Abstract
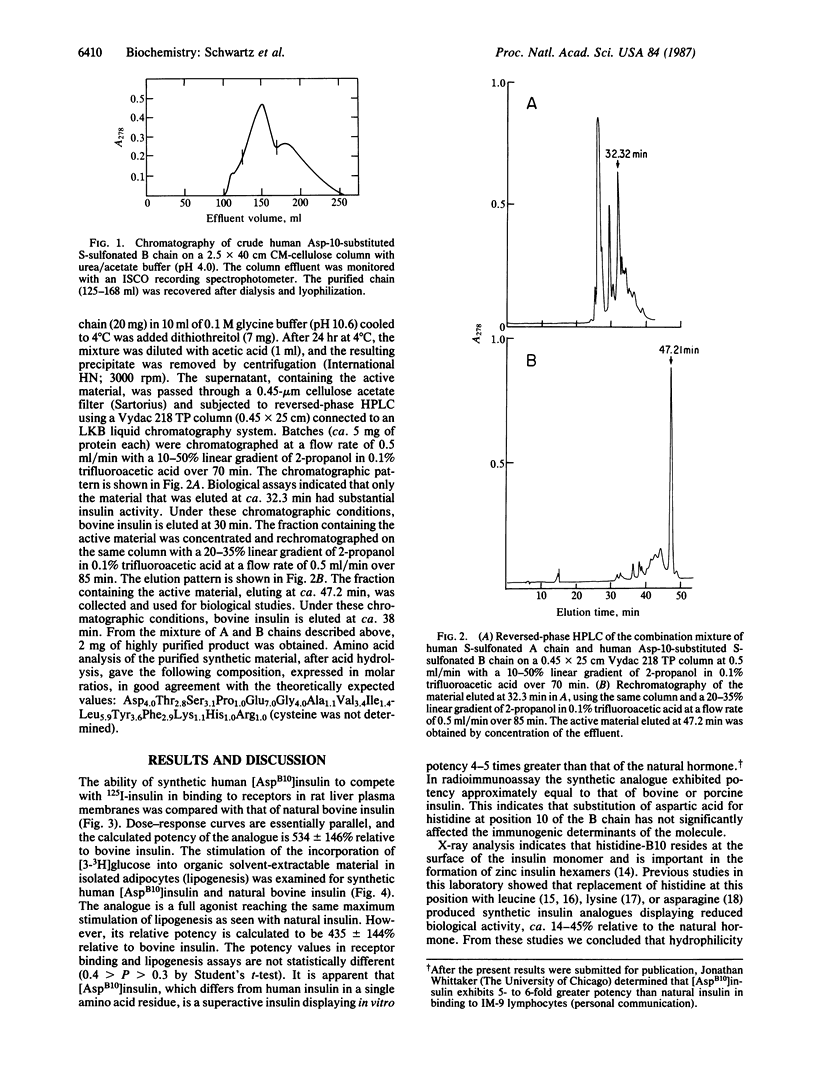
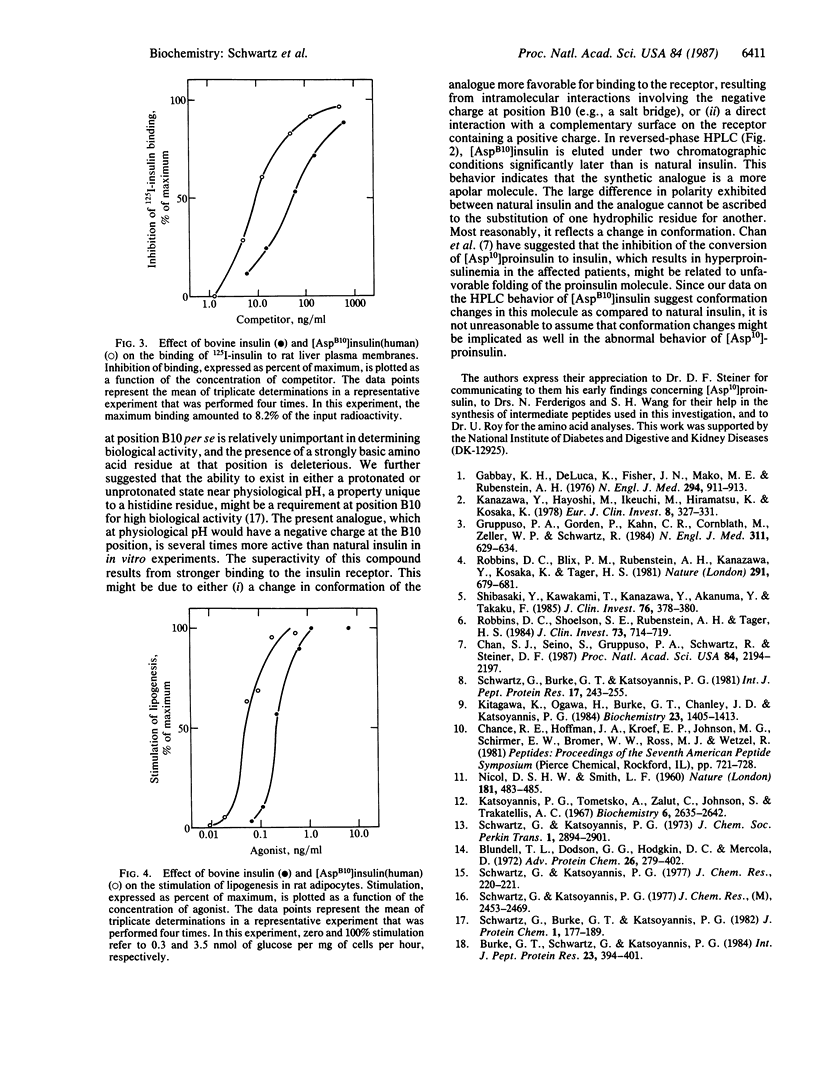
The genetic basis for a case of familial hyperproinsulinemia has been elucidated recently. It involves a single point mutation in the proinsulin gene resulting in the substitution of aspartic acid for histidine-10 of the B chain of insulin. We have synthesized a human insulin analogue, [AspB10]insulin, corresponding to the mutant proinsulin and evaluated its biological activity. [AspB10]Insulin displayed a binding affinity to insulin receptors in rat liver plasma membranes that was 534 +/- 146% relative to the natural hormone. In lipogenesis assays, the synthetic analogue exhibited a potency that was 435 +/- 144% relative to insulin, which is statistically not different from its binding affinity. Reversed-phase HPLC indicated that the synthetic analogue is more apolar than natural insulin. We suggest that the observed properties reflect changes in the conformation of the analogue relative to natural insulin, which result in a stronger interaction with the insulin receptor. Thus, a single substitution of an amino acid residue of human insulin has resulted in a superactive hormone.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke G. T., Schwartz G., Katsoyannis P. G. Nature of the B10 amino acid residue. Requirements for high biological activity of insulin. Int J Pept Protein Res. 1984 Apr;23(4):394–401. doi: 10.1111/j.1399-3011.1984.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Seino S., Gruppuso P. A., Schwartz R., Steiner D. F. A mutation in the B chain coding region is associated with impaired proinsulin conversion in a family with hyperproinsulinemia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2194–2197. doi: 10.1073/pnas.84.8.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay K. H., DeLuca K., Fisher J. N., Jr, Mako M. E., Rubenstein A. H. Familial hyperproinsulinemia. An autosomal dominant defect. N Engl J Med. 1976 Apr 22;294(17):911–915. doi: 10.1056/NEJM197604222941701. [DOI] [PubMed] [Google Scholar]
- Gruppuso P. A., Gorden P., Kahn C. R., Cornblath M., Zeller W. P., Schwartz R. Familial hyperproinsulinemia due to a proposed defect in conversion of proinsulin to insulin. N Engl J Med. 1984 Sep 6;311(10):629–634. doi: 10.1056/NEJM198409063111003. [DOI] [PubMed] [Google Scholar]
- Katsoyannis P. G., Tometsko A., Zalut C., Johnson S., Trakatellis A. C. Studies on the synthesis of insulin from natural and synthetic A and B chains. I. Splitting of insulin and isolation of the S-sulfonated derivatives of the A and B chains. Biochemistry. 1967 Sep;6(9):2635–2642. doi: 10.1021/bi00861a001. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Ogawa H., Burke G. T., Chanley J. D., Katsoyannis P. G. Critical role of the A2 amino acid residue in the biological activity of insulin: [2-glycine-A]- and [2-alanine-A]insulins. Biochemistry. 1984 Mar 27;23(7):1405–1413. doi: 10.1021/bi00302a011. [DOI] [PubMed] [Google Scholar]
- NICOL D. S., SMITH L. F. Amino-acid sequence of human insulin. Nature. 1960 Aug 6;187:483–485. doi: 10.1038/187483a0. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Blix P. M., Rubenstein A. H., Kanazawa Y., Kosaka K., Tager H. S. A human proinsulin variant at arginine 65. Nature. 1981 Jun 25;291(5817):679–681. doi: 10.1038/291679a0. [DOI] [PubMed] [Google Scholar]
- Robbins D. C., Shoelson S. E., Rubenstein A. H., Tager H. S. Familial hyperproinsulinemia. Two cohorts secreting indistinguishable type II intermediates of proinsulin conversion. J Clin Invest. 1984 Mar;73(3):714–719. doi: 10.1172/JCI111264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. P., Burke G. T., Katsoyannis P. G. [12-asparagine-B] human insulin. An analogue with modification in the hydrophobic core of insulin. Int J Pept Protein Res. 1981 Feb;17(2):243–255. doi: 10.1111/j.1399-3011.1981.tb01989.x. [DOI] [PubMed] [Google Scholar]
- Shibasaki Y., Kawakami T., Kanazawa Y., Akanuma Y., Takaku F. Posttranslational cleavage of proinsulin is blocked by a point mutation in familial hyperproinsulinemia. J Clin Invest. 1985 Jul;76(1):378–380. doi: 10.1172/JCI111973. [DOI] [PMC free article] [PubMed] [Google Scholar]



