kien P, Behar C, Romey MC, et al. Characterisation of a large complex intragenic re-arrangement in the FVII gene (F7) avoiding misdiagnosis in inherited factor VII deficiency. Br J Haematol. 2007;138:359–365. doi: 10.1111/j.1365-2141.2007.06660.x. [DOI] [PubMed] [Google Scholar]
- 5.Sellner LN, Taylor GR. MLPA and MAPH: new techniques for detection of gene deletions. Hum Mutat. 2004;23:413–419. doi: 10.1002/humu.20035. [DOI] [PubMed] [Google Scholar]
- 6.Laccone F, Junemann I, Whatley S, Morgan R, Butler R, et al. Large deletions of the MECP2 gene detected by gene dosage analysis in patients with Rett syndrome. Hum Mutat. 2004;23:234–244. doi: 10.1002/humu.20004. [DOI] [PubMed] [Google Scholar]
- 7.Rouleau E, Lefol C, Bourdon V, Coulet F, Noguchi T, et al. Quantitative PCR high-resolution melting (qPCR-HRM) curve analysis, a new approach to simultaneously screen point mutations and large rearrangements: application to MLH1 germline mutations in Lynch syndrome. Hum Mutat. 2009;30:867–875. doi: 10.1002/humu.20947. [DOI] [PubMed] [Google Scholar]
- 8.Steger G. Thermal denaturation of double-stranded nucleic acids: prediction of temperatures critical for gradient gel electrophoresis and polymerase chain reaction. Nucleic Acids Res. 1994;22:2760–2768. doi: 10.1093/nar/22.14.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou LS, Lyon E, Wittwer CT. A comparison of high-resolution melting analysis with denaturing high-performance liquid chromatography for mutation scanning: cystic fibrosis transmembrane conductance regulator gene as a model. Am J Clin Pathol. 2005;124:330–338. doi: 10.1309/BF3M-LJN8-J527-MWQY. [DOI] [PubMed] [Google Scholar]
- 10.Cuisset L, Drenth JP, Simon A, Vincent MF, van der Velde Visser S, et al. Molecular analysis of MVK mutations and enzymatic activity in hyper-IgD and periodic fever syndrome. Eur J Hum Genet. 2001;9:260–266. doi: 10.1038/sj.ejhg.5200614. [DOI] [PubMed] [Google Scholar]
- 11.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeru I, Hayrapetyan H, Duquesnoy P, Sarkisian T, Amselem S. PYPAF1 nonsense mutation in a patient with an unusual autoinflammatory syndrome: role of PYPAF1 in inflammation. Arthritis Rheum. 2006;54:508–514. doi: 10.1002/art.21618. [DOI] [PubMed] [Google Scholar]
- 14.Kalari KR, Casavant TL, Scheetz TE. A knowledge-based approach to predict intragenic deletions or duplications. Bioinformatics. 2008;24:1975–1979. doi: 10.1093/bioinformatics/btn370. [DOI] [PubMed] [Google Scholar]
- 15.Levy S, Sutton G, Ng PC, Feuk L, Halpern AL, et al. The diploid genome sequence of an individual human. PLoS Biol. 2007;5:e254. doi: 10.1371/journal.pbio.0050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Wang W, Li R, Li Y, Tian G, et al. The diploid genome sequence of an Asian individual. Nature. 2008;456:60–65. doi: 10.1038/nature07484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Smith AJ, Tsalenko A, Sampas N, Scheffer A, Yamada NA, et al. Array CGH analysis of copy number variation identifies 1284 new genes variant in healthy white males: implications for association studies of complex diseases. Hum Mol Genet. 2007;16:2783–2794. doi: 10.1093/hmg/ddm208. [DOI] [PubMed] [Google Scholar]